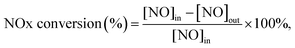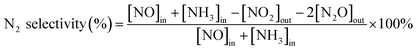Open Access Article
Open Access ArticleMesoporous MnOx–CeO2 composites for NH3-SCR: the effect of preparation methods and a third dopant†
Li Weiman abcd,
Liu Haidia and
Chen Yunfa
abcd,
Liu Haidia and
Chen Yunfa *ad
*ad
aState Key Laboratory of Multi-phase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: chenyf@ipe.ac.cn
bUniversity of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, China
cZhongke Langfang Institute of Process Engineering, Langfang Economic & Technical Development Zone, Fenghua Road No. 1, Hebei Province, China
dCAS Center for Excellence in Urban Atmospheric Environment, Xiamen 361021, China
First published on 16th April 2019
Abstract
In this study, different preparation methods including an oxalate route, a nano-casting strategy and a traditional co-precipitation route were applied to obtain MnOx–CeO2 mixed oxides for selective catalytic reduction (SCR) of NO with NH3. The catalyst prepared from the oxalate route showed improved performance for NOx conversion and SO2 + H2O durability. To further improve the SO2 and H2O resistance of catalysts, ternary oxides were prepared from the oxalate route. The catalysts were studied by X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET) surface area analysis, X-ray photoelectron spectroscopy (XPS), H2 temperature-programmed reduction (H2-TPR), NH3 temperature-programmed desorption (NH3-TPD), SO2 temperature-programmed desorption (SO2-TPD), and in situ diffuse reflectance infrared fourier transform spectroscopy (in situ DRIFTS). The nickel–manganese–cerium ternary oxide showed the best SO2 and H2O durability. The reason can be ascribed to its smaller pores, amorphous structure, and moderate amount of surface Mn3+/oxygen species, which could decrease chemical adsorption of SO2.
1 Introduction
Nitrogen oxides (NOx), emitted from combustion of fossil fuels in industrial stationary sources, have caused a series of environmental problems such as acid rain, haze, and ozone depletion. Selective catalytic reduction of NOx by ammonia (NH3-SCR) is one of the most promising NOx abatement technologies. The widely used catalyst in NH3-SCR is V2O5–WO3(MoO3)/TiO2, whose optimum working temperature is higher than 350 °C. In practical application, the SCR reactor is usually located downstream of dust and SO2 removing modules to avoid catalyst deactivation from dust, SO2 and other poisoners. As a result, the temperature of flue gas is usually lower than 300 °C, and the flue gas has to be reheated to meet the V–W–Ti working temperature, which will increase the cost of deNOx. Therefore, a better choice is to develop catalysts with high deNOx efficiency, high resistance to water vapor/SO2, and a low working temperature window.Ce–Mn composites have been reported widely for their excellent low temperature deNOx efficiency and high N2 selectivity. The catalytic ability of Ce–Mn composites is related to the pore structure, specific area, and redox behavior.1–3 For example, MnOx–CeO2 nanosphere catalyst showed superior performance compared to its counterpart MnOx–CeO2 without any defined structural morphology.4 MnOx–CeO2 hollow nanotube synthesized through the interfacial oxidation–reduction reaction showed 96% NOx conversion at 100 °C, and the authors attributed to the uniform distribution of active species and the hollow porous architectures which provided huge specific surface area and sufficient acidic sites.5 However, the activity of MnOx–CeO2 composites is still low in the presence of SO2 and H2O. Lots of efforts have been done to improve SO2 and H2O resistance of MnOx–CeO2 composites. For example, adding a third dopant (Sn, Cr, W, Eu etc.) into MnOx–CeO2 composites can further improve the deNOx efficiency and SO2 resistance.6–9 Cobalt or nickel doped MnOx–CeO2 catalysts showed high SCR activity and good tolerance of SO2, and the authors suggested different SCR reaction pathways and mechanisms of SO2 tolerance between Co/Ni doped and un-doped MnOx–CeO2.10 Mesoporous catalysts have been proved a good choice to improve SO2 resistance.11 Zha prepared a MnCeW catalyst on mesoporous TiO2 spheres, and the catalyst showed excellent SCR activity in a wide temperature range.9 Fe2O3 promoted halloysite-supported CeO2–WO3 catalysts showed improved NOx reduction in the presence of SO2, and the authors confirmed that the increase of Brønsted acid site derived from Fe2O3 promotion is the main cause.12
Based on previous studies, the catalytic performance of NH3-SCR activity, including NOx conversion efficiency, N2 selectivity and H2O + SO2 resistance are mainly determined by following factors: pore structure and surface area, the distribution of manganese and cerium states, and the active oxygen species including adsorbed oxygen, oxygen vacancy and lattice oxygen, as well as surface acidity. Inspired by the above studies, we have fabricated MnOx–CeO2 with different pore structures by various methods, and the relationship of NH3-SCR ability and structure was discussed. To further improve the H2O + SO2 resistance of the catalysts, nickel/cobalt was introduced to the MnOx–CeO2 catalysts, and the catalytic performance was evaluated.
2 Experimental section
2.1 Catalysts preparation
All chemical reagents used in this work including Mn(ac)2·4H2O (99.0%), Ce(ac)3·0.5H2O (99.0%), Co(ac)2·4H2O (99.0%), Ni(ac)2·4H2O (99.0%), C2H2O4·2H2O (99.5%), Mn(NO3)2 (50% wt), Ce(NO3)3·6H2O, Co(NO3)2·6H2O, Ni(NO3)2·6H2O and NaOH were purchased from Xilong Cop. (China) and used without further purification. To prepare MnOx–CeO2 composites with different pore structures, an oxalate route, a nano-casting strategy and a simple precipitation method were applied.In an oxalate route, calculated metal salts (the molar ratio of Mn and Ce was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) were dissolved in deionized water at room temperature, labeled as solution A. Then, C2H2O4·2H2O (the molar ratio of C2H2O4·2H2O to total metals is 1.2) was dissolved in ethanol, labeled as solution B. The mixtures of A and B were stirred for 24 h. The precipitates were washed, dried, and finally calcined at 550 °C for 4 h. The sample was labeled as 6Mn4Ce-O. Nickel or cobalt doped MnOx–CeO2 composite was prepared as the same procedure. The samples were denoted as 1Ni6Mn3Ce-O and 1Co6Mn3Ce-O, respectively.
4) were dissolved in deionized water at room temperature, labeled as solution A. Then, C2H2O4·2H2O (the molar ratio of C2H2O4·2H2O to total metals is 1.2) was dissolved in ethanol, labeled as solution B. The mixtures of A and B were stirred for 24 h. The precipitates were washed, dried, and finally calcined at 550 °C for 4 h. The sample was labeled as 6Mn4Ce-O. Nickel or cobalt doped MnOx–CeO2 composite was prepared as the same procedure. The samples were denoted as 1Ni6Mn3Ce-O and 1Co6Mn3Ce-O, respectively.
In a nano-casting route, KIT-6 silica was employed as a hard template, and the MnOx–CeO2 catalyst was synthesized by a simple “two-solvent” approach. KIT-6 silica was prepared as previously reported studies.13 In a typical procedure, 1.0 g KIT-6 was suspended in 80 ml n-hexane and stirred at room temperature for 2 h. Then a mixed solution of Mn(NO3)2 and Ce(NO3)3 was added slowly with vigorous stirring. After stirred overnight, the mixture was filtered and dried at 80 °C for 24 h. The obtained powder was calcined at 550 °C for 4 h, with a heating rate of 2 °C min−1 in air. Finally, the sample was treated three times with a 2 M NaOH solution, washed to pH ∼ 7 and dried at 80 °C. The product was denoted as 6Mn4Ce-N.
As a comparison, the third MnOx–CeO2 sample was synthesized by a co-precipitation method using NH3 H2O as precipitant, and the sample was labeled as 6Mn4Ce-C.
2.2 Catalytic activity tests
Before SCR activity test, the catalysts were crushed and sieved to 40–60 mesh. A fixed-bed quartz flow reactor was used to perform SCR activity test. The typical reactant gas contained 200 ppm NOx, 200 ppm NH3, 5%O2, 100 ppm SO2 (when used), 5 vol% H2O (when used) and balance of N2. In a typical experiment, 100 mg catalyst was used, corresponding to gas volume hourly space velocity (GHSV) of 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The temperature range was set to 100–300 °C. An FTIR spectrometer (Bruker Vertex 70 spectrometer, USA) equipped with a heated, multiple path gas cell (10 m) was used to collect the FTIR spectra. The NOx conversion and N2 selectivity were calculated as follows:
000 h−1. The temperature range was set to 100–300 °C. An FTIR spectrometer (Bruker Vertex 70 spectrometer, USA) equipped with a heated, multiple path gas cell (10 m) was used to collect the FTIR spectra. The NOx conversion and N2 selectivity were calculated as follows:2.3 Characterization
XRD patterns were recorded on a PANalytical X'Per PRO X-ray diffraction using Ni filtered Cu Kα (λ = 0.15418 nm) radiation at 40 kV and 30 mA, in 2θ from 5° to 90° with a scanning step of 0.0334°. The specific surface area and pore size distributions of all catalysts were obtained according to the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively, using N2 adsorption–desorption method on an automatic surface analyzer (SSA-7300, BJ-Builder, China) at 77 K. Each sample was pre-degassed at 150 °C for 3 h. Surface species of as-prepared catalysts were determined by X-ray photoelectron spectroscopy (XPS) using a XLESCALAB 250 Xi electron spectrometer (Thermo Scientific, USA) with monochromatic Al Kα radiation (1486.6 eV).H2 temperature-programmed reduction (H2-TPR) was conducted on a Micromeritics Chemisorb 2720 analyzer (Micromeritics, USA) at a heating rate of 10 °C min−1 with 5% H2/Ar gas. The H2 consumption was recorded continuously to investigate reduction abilities. NH3 temperature-programmed desorption (NH3-TPD) was performed on a Micromeritics Autochem II 2920 analyzer (Micromeritics, USA). The catalysts were firstly heated to 300 °C for 1 h in helium, and then cooled down to 50 °C. After saturated in pure NH3 for 30 min, the samples were purged in pure helium for 1 h to remove the physically adsorbed NH3. The TPD curve was finally recorded in helium from 50 °C to 600 °C at a heating rate of 10 °C min−1. SO2 temperature-programmed desorption (SO2-TPD) was performed on a TP5080B apparatus (Tianjin XQ, China). 50 mg of 40–60 mesh catalysts was firstly heated to 300 °C for 1 h in 30 ml min−1 helium, and then cooled to 200 °C. The catalysts was saturated in 2000 ppm SO2 for 1 h, and then purged with 30 ml min−1 helium for 1 h to remove residue SO2 at 200 °C. At last, the catalyst was heated from 200 °C to 850 °C in helium at 10 °C min−1.
A Bruker Vertex 70 spectrometer (Bruker, USA) equipped with diffuse reflectance accessory (PIKE, and MCT/A detector cooled by liquid nitrogen) was used for recording the in situ DRIFT spectra of the samples. Twenty mg KBr powder was placed under proper amount of samples, and a specially made steel stick was used to smash the sample to a flat surface. The sample was pretreated at 300 °C for 2 h in N2 (50 ml min−1). The reaction system was cooled to 200 °C in N2, and the spectra were collected as background. The spectra were recorded by accumulating 32 scans at a resolution of 4 cm−1.
3 Results and discussion
3.1 NH3-SCR performance
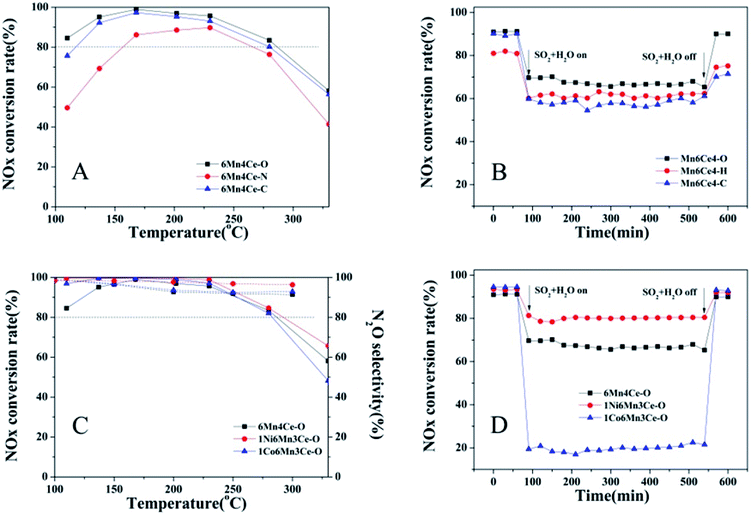
All composites prepared by different methods were tested for NH3-SCR in temperature range of 100–350 °C, as shown in Fig. 1A. The NOx conversion of all catalysts first increased and then decreased. The NOx conversion rates at the whole temperature range of the three catalysts followed the sequence of 6Mn4Ce-O > 6Mn4Ce-C > 6Mn4Ce-N. The NOx conversion of 6Mn4Ce-O reached its maximum of 99% at ∼170 °C; and it's NO conversion rate remained >80% at the temperature range of 120–275 °C. The NOx conversion of 6Mn4Ce-C was slightly lower than that of 6Mn4Ce-O. The catalyst prepared from nanocasting route showed the worst catalytic performance. Resistance to H2O and SO2 is an important factor that to be concerned in the evaluation of NH3-SCR catalysts, as there is more or less SO2 and H2O in gas that to be managed. The H2O + SO2 resistance of three samples is shown in Fig. 1B. After H2O + SO2 was introduced into flue gas, the NOx conversion rate of 6Mn4Ce-O, 6Mn4Ce-N and 6Mn4Ce-C deceased to ∼70%, ∼62.5% and ∼59%, respectively. This indicated that different structure of catalysts had a profound effect on resistance of H2O and SO2.Addition of transition metal oxides (CrOx,14 NiO,10,15–17 Co3O4,10 SnO2,18,19 et al.) into MnOx or CeO2 were shown to improve the SCR activity in presence of SO2 and H2O, by increasing the surface acidity concentration of Lewis or Brønsted acid sites.16,18,20 Based on above results, the H2O and SO2 resistance of sample prepared from oxalate route was better than other two samples. Therefore, we prepared Co/Ni doped MnOx–CeO2 composites by oxalate route to further improve their resistance to H2O and SO2. As shown in Fig. 1C, addition of nickel or cobalt can both increase the NOx conversion rate at temperature range of 100–280 °C. For example, the NOx conversion of 1Ni6Mn3Ce-O and 1Co6Mn3Ce-O were 99% at 200 °C, slightly higher than that of 6Mn4Ce-O (∼96% at 200 °C). Besides, the N2 selectivity of 6Mn4Ce-O was lower than those of 1Ni6Mn3Ce-O and 1Co6Mn3Ce-O (Fig. 1C). The above results showed that addition of nickel or cobalt slightly improves the SCR activity and N2 selectivity. The H2O and SO2 resistance of transition metal oxide doped composites were shown in Fig. 1D. With co-existence of H2O and SO2, NOx conversion decreased sharply to 70%, 80% and 20% over 6Mn4Ce-O, 1Ni6Mn3Ce-O and 1Co6Mn3Ce-O, respectively. When H2O and SO2 were cutoff, the deNOx activity of 6Mn4Ce-O, 1Ni6Mn3Ce-O and 1Co6Mn3Ce-O resumed. In previous studies, MnOx–CeO2 composite exhibited low SO2 tolerance because of sulfate species formation. Therefore, many efforts have been done to improve the SO2 resistance of MnOx–CeO2 catalysts. For example, novel MnOx–CeO2 nano-sphere catalyst showed improved SO2 tolerance than MnOx–CeO2 catalyst without defined structural morphology.4 CeO2–MnOx showed relatively good resistance to SO2 because of its core–shell structure.21 In our study, both preparation methods and addition of a third dopant were help to improve the resistance to SO2 and H2O. This phenomenon illustrated structure, as well as different interaction among third dopants and Mn–Ce composites may play important roles in NH3-SCR, which will further be discussed in following parts.
3.2 Structural properties
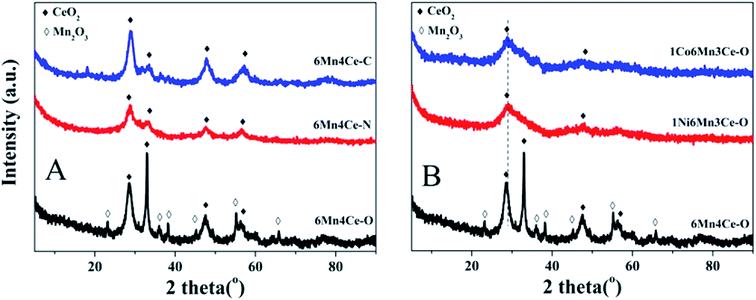 | ||
| Fig. 2 XRD patterns of as-prepared samples, (A) samples from different methods, and (B) nickel or cobalt doped samples from oxalate route. | ||
The diffraction peaks of 1Ni6Mn3Ce-O and 1Co6Mn3Ce-O exhibited broad reflections of CeO2, no obvious peaks related to MnOx, NiO or cobalt oxides. Similar results22 were reported in the case of Ni(0.4)–MnOx spinel. It is commonly accepted than amorphous structure is easy to embed and intercalate protons rapidly,23 therefore promoting adsorption/desorption and redox reaction on the surface of catalysts.23,24 In our study, although the deNOx efficiency and N2 selectivity of catalysts were similar, the SO2 + H2O resistance varied a lot. It can be concluded that amorphous structure caused by addition of a third dopant may be responsible for the good performance of catalysts.
The N2-adsorption–desorption isotherms and corresponding Barrett–Joyner–Halenda (BJH) pore size distribution curves for catalysts were shown in Fig. 3 (inset). The isotherms of all catalysts showed a characteristic type IV pattern, with a hysteresis loop of type H2 in the IUPAC classification, indicating the existence of meso-pores and micro-pores. At low P/P0, the isotherm curve of 6Mn4Ce-O had a relatively lower slope than those of other catalysts, indicating its higher degree of crystallinity, consistent with XRD results. As shown in Fig. 3 (A, inset) and Table 1, the specific surface area of 6Mn4Ce-N (152.116 m2 g−1) was higher than 6Mn4Ce-O (116.678 m2 g−1) and 6Mn4Ce-C (77.269 m2 g−1); while the pore diameter followed the sequence of 6Mn4Ce-N < 6Mn4Ce-O < 6Mn4Ce–C. This result showed that the specific surface area is not positively correlated with the NOx conversion, similar to the report from Qi.25 Mesoporous materials present lots of edges and corners for adsorption of reactants; and the pores facilitate mass transfer at the gas–solid phase boundary.26 Large surface area contributed more active sites, which would facilitate the adsorption of reactants. However, the SO2 and H2O in flue gas may also occupy the active sites of catalysts, leading to a drop of SCR conversion. The smaller pores in catalysts from oxalate routes may possibly inhibit the reaction of NOx, NH3 with SO2 and H2O. Although 6Mn4Ce-O has a higher degree of crystallinity, which may not facilitate the synergistic effect between elements, the smaller pores in it can balance its structure.
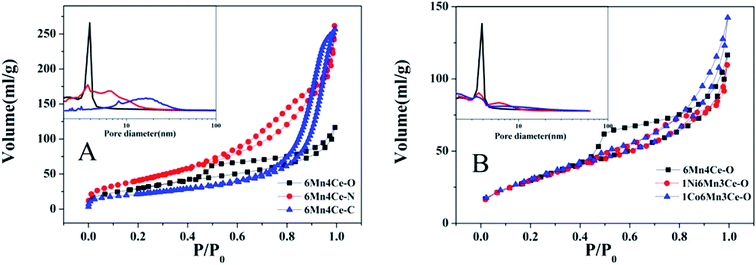 | ||
| Fig. 3 N2 adsorption/desorption isotherms and pore size distribution (inset) for samples (A) samples from different methods, and (B) nickel or cobalt doped samples from oxalate route. | ||
| BET (m2 g−1) | Pore volume (ml g−1) | Average pore diameter (nm) | |
|---|---|---|---|
| 6Mn4Ce-O | 116.678 | 0.1686 | 6.5 |
| 6Mn4Ce-N | 152.116 | 0.3955 | 10.7 |
| 6Mn4Ce-C | 77.269 | 0.4036 | 20.6 |
| 1Ni5Mn4Ce-O | 113.258 | 0.1428 | 6.0 |
| 1Co5Mn4Ce-O | 116.926 | 0.1937 | 7.5 |
For catalysts from a same oxalate route, a third dopant did not change the specific surface area significantly as shown in Table 1. However, the pore diameter of 6Mn4Ce-O and 1Co6Mn3Ce-O were among 1.5–2.5 nm. While for 1Ni6Mn3Ce-O, the pore size distribution was among 1.5–2 nm and 2.5–10 nm, similar to previous study.27 This indicated addition of nickel into MnOx–CeO2 could change its pore structure, leading to a combination of macropores and mesopores. According to literature,28 the release of gaseous H2O and COx will create pores during the decomposition of oxalate chains chelated in corresponding precursors. In our study, nickel may regulate gas release during the thermo-decomposition precursors.
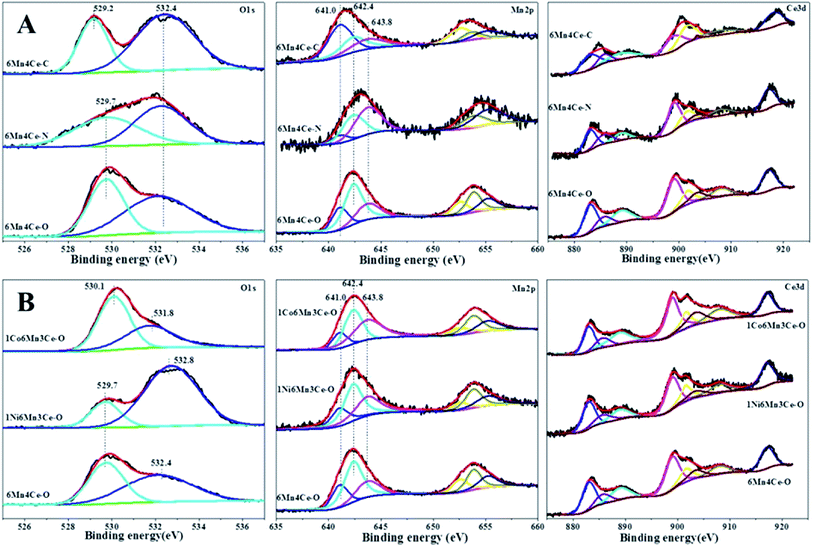 | ||
| Fig. 4 O1s, Mn2p and Ce3d spectra of (A) samples from different methods, and (B) nickel or cobalt doped samples from oxalate route. | ||
The Mn2p spectra were deconvoluted into three peaks located at 641.0, 642.4 and 643.8 eV, which can be assigned to Mn2+, Mn3+ and Mn4+, respectively.30,31 The percentage of Mn species with different valences was calculated, and the results were shown in Table 2. Although a higher oxidation state of manganese species was better for redox properties,32 moderate ratio of Mn3+/Mn4+ was help to improve SO2 + H2O resistance. In our study, the surface Mn3+ species was related to SO2 + H2O resistance. As shown in Table 2, the sequence of Mn3+ species was 6Mn4Ce-O > 6Mn4Ce-N > 6Mn4Ce-C, consistent with catalytic sequence in Fig. 1B. For samples from oxalate route, the sequence of Mn3+ species was 1Ni6Mn4Ce-O > 6Mn4Ce-O > 1Co6Mn4Ce-O, indicating a third dopant can regulate the surface manganese species. Based on the above various characterization studies, it was observed that different preparation method or a third dopant can regulate interaction among Mn, Ce, Ni/Co oxides, which would bring about an appropriate content of active Mn3+ and oxygen species on the surface of catalyst.
| Oads/Olatt | Mn2+ (%) | Mn3+ (%) | Mn4+ (%) | B/L | |
|---|---|---|---|---|---|
| 6Mn4Ce-O | 1.96 | 21.89 | 55.34 | 22.77 | 0.47 |
| 6Mn4Ce-N | 1.26 | 9.17 | 39.08 | 51.75 | 0.37 |
| 6Mn4Ce-C | 1.09 | 47.31 | 30.17 | 22.52 | 0.29 |
| 1Ni5Mn4Ce-O | 3.91 | 20.64 | 46.73 | 32.63 | 0.95 |
| 1Co5Mn4Ce-O | 0.71 | 14.56 | 39.98 | 45.46 | 0.39 |
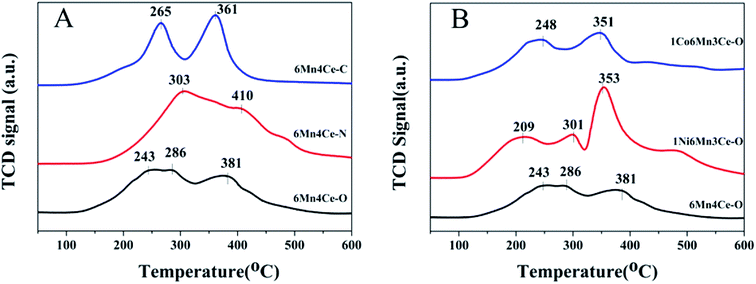 | ||
| Fig. 5 H2-TPR spectra of (A) samples from different methods, and (B) nickel or cobalt doped samples from oxalate route. | ||
NH3-TPD was employed to investigate NH3 adsorption, therefore to examine the acidic sites on different catalysts, as presented in Fig. 6. There were two main peaks on all spectra. The peak at the mid-temperature range (100–400 °C) is related to the desorption of NH3 coordinated to Lewis acid sites, and the peak at high-temperature range (400–550 °C) is considered to the desorption of NH3 coordinated to Brønsted acid sites.15,34 Compared with catalysts prepared from nano-casting and precipitation method, the peak related to Lewis acid sites of 6Mn4Ce-O moved toward lower temperature range, indicating the existence of weak Lewis adsorption sites. As described above, samples from oxalate route may have more acidic sites during the decomposition of precursors. The ratios of NH3 adsorption on Brønsted acid site to Lewis acid sites of different catalysts are shown in Table 2. Oxalate route was easier to produce more Brønsted acid sites than other two methods. And addition of nickel can greatly increase the ratio of Brønsted acid/Lewis acid sites. Based on the above various characterization studies, it was observed that different preparation method or a third dopant could regulate interaction among Mn, Ce, Ni/Co oxides, which would bring about an appropriate content of active Mn3+ and oxygen species on the surface of catalyst.
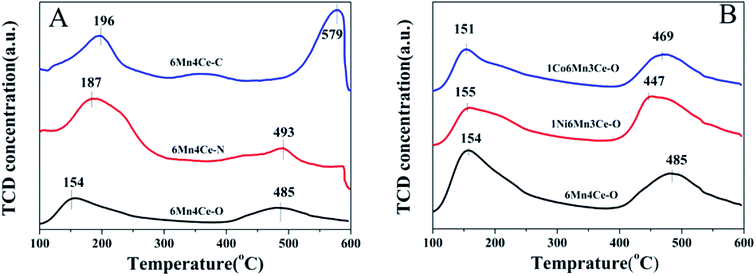 | ||
| Fig. 6 NH3-TPD spectra of (A) samples from different methods, and (B) nickel or cobalt doped samples from oxalate route. | ||
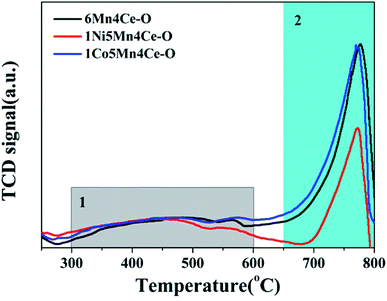
As samples from the oxalate route had different resistance to SO2 and H2O, SO2-TPD tests were performed on these three samples, and the results were shown in Fig. 7. There were two main desorption peaks in the temperature range of 300–600 °C, and 650–800 °C, labeled as 1 and 2, respectively. The small and broad peak in area 1 was reported to be assigned to desorption of SO2 adsorbed on the surface of catalysts.35 In this paper, the area labeled 1 of 1Ni5Mn4Ce was slightly smaller than the other two catalysts, which indicated less SO2 adsorption on it. The main peak at 650–580 °C was associated to desorption of SO2 released from the decomposition of metal sulfates. The area 2 of 1Ni5Mn4Ce was much smaller compared with others, which indicated nickel adding can inhabit formation of metal sulfates. Similar conclusion could be achieved from the TG-DTA test of samples after SO2 + H2O resistance experiments, as shown in ESI.†
3.3 In situ DRIFTs
To better understand gas adsorption on different catalysts from oxalate route, in situ DRIFTs was conducted, and the results were shown in Fig. 8. Fig. 8A showed in situ DRIFTS spectra of NH3 + O2 adsorbed on three samples. The bands at 1235, 1565, 1610 cm−1 were ascribed to NH3 coordinated on Lewis acid sites.36 The band at 1457 cm−1 was due to the symmetric bending vibration of NH4+ chemosorbed on the Brønsted acid sites.18 Meanwhile, a band at 3230 cm−1 was due to the N–H stretching vibrations of the NH4+.37 Comparing with 1Co6Mn3Ce-O, 6Mn4Ce-O and 1Ni6Mn3Ce-O revealed more Brønsted acid sites, consistent with NH3-TPD results. Wu obtained the same result that addition of nickel could significantly improve the Brønsted acid sites on FeVO4/TiO2.38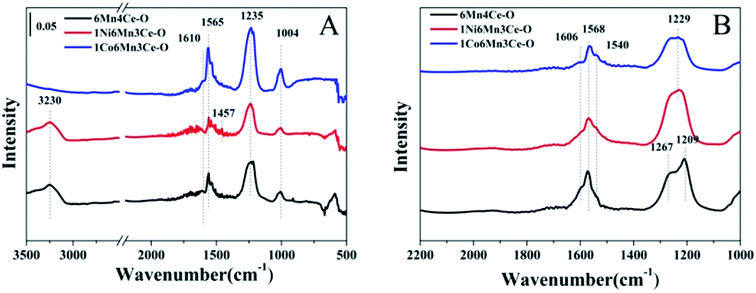 | ||
| Fig. 8 In situ DRIFTS of samples under NH3 + air (A) and NOx + air (B) for 1 h and then purged by helium for 30 min at 200 °C. | ||
Fig. 8B displayed the in situ DRIFTs spectra of NOx + O2 adsorption on catalysts at 200 °C. The peaks centered at 1606 cm−1 can be ascribed to bridging monodentate nitrates, and peaks centered at 1267, 1209, 1568 and 1540 cm−1 were assigned to bidentate nitrates.39 It was noteworthy that bidentate nitrates species on 1Ni6Mn3Ce-O were more than those on other catalysts, indicating nickel addition increased NO uptake. However, cobalt doping leaded to the decrease of bands related to bidentate nitrates, indicating its suppression to NO adsorption.
As 1Ni6Mn3Ce-O showed the best H2O and SO2 resistance, the in situ DRIFTs were further displayed to understand the mechanism, and the results were shown in Fig. 9. The 1Ni6Mn3Ce-O was firstly exposed to NH3 for 60 min, and then SO2 and H2O was introduced to the reaction gas. The bands corresponding to NH3(a) was quickly diminished, and new bands related to SO2 adsorption appeared, as shown in Fig. 9A. While 1Ni6Mn3Ce-O was firstly exposed to NO + O2 for 60 min, and then SO2 was introduced to the reaction gas, no new bands appeared, which indicated that NO was more easily adsorbed on the surface of catalysts. The 1Ni6Mn3Ce-O was exposed to NH3 + NO + O2 at 200 °C for 60 min, and there were peaks related to NH3 and NOx adsorption, indicating the co-adsorption of NH3 and NOx. After 100 ppm SO2 and H2O were added for another 60 min, no new bands appeared and the intensity of the original bands remained unchanged. This indicated that SO2 and H2O in the flue gas had no impact on NH3-SCR of 1Ni6Mn3Ce-O, which was in agreement with the results in Fig. 1. The NH3-SCR reaction on 1Ni6Mn3Ce-O may follow L–H mechanism, NH3(a) and NO(a) reacted to form N2 and H2O. When SO2 and H2O were introduced into the flue gas, NO(a) was more prone to react with NH3(a) than SO2. All of these contribute to excellent H2O and SO2 resistance.
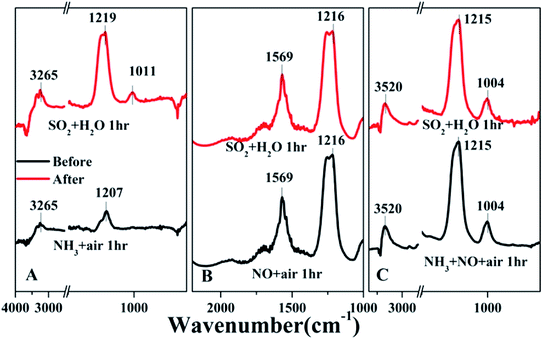 | ||
| Fig. 9 In situ DRIFTS of 1Ni6Mn3Ce-O under NH3 + air (A), NOx + air (B) and NOx + NH3 + O2 (C) for 1 h and then SO2 and H2O were introduced into the mixed gas for 1 h at 200 °C. | ||
4 Discussion and conclusions
It was commonly accepted that there was an intense correlation between catalytic performance and structural properties, such as morphologies, crystal plans, crystal phases and porous structures. A porous structure provided much surface area and makes the adsorption and diffusion of reactant molecules easy to conduct on the surface of catalysts. In this report, catalysts with different pore structures were created by oxalate route, nanocasting strategy and co-precipitation method. Based on above analysis, catalyst from oxalate route showed best NOx conversion, including NOx conversion and SO2 and H2O resistance. Catalyst from oxalate route presented proper ratio of Mn3+, surface oxygen species, surface acidic sites, moderate pore size and specific surface area.Because the catalytic process over single oxide catalysts was limited, the addition of another component into the system would be favorable.28 In this study, cobalt or nickel was added to Mn–Ce composites, which enhanced the interaction between different elements. However, the deNOx performances of catalysts were varied based on the added elements. The introduction of nickel leads to the co-existence of Lewis acid sites and Brønsted acid sites. Addition of cobalt leads to much more Lewis acid sites. It has been proved in previous analysis that Brønsted acid sites are better for the resistance of H2O and SO2.
Several methods have been developed to reduce the poisonous effect of H2O and SO2 to SCR, such as selecting most active support, metal modification, rational design of structure and morphology,1 and pre-sulfation.40,41 The mechanism of these methods was to make a balance among redox properties, effective acid sites, and the formation/decomposition of ammonium sulfate, therefore enhancing the deNOx properties and suppressing the blocking effect of H2O and SO2. In this study, introducing nickel into Mn–Ce composite gave rise to more Brønsted acid sites, which was responsible for good H2O and SO2 resistance.
Different preparation method including oxalate route, nanocasting strategy and traditional co-precipitation were applied to obtain MnOx–CeO2 mixed oxides. The mesoporous Mn–Ce base catalysts prepared from oxalate route showed high deNOx efficiency and good SO2 and H2O resistance in the low temperature range. A third dopant such as nickel or cobalt was introduced to the composite to increase SO2 and H2O durability. The nickel–manganese–cerium ternary oxides showed the best SO2 and H2O durability. The reason can be ascribed to its smaller pores and amorphous structure and proper amount of surface Mn3+/oxygen species, which could decrease the chemical adsorption of SO2.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was financially supported by the National Key Research and Development Plan (2016YFC0204100, 2016YFC0204103), Control Strategy and Technology Integrated Demonstration of Industrial Source Pollution in Guanzhong area of China (ZDRW-ZS-2017-6-2). The authors also acknowledge the analytical and testing center of Institute of Process Engineering, Chinese Academy of Sciences, for their extensive help in testing of samples.References
- C. Gao, J. W. Shi, Z. Y. Fan, G. Gao and C. M. Niu, Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: a Review, Catalysts, 2018, 8, 11 CrossRef.
- L. Q. Chen, F. L. Yuan, Z. B. Li, X. Y. Niu and Y. J. Zhu, Synergistic effect between the redox property and acidity on enhancing the low temperature NH3-SCR activity for NOx removal over the Co0.2CexMn0.8-xTi10 (x=0-0.40) oxides catalysts, Chem. Eng. J., 2018, 354, 393–406 CrossRef CAS.
- X. Hu, L. Huang, J. Zhang, H. Li, K. Zha and L. Shi, et al., Facile and template-free fabrication of mesoporous 3D nanosphere-like MnxCo3-xO4 as highly effective catalysts for low temperature SCR of NOx with NH3, J. Mater. Chem. A, 2018, 6, 2952–2963 RSC.
- L. Li, B. Sun, J. Sun, S. Yu, C. Ge and C. Tang, et al., Novel MnOx-CeO2 nanosphere catalyst for low-temperature NH3-SCR, Catal. Commun., 2017, 100, 98–102 CrossRef CAS.
- C. Li, X. Tang, H. Yi, L. Wang, X. Cui and C. Chu, et al., Rational design of template-free MnOx-CeO2 hollow nanotube as de-NOx catalyst at low temperature, Appl. Surf. Sci., 2018, 428, 924–932 CrossRef CAS.
- H. Chang, J. Li, X. Chen, L. Ma, S. Yang and J. W. Schwank, et al., Effect of Sn on MnOx-CeO2 catalyst for SCR of NOx by ammonia: Enhancement of activity and remarkable resistance to SO2, Catal. Commun., 2012, 27, 54–57 CrossRef CAS.
- J. Liu, R.-t Guo, M.-y Li, P. Sun, S.-m. Liu and W.-g. Pan, et al., Enhancement of the SO2 resistance of Mn/TiO2 SCR catalyst by Eu modification: A mechanism study, Fuel, 2018, 223, 385–393 CrossRef CAS.
- Z. Liu, X. Feng, Z. Zhou, Y. Feng and J. Li, Ce-Sn binary oxide catalyst for the selective catalytic reduction of NOx by NH3, Appl. Surf. Sci., 2018, 428, 526–533 CrossRef CAS.
- K. Zha, S. Cai, H. Hu, H. Li, T. Yan and L. Shi, et al., In Situ DRIFTs Investigation of Promotional Effects of Tungsten on MnOx-CeO2/meso-TiO2 Catalysts for NOx Reduction, J. Phys. Chem. C, 2017, 121, 25243–25254 CrossRef CAS.
- F. Gao, X. Tang, H. Yi, J. Li, S. Zhao and J. Wang, et al., Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature, Chem. Eng. J., 2017, 317, 20–31 CrossRef CAS.
- K. Zha, L. Kang, C. Feng, L. Han, H. Li and T. Yan, et al., Improved NOx reduction in the presence of alkali metals by using hollandite Mn–Ti oxide promoted Cu-SAPO-34 catalysts, Environ. Sci. Nano, 2018, 5, 1408–1419 RSC.
- L. Kang, L. Han, J. He, H. Li, T. Yan and G. Chen, et al., Improved NOx Reduction in the Presence of SO2 by Using Fe2O3-Promoted Halloysite-Supported CeO2–WO3 Catalysts, Environ. Sci. Technol., 2019, 53, 938–945 CrossRef CAS PubMed.
- F. Kleitz, S. Hei Choi and R. Ryoo, Cubic Ia3d large mesoporous silica: synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes, Chem. Commun., 2003, 2136–2137 RSC.
- H. Liu, L. Wei, R. Yue and Y. Chen, CrOx–CeO2 binary oxide as a superior catalyst for NO reduction with NH3 at low temperature in presence of CO, Catal. Commun., 2010, 11, 829–833 CrossRef CAS.
- J. Liu, X. Li, R. Li, Q. Zhao, J. Ke and H. Xiao, et al., Facile synthesis of tube-shaped Mn-Ni-Ti solid solution and preferable Langmuir-Hinshelwood mechanism for selective catalytic reduction of NOx by NH3, Appl. Catal., A, 2018, 549, 289–301 CrossRef CAS.
- Y. Wan, W. Zhao, Y. Tang, L. Li, H. Wang and Y. Cui, et al., Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3, Appl. Catal., B, 2014, 148–149, 114–122 CrossRef CAS.
- X. Li, Y. Du, X. Guo, R. Wang, B. Hou and X. Wu, Synthesis of a Novel NiMnTi Mixed Metal Oxides from LDH Precursor and Its Catalytic Application for Selective Catalytic Reduction of NOx with NH3, Catal. Lett., 2018, 149, 456–464 CrossRef.
- H. Chang, X. Chen, J. Li, L. Ma, C. Wang and C. Liu, et al., Improvement of Activity and SO2 Tolerance of Sn-Modified MnOx-CeO2 Catalysts for NH3-SCR at Low Temperatures, Environ. Sci. Technol., 2013, 47, 5294–5301 CrossRef CAS PubMed.
- B. Thirupathi and P. G. Smirniotis, Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures, Appl. Catal., B, 2011, 110, 195–206 CrossRef CAS.
- X. Tang, J. Li, L. Wei and J. Hao, MnOx-SnO2 catalysts synthesized by a redox coprecipitation method for selective catalytic reduction of NO by NH3, Chin. J. Catal., 2008, 29, 531–536 CrossRef CAS.
- S. Li, B. Huang and C. Yu, A CeO2-MnOx core-shell catalyst for low-temperature NH3-SCR of NO, Catal. Commun., 2017, 98, 47–51 CrossRef CAS.
- L. Chen, X. Niu, Z. Li, Y. Dong, Z. Zhang and F. Yuan, et al., Promoting catalytic performances of Ni-Mn spinel for NH3-SCR by treatment with SO2 and H2O, Catal. Commun., 2016, 85, 48–51 CrossRef CAS.
- W. Sun, X. Li, Q. Zhao, M. Tade and S. Liu, WαMn1–αOx Catalysts Synthesized by a One-Step Urea Co-precipitation Method for Selective Catalytic Reduction of NOx with NH3 at Low Temperatures, Energy Fuels, 2016, 30, 1810–1814 CrossRef CAS.
- N. Fang, J. Guo, S. Shu, H. Luo, Y. Chu and J. Li, Enhancement of low-temperature activity and sulfur resistance of Fe0.3Mn0.5Zr0.2 catalyst for NO removal by NH3-SCR, Chem. Eng. J., 2017, 325, 114–123 CrossRef CAS.
- G. Qi and R. T. Yang, Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst, J. Catal., 2003, 217, 434–441 CrossRef CAS.
- M. Qiu, S. Zhan, H. Yu and D. Zhu, Low-temperature selective catalytic reduction of NO with NH3 over ordered mesoporous MnxCo3−xO4 catalyst, Catal. Commun., 2015, 62, 107–111 CrossRef CAS.
- P. Zhang, Y. Sun, W. Su, Y. Wei and J. Liu, Low-temperature selective catalytic reduction of NO with NH3 over Ni-Mn-Ox catalysts, RSC Adv., 2016, 6, 107270–107277 RSC.
- W. Tang, Y. Deng, W. Li, J. Li, G. Liu and S. Li, et al., Importance of porous structure and synergistic effect on the catalytic oxidation activities over hierarchical Mn-Ni composite oxides, Catal. Sci. Technol., 2016, 6, 1710–1718 RSC.
- B. Jia, J. Guo, H. Luo, S. Shu, N. Fang and J. Li, Study of NO removal and resistance to SO2 and H2O of MnOx/TiO2, MnOx/ZrO2 and MnOx/ZrO2–TiO2, Appl. Catal., A, 2018, 553, 82–90 CrossRef CAS.
- Q. Shen, L. Zhang, N. Sun, H. Wang, L. Zhong and C. He, et al., Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation, Chem. Eng. J., 2017, 322, 46–55 CrossRef CAS.
- W. Sun, X. Li, Q. Zhao, J. Mu and J. Chen, Fe–Mn Mixed Oxide Catalysts Synthesized by One-Step Urea-Precipitation Method for the Selective Catalytic Reduction of NOx with NH3 at Low Temperatures, Catal. Lett., 2018, 148, 227–234 CrossRef CAS.
- D. S. Zhang, L. Zhang, L. Y. Shi, C. Fang, H. R. Li and R. H. Gao, et al., In situ supported MnOx-CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3, Nanoscale, 2013, 5, 1127–1136 RSC.
- Y. Niu, T. Shang, S. Hui, X. Zhang, Y. Lei and Y. Lv, et al., Synergistic removal of NO and N2O in low-temperature SCR process with MnOx/Ti based catalyst doped with Ce and V, Fuel, 2016, 185, 316–322 CrossRef CAS.
- J. Liu, X. Li, Q. Zhao, J. Ke, H. Xiao and X. Lv, et al., Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FTIR analysis for structure-activity correlation, Appl. Catal., B, 2017, 200, 297–308 CrossRef CAS.
- L. Chen, R. Li, Z. Li, F. Yuan, X. Niu and Y. Zhu, Effect of Ni doping in NixMn1-xTi10 (x = 0.1-0.5) on activity and SO2 resistance for NH3-SCR of NO studied with in situ DRIFTS, Catal. Sci. Technol., 2017, 7, 3243–3257 RSC.
- H.-D. Liu, W.-M. Li, R.-L. Yue and Y.-F. Chen, Reaction Mechanism of NH3-Selective Catalytic Reduction for NO on CrOx-CeO2 Binary Oxide, Chin. J. Inorg. Chem., 2013, 29, 2399–2404 CAS.
- T. Zhang, F. Qiu, H. Chang, Y. Peng and J. Li, Novel W-modified SnMnCeOx catalyst for the selective catalytic reduction of NOx with NH3, Catal. Commun., 2017, 100, 117–120 CrossRef CAS.
- G. Wu, X. Feng, H. Zhang, Y. Zhang, J. Wang and Y. Chen, et al., The promotional role of Ni in FeVO4/TiO2 monolith catalyst for selective catalytic reduction of NOx with NH3, Appl. Surf. Sci., 2018, 427, 24–36 CrossRef CAS.
- W. Shan, F. Liu, H. He, X. Shi and C. Zhang, Novel cerium-tungsten mixed oxide catalyst for the selective catalytic reduction of NOx with NH3, Chem. Commun., 2011, 47, 8046–8048 RSC.
- K. Wijayanti, S. Andonova, A. Kumar, J. Li, K. Kamasamudram and N. W. Currier, et al., Impact of sulfur oxide on NH3-SCR over Cu-SAPO-34, Appl. Catal., B, 2015, 166, 568–579 CrossRef.
- L. Zhang, H. Qu, T. Du, W. Ma and Q. Zhong, H2O and SO2 tolerance, activity and reaction mechanism of sulfated Ni–Ce–La composite oxide nanocrystals in NH3-SCR, Chem. Eng. J., 2016, 296, 122–131 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00731h |
| This journal is © The Royal Society of Chemistry 2019 |