Open Access Article
Open Access ArticleDissociative adsorption modes of TATB on the Al (111) surface: a DFT investigation †
Guo-zheng Zhao *a,
Hui-li Lia,
Jian-feng Jiaa,
Hai-shun Wua and
Ming Lub
*a,
Hui-li Lia,
Jian-feng Jiaa,
Hai-shun Wua and
Ming Lub
aKey Laboratory of Magnetic Molecules, Magnetic Information Materials Ministry of Education, The School of Chemistry and Material Science, Shanxi Normal University, Linfen 041004, PR China. E-mail: zhaoguozheng99@126.com
bSchool of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
First published on 15th April 2019
Abstract
Herein, the adsorption modes and electronic structures of TATB/Al (111) systems were investigated using the density functional theory (DFT) approach. We found that chemical adsorption led to the decomposition of the TATB molecule on the Al surface by four adsorption modes. All the adsorption configurations were accompanied by fractures of the N–O bonds in the nitro groups. In addition, there was a hydrogen atom transfer for 5P. For parallel and vertical adsorptions, the TATB molecules favored planar or quasi-planar structures. The order of total energy with BSSE correction matches well with the order of adsorption energy. The absolute values of energy and adsorption energy of 6P and 6V are highest in the parallel and vertical adsorption systems, respectively. Electrons are transferred from the Al (111) surface to the TATB molecule; this results in the activation of TATB on the Al (111) surface and obvious augmentation of the PDOS (partial density of states) peaks of the N and O atoms. From the Al (111) surface to the TATB molecule, the transfer of the electrons of 4P (14.00e) and 6V (9.04e) is largest for the parallel and vertical adsorptions, respectively.
1. Introduction
TATB was reported for the first time in 1888 by Jackson and Wing.1 Subsequently, the crystal structure (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group) of TATB was obtained and characterized by Cady et al.2 Over the past few decades, 1,3,5-triamino-2,4,6-trinitrobenzene (TATB), a major representative of nitro explosives,3,4 has been extensively used in many areas as an energetic material;5,6 the intramolecular and intermolecular hydrogen bonding interactions are relatively stronger in TATB, which contribute to its exceptional insensitivity to thermal, impact, friction, and shock, as compared to those in traditional energetic materials such as 2,4,6-trinitrotoluene (TNT), 1,3,5-trinitrohexahydro-s-triazine (RDX), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX). Thus, significant experimental and theoretical investigations have been focused on TATB.7–9 Numerous investigations have been focused on its thermal decomposition and structural details, and several possibilities have been introduced for the decomposition pathways of TATB; however, the universal mechanism of these reactions is still unknown.
space group) of TATB was obtained and characterized by Cady et al.2 Over the past few decades, 1,3,5-triamino-2,4,6-trinitrobenzene (TATB), a major representative of nitro explosives,3,4 has been extensively used in many areas as an energetic material;5,6 the intramolecular and intermolecular hydrogen bonding interactions are relatively stronger in TATB, which contribute to its exceptional insensitivity to thermal, impact, friction, and shock, as compared to those in traditional energetic materials such as 2,4,6-trinitrotoluene (TNT), 1,3,5-trinitrohexahydro-s-triazine (RDX), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX). Thus, significant experimental and theoretical investigations have been focused on TATB.7–9 Numerous investigations have been focused on its thermal decomposition and structural details, and several possibilities have been introduced for the decomposition pathways of TATB; however, the universal mechanism of these reactions is still unknown.
The transformation of intermolecular hydrogen is a possible initial step, which is suggested by theoretical calculations, in the thermal and shock decomposition process of crystal TATB.10–12 Based on quantum chemical methods, both molecular and crystalline processes have been analysed by Kuklja et al., who discovered that C–NO2 fission is the main pathway for the initial decomposition of TATB in the gas phase and on the (001) surface at high temperatures.13,14 The formation of free radicals, which was examined by Xu et al., probably occurred during the photolysis of TATB.15 Goddard et al. predicted that the formation of large carbonaceous clusters was initiated by the decomposition of TATB by applying the first-principle-based ReaxFF reactive force field.16 Their research was mainly focused on the TATB molecule and crystal under different conditions. However, explosives, propellants, and pyrotechnics are made up of a variety of components, which interact with each other, and due to the influence of other components, TATB shows different dissociation modes. Apart from the questionable state of TATB, it is believed that its dissociation mode is also related to its interface structures. Liu and Wang et al. investigated 1,1-diamino-2,2-dinitroethene (FOX-7)-solvent interfacial interactions and found that hydrogen bonds and vdW interactions play a dominant role in the interaction between the solvent and FOX-7 surfaces.17 The interactions between the 3-nitro-1,2,4-triazole-5-one (NTO) crystal and solvent were studied by Li et al., who reported that the solvent adsorption on NTO occurs mainly via the solvent-crystal face interactions of hydrogen bonding.18
To improve the performance of high energetic materials, aluminum (Al) powder is widely used as a metallic additive to explosives, propellants, and pyrotechnic compositions.19,20 As is known, Al can improve the energy toward the burning reaction in propellants and strengthen the blast effect of explosives.21,22 The size of Al particles plays a dominant role in the efficiency of these processes. Due to its large surface area, Al nanopowder can dramatically improve the performance of some energetic materials.23,24 Additionally, the burn rate is improved remarkably by incorporating Al nanopowder in propellants. Al is also widely used in formulations of propellants and explosives; thus, it is of great importance to explore its reactions with energetic molecules.25 Ammunition composed of TATB and aluminum powder shows excellent performance. To understand the interaction mode of TATB/Al systems, comprehensive knowledge about the interface and electronic structure of the system is necessary. The interface between RDX and Al with different oxide layers was studied by Jaramillo-Botero et al. to understand the effect of nanosized Al on the thermal decomposition of RDX.26 Bean et al. prepared Al enriched 1,1-diamino-2,2-dinitroethylene (Al-FOX-7) crystals to improve the friction sensitivity and detonation properties of the FOX-7 crystal.27 Zhou et al. studied the diffusion of NH2NO2 molecules on the surface of Al under different temperatures and pressures.28 The (111) surface of the Al crystal is easily exposed, decomposed and chemically corroded.29,30 In contrast, to date, no theoretical work has been reported on the dissociative adsorption of TATB on the Al (111) surface. In the present study, we focused our investigation on the adsorption mode of TATB/Al (111). We elucidated the interaction mechanism and discussed the electronic structures and charge distributions of TATB on Al (111) surface.
2. Computational details
DFT calculations were performed using the CP2K software package based on the gradient-corrected functional of a revised Perdew–Burke–Ernzerhof functional (PBEsol).31,32 The initial Al crystal was introduced by Cooper.33 To build the Al (111) surface, a 4 × 4 × 4 supercell of Al was optimized with the lattice parameters a = b = c = 4.057 Å, which match well with the reported values (a = b = c = 4.050 Å).33 A 5 × 5 slab was constructed based on the optimized Al cell, which ensured that the distance between TATB and its period replica was larger than 7 Å. Four Al layers were included and the vacuum region along the c direction was larger than 15 Å. Fig. 1 illustrates the top and lateral views of the 5 × 5 surface model. The detailed lattice parameters and atomic coordinates of the Al (111) surface are listed in the ESI.† With the aim of finding the most stable TATB/Al (111) configuration, many types of adsorption sites were analyzed. The calculation details are listed in the ESI.†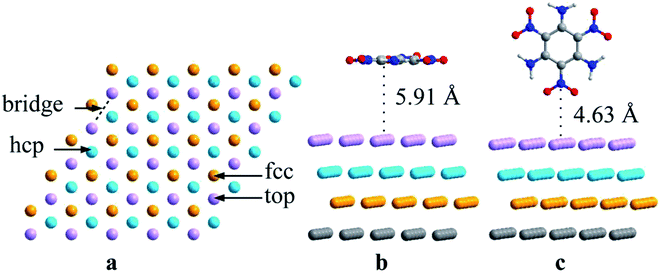 | ||
| Fig. 1 (a) Top view of the sites depicted on the surface, (b) parallel TATB on the Al (111) surface with no interactions and (c) vertical TATB on the Al (111) surface with no interactions. | ||
3. Results and discussion
3.1 Adsorption mode of TATB/Al (111)
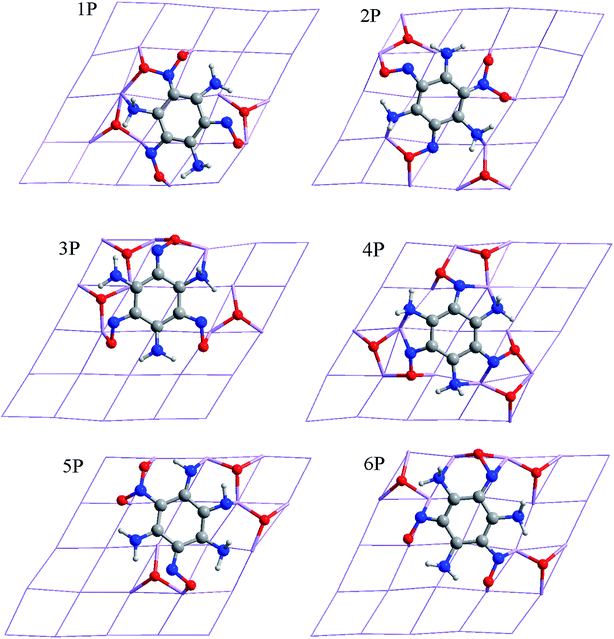
Chemical adsorption causes the decomposition of the TATB molecule on the Al surface, which makes the dissociative adsorption extremely complicated.34,35 There are four adsorption modes, as follows:(1) C6N3H6 + 3NO2 → C6N3H6 + 2NO + 2O + NO2 (Fig. 2 and 3; 1P, 2P, 6V).
(2) C6N3H6 + 3NO2 → C6N3H6 + 3NO + 3O (Fig. 2; 3P, 4P, 6P).
(3) C6N3H6 + 3NO2 → C6N3H6 + NO + N + 3O + NO2 (Fig. 2; 5P).
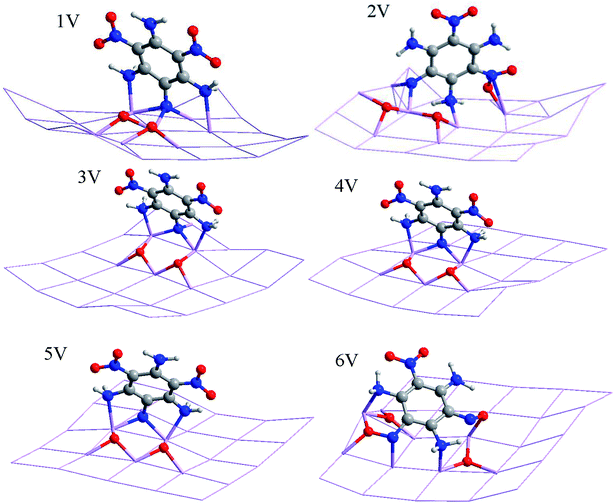
(4) C6N3H6 + 3NO2 → C6N3H6 + N + 2O + 2NO2 (Fig. 2 and 3; 1V, 2V, 3V, 4V, 5V).
According to the center and C–C symmetric axis of the benzene ring relative to the Al (111) surface, P and V denote parallel and vertical adsorptions of TATB, respectively, as listed in Fig. 2 and 3. Each stable form of TATB/Al (111) represents the top view of the optimized adsorption configuration after full relaxation of the atomic positions. Selected bond lengths, atomic distances and adsorption energies of different TATB/Al (111) are shown in Tables 1 and 2, respectively. For the parallel adsorption configurations, the dissociation of the TATB molecule can be shown in three ways, with the products of NO + O, NO + O + NO2, and NO + O + N + NO2, and for the vertical adsorption configurations, the dissociation of the TATB molecule can be shown in two ways, with the products of NO + O + NO2, and N + O + NO2. The TATB molecules in our study prefer planar structures, which lay flat on the Al (111) surfaces.
| Geometries | Pa | 1P | 2P | 3P | 4P | 5P | 6P |
|---|---|---|---|---|---|---|---|
| a Configuration P refers to Fig. 1(b), in which there is no interaction between TATB and the Al (111) surface.b Configuration V refers to Fig. 1(c), in which there is no interaction between TATB and Al the (111) surface. | |||||||
| N1–O11 | 1.261 | 2.753 | 1.387 | 1.422 | 1.456 | 1.422 | 2.647 |
| N1–O12 | 1.260 | 1.393 | 1.424 | 2.630 | 2.555 | 2.576 | 1.429 |
| N3–O31 | 1.260 | 1.473 | 1.416 | 2.671 | 1.456 | 2.593 | 1.504 |
| N3–O32 | 1.261 | 1.373 | 2.724 | 1.442 | 2.563 | 2.716 | 2.841 |
| N5–O51 | 1.259 | 1.422 | 3.510 | 2.738 | 1.458 | 1.405 | 1.419 |
| N5–O52 | 1.259 | 2.915 | 1.482 | 1.531 | 2.557 | 1.387 | 2.617 |
| Al–O | 1.823 | 1.831 | 1.816 | 1.811 | 1.788 | 1.806 | |
| 1.832 | 1.850 | 1.811 | 1.813 | 1.820 | 1.811 | ||
| 1.833 | 1.851 | 1.813 | 1.814 | 1.830 | 1.829 | ||
| 1.841 | 1.870 | 1.829 | 1.844 | 1.841 | 1.838 | ||
| 1.844 | 1.876 | 1.832 | 1.846 | 1.843 | 1.839 | ||
| 1.873 | 1.894 | 1.833 | 1.848 | 1.844 | 1.842 | ||
| 1.850 | 1.848 | 1.844 | 1.861 | ||||
| 1.863 | 1.854 | 1.852 | 1.895 | ||||
| 1.870 | 1.855 | 1.855 | 1.942 | ||||
| Al–N | 1.990 | 2.005 | 2.092 | 1.957 | 1.931 | 1.995 | |
| 2.054 | 2.097 | 1.958 | 1.961 | 1.995 | |||
| 1.965 | 2.080 | ||||||
| Geometries | Vb | 1V | 2V | 3V | 4V | 5V | 6V |
|---|---|---|---|---|---|---|---|
| N1–O11 | 1.265 | 2.558 | 2.960 | 2.997 | 3.078 | 3.037 | 3.149 |
| N1–O12 | 1.266 | 3.538 | 2.907 | 3.025 | 3.005 | 3.033 | 1.498 |
| N3–O31 | 1.259 | 1.260 | 1.263 | 1.260 | 1.259 | 1.258 | 1.446 |
| N3–O32 | 1.259 | 1.271 | 1.356 | 1.265 | 1.270 | 1.263 | 2.727 |
| N5–O51 | 1.260 | 1.270 | 1.254 | 1.266 | 1.265 | 1.267 | 1.272 |
| N5–O52 | 1.260 | 1.257 | 1.258 | 1.260 | 1.260 | 1.258 | 1.256 |
| Al–O | 1.791 | 1.825 | 1.843 | 1.850 | 1.848 | 1.843 | |
| 1.859 | 1.834 | 1.847 | 1.852 | 1.854 | 1.850 | ||
| 1.862 | 1.853 | 1.853 | 1.860 | 1.862 | 1.862 | ||
| 1.865 | 1.872 | 1.866 | 1.864 | 1.863 | 1.873 | ||
| 1.878 | 1.883 | 1.869 | 1.868 | 1.867 | 1.881 | ||
| 1.906 | 1.885 | 1.884 | 1.880 | 1.876 | 1.942 | ||
| Al–N | 1.901 | 1.868 | 1.967 | 1.987 | 1.945 | 1.982 | |
| 2.027 | 1.888 | 1.983 | 1.991 | 1.956 | |||
| 2.048 | 1.984 | 1.993 | 1.987 |
| Configurations | 1P | 2P | 3P | 4P | 5P | 6P |
| Adsorption sites | Bridge | Bridge | fcc | Top | hcp | Bridge |
| Eads (kJ mol−1) | −1415.67 | −1511.88 | −1518.61 | −1531.33 | −1646.86 | −1877.67 |
| Configurations | 1V | 2V | 3V | 4V | 5V | 6V |
| Adsorption sites | Top | Bridge | fcc | hcp | Bridge | Bridge |
| Eads (kJ mol−1) | −1125.26 | −1126.97 | −1142.18 | −1167.29 | −1175.35 | −1383.32 |
For the twelve TATB/Al (111) adsorption systems, the order of total energy with BSSE correction is consistent with the order of adsorption energy, indicating that the total energy is closely related to the adsorption energy. The energies of the parallel and vertical adsorption are in the range of −398.339594 a.u. to −398.163630 a.u. and −398.151305 a.u. to −398.053016 a.u., respectively, which indicate that the parallel adsorption configurations are more stable than the vertical adsorption configurations, and therefore, the adsorption modes of these systems are inclined to parallel adsorption. The adsorption configurations are the most stable in the dissociation mode (2), which are all parallel adsorption, and the adsorption configurations are the most unstable in the dissociation mode (4), which are all vertical adsorption. Since the TATB molecule decomposition products of 3P, 4P, 5P and 6P are four radical species, their corresponding adsorption energies are higher than that of three radical species. From Table 2, the adsorption energies of 1P–6P are much higher than that of P (−25.65 kJ mol−1), and the adsorption energies of 1V–6V are much higher than that of V (−48.66 kJ mol−1), indicating that the TATB/Al (111) adsorption systems are more stable than P and V. Therefore, the Al atoms are oxidized easily by the radical species, which form stable Al–O and Al–N bonds.
The lying structure of 6P in Fig. 2 is confirmed to be the lowest energy (−398.339594 a.u.) configuration for TATB/Al (111), and an oxygen atom is dissociated from each nitro group in dissociation mode (2). The first layer of aluminum is no longer flat, and two aluminum atoms are much closer to the TATB molecule. Four Al–N bonds and thirteen Al–O bonds are formed, which have bond lengths of 1.995–2.099 Å and 1.806–1.960 Å, respectively. In comparison to the isolated TATB structure in Fig. 1(b) (i.e., there is no interaction between the TATB molecule and Al atoms on the surface), there is an irregular change for the N–H bond of the amino group. For example, the N4–H41 bond is stretched from 1.042 Å to 1.049 Å and the N6–H61, N6–H62, and N4–H42 bonds are shortened to 1.039, 1.034, and 1.036 Å, respectively. The N–O bond lengths augment further to a certain degree, which why the N and O atoms interact strongly with the Al atoms on the surface. For 6P, the adsorption energy value is −1877.67 kJ mol−1, which is the lowest among the twelve TATB/Al (111) adsorption systems. For 3P, both the O and N atoms of –NO2 and N atoms of –NH2 with Al atoms form one Al–N bond (2.092 Å) and thirteen Al–O bonds (1.811–1.919 Å) and for 4P, both O and N atoms of –NO2 and N atoms of –NH2 with Al atoms form six Al–N bonds with the length of 1.957–2.089 Å and fifteen Al–O bonds with the length of 1.811–2.068 Å. The lengths of the N–H and N–O bonds of 3P and 4P are longer than that of the isolated TATB molecule. 1P and 2P in Fig. 2 describe the case in which TATB is detached into C6N3H6, 2NO, 2O, and NO2. For 1P, two Al–N and eleven Al–O bonds form with the lengths of 1.990–2.054 Å and 1.820–2.073 Å, respectively. For 2P, three Al–N and eleven Al–O bonds form with the lengths of 2.005–2.097 Å and 1.816–1.993 Å, respectively. The formation of a large number of Al–O bonds significantly activates the dissociated oxygen atoms.
As shown in Fig. 3, 6V shows vertical adsorption at the bridge site between the first layer of the Al surface. 6V is the most stable structure in the vertical adsorption system studied, and its energy value is −398.151305 a.u., which illustrates the dissociation of one of the O atoms from two nitro groups. The adsorption and dissociation mode of 6V agree better with that of 1P. One of the dissociated oxygen atoms is embedded in the aluminum surface, and the first layer of aluminum atoms is extremely uneven, showing that there is strong interaction between TATB and the Al surface. As a result, the TATB molecular skeleton is approximately parallel to the aluminum surface, which achieves the transition from vertical adsorption to parallel adsorption. This result corresponds well with the fact that the adsorption mode of the system is inclined to parallel adsorption. Nine Al–O and three Al–N bonds are formed with lengths of 1.832–1.942 Å and 1.982–2.020 Å, respectively. The undissociated N–H and N–O bonds are elongated to 1.032–1.069 Å and 1.256–1.498 Å, respectively, which is because the N and O atoms interact actively with the adjacent Al atoms.36 The corresponding adsorption energy of 6V is −1383.32 kJ mol−1, which is the highest energy in the vertical adsorption systems. 5V exhibits the C–C symmetric axis of the TATB molecule above the bridge site between the three layers of the Al surface. According to this site, chemical adsorption causes the dissociation of two O atoms from the same nitro group. 5V is the sub-stable structure in the vertical adsorption system studied, and its energy value is −398.072096 a.u. C6N5H6O4, with two dissociated O atoms, and one dissociated N atom, which are adsorbed on the Al surface, results in the formation of six Al–O and five Al–N bonds with the lengths of 1.848–1.876 Å and 1.945–2.117 Å, respectively. Furthermore, the N–H and N–O bonds are lengthened to 1.039–1.065 Å and 1.258–1.267 Å, respectively, in comparison with the initial values in Fig. 1(c). The adsorption energy of 5V is larger by 207.97 kJ mol−1 than that of 6V, which shows that vertical adsorption leads to a higher adsorption energy and unstable system. 1V, 2V, 3V, and 4V in Fig. 3 depict that the C–C symmetric axis of the TATB molecule is above an on-top site, a bridge site between the second layer of the Al surface, an fcc site, and an hcp site, respectively. The adsorption and dissociation modes of 1V, 2V, 3V, and 4V are in full agreement with that of 5V. At the on-top site, the formation of six Al–O and four Al–N bonds is caused by chemical adsorption, which have lengths of 1.791–1.906 Å and 1.901–2.048 Å, respectively. At the hcp site, six Al–O and five Al–N bonds form with the lengths of 1.850–1.880 Å and 1.987–2.120 Å, respectively. In addition, the chemical adsorption at the fcc site induces the formation of six Al–O bonds with the length of 1.843–1.884 Å and five Al–N bonds with the length of 1.967–2.083 Å. Finally, seven Al–O bonds (1.825–1.898 Å) and three Al–N bonds (1.868–2.079 Å) are formed by chemical adsorption at a bridge site between the second layer of the Al surface.
The structures of TATB are strongly affected by the surfaces of metal aluminum. The Eads of all the TATB/Al (111) systems are less than zero, indicating that the adsorption interaction process is exothermic between TATB and the Al surface. For parallel and vertical adsorption, TATB favors planar or quasi-planar structures. Moreover, TATB significantly changes the structures of the Al (111) surfaces. For the different adsorption sites, there are different decomposition products. Besides the stable Al–O bonds, Al–N bonds are also created by the active adsorption interaction between the Al atoms on the surface and N atoms in either the nitro group or the amino group. The fracture of N–O bonds in the nitro groups and the formation of Al–N and Al–O bonds between dissociative N and O atoms and Al surface are the main way of dissociation adsorption (Fig. 4 and 5).
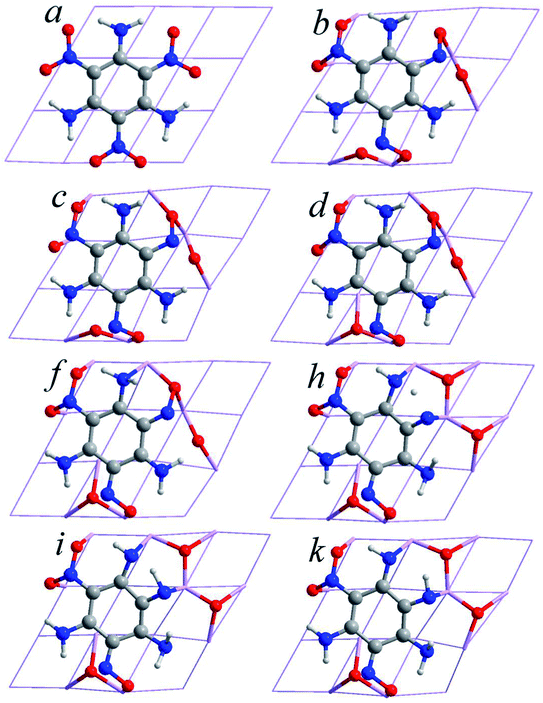 | ||
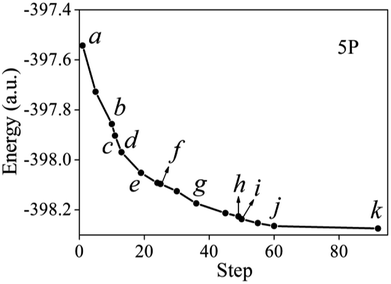
| Fig. 5 Snapshots of points b, c, d, f, h, and i, corresponding to various stages of geometry optimization, where a and k represent the initial and final geometry of 5P, respectively. | ||
5P is the second most stable configuration, which is higher in energy than 6P by 0.08791 a.u., and TATB is dissociated to C6N3H6, NO2, NO, N, and 3O. The two Al–N and twelve Al–O bonds lengths are 1.931–1.961 Å and 1.788–1.882 Å, respectively. The Al–N and Al–O bond lengths are less than that of 6P, which leads to an increase in its configuration energy. The elongation tendency of all the undissociated N–O bonds is exactly the same as that of 6P. The adsorption energy of 5P is the second highest among the adsorption energies (−1646.86 kJ mol−1), which is consistent with the results of the configuration energy. All the adsorption configurations are accompanied by fractures of the N–O bonds in the nitro groups. In addition, there is also a hydrogen atom transfer for 5P. Fig. 6 illustrates the optimized structures of 5P during the various minimization steps, which was calculated using the PBEsol functional. From a to e, with the energy optimization process, it is obvious that the total energy of the optimized structure of 5P drops significantly. Before b, there is no fracture of the chemical bonds for the TATB molecule, and only its molecular conformation is changed. Fig. 6 shows that the change in molecular conformation contributes to the greatest reduction in the energy of the system. At b, with a decrease in energy (72.87 kJ mol−1), two N–O bonds (N1–O12, N3–O32) are broken, and the dissociated oxygen atoms form two Al–O bonds with aluminum atoms. From b to c, the N5–O51 bond breaks in the third nitro group and an Al–O bond forms. Furthermore, at d, the N5–O51 bond forms again. From e to g, with the optimization of the adsorption configuration, the N1, N3, O12, O31, and O32 atoms interact with the aluminum atoms on the surface. At f, ten Al–O and one Al–N bonds form. Except the breakage of the N1–O1 and N3–O32 bonds, the N3–O31 bond also breaks with a reduction in energy (5.50 kJ mol−1), which means that the two N–O bonds from the same nitro group break. Compared with the fracture of the first N–O bond, the second N–O bond is not subjected to break in the same nitro group. After g, the energy reduces rapidly again. Furthermore, the energy is reduced by 19.28 kJ mol−1 from h to i. A hydrogen atom from the N4 site of TATB detaches and reattaches to the nearby N3. Hydrogen transfer is also an important way to reduce the energy of the system. For 5P, the main form of dissociation is the fracture of the first N–O bond in each nitro group, accompanied by the transfer of hydrogen atoms.
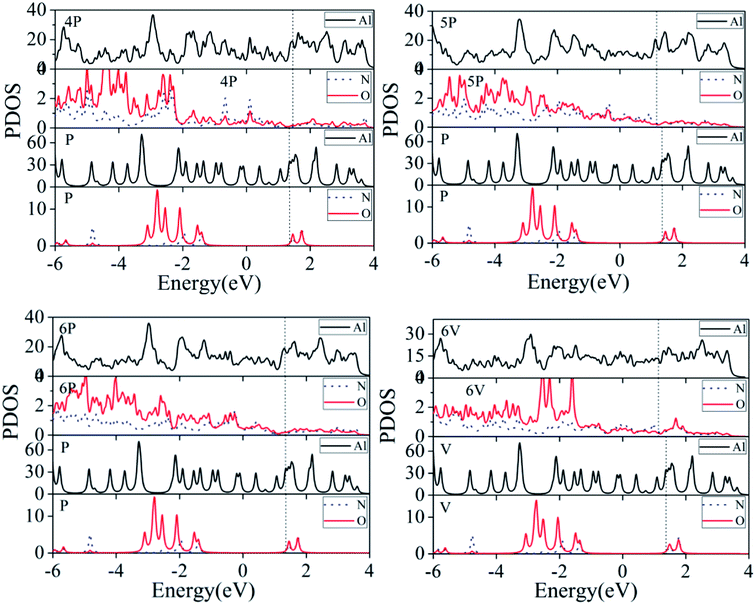
3.2 Electronic structure
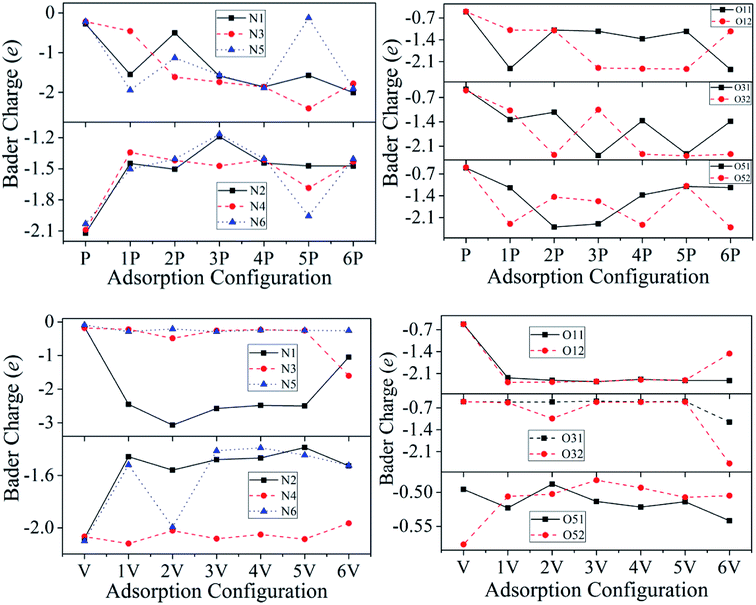 | ||
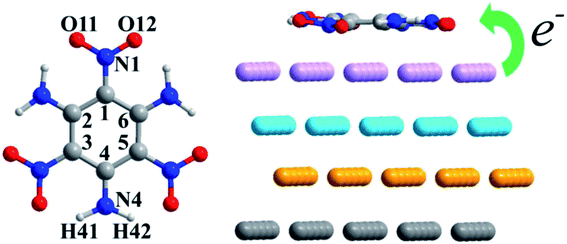
| Fig. 7 Bader charges on the individual N, and O atoms. Configurations P and V refer to Fig. 1(b) and (c), respectively, in which there is no interaction between TATB and the Al (111) surface. | ||
The largest number of electrons is obtained by 6P, which is 2.01e, 2.35e, and 2.41e for N1, O11, and O52, respectively. Each nitro group in the TATB molecule breaks one N–O bond, which causes the largest degree of bond interaction between TATB and the Al (111) surface and charge transfer from the Al (111) surface to TATB. For 1P, the N5–O52 bond breaks and an N5–Al bond forms, which indicated that the Al surface transfers the most electrons to N5 (1.94e). Furthermore, N5 atom of 5P only obtains 0.12e, which is less than that of P (0.22e). This is because the N5–O51 and N5–O52 bonds do not break. The N5–O51 and N5–O52 bond lengths (1.405 Å and 1.387 Å, respectively) of 5P are longer than that of P (both are 1.259 Å). In addition, the N5 of 5P is farther away from the aluminum surface in comparison with that of P, and has a weaker interaction force; therefore, the number of transferred electrons in 5P is less than that in P. Similarly, for N2, N4, and N6, the obtained electrons of 1P–6P are less than that of P.
The charge evolution for vertical adsorption is remarkably different from that for parallel adsorption. 6V obtained the largest number of electrons for N3, O31, and O32, which is 1.60e, 1.15e, and 2.48e, respectively. The fracture of the covalent bond of N3–O32 leads to the formation of four Al–O bonds and the transformation of 2.41e. Also, the other TATB/Al (111) systems have little change in electron transfer. For 1V–6V, the change rules of N5, O51, and O52 are consistent with that of N3, O31, and O32, and the electrons obtained in N2 and N6 are less than that of V. For example, for 5V, the lengths of the N2–H21, N2–H22, N6–H61, and N6–H62 bonds are 1.062 Å, 1.051 Å, 1.056 Å, and 1.067 Å, respectively, which are longer than that of V (all 1.043 Å), demonstrating weaker valence bond interaction and less transferred electrons.
The number of transferred electrons in parallel adsorptions is larger than that of vertical adsorptions. The strong interaction between the N and O atoms in –NO2 and aluminum surface results in a nonuniform distribution of charges. Some N and O atoms get the largest number of electrons from the surface of aluminum, and simultaneously, the N–O bond breaks, forming the internal hot spot of the system. The charge nonuniformity of TATB may be important for the activation of the TATB molecule adsorbed on the Al (111) surface.
4. Conclusions
In summary, the adsorption modes and electronic structures of TATB/Al (111) systems were studied based on DFT calculations. The results indicated that the TATB molecule rotates to maximize its interaction with the Al surface during the optimization. The TATB molecules prefer planar structures, which lay flat on the Al (111) surfaces. The dissociated oxygen atom in each nitro group interacts strongly with the aluminum atoms on the surface. In addition to the fractures of the N–O bonds, the transformation of the hydrogen atom is also a form of dissociation adsorption of TATB on the Al (111) surface. In comparison with the fractures of chemical bonds, the changes of molecular conformation are easier to decrease the energy of the TATB/Al (111) surface. For the parallel adsorption configurations, the energy and adsorption energy of 6P are the lowest, which is −398.339594 a.u. and −1877.67 kJ mol−1, respectively. For the vertical adsorption configurations, the energy and adsorption energy of 6V are the lowest, which are −398.151305 a.u. and −1383.32 kJ mol−1, respectively. The dissociative adsorption of TATB on the Al (111) surface is an exothermic process due to the negative adsorption energy. The parallel adsorption systems are more stable than the vertical adsorption systems. The TATB molecular skeleton of 6V is approximately parallel to the Al surface, which also proves that the mode is more inclined to parallel adsorption. The adsorption systems are the most stable in dissociation mode (2), which are all parallel adsorption, and the adsorption systems are the most unstable in the dissociation mode (4), which are all vertical adsorption. The activation of TATB on the Al (111) surface and augmentation of the N and O atom PDOS peaks are caused by the charge transfer from the Al (111) surface to the TATB molecule.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2016157), and Natural Science Foundation of Shanxi Normal University Modern College of Humanities and Sciences (No. 2018JCYJ03).References
- C. L. Jackson and J. F. Wing, Am. Chem. J., 1888, 10, 283 Search PubMed.
- H. H. Cady and A. C. Larson, Acta Crystallogr., 1965, 18, 485 CrossRef CAS.
- M. P. Kroonblawd and T. D. Sewell, J. Phys. Chem. C, 2016, 120, 17214 CrossRef CAS.
- T. D. Aslam, J. Appl. Phys., 2017, 122, 035902 CrossRef.
- G. Z. Zhao and D. F. Yang, RSC Adv., 2018, 8, 32241 RSC.
- S. Elbasuney, M. G. Zaky, M. Radwan and S. F. Mostafa, Appl. Surf. Sci., 2017, 419, 328 CrossRef CAS.
- D. Picart and A. Junqua-Moullet, Propellants, Explos., Pyrotech., 2017, 42, 1431 CrossRef CAS.
- C. Y. Zhang, Y. S. Wen, X. G. Xue, J. Liu, Y. Ma, X. D. He and X. P. Long, J. Phys. Chem. C, 2016, 120, 25237 CrossRef CAS.
- Y. S. Wen, C. Y. Zhang, X. G. Xue and X. P. Long, Phys. Chem. Chem. Phys., 2015, 17, 12013 RSC.
- Z. H. He, J. Chen and Q. Wu, J. Phys. Chem. C, 2017, 121, 8227 CrossRef CAS.
- Y. S. Wen, X. G. Xue, X. P. Long and C. Y. Zhang, J. Phys. Chem. A, 2016, 120, 3929 CrossRef CAS PubMed.
- Q. Wu, H. Chen, G. L. Xiong, W. H. Zhu and H. M. Xiao, J. Phys. Chem. C, 2015, 119, 16500 CrossRef CAS.
- R. V. Tsyshevsky, O. Sharia and M. M. Kuklja, Molecules, 2016, 21, 236 CrossRef PubMed.
- W. H. Zhu, X. M. Zhang, T. Wei and H. M. Xiao, J. Mol. Struct.: THEOCHEM, 2009, 900, 84 CrossRef CAS.
- Y. Xiong, J. Liu, F. C. Zhong, T. Xu and K. M. Cheng, J. Phys. Chem. A, 2014, 118, 6858 CrossRef CAS PubMed.
- L. Z. Zhang, S. V. Zybin, A. C. T. van Duin, S. Dasgupta, W. A. Goddard and E. M. Kober, J. Phys. Chem. A, 2009, 113, 10619 CrossRef CAS PubMed.
- N. Liu, C. Zhou, Z. K. Wu, X. M. Lu, B. Z. Wang and W. L. Wang, J. Mol. Graphics Modell., 2018, 85, 262 CrossRef CAS PubMed.
- J. Li, S. H. Jin, G. C. Lan, X. Ma, J. Ruan, B. Zhang, S. S. Chen and L. J. Li, CrystEngComm, 2018, 20, 6252 RSC.
- N. Zakiyyan, A. Q. Wang, R. Thiruvengadathan, C. Staley, J. Mathai, K. Gangopadhyay, M. R. Maschmann and S. Gangopadhyay, Combust. Flame, 2018, 187, 1 CrossRef CAS.
- A. L. Shoaf and C. A. Bayse, J. Comput. Chem., 2018, 39, 1236 CrossRef CAS PubMed.
- J. Y. Wu, Y. X. Huang, L. J. Yang, D. S. Geng, F. P. Wang, H. Q. Wang and L. Chen, ChemPhysChem, 2018, 19, 2683 CrossRef CAS PubMed.
- J. B. Delisio, X. L. Hu, T. Wu, G. C. Egan, G. Young and M. R. Zachariah, J. Phys. Chem. B, 2016, 120, 5534 CrossRef CAS PubMed.
- D. M. Zhang, H. Zou and S. Z. Cai, Propellants, Explos., Pyrotech., 2017, 42, 953 CrossRef CAS.
- H. Robatjazi, H. Q. Zhao, D. F. Swearer, N. J. Hogan, L. N. Zhou, A. Alabastri, M. J. Mcclain, P. Nordlander and N. J. Halas, Nat. Commun., 2017, 8, 27 CrossRef PubMed.
- J. W. Bennett, J. L. Bjorklund, T. Z. Forbes and S. E. Mason, Inorg. Chem., 2017, 56, 13014 CrossRef CAS PubMed.
- N. Wang, J. H. Peng, A. M. Pang, T. S. He, F. Du and A. Jaramillo-Botero, J. Phys. Chem. C, 2017, 121, 14597 CrossRef CAS.
- L. Bian, Y. J. Shu, J. B. Xu and L. Wang, J. Mol. Model., 2013, 19, 131 CrossRef CAS PubMed.
- S. Q. Zhou, D. H. Li, W. Zhou, X. H. Ju and D. Y. Jiang, Surf. Rev. Lett., 2016, 23, 1650048 CrossRef CAS.
- D. C. Sorescu, J. A. Boatz and D. L. Thompson, J. Phys. Chem. B, 2003, 107, 8953 CrossRef CAS.
- F. G. Wang, R. Tsyshevsky, A. Zverev, A. Mitrofanov and M. M. Kuklja, J. Phys. Chem. C, 2017, 121, 1153 CrossRef CAS.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Phys. Rev. Lett., 2008, 100, 136406 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- A. S. Cooper, Acta Crystallogr., 1962, 15, 578 CrossRef CAS.
- M. K. Shukla and F. Hill, J. Phys. Chem. C, 2014, 118, 310 CrossRef CAS.
- S. Q. Zhou, F. Q. Zhao, X. H. Ju, X. C. Cheng and J. H. Yi, J. Phys. Chem. C, 2010, 114, 9390 CrossRef CAS.
- L. N. Wu, Z. Y. Tian and W. Qin, J. Phys. Chem. C, 2018, 122, 16733 CrossRef CAS.
- E. Sanville, S. D. Kenny, R. Smith and G. Henkelman, J. Comput. Chem., 2007, 28, 899 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00989b |
| This journal is © The Royal Society of Chemistry 2019 |