Open Access Article
Open Access ArticlePd nanoparticle supported reduced graphene oxide and its excellent catalytic activity for the Ullmann C–C coupling reaction in a green solvent†
Surjyakanta Rana*,
G. Bishwa Bidita Varadwaj and
Sreekantha B. Jonnalagadda *
*
School of Chemistry & Physics, College of Agriculture, Engineering & Science, University of KwaZulu-Natal, Durban, South Africa. E-mail: jonnalagaddas@ukzn.ac.za; surjya.nou@gmail.com; Fax: +27 31 260 3091; Tel: +27 31 260 7325 Tel: +27 31 260 3090
First published on 30th April 2019
Abstract
An efficient and easy route to synthesize reduced graphene oxide with well dispersed palladium (Pd) nanoparticles (Pd(0)-RGO) is described. The synthesized materials were fully characterized by different techniques such as: XRD, FTIR, Raman, SEM, and TEM. An average particle size of 7.5 nm for the metal particles was confirmed by TEM analysis. Pd(0)-RGO demonstrated outstanding catalytic activity for Ullmann coupling with 97% yield and good reusability (4 cycles).
Nanoparticles (NPs) proved to be efficient materials with wide applications in the fields of energy, environment, fine chemical synthesis, adsorption and sensors.1–3 Nano catalysts are more efficacious than traditional catalysts because of their higher surface to volume ratio, as well as increased number of active sites.4,5 Among all the nano catalysts, palladium and palladium based nanoparticles with many applications have gained importance in the last decade.6–8 Various types of Pd nanoparticle have been employed as catalysts for different coupling reactions. Due to the recovery and reusability limitations of pure nanoparticles, the use of supported Pd nanoparticles is more cost effective and eco-friendly.
Over the period, numerous carbon based materials such as activated carbon, carbon nanotubes, graphite and graphene have been explored as supports appropriate to the catalyst loading. Among the carbon supports, reduced graphene oxide has evolved as attractive option to be active support, due to its remarkable properties like optical properties, high surface area and electrical conductivity.9–17 Graphene and graphene oxide (GO) have also gained importance as prospective support materials for palladium-catalysed C–C coupling reactions.18 Ullmann C–C coupling reaction, involving two aryl halides yielding biphenyl as a selective product, has attracted researchers' attention in the recent past. Wang et al. reported that palladium modified ordered mesoporous carbon (Pd/OMC) as catalyst gave 43% biphenyl yield at 100 °C in water medium at 6 h.19 Yuan et al. reported excellent yields (96%) towards C–C coupling reaction at 80 °C in 20 h using Pd/MIL-101 as catalyst.20 Liyu et al. showed that MOF-253·0.05PdCl2 as catalyst, the reaction gives 99% yield in DMSO/EtOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 120 °C in 10 h.21 Karimi et al., obtained 95% yield of biphenyl by using Au supported mesoporous silica at 100 °C for 16 h.22 Varadwaj et al., obtained 96% yield of biphenyl in water medium at 80 °C in 6 h, employing Pd(0) nanoparticles supported organ functionalized clay.23
1) at 120 °C in 10 h.21 Karimi et al., obtained 95% yield of biphenyl by using Au supported mesoporous silica at 100 °C for 16 h.22 Varadwaj et al., obtained 96% yield of biphenyl in water medium at 80 °C in 6 h, employing Pd(0) nanoparticles supported organ functionalized clay.23
In this communication, we describe a facile and efficient route for synthesis of Pd nanoparticles supported on reduced graphene oxide and its efficacy as catalyst for Ullmann reaction in water with exceptional yields (97%). Reusability test confirms that the material is perpetual and recyclable up to four cycles.
The graphene oxide was prepared according to modified Hummers' method.24 For the preparation of Pd reduced graphene oxide (Pd(0)-RGO) catalyst: 1.0 g of GO and 50 ml of distilled water was taken in a flask and sonicated for 30 min. Then palladium nitrate was added in the solution with GO, to prepare 5 and 7 wt% of Pd loaded materials. The mixture was stirred for 2 h. Then, 12 mmol of NaBH4 with tetrahydrofuran (10 ml) solvent was added to the mixture, which was constantly stirred for 1 h. The material was filtered and washed, followed by drying at 100 °C overnight in a vacuum oven to obtain 5-Pd(0)-RGO and 7-Pd(0)-RGO materials.
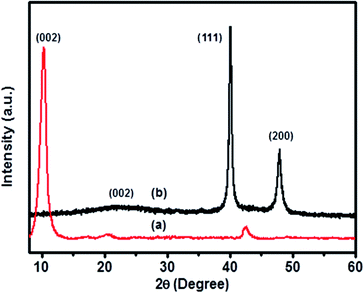
Fig. 1 illustrates the XRD spectra of GO (a) and 5-Pd(0)@RGO (b). In Fig. 1(a), the spectrum represents 2θ ≈ 10.75 corresponding to (002) plane of GO.25,26 In case of the Pd(0) metal modified graphene oxide material converted to Pd(0) reduced graphene oxide [Fig. 1(b)], plane (002) at 2θ ≈ 23.11 is due to the reducing agent.27 The angle at 2θ ≈ 40.11 and 46.79 correspond to (111), (200) planes of Pd metal particles.26 This confirms the presence of Pd(0) metal particles on the RGO surface.
The stretching and bending frequencies of the FT-IR spectra of GO (a) and 5-Pd(0)-RGO (b) samples are illustrated in ESI Fig. S1.† In these spectra, 3400 cm−1, 1740 cm−1 and 1385 cm−1 represent the O–H stretching, O–H bending vibration of C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of –COOH groups respectively, which were clearly attributed to graphene oxide material.28 After modification of Pd metal on the GO surface, maximum number of functional groups disappeared, which is because of the reducing agent.
O stretching of –COOH groups respectively, which were clearly attributed to graphene oxide material.28 After modification of Pd metal on the GO surface, maximum number of functional groups disappeared, which is because of the reducing agent.
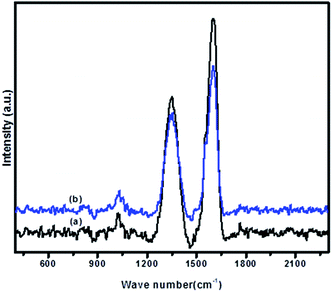
Fig. 2 illustrates the Raman spectra of GO (a) and 5-Pd(0)-RGO (b) samples. In the Raman spectra, all the samples showed the characteristic D-bands at 1342 cm−1 and G-bands at 1595 cm−1.29 The intensity of the ID/IG ratio of normal GO sample is 0.63, but in case of 5-Pd(0)-RGO sample, the intensity of the ID/IG ratio increased to 0.69.
The SEM, TEM and particle size distribution images of 5-Pd(0)-RGO sample are shown in Fig. 3. The SEM and TEM images give the details about the layered sheets of the Pd(0)-RGO sample. The uniform distribution of Pd nanoparticles on the RGO surface was confirmed by transmitted electron microscope monograph. The average particle size of the nanoparticles was 7.5 nm as calculated from TEM image (Fig. 3(d)).
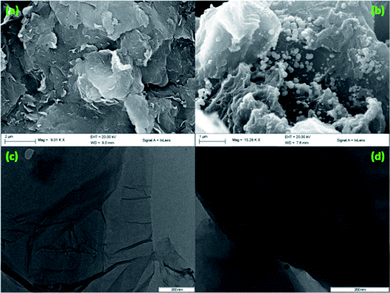 | ||
| Fig. 3 SEM image of GO, scale bar = 2 μm (a), 5-Pd(0)-RGO, scale bar = 1 μm (b) and TEM image of GO, scale bar = 200 nm (c), 5-Pd(0)-RGO, scale bar = 200 nm (d) catalyst. | ||
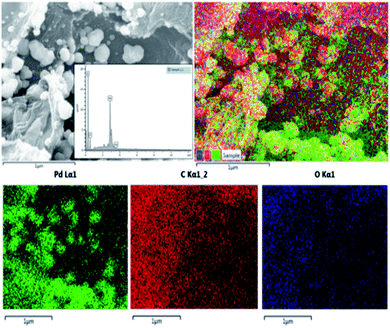
The SEM/EDX analysis provides information on elements present on the material. Fig. 4 illustrates the SEM/EDX and colour mapping images of 5-Pd(0)-RGO catalyst. The images validate the presence of Pd, C and O on the catalyst, which is also highlighted through their colour mapping. Fig. S2, in the ESI† shows the binding energy of Pd. Binding energy of Pd 3d5/2 and Pd 3d3/2 were 335.7 eV and 341.08 eV, which represent the zero-oxidation state of Pd metal. The exact amount of the Pd metal loaded on the support surface was confirmed by ICP-MS analysis indicating the Pd content in materials was 4.5 wt% and 5.1 wt% respectively.
Ullmann C–C coupling is one of the valuable procedures to produce biaryls and biaryls derivatives. C–C coupling reactions are known to be accelerated by various Pd-based catalysts together with organic solvents30 and aqueous inorganic bases.31 As reported by Li et al., Pd/Ph-SBA-15 catalyst gave 75% yield towards coupling product at 100 °C for 10 h.32 Wan et al. reported that, silica-carbon supported palladium catalyst gave 64% yields towards coupling product and also the reaction was performed under water medium for 6 h.33 Gadda et al. reported 46% conversion and 91% selectivity towards biphenyl at 150 °C in water medium with Pd/C catalyst for Ullmann coupling reaction.34 The inherent drawbacks in these reports were essentially long reaction times and high temperature requirement, which have negative impact both fiscally and environmentally.
We report an efficient Ullmann C–C coupling reaction of two molecules of iodobenzene with excellent yields, using potassium carbonate as base and Pd(0)-RGO as catalyst. No reaction was observed in absence of catalyst. In the preliminary studies, when the coupling reaction was performed in presence of graphene oxide (GO) for 5 h at 80 °C, reaction gave 4% yield. Results with reduced graphene oxide (RGO) and different wt% of Pd(0) modified RGO as catalysts, under similar conditions are summarized in Table 1. Due to the less number of functional group, the RGO gave 11% yields, but the 5-Pd(0)-RGO catalyst, showed excellent yield (97%). In the coupling reaction, the aryl radicals get trapped by the oxygen containing functional group of GO materials compared to RGO materials. So, the catalytic activity of GO is less compared to RGO materials. Using 7-Pd(0)-RGO catalyst, 1% higher activity relative to 5-Pd(0)-RGO catalyst was observed. Based on the cost effective, we choose only 5-Pd(0)-RGO catalyst for optimization of the reaction.
| Entry | Catalyst | Yield (%) |
|---|---|---|
| a Reaction conditions: time, 5 h; temperature, 80 °C; catalyst, 0.03 g; solvent (DD water), 10 ml. Reactants: aryl halides (4.5 mmol); HCOONa (1.10 g); KOH (1.40 g). | ||
| 1 | Without catalyst | — |
| 2 | GO | 4 |
| 3 | RGO | 11 |
| 4 | 5-Pd(0)-RGO | 97 |
| 5 | 7-Pd(0)-RGO | 98 |
We investigated efficiency of C–C coupling reactions using different halo benzenes (ArX) with 5-Pd(0)-RGO catalyst (Table 2). An observation the results show that iodobenzene gave best yield (97%) compared with the chloro- and bromo benzenes. The yield of the reaction product depended upon the C–X, bond energy. The C–I bond easily breakdowns facilitating higher yield of the C–C product, than with the other halides, based on bond energies, C–I < C–Br < C–Cl.
| Entry | Aryl halide | Time (h) | Temp (°C) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: time, 5 h; temperature, 80 °C; catalyst 0.03 g; solvent (DD water), 10 ml. Reactants: different aryl halides (4.5 mmol); HCOONa (1.10 g); KOH (1.40 g). | ||||
| 1 | C6H5I | 5 | 80 | 97 |
| 2 | C6H5Cl | 5 | 80 | 79 |
| 3 | C6H5Br | 5 | 80 | 88 |
Solvent plays a vital role in improving the catalytic activity. The effect on different solvents on the reaction yield was investigated (Table 3). While, the polar solvents THF and DMF gave excellent yields, nonpolar solvents like toluene gave lower yields. With water solvent, the yield was nearly same as with polar solvents. Therefore, water the inexpensive green solvent, was chosen as medium for the study.
| Entry | Solvent | Time (h) | Temp (°C) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: time, 5 h; temperature, 80 °C; catalyst, 0.03 g; solvent, 10 ml. Reactants: aryl halides (4.5 mmol); HCOONa (1.10 g); KOH (1.40 g). | ||||
| 1 | Toluene | 5 | 80 | 84 |
| 2 | Water | 5 | 80 | 97 |
| 3 | DMF | 5 | 80 | 98 |
| 4 | THF | 5 | 80 | 96 |
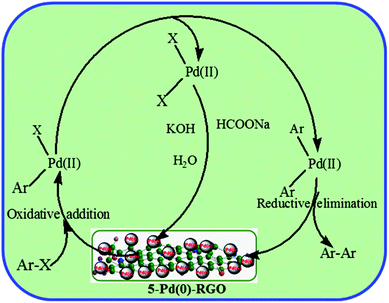
The reaction mechanism of Ullmann C–C coupling reaction over 5-Pd(0)-RGO catalyst is represented in Scheme 1. In the first step followed by oxidative addition, the aryl halide reacts with Pd(0) species on catalyst surface to form a (Ar–Pd(II)–I) complex as a reactive intermediate. Then, the (I–Pd(II)–Ar) complex reacts with another aryl halide molecule to produce a Ar–Pd(II)–Ar and I–Pd(II)–I complex. In the intermediate state, dihydrogen was generated by sodium formate and water in presence of Pd(0). Dihydrogen can reduce I–Pd(II)–I complex to Pd(0).35 Further, the Ar–Pd(II)–Ar complex gives Ar–Ar product and Pd(II) to Pd(0) through reductive elimination process.
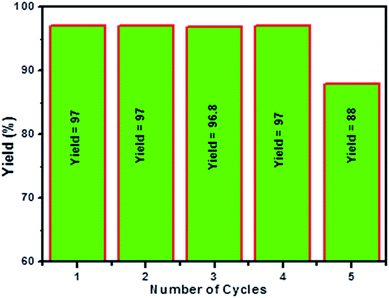
Fig. 5 illustrates the results of reusability test for Pd(0)-RGO heterogeneous catalyst. After completion of each reaction, the used catalyst was filtered, and washed several times in ethanol, and dried at 100 °C. There was no loss of catalytic activity and it is repeatedly used for four times. The catalytic activity reduced after fourth run, due to partial leaching the metal particles.
Using a simple procedure, material with Pd nanoparticles supported on reduced graphene was successfully prepared. Pd(0)-RGO proved to be effective, stable and recyclable material for Ullmann coupling reaction with excellent yields (79–97%) in water medium. The particle size of metal particles was confirmed by TEM analysis. XPS spectra validated the zero-valent state of Pd metal.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the support received from the School of Chemistry & Physics and College of Agriculture, Engineering & Science, University of KwaZulu-Natal, Durban, South Africa in the form of research facilities and financial support.Notes and references
- A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973 RSC.
- A. Fihri, M. Bouhrara, B. Nekoueishahraki, J. M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181 RSC.
- M. Stratakis and H. Garcia, Chem. Rev., 2012, 112, 4469 CrossRef CAS PubMed.
- K. N. Sharma, H. Joshi, V. V. Singh, P. Singh and A. K. Singh, Dalton Trans., 2013, 42, 3908 RSC.
- K. N. Sharma, H. Joshi, A. K. Sharma, O. Prakash and A. K. Singh, Organometallics, 2013, 32, 2443 CrossRef CAS.
- S. W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642 CrossRef CAS PubMed.
- L. Piccolo, A. Valcarcel, M. Bausach, C. Thomazeau, D. Uziob and G. Berhault, Phys. Chem. Chem. Phys., 2008, 10, 5504 RSC.
- J. Watt, S. Cheong, M. F. Toney, B. Ingham, J. Cookson, P. T. Bishop and R. D. Tilley, ACS Nano, 2010, 4, 396 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132 CrossRef CAS PubMed.
- X. Huang, Z. Yin, S. Wu, X. Qi, Q. He, Q. Zhang, Q. Yan, F. Boey and H. Zhang, Small, 2011, 7, 1876 CrossRef CAS PubMed.
- L. T. Qu, Y. Liu, J. B. Baek and L. Dai, ACS Nano, 2010, 4, 1321 CrossRef CAS PubMed.
- D. Yu and L. Dai, J. Phys. Chem. Lett., 2010, 1, 467 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906 CrossRef CAS PubMed.
- Z. Wei, H. Liu, Y. Chen, D. Guo, R. Pan and Y. liu, Chin. J. Chem. Eng., 2018, 26, 2542–2548 CrossRef.
- L. Pan, H. Zhao, W. Shen, X. Dong and J. Xu, J. Mater. Chem. A, 2013, 1(24), 7159–7166 RSC.
- G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mülhaupt, J. Am. Chem. Soc., 2009, 131, 8262 CrossRef CAS PubMed.
- H. Wang and Y. Wan, J. Mater. Sci., 2009, 44, 6553 CrossRef CAS.
- B. Yuan, Y. Pan, Y. Li, B. Yin and H. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4054 CrossRef CAS PubMed.
- L. Chen, Z. Gao and Y. weil, Catal. Today, 2015, 245, 122 CrossRef CAS.
- B. Karimi and F. K. Esfahani, Chem. Commun., 2011, 47, 10452 RSC.
- G. Bishwa Bidita Varadwaj, S. Rana and K. M. Parida, J. Phys. Chem. C, 2014, 118, 1640 CrossRef.
- G. Bishwa Bidita Varadwaj, O. A. Oyetade, S. Rana, B. S. Martincigh, S. B. Jonnalagadda and V. O. Nyamori, ACS Appl. Mater. Interfaces, 2017, 9, 17290 CrossRef PubMed.
- Y. J. Li, W. Gao, L. J. Ci, C. M. Wang and P. M. Ajayan, Carbon, 2010, 48, 1124–1126 CrossRef CAS.
- H. K. Jeong, Y. P. Lee, R. J. W. E. Lahaye, M. H. Park, K. H. An, I. J. Kim, C.-W. Yang, C. Y. Park, R. S. Ruoff and Y. H. Lee, J. Am. Chem. Soc., 2008, 130, 1362 CrossRef CAS PubMed.
- R. N. Singh and R. Awasthi, Catal. Sci. Technol., 2011, 1, 778 RSC.
- S. Rana, S. Maddila, K. Yalagala and S. B. Jonnalagadda, Appl. Catal., A, 2015, 505, 539 CrossRef CAS.
- S. Rana and S. B. Jonnalagadda, Catal. Commun., 2017, 92, 31 CrossRef CAS.
- J. A. Corral, M. I. López, D. Esquivel, M. Mora, S. Jiménez-Sanchidrián and F. J. Romero-Salguero, Materials, 2013, 6, 1554 CrossRef CAS PubMed.
- S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef CAS.
- H. Li, W. Chai, F. Zhang and J. Chen, Green Chem., 2007, 9, 1223 RSC.
- Y. Wan, H. Wang, Q. Zhao, M. Klingstedt, O. Terasaki and D. Zhao, J. Am. Chem. Soc., 2009, 131, 4541 CrossRef CAS PubMed.
- T. M. Gadda, Y. Kawanishi and A. Miyazawa, Synth. Commun., 2012, 42, 1259 CrossRef.
- S. Mukhopadhyay, G. Rothenberg, D. Gitis, H. Wiener and Y. Sasson, J. Chem. Soc., Perkin Trans. 2, 1999, 2, 2481 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Reaction procedure and instrumentation, FTIR figures. See DOI: 10.1039/c9ra01715a |
| This journal is © The Royal Society of Chemistry 2019 |