Open Access Article
Open Access ArticleThe effect of different surface treatment methods on the physical, chemical and biological performances of a PGA scaffold
Yimin Songa,
Minghua Renb,
Yadong Wu *c,
Siyu Lib,
Chun Songb,
Fang Wangc and
Yudong Huangc
*c,
Siyu Lib,
Chun Songb,
Fang Wangc and
Yudong Huangc
aDepartment of Health Medicine, Peking Union Medical College Hospital, Beijing 100730, China
bSurgery Department, the Key Laboratory of Cell Transplantation of Ministry of Health of the First Affiliated Hospital of Harbin Medical University, Harbin, 150001 China
cMIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, 150001, China. E-mail: wuyadong@hit.edu.cn; Fax: +86-451-86414806; Tel: +86-451-86414806
First published on 28th June 2019
Abstract
In order to improve the adhesion between a PGA scaffold and islet cells, it is necessary to find a suitable method to modify the scaffold. In this study, the PGA scaffold surface was modified by plasma, polylysine coating and plasma combined with polylysine coating (P–P-PGA). The surface adhesion of the modified PGA scaffold was examined, and the stretchability and infiltration of the PGA scaffold were also tested. Then, the PGA scaffold treated under the optimal treatment conditions was selected to co-culture with rat islet cells, and the survival activity of the rat islet cells on the untreated PGA scaffold and the P–P-PGA scaffold was examined via the MTT method. Rhodamine staining and DAPI staining were used to detect the number of islet cells adhered to four groups of scaffolds at different culture time points. The PGA-islet graft in the leg muscle of rats was stained with HE to perform the PGA-islet graft pathological examination. The experimental results showed that when the plasma treatment power was 240 W, the processing time was 4 min; the concentration of the polylysine coating solution was 2 mg ml−1, the tensile strength of the PGA scaffold was 320.45 MPa and the amount of infiltration of the PGA scaffold by the serum medium presented the maximum value: 3.17 g g−1. The MTT survival activity test results showed that after 3 d of culture, the survival activity of the islet cells of the treated PGA scaffold culture group (2.02 ± 0.13) was significantly different from the survival activity of the islet cells of the untreated PGA scaffold culture group (1.93 ± 0.10). The survival activities of the islet cells in the experimental groups (1.60 ± 0.13, 1.40 ± 0.12) were still higher than those of the control groups (0.96 ± 013, 0.69 ± 0.09) at 15 and 21 d. The results of the rhodamine and DAPI staining showed that with the increase in culture time, the number of the adherent cells in each group increased, and the number of the adherent islet cells in the experimental group was higher than that in the untreated group. The HE staining results showed that the islet cells on the P–P-PGA scaffold were more than those on the untreated PGA scaffold. After modification of the PGA scaffold, the adhesion of the islet cells improved, which was conducive to the growth of islet cells. These results confirmed that the plasma combined with polylysine coating treatment could enhance the adhesion of the PGA scaffold surface, so that the scaffold and the islet cells exhibited better adhesion and biocompatibility, and the modified PGA scaffold (P–P-PGA) could be used as a promising islet cell scaffold.
1. Introduction
Polyglycolic acid (PGA)1 is the simplest linear aliphatic polyester,2 and the lack of toxicity, immunogenicity and accumulation in the cells and tissues3,4 is advantageous. Therefore, it is the most actively researched biomaterial,5,6 which has been approved by the Food and Drug Administration (FDA).7,8 Due to its superior biodegradability9–11 and biocompatibility12–14 and other excellent properties, PGA can be a potential and promising candidate for biological cell scaffolds.15–18Scaffolds with a specific shape and a linked porous structure can be beneficial for the cell adherence and growth,19–21 and they have been widely applied in bone repair and bone tissue engineering.22–24 In recent years, some researchers have made great efforts to improve the adhesion properties of the PGA scaffold. Some researchers applied a polyurethane coating to the surface of the PGA scaffold materials for modification to promote cell adhesion, growth and proliferation.25 Others dissolved biodegradable polymers, such as polyglycolic acid (PGA), poly-L-lactic acid (PLLA), and polylactic acid (PLGA), in suitable solvents to prepare nanofiber scaffolds; then, they used the oxygen plasma treatment and hydrophilic acrylic acid (AA) in situ grafted on the surface to modify the surface of the scaffold. Thus, the cell adhesion and proliferation capability improved for co-culture.26 Others precoated the polymer scaffold with fibronectin, which is an important protein that can improve the efficiency of cell adhesion.27,28 In addition, the plasma treatment is an effective and widely used method for modifying the material surface,29–31 and polymer surfaces can be modified by the plasma treatment to improve cell adhesion and promote cell growth.32 At present, in order to improve cell adhesion, a variety of surface modification treatment methods have been used in tissue engineering polymer scaffolds.33–35
Islet cell transplantation is an ideal treatment for diabetes36–38 because the immunogenicity of the graft can be reduced and the immunological rejection of the receptor can be eliminated if the cells are transplanted after in vitro culture.39,40 However, it is difficult for the islet cells to survive in an in vitro culture; thus, it is extremely important to select an appropriate scaffold for the islet cell growth. As polylysine exhibits strong adhesion to the surface of the cell membrane, it can promote cell adhesion and growth.41,42
In this study, the PGA scaffold was modified by plasma, polylysine coating and plasma combined with the polylysine coating to improve the adhesion between the PGA scaffold and the islet cells. The effects of plasma treatment with polylysine coating and plasma combined with polylysine coating on the PGA scaffold adhesion were studied by stretchability and infiltration tests. Finally, better modification conditions were selected for the surface treatment of the PGA scaffold, making it more suitable for the islet cell adhesion and growth.
2. Materials and methods
2.1 Materials
PGA scaffolds were obtained from Synthecon, USA. Polylysine was purchased from Sigma, USA. RPMI-1640 medium was supplied by Hyclone, USA. Healthy adult Wistar rats were provided by the experimental animal center of the hospital affiliated to Harbin Medical University. Fetal bovine serum was supplied by Hyclone, USA. Penicillin and streptomycin were purchased from Hyclone, USA. Hank's solution was provided by Gibco, USA. MTT and DMSO were purchased from Amresco, USA. All chemicals were used without further purification.2.2 Plasma treatment on PGA scaffold
PGA scaffolds were cut into 2 cm × 2 cm samples and placed inside the plasma cavity. The vacuum pump and the high frequency power supply were turned on and tuned to produce air plasma in the plasma cavity. The power and the reaction time were recorded after the completion of the air cooled plasma activation treatment and then, the energetic power and the vacuum pump were turned off. Finally, the scaffold was removed and set aside.2.3 Coating treatment on the surface of PGA scaffold
Prior to the experiment, polylysine mixtures in aqueous solutions at different concentrations (0.5 mg ml−1, 1 mg ml−1, 2 mg ml−1, and 4 mg ml−1) were prepared. The PGA scaffold (sample of 2 cm × 2 cm) was then immersed into the above solution for coating treatment, and the soaking time was recorded. After the reaction, the scaffold was dried for 24 h for backup use.2.4 Plasma combined with coating treatment
After the air-cooled plasma activation, the high-energy power supply and the vacuum pump were turned off; the sample was quickly removed and immersed into a polylysine coating solution of a certain concentration, and the dip time was recorded. After the reaction, the support was removed and the scaffold was air dried for 24 h for backup use.2.5 Stretchability test of PGA scaffold
The PGA scaffold was cut into 1 cm × 5 cm pieces and then, it was clamped between the two parallel beams of the CMT8102 electronic universal testing machine (New think again co., LTD., China). The stretchability was tested at the loading speed of 2 mm s−1.2.6 Infiltration performance test of PGA scaffold
The PGA scaffold was cut into pieces and immersed into the serum culture medium (RPMI-1640 medium). Then, this mixture was transferred into a pipe, which was 50 mm in length and 0.35 mm in diameter. After that, the pipe was placed vertically in a HB-312 type fiber infiltration meter (Chemical Research Institute in Beijing, China) to test the infiltration performance, and the test was automatically terminated when the difference between two measurements was less than 0.1 mg.2.7 Cell culture
Healthy adult Wistar rats, weighing 150–200 g, were randomly selected from male and female rats, and this was approved by the animal ethics committee of the hospital affiliated to Harbin Medical University. The rats were isolated to culture the islet cells. Prior to the assay, the scaffolds were sterilized under the ultraviolet light. The experiment was divided into two groups, i.e., the islet cells cultured on the untreated PGA scaffold (control group) and the scaffold treated under the optimal surface modification conditions. RPMI-1640 served as the culture medium; then, 10% fetal bovine serum (FBS), 1% penicillin (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per ml) and 1% streptomycin (10
000 units per ml) and 1% streptomycin (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg ml−1) were added. After that, the cells were cultured at 37 °C under 5% CO2 in a thermostat incubator (McO-15ac, Sanyo, Japan).
000 μg ml−1) were added. After that, the cells were cultured at 37 °C under 5% CO2 in a thermostat incubator (McO-15ac, Sanyo, Japan).
2.8 MTT assay
The islet cells of the two culture groups were tested by an MTT assay at 0, 3, 7, 11, 15 and 21 d. The method is described as follows: the islet cells on the scaffold were eluted by Hank's solution, and 10 holes in the first row of the 96-well plate were put into the islet cell suspension of the control group (untreated PGA scaffold); each hole was inoculated with 180 μL cell suspension (∼600 islet cells). The islet cells of the experimental group (scaffold modified by plasma combined with polylysine coating) were inoculated into the second row of 10 holes with the same concentration of the cell suspension. After the cells were inoculated, the 96-well plates were incubated in an incubator with 5% CO2 at 37 °C for 24 h. Then, 20 μL of MTT with the final concentration of 5 mg ml−1 was added to each hole and cultured at 37 °C for additional 4 h. After that, the mixture was centrifuged and the supernatant was discarded. Then, 150 μL DMSO was added and the suspension was mixed for 5–10 min. Finally, colorimetry was performed using the MK3 type automatic multi-function enzyme marker (Thermo Labsystems, USA), measuring the light absorption value (OD490 nm) of each hole.2.9 Islet cell adhesion experiment
The experiment was divided into four groups: (1) original PGA scaffold, (2) PGA scaffold treated with plasma, (3) PGA scaffold treated with polylysine, and (4) PGA scaffold treated with plasma combined with polylysine. Ten thousand islet cells were inoculated into the four scaffold groups and incubated in 37 °C with 5% CO2 in the incubator. The number of the adherent islet cells after they were cultured for 12, 24 and 48 h was measured. Then, the islet cells in each group were eluted from the scaffold, and rhodamine and DAPI staining was carried out as follows. The cell culture fluid was discarded and the cells were washed with PBS 3 times (each time for 5 min). Then, the cells were fixed by using 4% PFA (paraformaldehyde) for 15–30 min at room temperature. Next, the cells were washed with PBS 3 times (each time 5 min). Then, 0.1% Triton (Triton X-100) was added in order to perforate the cells for 15 min at room temperature. After that, the cells were washed with PBS 3 times (each time for 5 min) and then, they were blocked with 0.5% BSA at room temperature for 15 min. Rhodamine was then added until the fluid could cover the cells. After incubation for 1 h in the dark, the cells were washed with PBS 3 times (each time for 5 min). Then, DAPI was added and the amount of the fluid should be enough to immerse the cells. After being stained at room temperature for 15 min, the cells were washed for three times with PBS (5 min for each time). The observations of islet cells in the four groups were performed using an inverted fluorescence microscope (Olympus, Japan). Five observation points in each group were randomly selected to calculate the mean value of the adhesion degree, and each experimental group was repeated in three holes.2.10 In vivo experiment
The PGA-islet cells were embedded into the leg muscles of the healthy adult Wistar rats. Eleven days after the surgery, the specimens were taken out, embedded in paraffin and sectioned. Then, wood grain eosin (HE) staining was performed, followed by a water rinse and then dehydration with 80%, 90%, 95% and 100% ethanol, respectively. Xylene was used to produce a transparent section and then, the section was sealed with neutral gum. After that, the nuclei were stained with hematoxylin, and the cell plasma and other connective tissues were stained with eosin.2.11 Statistical analysis
The MTT test results of the rat islet cells in different groups were expressed as mean ± standard deviation (SD) at five time points and were completed by SAS 9.1 software. All quantitative data of the islet cell adhesion test were expressed as mean ± standard deviation (SD). ANOVA was carried out to compare the differences among four groups. Statistical comparisons were performed using the SPSS 17.0 software. A statistical significance was considered when p < 0.05.3. Results and discussion
3.1 Plasma treatment
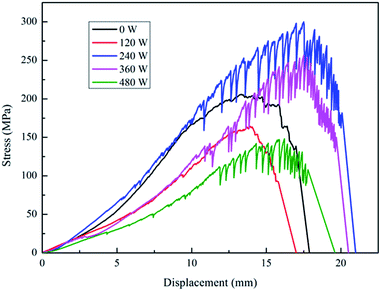
The stress-displacement curves (Fig. 1) show a large fluctuation of the force value at the maximum point. This may be caused by the loosening of the plasma-treated PGA materials, which could be found by the naked eye observation of the treated PGA scaffolds. When the treatment power was 120 W, the PGA scaffold is not very loose, and a continuous fracture can be basically guaranteed when a fracture occurs. Therefore, there is no substantial swing phenomenon of the force value, which is similar to that of the untreated samples. When the treatment power continued to increase, the degree of loosening of the PGA scaffold increased. Because the tested material is made of intertwined PGA scaffolds, when the material became loose, the time difference between the initial fracture and the next resulted in an up and down swing of the force value. The displacement of the samples treated with 240 W, 360 W and 480 W plasma powers could also be explained in the same way. The displacements of the samples treated with the plasma powers of 240 W, 360 W and 480 W were larger than that of the untreated and the 120 W samples.
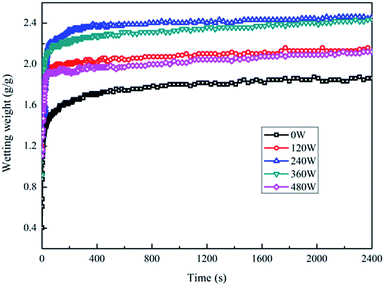
The above conclusions are basically the same as that of the infiltration of the PGA scaffolds by the serum culture medium treated with different powers. However, the difference is that the infiltration of the PGA scaffolds by the serum culture medium treated with plasma for different times showed no obvious decreasing trend. This may be due to the fact that the energy of the particles is basically the same under a fixed power, but the difference is only the contact times between the particles and the PGA scaffold. A long low-energy particle treatment is not equivalent to the short high-energy particle treatment. Therefore, when the treatment power was determined, the prolongation of the treatment time of the low-energy particles produced less effect than the short treatment of the high-energy particles on the surface of the fibers; thus, the decreasing trend of infiltration of the plasma-treated PGA scaffolds by the serum culture medium was not particularly obvious. After the above research, the optimum technological parameters of the plasma treatment of PGA scaffolds could be determined as follows: the treating power was 240 W, and the treating time was 4 min.
3.2 Surface coating treatment of PGA scaffold
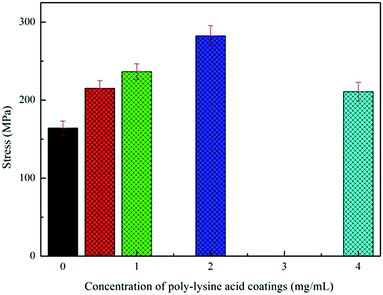 | ||
| Fig. 5 Influence of polylysine coating solution concentration on the mechanical performance of PGA scaffold. | ||
This observation could be explained by the moderate hardness of the polylysine coating and the strong bonding force to the PGA scaffold. Although some of the entangled fibers were separated by the introduction of the coating, the coated fibers were still bonded together by the coating. In addition, there were winding points bonded by the coating, which were also very important to enhance the tensile strength of the PGA scaffolds. Therefore, through the analysis of the tensile strength, it could be confirmed that the polylysine coatings with moderate hardness and viscosity bind the PGA scaffolds formed by filament winding, significantly improving the tensile strength of the PGA scaffolds. However, the polylysine solution exhibited poor fluidity when the concentration of the coating solution was very high, so that it was not easy to form a uniform coating on the surface of the fiber. Therefore, the tensile strength of the scaffold became worse, and the high concentration of polylysine also worsened the flexibility of the scaffold.
3.3 Plasma combined with polylysine surface coating treatment
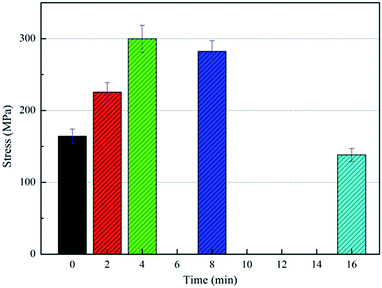
The effects of the plasma treatment and the polylysine coating treatment on the performance of PGA scaffolds were studied, and the appropriate process parameters were selected. These treatments could improve the tensile strength of the PGA scaffolds and the infiltration of the PGA scaffolds. Therefore, we combined the two methods to achieve the best treatment effect. We used the plasma power of 240 W to treat the scaffold for 4 min and the polylysine coating concentration of 2 mg ml−1 to modify the PGA scaffold. The stress-displacement curve of the PGA scaffolds treated with 240 W combined with 2 mg ml−1 polylysine coating shows that the tensile strength of the PGA scaffolds reaches 320.45 MPa, which is higher than the tensile strengths of the PGA scaffolds treated with plasma alone (299.78 MPa) and the coating alone (282.62 MPa). This suggests that plasma combined with the polylysine coating can greatly improve the tensile strength of the PGA scaffolds.At the same time, it can be observed in Fig. 7a that the fracture of the PGA scaffold after plasma bonding with the polylysine coating is basically a ductile fracture, and the tensile displacement is also very long. This implies that the polylysine coating makes a strong interfacial bond with the PGA scaffold. Therefore, the mechanical performance of the PGA scaffolds treated by plasma combined with the polylysine coating meets the expected requirements.
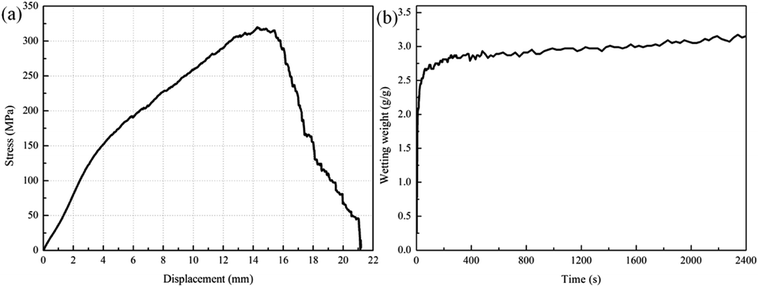 | ||
| Fig. 7 Influence of plasma treatment combined with polylysine surface coating treatment on (a) the mechanical performance and (b) wettability of PGA scaffold. | ||
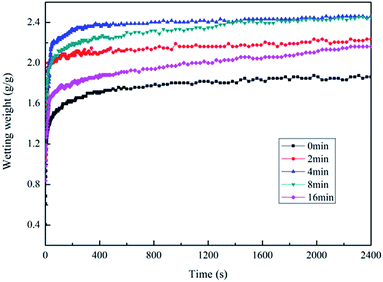
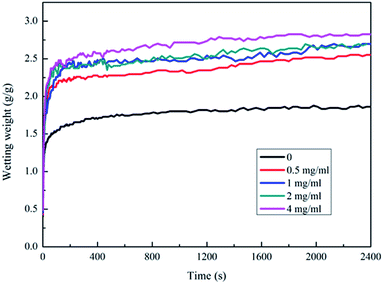
The infiltration curve of the plasma combined with polylysine coating PGA scaffold is exhibited in Fig. 7b. The maximum infiltration of the PGA scaffold by the serum culture medium is 3.17 g g−1, which is significantly higher than that of the PGA scaffold treated with plasma alone or the polylysine coating alone. This indicates that plasma combined with polylysine coating is useful to improve the infiltration of the PGA scaffold.
Many polar functional groups emerged on the surface of PGA after the plasma treatment, and these groups could improve the surface energy of the PGA scaffolds, so that the tensile strength and wettability of the scaffolds could be improved. At the same time, these polar functional groups could also interact with the polylysine coatings that infiltrated the PGA scaffolds in various physical and chemical ways, thus improving the interfacial bonding between the PGA scaffolds and the polylysine coatings and the infiltration of the PGA scaffolds. Besides, the polylysine coatings could increase the tensile strength of the PGA scaffolds. Furthermore, the coatings exhibited excellent adhesion to and infiltration of the PGA scaffolds, which helped improve the wettability of the polylysine coatings in the serum culture medium.
The application of the plasma treatment combined with the polylysine coating on PGA scaffolds significantly improved the mechanical performance and wettability. The experimental results reveal that the plasma treatment combined with the polylysine coating treatment is an ideal method for the modification of PGA, which can provide an adequate scaffold for cell adhesion and growth in vitro.
3.4 Analysis of MTT detection results
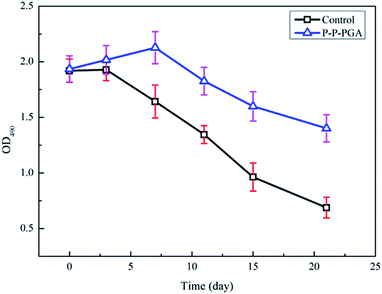
As mentioned above, the optimal PGA scaffold modified by plasma combined with polylysine coating was selected to carry out the subsequent analysis. The MTT assay is a method for detecting cell activity and growth. Succinic acid dehydrogenase in the mitochondria of the living cells can reduce exogenous MTT to insoluble blue-purple crystals (formazan), which will deposit in cells. However, dead cells cannot exhibit this feature. Thus, we could use DMSO to dissolve the purple crystals and then measure OD490 nm to indirectly determine the number of living cells. Within a certain range of cells, the amount of MTT crystals formed is proportional to the number of cells. Islet cells were non-renewable in this study; thus, the MTT method could be used to detect the survival activity of the islet cells in rats, so that the biological adhesion between the islet cells and the PGA scaffold material could be determined. As the PGA scaffold was modified by plasma combined with the polylysine coating, improving its adhesion performance, the islet cells could adhere to the scaffold and grow. The more the islet cells adhering to the scaffold, the higher the survival activity of islet cells, indicating better biocompatibility between the islet cells and the scaffold.As displayed in Fig. 8, the islet cells in the control group (untreated PGA scaffold) and the experimental group (PGA scaffold modified by plasma combined with polylysine coating, P–P-PGA) at different times determined by the MTT test showed significant differences (p < 0.05). The survival activity of the islet cells in the experimental group after 3 d (2.02 ± 0.13) was significantly higher than that in the control group (1.93 ± 0.10). After 15 and 21 d, the islet cell survival activities in the experimental groups (1.60 ± 0.13, 1.40 ± 0.12) were still higher than those in the control groups (0.96 ± 0.13, 0.69 ± 0.09). The MTT curve of the control group exhibited a rapid declining stage, which indicated that the survival activity of islet cells decreased obviously. However, the decrease in the MTT curve of the experimental group was slow, implying that the survival activity of the cells slowly decreased, which was obviously higher than that of the control group. It is worth noting that the islet cell survival activity on the scaffold modified by plasma combined with the polylysine coating was significantly higher than that of the cells on the untreated PGA scaffold.
3.5 Experimental results of islet cell adhesion
Rhodamine and DAPI staining was used to detect the number of the islet cells adhered to the scaffolds of the 4 groups at different culture times. DAPI stains the nuclei blue and rhodamine stains the cytoskeleton red. The islet cells are classified into single islet cells (represented by a) and a larger islet cell mass (represented by b) in Fig. 9.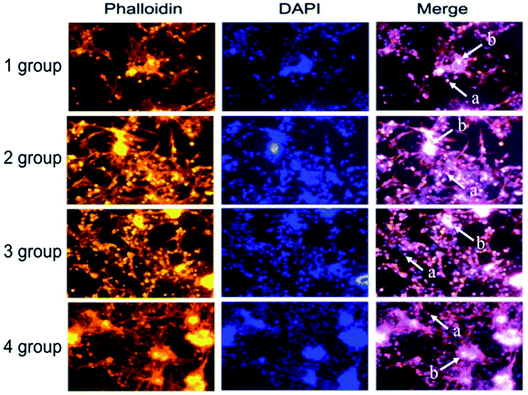 | ||
| Fig. 9 Images of the stained islet cells in different experimental groups (a represents individual islet cells and b is the larger islet cell mass). | ||
The adhesion of the islet cells on the scaffolds in 4 groups (1: original PGA scaffold, 2: plasma-treated PGA scaffold, 3: polylysine-coated PGA scaffold, and 4: P–P-PGA) at different culture times is shown in Fig. 10. After being cultured for 12 h, islet cell adhesion numbers were 154 ± 6.25 (original PGA scaffold), 191 ± 8.08 (plasma-treated PGA scaffold), 243.67 ± 6.12 (polylysine-coated PGA scaffold), and 282.67 ± 6.09 (P–P-PGA). The numbers of the adhesive islet cells in the experimental group were greater than that in the untreated group, and the difference was statistically significant (p < 0.05). When cultured for 24 h, the numbers of the adhered islet cells in the four groups were 169.67 ± 11.59 (original PGA scaffold), 343.3 ± 7.41 (plasma-treated PGA scaffold), 453 ± 5.67 (polylysine-coated PGA scaffold), and 519.3 ± 16.11 (P–P-PGA). As the time increased, the number of the adhered islet cells in each group increased, and the number of the adhered islet cells in the experimental group was higher than that in the untreated group. The number of the adhered islet cells in the fourth group was the highest, and the difference was statistically significant (p < 0.01). After being cultured for 48 h, the numbers of the adhered islet cells in the four groups were 229.67 ± 11.59 (original PGA scaffold), 343.3 ± 7.41 (plasma-treated PGA scaffold), 453 ± 5.67 (polylysine-coated PGA scaffold), and 519.3 ± 16.11 (P–P-PGA). The adhesion number of the islet cells on the polylysine-coated PGA scaffold was higher than that on the plasma-treated PGA scaffold. The number on P–P-PGA was still the highest, and the numbers of the islet cells on the three modified scaffolds were significantly higher than that on the untreated scaffold (p < 0.001).
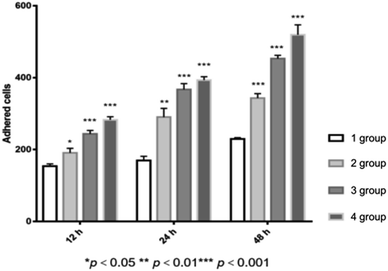 | ||
| Fig. 10 The adhesion study of islet cells on different PGA scaffolds for 12 h, 24 h and 48 h, respectively (*p < 0.05, **p < 0.01, ***p < 0.001). | ||
Thus, it could be concluded that the plasma treatment or the polylysine coating is able to enhance the adhesion of the PGA scaffold to the islet cells. However, the two methods exhibit different actions and mechanisms to enhance adhesion. The plasma treatment could increase the number of the oxygen-containing groups on the surface of the scaffold, and the polylysine coating exhibited strong adhesion to the surface of the cell membrane. Besides, the enhancement in the adhesion of the PGA scaffold brought by the polylysine coating was more obvious than that caused by the plasma treatment. For the P–P-PGA scaffold, we combined the two methods to modify the scaffold, i.e., we applied two individual methods to modify one PGA scaffold in sequence. The results indicated that the P–P-PGA scaffold exhibited optimal adhesion. Thus, we can say that the link between the modification methods, from plasma, polylysine coating to plasma combined with the polylysine coating, is a progressive relationship.
3.6 Pathology results
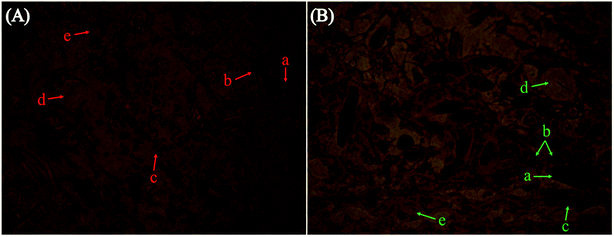
The HE staining images of the PGA-islet cell graft embedded in the rat leg muscle were obtained using a light microscope (LM). Fig. 11A shows the PGA scaffold without modification, and Fig. 11B exhibits the PGA scaffold modified by plasma combined with the polylysine coating. In the pictures, (a) is the tubular PGA scaffold, (b) corresponds to the purple dark islet cells (islet cells form round, irregular shapes or cells clumps), (c) corresponds to the lilac striated muscle fiber cells, (d) is fat, and (e) corresponds to the capillaries. The pathological results showed that the islet cells on the PGA-islet graft were in good shape with close adhesion on the PGA scaffolds. The PGA scaffold simulates the extracellular matrix for supporting the islet cells, making the cells form a three-dimensional growth pattern and keeping some space between the pancreatic islet cells. This can protect the islet cells from the backlog of the muscle fibers, which contain abundant capillaries. Thus, the PGA scaffold-islet grafts could absorb sufficient nutrients to maintain the growth of PGA scaffold-islet grafts in the muscle tissues. Inflammatory cells could not be observed from the pathological results, indicating that the PGA scaffold and muscle fiber tissues have good biocompatibility.As shown in Fig. 11B, many islet cells adhere and grow around the PGA scaffold modified by plasma combined with polylysine coating. However, the islet cells in the PGA scaffold-islet cell graft in Fig. 11A are not as abundant as those seen in Fig. 11B. The results indicate that cell adhesion on the modified PGA scaffolds is better than that of the untreated PGA scaffolds. This is because the adhesion of the PGA scaffold is improved after the modification by plasma combined with polylysine, which is beneficial to the islet cell adhesion and can create a favorable environment for the islet cells within the muscle tissue.
In recent years, with the continuous development of tissue engineering, the macromolecular biomaterials that can be used as cell scaffolds have been widely used.15,43,44 Cell scaffolds can support cell infiltration and protect the cells,45,46 and they can also provide a three-dimensional structure for the cell culture in vitro.47 In this study, the PGA scaffold was treated with three different surface modification methods, and the optimal conditions for the modification were 240 W plasma for 4 min and the polylysine impregnation concentration of 2 mg ml−1. After modification, the adhesion performance of the PGA scaffold could be increased. Besides, it could increase the ingrowth access and the surface adhesion area for the islet cells; thus, the PGA scaffold provides an excellent condition for the adhesion of islet cells.
4. Conclusion
This study used three methods, namely, plasma, polylysine coating and plasma combined with polylysine coating to modify PGA scaffolds. The results confirmed that plasma combined with the polylysine coating is the best treatment method for the modification of the PGA scaffold. When the plasma processing power was 240 W, the treatment time was 4 min, and the polylysine coating liquid concentration was 2 mg ml−1, the tensile strength of the PGA scaffold was 320.45 MPa and the infiltration amount of the scaffold with the serum culture was up to 3.17 g g−1. The PGA scaffold treated under the optimal conditions was co-cultured with islet cells. The results of the MTT test indicated that the survival activity of the islet cells in the modified scaffold was higher than that of the untreated scaffold. Rhodamine with DAPI staining results showed that with the increase in the incubation time, the number of the adhesive cells increased in each group, and it was higher in the experimental group than in the untreated group. However, the number of the islet cells adhered in the plasma-conjugated polylysine treatment (P–P-PGA) group was the highest among the four groups, and the difference was statistically significant (p < 0.01). The HE staining results also proved that the adhesion of the modified scaffold was better than that of the original scaffold. Therefore, it can be concluded that the PGA scaffold modified by plasma combined with the polylysine coating can be potentially used as a scaffold for islet cells.Statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the US National Institutes of Health (NIH) and approved by the Animal Ethics Committee of Harbin Medical University.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (No. 30570931). This study was also financially supported by China Postdoctoral Science Foundation (2013M541372). We would like to acknowledge the financial support from Heilongjiang Postdoctoral Fund (LBH-Z13086). This study was also supported by the Fundamental Research Funds for the Central Universities (Grant No. HIT. NSRIF. 2015047).References
- L. E. Niklason, J. Gao, W. M. Abbott, K. K. Hirschi, S. Houser, R. Marini and R. Langer, Functional arteries grown in vitro, Science, 1999, 284, 489–493 CrossRef CAS PubMed.
- H. F. Liu, X. M. Li, X. F. Niu, G. Zhou, P. Li and Y. B. Fan, Improved hemocompatibility and endothelialization of vascular grafts by covalent immobilization of sulfated silk fibroin on poly(lactic-co-glycolicacid) scaffold, Biomacromolecules, 2011, 12, 2914–2924 CrossRef CAS PubMed.
- K. A. Athanasiou, G. G. Niederauer and C. M. Agrawal, Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid polyglycolic acid copolymers, Biomaterials, 1996, 17, 93–102 CrossRef CAS PubMed.
- J. Nikolovski and D. J. Mooney, Smooth muscle cell adhesion to tissue engineering scaffolds, Biomaterials, 2000, 21, 2025–2032 CrossRef CAS PubMed.
- H. Cao and N. Kuboyama, A biodegradable porous composite scaffold PGA/beta-TCP for bone tissue engineering, Bone, 2010, 46, 386–395 CrossRef CAS PubMed.
- Y. C. Wang, M. C. Lin, D. M. Wang and H. J. Hsieh, Fabrication of a novel porous PGA-chitosan hybrid matrix for tissue engineering, Biomaterials, 2003, 24, 1047–1057 CrossRef CAS PubMed.
- J. P. Jarow, H. U. Ahmed, P. L. Choyke, S. S. Taneja and P. T. Scardino, Partial gland ablation for prostate cancer: Report of a Food and Drug Administration, American Urological Association, and Society of Urologic Oncology Public Workshop, Urology, 2016, 88, 8–13 CrossRef PubMed.
- M. F. Meek and J. H. Coert, US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves, Ann. Plast. Surg., 2008, 60, 466–472 CrossRef CAS PubMed.
- L. Yu, Z. Zhang, H. Zhang and J. Ding, Biodegradability and biocompatibility of thermoreversible hydrogels formed from mixing a sol and a precipitate of block copolymers in water, Biomacromolecules, 2010, 11, 2169–2178 CrossRef CAS PubMed.
- A. P. Acharya, J. S. Lewis and B. G. Keselowsky, Combinatorial co-encapsulation of hydrophobic molecules in poly(lactide-co-glycolide) microparticles, Biomaterials, 2013, 34, 3422–3430 CrossRef CAS PubMed.
- I. B. Bajaj and R. S. Singhal, Enhanced production of poly(γ-glutamic acid) from bacillus licheniformis NCIM2324 by using metabolic precursors, Appl. Biochem. Biotechnol., 2009, 159, 133–141 CrossRef CAS PubMed.
- K. G. Battiston, R. S. Labow and J. P. Santerre, Protein Binding Mediation of Biomaterial-Dependent Monocyte Activation on a Degradable Polar Hydrophobic Ionic Polyurethane, Biomaterials, 2012, 33, 8316–8328 CrossRef CAS PubMed.
- R. Langer and J. P. Vacanti, Tissue engineering, Science, 1993, 260, 920–926 CrossRef CAS PubMed.
- W. H. Ryu, S. W. Min, K. E. Hammerick, M. Vyakarnam, R. S. Greco, F. B. Prinz and R. J. Fasching, The construction of three-dimensional micro-fluidic scaffolds of biodegradable polymers by solvent vapor based bonding of micro-molded layers, Biomaterials, 2007, 28, 1174–1184 CrossRef CAS PubMed.
- L. D. Zhang and L. J. Hui, Bioengineering: Bile ducts regenerated, Nature, 2017, 547, 171–172 CrossRef CAS PubMed.
- P. Bianco and P. G. Robey, Stem cells in tissue engineering, Nature, 2001, 414, 118–121 CrossRef CAS PubMed.
- T. Z. Li, C. Z. Jin, B. H. Choi, M. S. Kim, Y. J. Kim, S. R. Park, J. H. Yoon and B. H. Min, Using cartilage extracellular matrix (CECM) membrane to enhance the reparability of the bone marrow stimulation technique for articular cartilage defect in canine Model, Adv. Funct. Mater., 2012, 22, 4292–4300 CrossRef CAS.
- K. H. Choi, B. H. Choi, S. R. Park, B. J. Kim and B. H. Min, The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes, Biomaterials, 2010, 31, 5355–5365 CrossRef CAS PubMed.
- C. L. Zhu, S. Pongkitwitoon, J. C. Qiu, S. Thomopoulos and Y. N. Xia, Design and fabrication of a hierarchically structure scaffold for tendon-to-bone repair, Adv. Mater., 2018, 30, 1707306 CrossRef PubMed.
- K. Wang, X. Wang, C. S. Han, W. D. Hou, J. Y. Wang, L. Y. Chen and Y. Luo, From micro to macro: the hierarchical design in a micropatterned scaffold for cell assembling and transplantation, Adv. Mater., 2017, 29, 1604600 CrossRef PubMed.
- Y. Sumita, M. J. Honda, T. Ohara, S. Tsuchiya, H. Sagara, H. Kagami and M. Ueda, Performacne of collagen sponge as a 3-D scaffold for tooth-tissue engineering, Biomaterials, 2006, 27, 3238–3248 CrossRef CAS PubMed.
- P. Feng, J. Y. He, S. P. Peng, C. D. Gao, Z. Y. Zhao, S. X. Xiong and C. J. Shaui, Characterizations and interfacial reinforcement mechanisms of multicomponent biopolymer based scaffold, Mater. Sci. Eng. Carbon, 2019, 100, 809–825 CrossRef CAS PubMed.
- P. Feng, P. Wu, C. D. Gao, Y. W. Yang, W. Guo, W. J. Yang and C. J. Shuai, A multimaterial scaffold with tunable properties: toward bone tissue repair, Adv. Sci., 2018, 5, 1700817 CrossRef PubMed.
- C. D. Gao, S. P. Peng, P. Feng and C. J. Shuai, Bone biomaterials and interactions with stem cells, Bone Res., 2017, 5, 17059 CrossRef CAS PubMed.
- R. S. Tare, F. Khan, G. Tourniaire, S. M. Morgan, M. Bradley and R. O. C. Oreffo, A microarray approach to the identification of polyurethanes for the isolation of human skeletal progenitor cells and augmentation of skeletal cell growth, Biomaterials, 2009, 30, 1045–1055 CrossRef CAS PubMed.
- K. Park, Y. M. Ju, J. S. Son, K. D. Ahn and D. K. Han, Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts, J. Biomater. Sci., Polym. Ed., 2007, 18, 369–382 CrossRef CAS PubMed.
- H. H. Lu, J. A. Cooper, S. Manuel, J. W. Freeman, M. A. Attawia, F. K. Ko and C. T. Laurencin, Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies, Biomaterials, 2005, 26, 4805–4816 CrossRef CAS PubMed.
- R. S. Bhati, D. P. Mukherjee, K. J. McCarthy, S. H. Rogers, D. F. Smith and S. W. Shalaby, The growth of chondrocytes into a fibronectin-coated biodegradable scaffold, J. Biomater. Sci., Polym. Ed., 2001, 56, 74–82 CAS.
- F. Mwale, H. T. Wang, V. Nelea, L. Luo, J. Antoniou and M. R. Wertheimer, The effect of glow discharge plasma surface modification of polymers on the osteogenic differentiation of committed human mesenchymal stem cells, Biomaterials, 2006, 27, 2258–2264 CrossRef CAS PubMed.
- Y. Yang, K. Kulangara, R. T. S. Lam, R. Dharmawan and K. W. Leong, Effects of topographical and mechanical property alterations induced by oxygen plasma modification on stem cell behavior, ACS Nano, 2012, 6, 8591–8598 CrossRef CAS PubMed.
- Y. Guo, D. L. Shi, H. S. Cho, Z. Y. Dong, A. Kulkarni, G. M. Pauletti, W. Wang, J. Lian, W. Liu, L. Ren, Q. Q. Zhang, G. K. Liu, C. Huth, L. M. Wang and R. C. Ewing, In vivo imaging and drug storage by quantum-dot-conjugated carbon nanotubes, Adv. Funct. Mater., 2008, 18, 2489–2497 CrossRef CAS.
- Y. Q. Wan, J. Yang, J. L. Yang, J. Z. Bei and S. G. Wang, Cell adhesion on gaseous plasma modified poly-(L-lactide) surface under shear stress field, Biomaterials, 2003, 24, 3757–3764 CrossRef CAS PubMed.
- H. Shin, S. Jo and A. G. Mikos, Biomimetic materials for tissue engineering, Biomaterials, 2003, 24, 4353–4364 CrossRef CAS PubMed.
- Z. W. Ma, M. Kotaki, R. Inai and S. Ramakrishna, Potential of nanofiber matrix as tissue-engineering scaffolds, Tissue Eng., 2005, 11, 101–109 CrossRef CAS PubMed.
- T. Desmet, R. Morent, N. De Geyter, C. Leys, E. Schacht and P. Dubruel, Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: a review, Biomacromolecules, 2009, 10, 2351–2378 CrossRef CAS PubMed.
- E. A. Ryan, J. R. T. Lakey, B. W. Paty, S. Imes, G. S. Korbutt, N. M. Kneteman, D. Bigam, R. V. Rajotte and A. M. J. Shapiro, Successful islet transplantation – continued insulin reserve provides long-term glycemic control, Diabetes, 2002, 51, 2148–2157 CrossRef CAS PubMed.
- R. P. Robertson, Medical progress: islet transplantation as a treatment for diabetes – a work in progress, N. Engl. J. Med., 2004, 350, 694–705 CrossRef CAS PubMed.
- P. Fiorina, A. M. J. Shapiro, C. Ricordi and A. Secchi, The clinical impact of islet transplantation, Am. J. Transplant., 2008, 8, 1990–1997 CrossRef CAS PubMed.
- L. P. Rutzky, S. Bilinski, M. Kloc, T. Phan, H. M. Zhang, S. M. Katz and S. M. Stepkowski, Microgravity culture condition reduces immunogenicity and improves function of pancreatic islets1, Transplantation, 2002, 74, 13–21 CrossRef PubMed.
- J. Park, B. Andrade, Y. Seo, M. J. Kim, S. C. Zimmerman and H. Kong, Engineering the surface of therapeutic “living” cells, Chem. Rev., 2018, 118, 1664–1690 CrossRef CAS PubMed.
- P. Orning, K. S. Hoem, A. E. Coron, G. Skjak-Braek, T. E. Mollnes, O. L. Brekke, T. Espevik and A. M. Rokstad, Alginate microsphere compositions dictate different mechanisms of complement activation with consequences for cytokine release and leukocyte activation, J. Controlled Release, 2015, 229, 58–69 CrossRef PubMed.
- K. E. Crompton, J. D. Goud, R. V. Bellamkonda, T. R. Gengenbach, D. I. Finkelstein, M. K. Horne and J. S. Forsythe, Polylysine-functionalised thermoresponsive chitosan hydrogel for neural tissue engineering, Biomaterials, 2007, 28, 441–449 CrossRef CAS PubMed.
- A. Hokugo, T. Takamoto and Y. Tabata, Preparation of hybrid scaffold from fibrin and biodegradable polymer fiber, Biomaterials, 2006, 27, 61–67 CrossRef CAS PubMed.
- W. J. Li, J. A. Cooper, R. L. Mauck and R. S. Tuan, Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications, Acta Biomater., 2006, 2, 377–385 CrossRef PubMed.
- Y. Luo and M. S. Shoichet, A photolabile hydrogel for guided three-dimensional cell growth and migration, Nat. Mater., 2004, 3, 249–253 CrossRef CAS PubMed.
- I. Kreiser-Saunders and H. R. Kricheldorf, Polyactones, 39 – Zn lactate-catalyzed copolymerization of L-lactide with glycolide or epsilon-caprolactone, Macromol. Chem. Phys., 1998, 199, 1081–1087 CAS.
- H. J. Sung, C. Meredith, C. Johnson and Z. S. Galis, The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis, Biomaterials, 2004, 25, 5735–5742 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |