Open Access Article
Open Access ArticleImproved antibacterial performance using hydrogel-immobilized lysozyme as a catalyst in water†
Yuemei Ye ,
Stanislav Klimchuk,
Mingwei Shang
,
Stanislav Klimchuk,
Mingwei Shang and
Junjie Niu
and
Junjie Niu *
*
Department of Materials Science and Engineering, CEAS, University of Wisconsin-Milwaukee, Milwaukee, WI 53211, USA. E-mail: niu@uwm.edu
First published on 28th June 2019
Abstract
Silver nanoparticle-based catalysts are used extensively to kill bacteria in drinking water treatment. However secondary contamination and their high cost require scientists to seek alternatives with non-toxicity, high activity and low cost. In this article, we develop a new hydrogel-immobilized lysozyme (h-lysozyme) that shows excellent antibacterial performance, including high activity duration of up to 55 days, inhibition efficiency as high as 99.4%, good recycling capability of up to 11 cycles, a wide temperature window and extremely low concentration. The immobilized lysozyme displayed greatly improved bacterial inhibition with both Gram-negative E. coli and Gram-positive B. subtilis, which enables broad antibacterial applications in various water systems. In parallel, the non-toxic structure and high stability of the h-lysozyme without additional contamination make it a promising alternative to nanoparticle catalysts fur use in drinking water purification.
Conventional disinfection technologies for drinking water include physical treatments, such as microfiltration, ultrafiltration and UV irradiation, and chemical methods using chlorine, bromine, silver, copper, zinc and ozone.1–3 However, most technologies generate by-products and heavy metal residues along with high cost, which largely limit their further applications.4,5 Recently lysozyme has been attracting attention in the preservation of foods and the inhibition of bacterial and virus growth.6–8 Lysozyme is an antimicrobial enzyme produced by animals that forms part of the innate immune system,9–11 and which exists in secretions, including tears, saliva, human milk, and mucus. In addition to its antimicrobial activity,12,13 lysozyme also shows potential in anti-biofouling and water disinfection.13–15
Most isolated enzymes pose a challenge in terms of stability, sensitivity to environmental change and recycling.16,17 In particular, applying lysozyme onto a membrane using chemical methods drastically reduces its activity.18–20 Scientists thus attempt to immobilize enzymes to improve their performance and expand their applications.21,22 Saeki et al. immobilized lysozyme on a reverse osmosis (RO) membrane via an amine coupling reaction with a reduced water flux.13 Forming a robust chemical cross-linking configuration using glutaraldehyde molecules17 and Lentikats® polymers19 can improve the stability, but it will deform the initial structure and thus lose some activity. Immobilizing lysozyme into a polymer network can enhance the interaction area with biological contaminants and thus improve the removal efficiency at a low dosage.23–25
Here, we developed a new hydrogel-immobilized lysozyme (h-lysozyme) to inhibit both Gram-negative (E. coli) and Gram-positive (B. subtilis) bacterial growth in water. The immobilized lysozyme showed greatly improved activity, efficiency and recycling capability at room temperature.26–28 Owing to the strong bonding to the hydrogel and the porous structure of the cross-linked network, the antibacterial activity can be retained for a long period, e.g., months to years.28,29 The large surface area of the hydrogel also increases the bacterial molecular capture ability and thus increases the reaction opportunities with lysozyme. The excellent antibacterial performance along with the non-toxicity and low cost of the h-lysozyme make it a promising alternative to replace the current silver particles in various water environments.30
As illustrated in Fig. 1a, a cross-linked porous hydrogel network is formed after the polarization of poly(ethylene glycol)methyl ether acrylate (PEGMA) monomer via UV irradiation. During this process, the lysozyme molecules are immobilized into the matrix. The largely exposed surface increases the interaction with bacteria while the porous structure provides sufficient space to capture bacteria (Fig. 1b). Then the presence of lysozyme introduces a catalytic reaction of the hydrolysis of peptidoglycan, which is the major component of the cell wall of most bacteria, as well as counteracting the osmotic pressure of the cytoplasm and binary fission during bacterial cell reproduction. Upon the reaction, the 1,4-beta-linkages between N-acetyl-D-glucosamine (NAG) and N-acetylmuramic acid (NAM) in peptidoglycan are broken (Fig. 1c). As a result, the hydrolyzed NAG and NAM molecules lead to the lysis of the bacteria.31,32
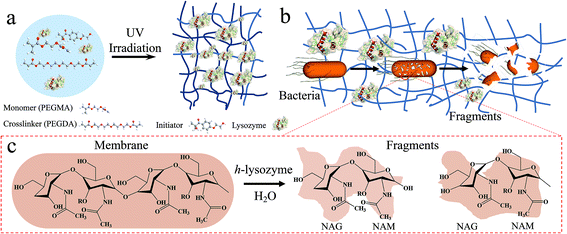 | ||
| Fig. 1 Schematic diagram of hydrogel-immobilized lysozyme for antibacterial membrane modification. (a) The synthesis process, (b) the antibacterial process and (c) mechanism for killing bacteria. | ||
A lysozyme with high activity and long stability is critical in antibacterial applications. It was found that the activity of lysozyme powder can remain for as long as two years.24,32 However, the activity of freestanding lysozyme will begin to be mitigated after being dispersed in water solution over a long time.33,34 In order to compare their activity, pure lysozyme powder, lysozyme in deionized (DI) water and h-lysozyme samples were checked by an ultraviolet (UV) spectrophotometer. In parallel, the samples under −20 °C, −4 °C, room temperature (RT) and high temperatures of 60 °C, 65 °C, 70 °C, 80 °C were also measured.
Lysozyme activity was checked via a typical process using whole cells of Micrococcus lysodeikticus (ML) as substrate.35,36 The ML was dissolved in 0.1 M phosphate buffer saline (PBS) solution (pH = 6.24) at RT. Then the concentration was adjusted until the measured UV density at 450 nm reached approximately 1.3. The optical density (OA) evolution vs. time was recorded to calculate the activity:
| Activity (U mg−1) = ΔOD450 × 1000/m | (1) |
Here ΔOD450 is the difference in optical density between 15 s and 75 s, and m is the mass (mg) of lysozyme in 0.5 mL solution. The relative activity (R%) is obtained:
| R% = (Ai/Af) × 100 | (2) |
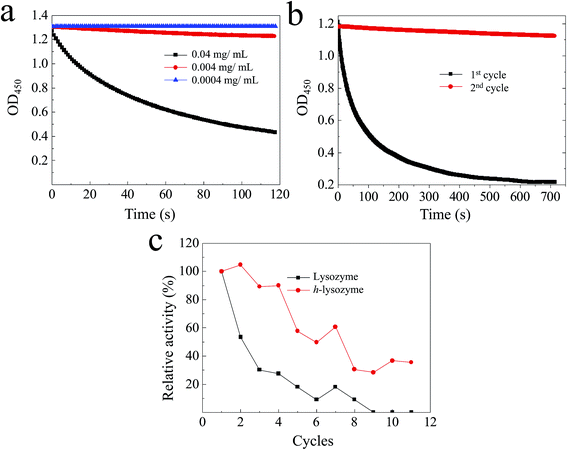
It was found that h-lysozyme exhibits a 120–250% higher relative activity compared to lysozyme powder or lysozyme in water solution (less than 100%) at RT (Fig. 2a), −4 °C (Fig. 2b) and −20 °C (Fig. 2c), respectively. Activity retention of up to 55 days was also recorded, indicating long-term stability. The fluctuation in the relative activity of the immobilized lysozyme is due to variations during sample preparation and UV measurement. At high temperature, 60 °C, the h-lysozyme displayed similar activity to lysozyme powder while it was higher than that of lysozyme in water (Fig. 2d). A relatively high activity at higher temperatures of 65, 70 and even 80 °C was still maintained, which demonstrates that h-lysozyme has good temperature tolerance. The hydrophilicity and large surface area of the PEG polymer based hydrogel attract plenty of bacteria, thus increasing the contact interface between the lysozyme and the ML molecules. As a result, a clearly improved activity under varying environments and temperatures was achieved.
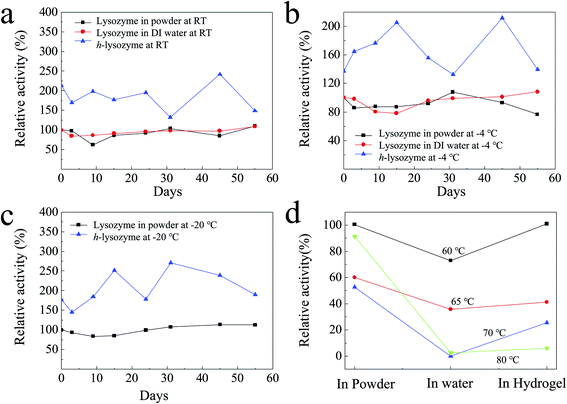 | ||
| Fig. 2 The activity of h-lysozyme at (a) room temperature, (b) −4 °C, (c) −20 °C and (d) high temperatures (60 °C, 65 °C, 70 °C, 80 °C). | ||
The activity of the lysozyme in water at different concentrations was investigated.37,38 It was found that the lysozyme has high activity at 0.04 mg mL−1 (Fig. 3a, black). The activity of killing bacteria was decreased until an extremely low concentration of 0.0004 mg mL−1 (Fig. 3a, blue). The recycling activity of the pure lysozyme was studied, as shown in Fig. 3b. It can be observed that most activity was lost after two cycles, which was caused by the decreased contact between lysozyme and ML due to the accumulation of debris on the surface. As a comparison, the hydrogel network with large spaces enhances the accommodation of debris and dead bacteria, resulting in a large exposed interface remaining. The recycling activity of both lysozyme and h-lysozyme is shown in Fig. 3c. It can clearly be seen that the immobilized lysozyme showed a high activity of about 30% even after 11 cycles, while the activity of the lysozyme without immobilization dropped to less than 10% after 8 cycles. These results further confirm that the porous hydrogel framework delivers sufficient contact interface between the lysozyme and bacteria to drastically improve the activity. In addition, the cross-linked net matrix also expands the acceptance capacity of the killed bacteria, avoiding surface coverage causing a loss of activity, which is a generic problem in the traditional silver particle killing process.
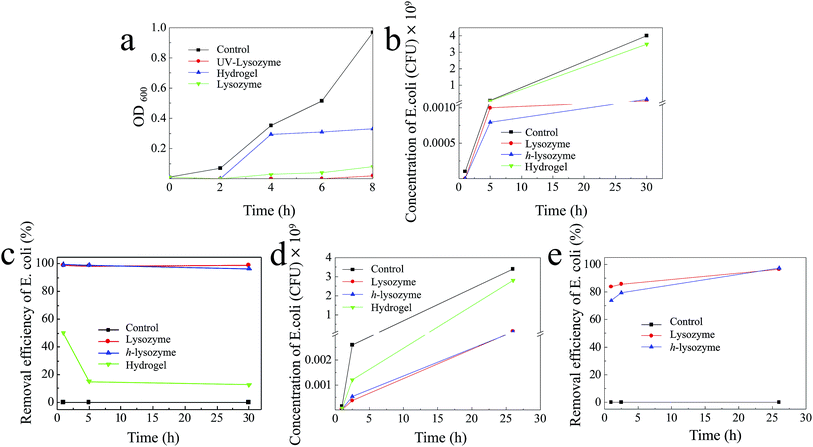
The antibacterial capability was first tested by exposing the h-lysozyme to the Gram-negative bacterial module E. coli. In order to fast check the bacterial regrowth, a lysogeny broth media (LB) bacterial culture solution was used.39 Fig. 4a illustrates that UV-lysozyme (red) has the highest inhibition compared to hydrogel (blue), lysozyme (green) or control (black) samples. Specifically the E. coli was completely inhibited by lysozyme in the first 2 hours and showed a negligible increase even after 8 hours, which indicates a remarkable inhibition of E. coli. It should be noted that UV-irradiated lysozyme showed slightly improved inhibition due to the activation of lysozyme under irradiation. Interestingly, the hydrogel also displayed certain inhibition to E. coli growth (blue). Large amounts of bacteria were trapped inside the matrix and thus further growth was prevented due to the change of environment.
In order to mimic the real environment in drinking water, a solution with an initial E. coli concentration of 105 CFU mL−1 was prepared and exposed to different samples of control (E. coli), lysozyme, h-lysozyme and hydrogel, respectively. It was observed that h-lysozyme showed excellent E. coli inhibition similar to that of pure lysozyme (Fig. 4b). The E. coli inhibition efficiency was calculated based on the measured concentration vs. the control concentration (Fig. 4c). The inhibition efficiencies of h-lysozyme and lysozyme were 99.4% and 99.0% in the first hour and they were still 96.3% and 98.8% after 30 hours, respectively. The E. coli inhibition with a low lysozyme concentration of 1 mg mL−1 was also studied, as shown in Fig. 4d. As can be seen from the figure, h-lysozyme shows the best inhibition performance in contrast to pure lysozyme, hydrogel or control samples. The inhibition efficiency of h-lysozyme after 26 hours was as high as 97.3%, which is higher than that of pure lysozyme of 96.4% (Fig. 4e), indicating promising potential in long-term antibacterial capability.
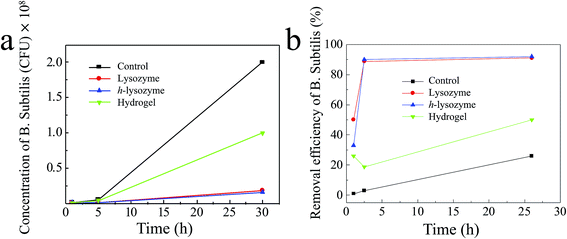
In order to expand the antibacterial applications, the Gram-positive bacteria Bacillus subtilis (B. subtilis) in water were also tested.30,40 As shown in Fig. 5a, h-lysozyme exhibited the lowest concentration, 1.6 × 107 CFU mL−1, of bacteria after 30 hours (blue). A corresponding inhibition efficiency of >92% after 26 hours was achieved (Fig. 5b), enabling better antibacterial durability.
In this work, we successfully developed a hydrogel-immobilized lysozyme that showed greatly improved antibacterial capability with high inhibition activity, wide concentration and temperature ranges and long recycling performance in drinking water. The increased interfaces between the lysozyme and bacteria molecules due to the large surface area from the porous structure of the hydrogel improve the inhibition of both Gram-negative and Gram-positive bacteria such as E. coli and B. subtilis. In addition, the industrial availability and non-toxicity of the lysozyme with hydrogel provide a promising alternative to the existing silver-based antibacterial catalysts for drinking water treatment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
All authors acknowledge the support from industry partners.Notes and references
- J. W. Patterson, Industrial Wastewater Treatment Technology, Butterworth-Heinemann, 1985, ISBN-13: 978-0409900026 Search PubMed.
- R. A. Crane and T. B. Scott, J. Hazard. Mater., 2012, 211, 112–125 CrossRef PubMed.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nanosci. Technol., World Scientific, 2010, pp. 337–346 Search PubMed.
- J. Lalley, D. D. Dionysiou, R. S. Varma, S. Shankara, D. J. Yang and M. N. Nadagouda, Curr. Opin. Chem. Eng., 2014, 3, 25–29 CrossRef.
- T. Shintani, H. Matsuyama and N. Kurata, Desalination, 2007, 207, 340–348 CrossRef CAS.
- T. Kawaguchi and H. Tamura, J. Appl. Polym. Sci., 1984, 29, 3359–3367 CrossRef CAS.
- L. Duan, Y. Wang, Y. Zhang and J. Liu, Appl. Surf. Sci., 2015, 355, 436–445 CrossRef CAS.
- J. Hankiewicz and E. Swierczek, Clin. Chim. Acta, 1974, 57, 205 CrossRef CAS.
- B. Porstmann, K. Jung, H. Schmechta, U. Evers, M. Pergande, T. Porstmann, H. Kramm and H. Krause, Clin. Biochem., 1989, 22, 349 CrossRef CAS PubMed.
- J. Wang, L. Tang, P. Somasundaran, W. Fan, G. Zeng, Y. Deng, Y. Zhou, J. Wang and Y. Shen, J. Colloid Interface Sci., 2017, 503, 131–141 CrossRef CAS PubMed.
- S. Bel, M. Pendse, Y. Wang, Y. Li, K. A. Ruhn, B. Hassell, T. Leal, S. E. Winter, R. J. Xavier and L. V. Hooper, Science, 2017, 357, 1047–1052 CrossRef CAS PubMed.
- D. Saeki, S. Nagao, I. Sawada, Y. Ohmukai, T. Maruyama and H. Matsuyama, J. Membr. Sci., 2013, 428, 403–409 CrossRef CAS.
- J. Wang, L. Qin, J. Lin, J. Zhu, Y. Zhang, J. Liu and B. Van der Bruggen, Chem. Eng. J., 2017, 323, 56–63 CrossRef CAS.
- T. Wu, C. Wu, S. Fu, L. Wang, C. Yuan, S. Chen and Y. Hu, Carbohydr. Polym., 2017, 155, 192–200 CrossRef CAS PubMed.
- C. Liu, L. Chen and L. Zhu, Water Res., 2017, 119, 33–46 CrossRef CAS PubMed.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
- P. Appendini and J. H. Hotchkiss, Packag. Technol. Sci., 1997, 10, 271–279 CrossRef CAS.
- L. Wilson, A. Illanes, B. C. C. Pessela, O. Abian, R. Fernández-Lafuente and J. M. Guisán, Biotechnol. Bioeng., 2004, 86, 558–562 CrossRef CAS PubMed.
- V. Figueira, I. Vaz-Moreira, M. Silva and C. M. Manaia, Water Res., 2011, 45, 5599–5611 CrossRef CAS PubMed.
- T. Su, Z. Tang, H. He, W. Li, X. Wang, C. Liao, Y. Sun and Q. Wang, Chem. Sci., 2014, 5, 4204–4209 RSC.
- G. Lesnierowski, J. Kijowski and R. Cegielska-Radziejewska, J. Food Sci. Technol., 2009, 44, 305–311 CrossRef CAS.
- Q. Z. Yang, Y. Y. Chang and H. Z. Zhao, Water Res., 2013, 47, 6712–6718 CrossRef CAS PubMed.
- M. Yuceer and C. Caner, J. Sci. Food Agric., 2014, 94, 153–162 CrossRef CAS PubMed.
- A. Zimoch-Korzycka and A. Jarmoluk, J. Food Sci. Technol., 2015, 52, 5996–6002 CrossRef CAS PubMed.
- H. Yuk, T. Zhang, S. Lin, G. A. Parada and X. Zhao, Nat. Mater., 2016, 15, 190 CrossRef CAS PubMed.
- T. Su, D. Zhang, Z. Tang, Q. Wu and Q. Wang, Chem. Commun., 2013, 49, 8033–8035 RSC.
- Q. Zhao, C. Liu, J. Liu and Y. Zhang, RSC Adv., 2015, 5, 38646–38653 RSC.
- T. Wu, J. Huang, Y. Jiang, Y. Hu, X. Ye, D. Liu and J. Chen, Food Chem., 2018, 240, 361–369 CrossRef CAS PubMed.
- N. R. Srinivasan, P. A. Shankar and R. Bandyopadhyaya, Carbon, 2013, 57, 1–10 CrossRef CAS.
- D. M. Chipman and N. Sharon, Science, 1969, 165, 454–465 CrossRef CAS PubMed.
- H. A. McKenzie and F. H. White, Adv. Protein Chem., 1991, 41, 173–315 CrossRef CAS PubMed.
- A. Malzert, F. Boury, D. Renard, P. Robert, J. E. Proust and J. P. Benoît, Int. J. Pharm., 2002, 242, 405–409 CrossRef CAS PubMed.
- M. van de Weert, J. Hoechstetter, W. E. Hennink and D. J. A. Crommelin, J. Controlled Release, 2000, 68, 351–359 CrossRef CAS PubMed.
- S. I. Park, M. A. Daeschel and Y. Zhao, J. Food Sci., 2004, 69, M215–M221 CrossRef CAS.
- F. Zhang, Y. Y. Zhao, H. Chen, X. H. Wang, Q. Chen and P. G. He, Chem. Commun., 2015, 51, 6613–6616 RSC.
- Q. Wang, Q. Gao and J. Shi, J. Am. Chem. Soc., 2004, 126, 14346–14347 CrossRef CAS PubMed.
- S. Priyadarshini and V. K. Kansal, J. Dairy Res., 2002, 69, 419–431 CrossRef CAS PubMed.
- Y. Ye, L. Xiao, B. He, Q. Zhang, T. Nie, X. Yang, D. Wu, H. Cheng, P. Li and Q. Wang, J. Mater. Chem. B, 2017, 5, 1518–1524 RSC.
- J. A. M. Delezuk, D. E. Ramírez-Herrera, B. E. F. de Ávila and J. Wang, Nanoscale, 2017, 9, 2195–2200 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures including sample preparation, characterization and antibacterial measurements. See DOI: 10.1039/c9ra02464f |
| This journal is © The Royal Society of Chemistry 2019 |