Open Access Article
Open Access ArticleOrganic–inorganic hybrid perovskite quantum dot light-emitting diodes using a graphene electrode and modified PEDOT:PSS†
Qing Zhanga,
Hongtao Yua,
Ziwei Liua,
Yao Lua,
Danqing Yea,
Jie Qiana,
Yanan Wua,
Wenwen Gua,
Ben Maa,
Liuquan Zhanga,
Yu Duan b,
Lihui Liua,
Kun Caoa,
Shufen Chen
b,
Lihui Liua,
Kun Caoa,
Shufen Chen *ac and
Wei Huang
*ac and
Wei Huang *ac
*ac
aKey Laboratory for Organic Electronics and Information Displays & Jiangsu Key Laboratory for Biosensors, Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications (NUPT), 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamsfchen@njupt.edu.cn; iamdirector@fudan.edu.cn
bState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, China
cInstitute of Flexible Electronics (SIFE), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an 710072, Shaanxi, China. E-mail: provost@nwpu.edu.cn
First published on 4th July 2019
Abstract
Perovskite quantum dot (PQD) light-emitting diodes (LEDs) have rapidly developed in the past several years due to the excellent optoelectronic properties of lead halide perovskites. However, PQD LEDs using graphene electrodes have not been reported despite their huge potential for applications in flexible displays and lighting sources. Herein, graphene was first used as the electrode of PQD LEDs. To overcome graphene's limitations such as hydrophobicity and graphene-induced film nonuniformity, the modification of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) with Triton X-100 and dimethyl sulfoxide (DMSO) codoping was reported, which not only improved the wettability of the graphene surface and the sequent film quality, but also reduced the dissolution of the PQD solvent to the bottom poly[N,N′-bis(4-butylphenyl)-N,N′-bis(phenyl)-benzidine] and PEDOT:PSS. More importantly, the synergistic effect of Triton X-100 and DMSO altered the PEDOT:PSS morphology from a coiled structure to a nanofibril conductive network, sufficiently enhancing the electrical conductivity of PEDOT:PSS. With this modification strategy, green PQD LEDs with CH3NH3PbBr3 emission layers were successfully fabricated on graphene anodes, with 3.7- and 4.4-fold enhancements in luminance and current efficiency, respectively, compared to those of their counterparts without PEDOT:PSS modification. The film modification strategy and graphene-based PQD LEDs in this work are expected to shed light on the further design and manufacture of flexible highly efficient PQD display and lighting devices.
1. Introduction
Perovskite quantum dot (PQD) light-emitting diodes (LEDs) have rapidly developed in the past several years due to the excellent optoelectronic properties of lead halide perovskites including facile tunable luminance over the entire visible spectrum, a high photoluminescence quantum yield (PLQY) of up to 100% and narrow full width at half maximum (FWHM).1–15 By using the strategy of anion exchange and quantum confinement effect of these PQDs, an ultrawide color gamut of >120 is achieved in PQD LEDs, exceeding that of organic light-emitting diodes (OLEDs), bulk perovskite light-emitting diodes (PeLEDs) and Cd/Zn-based quantum dot LEDs.16 These obvious advantages endow PQD LEDs with a huge potential for applications in next-generation high-definition displays and high-quality lighting sources.ITO has been widely used as the electrode of OLEDs, organic solar cells (OSCs) and perovskite photovoltaic devices due to its high intrinsic transmissivity and good conductivity. However, the brittle nature makes it unsuitable for future applications in flexible optoelectronic devices.17–19 Moreover, indium ion diffusion into the bottom transport layer triggers the degradation of a device's performance and lifetime.20 Therefore, the development of new transparent conductive materials to replace ITO is considered as a major resolution strategy. Representative flexible electrodes containing conducting polymers,21,22 carbon nanotubes,23,24 graphene,25,26 and metal nanowires27,28 have been extensively studied in the past decade. Among them, metal nanowires and graphene flexible electrodes have shown excellent optical and electrical performances in flexible OLED and OSC applications owing to their fine conductivity, high transmittance and good mechanical flexibility.29,30 However, to the best of our knowledge, they are rarely reported in flexible PeLEDs and PQD LEDs. In 2016, Bade et al. reported printed PeLEDs based on carbon nanotube anodes and Ag nanowire cathodes, which possessed the advantages of flexibility and roll-to-roll processability.31 However, the devices showed relatively low current efficiency (CE) of 0.6 cd A−1 and external quantum efficiency (EQE) of 1.1% together with low luminance (L) of 200 cd m−2. Zhong et al. first reported highly flexible CH3NH3PbBr3 (MAPbBr3) PQD LEDs on the AgNW/polymer composite anodes with the maximum current and power efficiencies of 10.4 cd A−1 and 8.1 lm W−1, respectively.32 Lee et al. fabricated flexible organic/inorganic hybrid PeLEDs on a graphene anode and obtained high CE of 18.0 cd A−1.33 It is noteworthy that this is the first work of the successful application of graphene in bulk perovskite LEDs, demonstrating that the graphene anode is also suitable for perovskite optoelectronic devices. Even so, to the best of our knowledge, PQD LEDs using graphene as electrodes have not been reported despite the huge potential for applications in next-generation flexible high-definition displays and high-quality lighting sources.
During continuous applications of a graphene electrode in optoelectronic devices, some of its intrinsic shortcomings such as high sheet resistance,34 low work function,35 and surface hydrophobicity36 have been found to negatively affect the device performances, and these problems need to be resolved to realize excellent electroluminescence (EL) performances. A p-doping treatment on graphene is considered as a useful approach to decrease the sheet resistance and increase the work function of graphene.37–39 In addition, we proposed an interfacial modification method of graphene with an ultrathin insulated poly(sodium 4-styrenesulfonate) film, with which we significantly improved the performances of OLEDs and OSCs by reducing the hole injection barrier from graphene to the PEDOT:PSS hole transport layer (HTL).40,41 To overcome the surface hydrophobicity problem, a conventional UV–ozone approach is used to treat the graphene's surface to achieve improved wettability, but this method usually breaks the pristine atomic ordering of graphene and leads to significantly enlarged sheet resistance by changing sp2-bonded carbons to sp3 carbons. An alternative method is the modification of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS, one of the most widely used commercial hole conductive materials) with IPA or zonyl to improve the wettability of hydrophobic graphene electrodes.42,43 The modification of PEDOT:PSS with the above-mentioned organic solvents or nonionic surfactants not only improves the wettability of graphene and forms a uniform film on the graphene surface, but also increases the conductivity of PEDOT:PSS by several orders of magnitude.44 More importantly, the PEDOT:PSS coating can sufficiently reduce the graphene electrode's sheet resistance and simultaneously improve its work function via forming p-doped graphene,45,46 which is more suitable for device applications as an anode.
Herein, we reported the first application of graphene into PQD LEDs as an anode and we used small-molecule nonionic surfactant Triton X-100 and DMSO to modify PEDOT:PSS. The addition of Triton X-100 and DMSO into PEDOT:PSS not only improved the wettability of the graphene surface and the sequent film quality containing PEDOT:PSS and poly[N,N′-bis(4-butylphenyl)-N,N′-bis(phenyl)-benzidine] (poly-TPD), but also reduced the dissolution of toluene (PQD solvent) to the bottom poly-TPD and PEDOT:PSS films. More importantly, the synergistic effect of Triton X-100 and DMSO altered the PEDOT:PSS morphology from a coiled structure to a nanofibril conductive network, sufficiently enhancing the electrical conductivity of PEDOT:PSS. With this modification strategy, green PQD LEDs with MAPbBr3 emission layers were successfully fabricated on graphene anodes, with 3.7- and 4.4-fold enhancements in luminance and current efficiency, respectively, compared to those of the counterpart without PEDOT:PSS modification. The best EL performance of our green PQD LEDs using graphene anodes is very close to that of the device with an ITO anode.
2. Experimental
2.1 Materials
The monolayer graphene films were purchased from Hangzhou Gelanfeng Nanotechnology Co. Ltd., which were synthesized on Cu foils with chemical vapor deposition (CVD) approach. Patterned indium-tin oxide (ITO) glasses with a sheet resistance of 7 Ω sq−1 were purchased from Hua Yu United Technology Co. Ltd. Polymethylmethacrylate (PMMA) with a molecular weight of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was dissolved in anisole with 6 wt% concentration. PEDOT:PSS (Clevios P VP AI 4083) was purchased from Heraeus Materials Technology Co. Ltd. Poly-TPD and TPBi were purchased from Lumtec Tech. Corp. PbBr2 and MABr were purchased from Shanghai MaterWin New Materials Co. Ltd. Oleic acid, octylamine, Triton X-100, DMSO, DMF and other solvents were purchased from Sigma-Aldrich. All materials were used as obtained without further purification.
000 was dissolved in anisole with 6 wt% concentration. PEDOT:PSS (Clevios P VP AI 4083) was purchased from Heraeus Materials Technology Co. Ltd. Poly-TPD and TPBi were purchased from Lumtec Tech. Corp. PbBr2 and MABr were purchased from Shanghai MaterWin New Materials Co. Ltd. Oleic acid, octylamine, Triton X-100, DMSO, DMF and other solvents were purchased from Sigma-Aldrich. All materials were used as obtained without further purification.
2.2 Transfer of graphene electrode
We used a conventional wet transfer process to transfer graphene. First, PMMA anisole solution was spin-coated onto the graphene-covered copper foil substrate, forming a ∼550 nm protective layer after heating to 120 °C and annealing for 10 min. Then, the Cu/graphene/PMMA sheet was placed into 0.1 mol (NH4)2S2O8 solution to slowly etch the copper foil. After 10 h, graphene was transferred into deionized water for cleaning (NH4)2S2O8 and Cu2+, and this step was repeated several times. Next, graphene/PMMA was transferred onto the target substrate by a high-temperature thermal annealing process to remove residual water. Finally, the substrate/graphene/PMMA was placed into boiling acetone to remove residual PMMA. Similarly, this step was repeated several times to eventually obtain the MLG electrode.2.3 Preparation of modified PEDOT:PSS films
The PEDOT:PSS aqueous solution was filtered using a filter (0.45 μm pore size) prior to use. Then, different amounts of Triton X-100 and DMSO were added into the PEDOT:PSS solution, followed by a six-hour magnetic stirring process for fully mixing of the above-mentioned raw materials. After a 5 min UV ozone treatment to the graphene sheets, the pristine and modified PEDOT:PSS solutions were spin-coated onto graphene anodes at 2000 rpm for 45 s. The resulting films were thermally annealed at 120 °C for 30 min in air.2.4 Characterization of graphene electrode and PEDOT:PSS solution
The Raman spectra of graphene were measured using a Raman microscope (InVia, Renishaw). The sheet resistance of graphene and conductivity of PEDOT:PSS films were measured with a four-point probe (RTS-9, China). The surface topographies of graphene and graphene/PEDOT:PSS were characterized using an optical microscope. The transmissivities were measured using an ultraviolet-visible spectrophotometer (Shimadzu, UV-3600). The surface morphologies of various HTL films were investigated using an atomic force microscope (BRUKER Dimension icon).2.5 Synthesis of MAPbBr3 PQD solution
To prepare MAPbBr3 PQD solution, 0.40 mmol of PbBr2 and 0.44 mmol of MABr were dissolved in 2 mL of DMF under vigorous stirring. Then, 220 μL of oleic acid and 30 μL of octylamine were added into the solution, followed by continuous stirring for 3 hours to obtain the transparent precursor solution. Following this, 0.25 mL of precursor solution was injected into 10 mL of toluene solvent with vigorous stirring, and the colorless mixed solution turned bright yellowish-green immediately. At last, the PQD solution was collected and used for subsequent studies and LED fabrication.2.6 Characterization of MAPbBr3 PQDs
The TEM image of MAPbBr3 PQDs was characterized using a transmission electron microscope (Hitachi, HT7700). The PL spectra of PQDs were tested with an IK series (Kimmon Koha, Japan). The UV-vis absorption spectra of PQD films were obtained using a PerkinElmer Lambda 650 S spectrophotometer with an excitation wavelength of 425 nm. The crystal structures of PQD film were measured using a Bruker D8 ADVANCE X-ray diffraction equipment.2.7 Device fabrication and measurement
The PEDOT:PSS and mixed PEDOT:PSS solutions were spin-coated onto graphene or ITO anode to form a ∼40 nm-thick HTL. Then, these PEDOT:PSS-covered samples were annealed at 120 °C in air for 30 min. After this, they were transferred into an N2 glove box to spin-coat the following poly-TPD HTL layer (at a rotation speed of 1500 rpm for 60 s and then annealing at 140 °C for 30 min) and MAPbBr3 PQD emitting layer (at a rotation speed of 2000 rpm for 45 s and then annealing at 50 °C for 5 min). Notably, spin-coating of the emitting layer was repeated five times. Finally, all samples were loaded into a high-vacuum chamber (5 × 10−7 Torr) for film depositions of TPBi (45 nm), LiF (1 nm) and Al (100 nm) as the electron transportation layer, the electron injection layer and the cathode, respectively. The EL spectra and luminance–current density–voltage characteristics were tested using a Keithley 2400 source, a calibrated luminance meter, and a PR-655 spectra scan spectrophotometer (Photo Research) in air without further encapsulation.3. Results and discussion
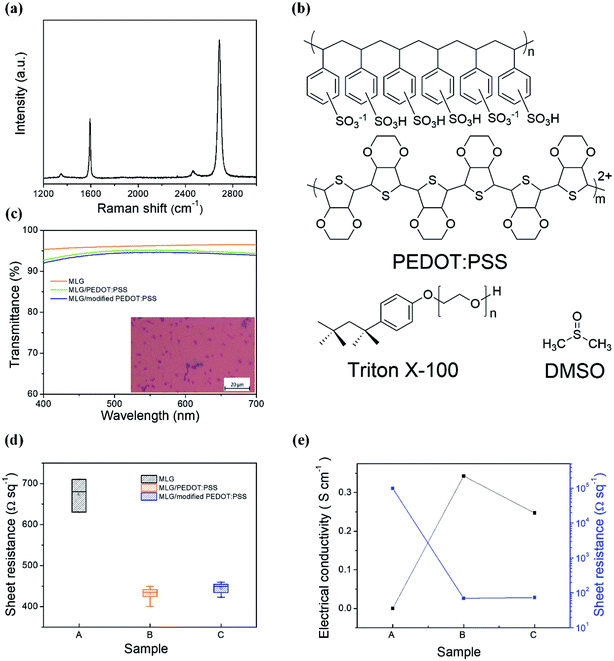
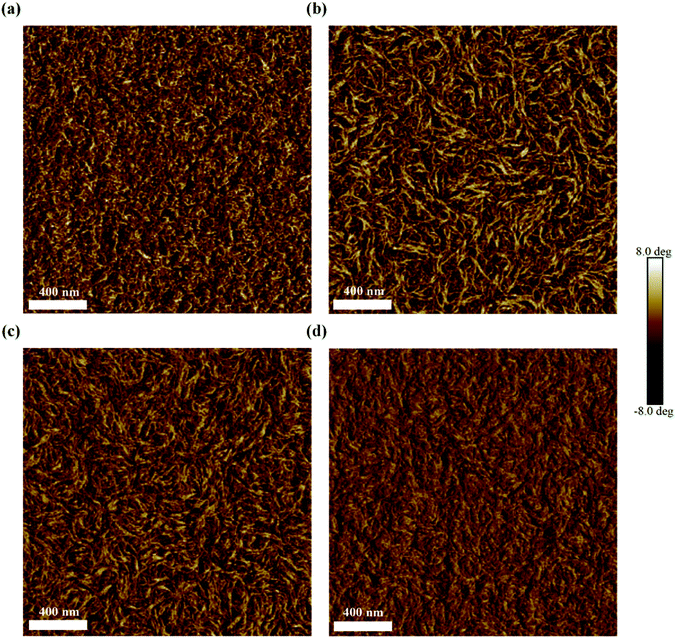
The graphene anode used in this work was obtained from the chemical vapor deposition-grown monolayer graphene (MLG) and it was transferred via a wet process prior to use, with details presented in the Experimental section. In order to observe the quality of the as-obtained MLG, we measured its Raman spectrum. From the result shown in Fig. 1(a), we clearly observe the G and 2D peaks of graphene at 1593 and 2687 cm−1, respectively. The weak D peak and the low G peak-to-2D peak intensity ratio (IG/I2D < 0.5) suggest high crystalline quality of the as-used MLG film.47 From Fig. S1(a),† the contact angle (CA) of the pristine graphene remains at 56.1°; thus, it is difficult to form a uniform film by directly spin-coating the PEDOT:PSS aqueous solution onto the graphene electrode. To improve the wettability of the graphene surface and the subsequent film quality, a nonionic surfactant Triton X-100 and DMSO (with chemical structures shown in Fig. 1(b)) were added into the PEDOT:PSS aqueous solution; after sufficient stirring, the mixing solutions with Triton X-100, DMSO or both were dropped onto graphene to test CAs. Compared to large CA of 56.1°, the addition of DMSO into PEDOT:PSS slightly reduced the CA to 49.5° (Fig. S1(b)†), while the introduction of Triton X-100 obviously reduced the CA to 16.9° (Fig. S1(c)†), which is beneficial for the formation of a uniform film on graphene. From the photographs and optical microscopy images of various PEDOT:PSS films shown in Fig. S2,† it can be observed that adding Triton X-100 significantly improves the film uniformity of PEDOT:PSS. In addition, the effect of doping DMSO and Triton X-100 on the transmittance of PEDOT:PSS was investigated (Fig. 1(c)). Compared to ∼96.2% for pristine MLG over the visible spectral range, the transmittance of PEDOT:PSS-covered graphene reduced to ∼95.2%, while the addition of DMSO and Triton X-100 into PEDOT:PSS resulted in loss of only ∼0.5% in transmissivity, indicating the negligible effects of the additives on the optical properties of the graphene film.The sheet resistance is also an important parameter to evaluate an electrode's quality. Fig. 1(d) illustrates the effect of different PEDOT:PSS solutions on the sheet resistance of MLG. The sheet resistance was 680 Ω sq−1 for pristine MLG, while it reduced to 430 Ω sq−1 for PEDOT:PSS-coated MLG, indicating the p-type doping of PEDOT:PSS to graphene.40,41 However, with the simultaneous addition of Triton X-100 and DMSO into the PEDOT:PSS solution, the sheet resistance slightly increased due to the insulated nature of Triton X-100. Here, DMSO is considered to be eventually evaporated from the film after the drying process at 120 °C.48 In order to ensure the effect of adding DMSO and Triton X-100 on the electrical properties of the PEDOT:PSS film, the electrical conductivities of various PEDOT:PSS films were measured, as exhibited in Fig. 1(e). The conductivity of the resulting PEDOT:PSS films is highly dependent on the ratio of PEDOT to PSS and the morphology of the films upon drying.49 A pure PEDOT:PSS film (Clevios P VP AI4083) has low conductivity of only ∼10−5 S cm−1. Once DMSO and Triton X-100 are added to the PEDOT:PSS aqueous solution, the interactions induce conformational changes of the PEDOT chains from coiled to linear conformation, resulting in the formation of PEDOT nanofibrils with a good conducting network and better electrical conductivity (0.34 S cm−1). The atomic force microscopy (AFM) phase images in Fig. 2 reflect the morphological changes of various PEDOT:PSS films with the increase in Triton X-100 concentration. As can be seen from Fig. 2, the bright and dark regions correspond to the PEDOT-rich and PSS-rich zones, respectively. It should be noted that for polymer systems, a lower phase angle (darker region) corresponds to a softer material, while a large phase angle (bright region) represents the rigid conjugated polymer PEDOT with higher conductivity.50 From Fig. 2, we can observe that the PEDOT:PSS films show obvious phase separation with the addition of Triton X-100 and DMSO. When the concentration of Triton X-100 changes from 0.01 to 0.05 wt%, the coiled PEDOT structure gradually turns into a nanofibril structure, revealing the formation of a better conductive pathway. However, the continuous increase in Triton X-100 concentration begins to interfere with the conductive PEDOT pathways due to the insulating nature of Triton X-100. As a result, the optimal doping weight ratio for Triton X-100 is about 0.01–0.05 wt%. To better understand the effects of Triton X-100 and DMSO on the formation of conductive networks, we also observed the AFM phase diagrams of the PEDOT:PSS films with only Triton X-100 or DMSO added. As shown in Fig. S3,† the films with only one additive do not exhibit an obvious nanofibril network, implying that a small fraction of Triton X-100 (0.01–0.05 wt%) has a synergistic effect with DMSO on forming a conductive network.
We also accidently found that the toluene solvent of PQDs not only re-dissolves the bottom poly-TPD HTL, but also partially removes PEDOT:PSS (please see thickness statistics in Fig. S4(a)†). Interestingly, the dissolution to both poly-TPD and PEDOT:PSS films is greatly reduced with the doping of DMSO and Triton X-100 (Fig. S4(b)†). About 8 nm poly-TPD can be reserved on the DMSO- and Triton X-100-modified PEDOT:PSS film, which is in favor of hole injection and transportation in PQD LEDs. In addition, after toluene treatment, the modified PEDOT:PSS/poly-TPD film showed slightly declined surface roughness (Rq) of 1.40 nm compared to that of the PEDOT:PSS/poly-TPD counterpart (1.70 nm) (Fig. S4(c) and (d)†).
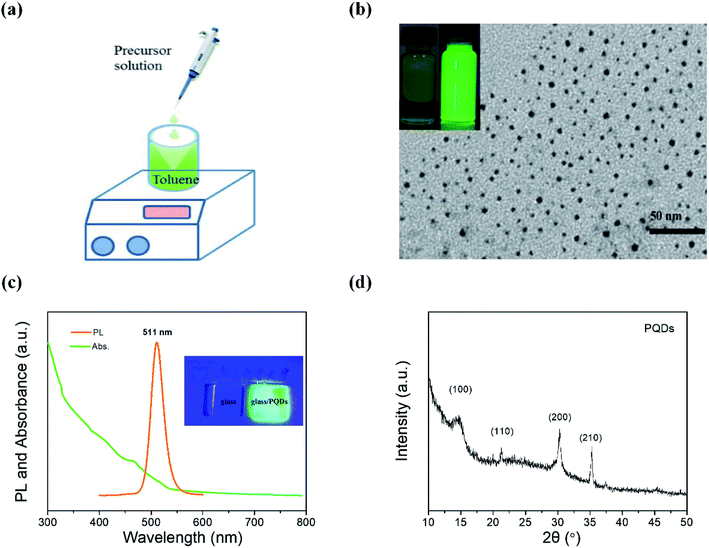
Prior to the application of the above-mentioned modified PEDOT:PSS/graphene sheets in PQD LEDs, we synthesized and characterized MAPbBr3 PQDs. Zero-dimensional MAPbBr3 PQDs were synthesized with the conventional ligand-assisted reprecipitation technique; the related details are described in the Experimental section.14 The as-prepared DMF precursor solution containing lead bromine (PbBr2), methylammonium bromine (MABr), oleic acid and octylamine was injected into the toluene solvent, and the colorless solution immediately turned yellowish-green under vigorous stirring, indicating the formation of MAPbBr3 quantum dots. The diagram of the synthesis process of MAPbBr3 PQDs and the photograph of the as-generated PQD solution are shown in Fig. 3(a) and the inset figure of Fig. 3(b), respectively. Under the illumination of a UV light, MAPbBr3 PQDs emit bright green light with an absolute PLQY of 43 ± 1%. The transmission electron microscopy (TEM) image in Fig. 3(b) illustrates MAPbBr3 PQDs with an average diameter of 4 ± 1.5 nm. Fig. 3(c) shows the UV-visible absorption and PL spectra. The PL spectrum exhibits a sharp emission peak at 511 nm with narrow FWHM of 28 nm. The X-ray diffraction (XRD) patterns in Fig. 3(d) show four diffraction peaks of 14.69°, 21.31°, 30.29° and 35.27°, which represent the (100), (110), (200) and (210) crystal planes, respectively, indicating a cubic structure for our products.
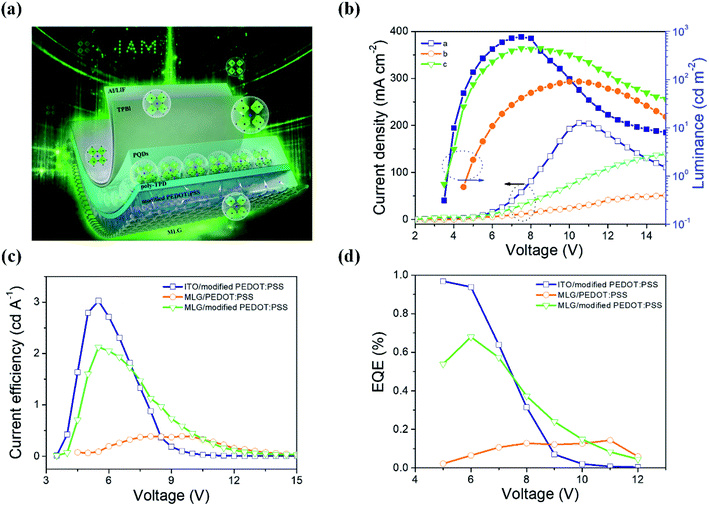
For the purpose of anode application, the pristine Fermi level (or called work function) of graphene was low, which remained at ∼4.3–4.5 eV in our previous measurements.40,51 The low work function and high sheet resistance of graphene will restrain hole injection in LED applications. At the same time, the low conductivity (∼10−5 S cm−1) of PEDOT:PSS also limited hole transport inside the devices. A small amount of Triton X-100 additive (0.01 wt%) with different amounts of DMSO was doped into PEDOT:PSS to improve its hole injection/transport ability and film uniformity. The as-used device structure was MLG/PEDOT:PSS (40 nm)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Triton X-100 (0.01 wt%)
Triton X-100 (0.01 wt%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (x vol%)/poly-TPD (45 nm)/PQDs (20 nm)/TPBi (45 nm)/LiF (1 nm)/Al (100 nm), as shown in Fig. 4(a). As illustrated in Fig. S5,† the current density and luminance of the fabricated device obviously improve with DMSO being added into PEDOT:PSS. The luminance increased from 12.6 cd m−2 to 100.8, 191.6 and 79.7 cd m−2 at 8 V on increasing the DMSO doping amount to 1, 2, and 3 vol%. The turn-on voltage (Von) was also reduced significantly from ∼4.9 to ∼4.0 V. The ultraviolet photoelectron spectroscopy (UPS) measurements for pristine PEDOT:PSS/MLG and modified PEDOT:PSS/MLG demonstrated that the addition of DMSO and Triton X-100 slightly reduced the work function of PEDOT:PSS from 4.81 to 4.56 eV (Fig. S6†), revealing that the increasing EL performances are closely related to the conductivity and film morphology of PEDOT:PSS. This point is well coincident with the observed results of increased injection current densities in all devices with DMSO (Fig. S5(a)†). The optimum doping concentration of DMSO was 2 vol%. Even so, the luminous efficiency of the best-performing device was still low: only about 0.3 cd A−1.
DMSO (x vol%)/poly-TPD (45 nm)/PQDs (20 nm)/TPBi (45 nm)/LiF (1 nm)/Al (100 nm), as shown in Fig. 4(a). As illustrated in Fig. S5,† the current density and luminance of the fabricated device obviously improve with DMSO being added into PEDOT:PSS. The luminance increased from 12.6 cd m−2 to 100.8, 191.6 and 79.7 cd m−2 at 8 V on increasing the DMSO doping amount to 1, 2, and 3 vol%. The turn-on voltage (Von) was also reduced significantly from ∼4.9 to ∼4.0 V. The ultraviolet photoelectron spectroscopy (UPS) measurements for pristine PEDOT:PSS/MLG and modified PEDOT:PSS/MLG demonstrated that the addition of DMSO and Triton X-100 slightly reduced the work function of PEDOT:PSS from 4.81 to 4.56 eV (Fig. S6†), revealing that the increasing EL performances are closely related to the conductivity and film morphology of PEDOT:PSS. This point is well coincident with the observed results of increased injection current densities in all devices with DMSO (Fig. S5(a)†). The optimum doping concentration of DMSO was 2 vol%. Even so, the luminous efficiency of the best-performing device was still low: only about 0.3 cd A−1.
The surfactant Triton X-100 can fully improve the PEDOT:PSS film uniformity and promote the formation of nanofibril-shaped structures; however, introducing more amounts of Triton X-100 into PEDOT:PSS will inevitably induce low conductivity of the PEDOT:PSS film due to the insulating property of Triton X-100, thus generating decreased luminance and CE with increased Von. Thus, in the following part, the doping concentration of Triton X-100 in PEDOT:PSS was adjusted from 0.01 to 0.25 wt% to find the optimal concentration of Triton X-100. Herein, we simultaneously doped 2 vol% DMSO into PEDOT:PSS by referring to the experimental results in Fig. S5(a).† From the luminance, current density and CE curves shown in Fig. S7† and the summarized parameters in Table S1,† we ensured that the optimal doping concentration was 0.05 wt% for Triton X-100. Higher brightness and CE values of 431.2 cd m−2 (8 V) and 2.12 cd A−1 were acquired at 0.05 wt% Triton X-100, which were 8 and 3 times higher than the corresponding values with 0.01 wt% Triton X-100. To analyze the above-mentioned alteration trend, we fabricated hole-only devices with the structures of MLG/PEDOT:PSS (40 nm)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (2 vol%)
DMSO (2 vol%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
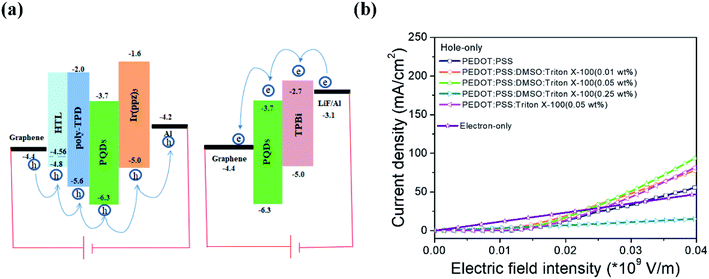
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Triton X-100 (0.01, 0.05 and 0.25 wt%)/poly-TPD (45 nm)/MAPbBr3 (20 nm)/Ir(ppz)3 (70 nm)/Al (100 nm). As shown in the schematics of the energy levels depicted in Fig. 5(a), Ir(ppz)3 is used as an effective electron blocking layer owing to its low lowest unoccupied molecular orbital (LUMO) level of −1.6 eV, which can sufficiently block electron injection into the device. Fig. 5(b) compares the injected current densities of the hole-dominated devices with different PEDOT:PSS films, from which the observable increase in current density with a small amount of Triton X-100 added into PEDOT:PSS is mainly due to the formation of the nanofibril-shaped PEDOT conducting network with increasing film hole conductivity. The largest injected hole current is obtained at 0.05 wt% Triton X-100 and this result is well consistent with the AFM phase image in Fig. 2(c). When more Triton X-100 was doped into PEDOT:PSS, the insulated nature of Triton X-100 led to serious deterioration of film conductivity and significantly declined the injected hole current. Using a moderate Triton X-100 doping ratio of 0.05 wt% was helpful to improve the balance between holes and electrons, particularly in the electric field intensity range of 0–3 × 107 V m−1 (Fig. 5(b), the electron-only device is fabricated with the structure of MLG/MAPbBr3 (20 nm)/TPBi (45 nm)/LiF (1 nm)/Al (100 nm)).
Triton X-100 (0.01, 0.05 and 0.25 wt%)/poly-TPD (45 nm)/MAPbBr3 (20 nm)/Ir(ppz)3 (70 nm)/Al (100 nm). As shown in the schematics of the energy levels depicted in Fig. 5(a), Ir(ppz)3 is used as an effective electron blocking layer owing to its low lowest unoccupied molecular orbital (LUMO) level of −1.6 eV, which can sufficiently block electron injection into the device. Fig. 5(b) compares the injected current densities of the hole-dominated devices with different PEDOT:PSS films, from which the observable increase in current density with a small amount of Triton X-100 added into PEDOT:PSS is mainly due to the formation of the nanofibril-shaped PEDOT conducting network with increasing film hole conductivity. The largest injected hole current is obtained at 0.05 wt% Triton X-100 and this result is well consistent with the AFM phase image in Fig. 2(c). When more Triton X-100 was doped into PEDOT:PSS, the insulated nature of Triton X-100 led to serious deterioration of film conductivity and significantly declined the injected hole current. Using a moderate Triton X-100 doping ratio of 0.05 wt% was helpful to improve the balance between holes and electrons, particularly in the electric field intensity range of 0–3 × 107 V m−1 (Fig. 5(b), the electron-only device is fabricated with the structure of MLG/MAPbBr3 (20 nm)/TPBi (45 nm)/LiF (1 nm)/Al (100 nm)).
We then compared our graphene-based PQD LEDs with a traditional ITO device, which uses the same structure as the graphene counterpart, that is, ITO/PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Triton X-100 (0.05 wt%)
Triton X-100 (0.05 wt%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (2 vol%)/poly-TPD/MAPbBr3/TPBi/LiF/Al. Fig. 4(b) displays the current density and luminance data obtained from each type of device. The injected current density of the graphene-based LEDs was still low relative to that of the ITO counterpart, while the luminance and Von were comparable to those of the ITO device. As shown in Fig. 4(c) and (d), the optimized CE and EQE values are 2.12 cd A−1 and 0.67%, respectively, in the graphene-based device, which are very close to those of the ITO contrast one (3.0 cd A−1 and 0.96%); both of these are far larger than those of the control graphene device without any modification (0.39 cd A−1 and 0.14%). In addition, these data demonstrate that good EL performances are observed in our present graphene-based PQD LEDs.
DMSO (2 vol%)/poly-TPD/MAPbBr3/TPBi/LiF/Al. Fig. 4(b) displays the current density and luminance data obtained from each type of device. The injected current density of the graphene-based LEDs was still low relative to that of the ITO counterpart, while the luminance and Von were comparable to those of the ITO device. As shown in Fig. 4(c) and (d), the optimized CE and EQE values are 2.12 cd A−1 and 0.67%, respectively, in the graphene-based device, which are very close to those of the ITO contrast one (3.0 cd A−1 and 0.96%); both of these are far larger than those of the control graphene device without any modification (0.39 cd A−1 and 0.14%). In addition, these data demonstrate that good EL performances are observed in our present graphene-based PQD LEDs.
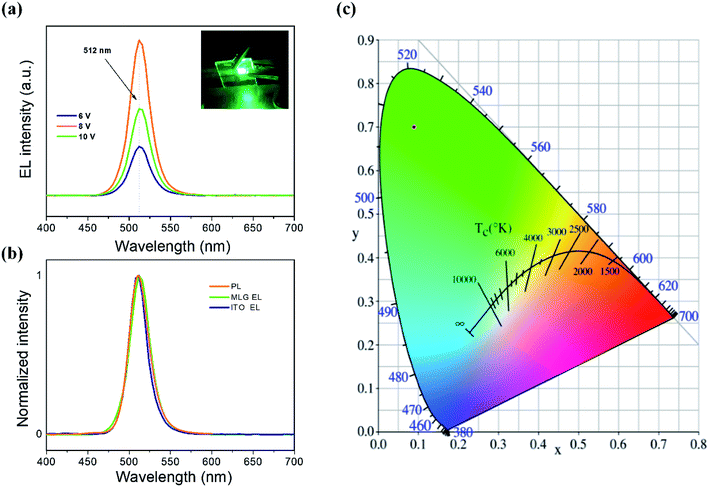
Fig. 6(a) shows the EL spectra of our as-fabricated PQD LEDs with a graphene anode and modified PEDOT:PSS at different voltages. The emission peaks appear at around 512 nm with narrow FWHM of ∼28 nm, and they are consistent with the PQD's PL spectrum with a peak centered at 511 nm and FWHM of 28 nm (Fig. 6(b)). The inset of Fig. 6(a) illustrates an emission photograph from a working PQD LED under driving bias of 8 V, showing a pure green light from MAPbBr3, which corresponds to the Commission Internationale de I'Eclairage (CIE) color coordinates of (0.09, 0.70), as displayed in Fig. 6(c).
4. Conclusion
In summary, in this work, we first developed organic/inorganic hybrid PQD LEDs on graphene electrodes. To overcome pristine hydrophobicity of graphene-induced film nonuniformity, we provided a modification approach to PEDOT:PSS by codoping DMSO and surfactant Triton X-100 into PEDOT:PSS. The modified PEDOT:PSS not only improved the wettability of the graphene anode and the sequent film quality, but also reduced the dissolution of the PQD solvent to the bottom poly-TPD and PEDOT:PSS. More importantly, the synergistic effect of Triton X-100 and DMSO altered the PEDOT:PSS morphology from a coiled structure to a nanofibril conductive network, sufficiently enhancing the electrical conductivity of PEDOT:PSS HTL. With this modification strategy, green LEDs with MAPbBr3 PQDs as the light-emitting layers were successfully fabricated on graphene anodes. The luminance and CE values exhibited 3.7- and 4.4-fold enhancements, respectively, over those of the control counterpart without PEDOT:PSS modification; these were close to the values of a conventional ITO device. The first graphene electrode-based PQD LED and the film modification strategy offered in this work are expected to shed light on the further design and manufacture of flexible highly efficient PQD display and lighting devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Foundation for Science and Technology Development of China (973 project, Grant No. 2015CB932203), the National Key Research and Development Program of China (Grant No. 2017YFB0404501), the National Major Fundamental Research Program of China (Grant No. 91833306), the National Natural Science Foundation of China (Grant No. 61505086, 61675088, 61705111 and 61704091), the Science Fund for Distinguished Young Scholars of Jiangsu Province of China (Grant No. BK20160039), the Natural Science Foundation of Jiangsu Province (Grant No. BM2012010 and BK20170899), the Priority Academic Program Development of Jiangsu Higher Education Institutions (Grant No. YX030002), the Synergetic Innovation Center for Organic Electronics and Information Displays, the National Postdoctoral Program for Innovative Talents (Grant No. BX201700122), and the Open Foundation from Jilin University (Grant No. IOSKL2017KF04 and IOSKL2018KF01), and International Science & Technology Cooperation Program of Jilin (Grant No. 20190701023GH).Notes and references
- L. C. Schmidt, A. Pertegás, S. González-Carrero, O. Malinkiewicz, S. Agouram, G. Mínguez Espallargas, H. J. Bolink, R. E. Galian and J. Pérez-Prieto, J. Am. Chem. Soc., 2014, 136, 850 CrossRef CAS PubMed.
- M. Y. Leng, Z. W. Chen, Y. Yang, Z. Li, K. Zeng, K. H. Li, G. D. Niu, Y. S. He, Q. C. Zhou and J. Tang, Angew. Chem., Int. Ed., 2016, 55, 1 CrossRef PubMed.
- J. Z. Song, J. H. Li, X. M. Li, L. M. Xu, Y. H. Dong and H. B. Zeng, Adv. Mater., 2015, 27, 7162 CrossRef CAS PubMed.
- M. Mittal, A. Jana, S. Sarkar, P. Mahadevan and S. Sapra, J. Phys. Chem. Lett., 2016, 7, 3270 CrossRef CAS PubMed.
- X. L. Zhang, H. Liu, W. G. Wang, J. B. Zhang, B. Xu, K. L. Karen, Y. J. Zheng, S. Liu, S. M. Chen, K. Wang and X. W. Sun, Adv. Mater., 2017, 29, 1606405 CrossRef PubMed.
- A. Perumal, S. Shendre, M. J. Li, Y. K. E. Tay, V. K. Sharma, S. Chen, Z. H. Wei, Q. Liu, Y. Gao, P. J. S. Buenconsejo, S. T. Tan, C. L. Gan, Q. H. Xiong, T. C. Sum and H. V. Demir, Sci. Rep., 2016, 6, 36733 CrossRef CAS PubMed.
- H. L. Huang, F. C. Zhao, L. G. Liu, F. Zhang, X. G. Wu, L. J. Shi, B. S. Zou, Q. B. Pei and H. Z. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 28128 CrossRef CAS PubMed.
- S. A. Veldhuis, Y. K. E. Tay, A. Bruno, S. S. H. Dintakurti, S. Bhaumik, S. K. Muduli, M. Li, N. Mathews, T. C. Sum and S. G. Mhaisalkar, Nano Lett., 2017, 17, 7424 CrossRef CAS PubMed.
- F. Liu, Y. H. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase, T. Minemoto, K. Yoshino, S. Y. Dai and Q. Shen, ACS Nano, 2017, 11, 10373 CrossRef CAS PubMed.
- B. A. Koscher, J. K. Swabeck, N. D. Bronstein and A. P. Alivisatos, J. Am. Chem. Soc., 2017, 139, 6566 CrossRef CAS PubMed.
- J. Z. Song, J. H. Li, L. M. Xu, J. H. Li, F. J. Zhang, B. Han, Q. S. Shan and H. B. Zeng, Adv. Mater., 2018, 1800764 CrossRef PubMed.
- X. M. Li, Y. Wu, S. L. Zhang, B. Cai, Y. Gu, J. Z. Song and H. B. Zeng, Adv. Funct. Mater., 2016, 26, 2435 CrossRef CAS.
- F. Zhang, S. Huang, P. Wang, X. M. Chen, S. L. Zhao, Y. P. Dong and H. Z. Zhong, Chem. Mater., 2017, 29, 3793 CrossRef CAS.
- F. Zhang, H. Z. Zhong, C. Chen, X. G. Wu, X. M. Hu, H. L. Huang, J. B. Han, B. S. Zou and Y. P. Dong, ACS Nano, 2015, 9, 4533 CrossRef CAS PubMed.
- N. K. Kumawat, A. Dey, A. Kumar, S. P. Gopinathan, K. L. Narasimhan and D. Kabra, ACS Appl. Mater. Interfaces, 2015, 7, 13119 CrossRef CAS PubMed.
- Q. C. Zhou, Z. L. Bai, W. G. Lu, Y. T. Wang, B. S. Zou and H. Z. Zhong, Adv. Mater., 2016, 28, 9163 CrossRef CAS PubMed.
- A. Kumar and C. Zhou, ACS Nano, 2010, 4, 11 CrossRef CAS PubMed.
- K. Ellmer, Nat. Photonics, 2012, 6, 809 CrossRef CAS.
- K. Alzoubi, M. M. Hamasha, S. S. Lu and B. Sammakia, J. Disp. Technol., 2011, 7, 593 CAS.
- S. T. Lee, Z. Q. Gao and L. S. Hung, Appl. Phys. Lett., 1999, 75, 1404 CrossRef CAS.
- K. Fehse, K. Walzer, K. Leo, W. Lovenich and A. Elschner, Adv. Mater., 2007, 19, 441 CrossRef CAS.
- W. H. Kim, A. J. Makinen, N. Nikolov, R. Shashidhar, H. Kim and Z. H. Kafafi, Appl. Phys. Lett., 2002, 80, 3844 CrossRef CAS.
- S. L. Hellstrom, H. W. Lee and Z. Bao, ACS Nano, 2009, 3, 1423 CrossRef CAS PubMed.
- C. Feng, K. Liu, J. S. Wu, L. Liu, J. S. Cheng, Y. Zhang, Y. Sun, Q. Li, S. Fan and K. Jiang, Adv. Funct. Mater., 2010, 20, 885 CrossRef CAS.
- L. Yao, X. Fang, W. Gu, W. H. Zhai, Y. Wan, X. X. Xie, W. J. Xu, X. D. Pi, G. Z. Ran and G. G. Qin, ACS Appl. Mater. Interfaces, 2017, 9, 24005 CrossRef CAS PubMed.
- N. Li, S. Oida, G. S. Tulevski, S. J. Han, J. B. Hannon, D. K. Sadana and T. C. Chen, Nat. Commun., 2013, 4, 2294 CrossRef PubMed.
- H. Wu, L. Hu, M. W. Rowell, D. Kong, J. J. Cha, J. R. McDonough, J. Zhu, Y. Yang, M. D. McGehee and Y. Cui, Nano Lett., 2010, 10, 4242 CrossRef CAS PubMed.
- Z. B. Yu, Q. W. Zhang, L. Li, Q. Chen, X. F. Niu, J. Liu and Q. B. Pei, Adv. Mater., 2011, 23, 664 CrossRef CAS PubMed.
- T. H. Han, Y. B. Lee, M. R. Choi, S. H. Woo, S. H. Bae, B. H. Hong, J. H. Ahn and T. W. Lee, Nat. Photonics, 2012, 6, 105 CrossRef CAS.
- D. D. Li, W. Y. Lai, Y. Z. Zhang and W. Huang, Adv. Mater., 2018, 30, 1704738 CrossRef PubMed.
- S. G. R. Bade, J. Q. Li, X. Shan, Y. C. Ling, Y. Tian, T. Dilbeck, T. Besara, T. Geske, H. W. Gao, B. W. Ma, K. Hanson, T. Siegrist, C. Y. Xu and Z. B. Yu, ACS Nano, 2016, 10, 1795 CrossRef CAS PubMed.
- F. C. Zhao, D. Chen, S. Chang, H. L. Huang, K. Tong, C. T. Xiao, S. Y. Chou, H. Z. Zhong and Q. B. Pei, J. Mater. Chem. C, 2017, 5, 531 RSC.
- H. K. Seo, H. Kim, J. Lee, M. H. Park, S. H. Jeong, Y. H. Kim, S. J. Kwon, T. H. Han, S. Yoo and T. W. Lee, Adv. Mater., 2017, 29, 1605587 CrossRef PubMed.
- J. B. Wu, M. Agrawal, H. A. Becerril, Z. N. Bao, Z. F. Liu, Y. S. Chen and P. Peumans, ACS Nano, 2010, 4, 43 CrossRef CAS PubMed.
- D. Kim, D. Lee, Y. Lee and D. Y. Jeon, Adv. Funct. Mater., 2013, 23, 5049 CrossRef CAS.
- S. R. Wang, Y. Zhang, N. Abidi and L. Cabrales, Langmuir, 2009, 25, 11078 CrossRef CAS PubMed.
- T. H. Han, S. J. Kwon, N. N. Li, H. K. Seo, W. T. Xu, K. S. Kim and T. W. Lee, Angew. Chem., Int. Ed., 2016, 128, 6305 CrossRef.
- C. L. Hsu, C. T. Lin, J. H. Huang, C. W. Chu, K. H. Wei and L. J. Li, ACS Nano, 2012, 6, 5031 CrossRef CAS PubMed.
- K. C. Kwon, K. S. Choi and S. Y. Kim, Adv. Funct. Mater., 2012, 22, 4724 CrossRef CAS.
- Y. F. Zhou, M. Wang, L. Wang, S. L. Liu, S. F. Chen, K. Cao, W. J. Shang, J. Q. Mai, B. M. Zhao, J. Feng, X. H. Lu and W. Huang, Appl. Phys. Lett., 2017, 111, 113302 CrossRef.
- S. F. Chen, Q. Zhang, W. J. Shang, L. H. Liu, H. T. Yu, S. Zhang, L. L. Deng, M. Wang, M. H. Wang, X. Li, B. X. Mi and W. Huang, Sci. Rep., 2018, 8, 8155 CrossRef PubMed.
- H. Park, Y. Shi and J. Kong, Nanoscale, 2013, 5, 8934 RSC.
- J. H. Chu, D. H. Lee, J. Jo, S. Y. Kim, J. W. Yoo and S. Y. Kwon, Adv. Funct. Mater., 2016, 26, 7234 CrossRef CAS.
- M. Vosgueritchian, D. J. Lipomi and Z. N. Bao, Adv. Funct. Mater., 2012, 22, 421 CrossRef CAS.
- X. Z. Zhu, Y. Y. Han, Y. Liu, K. Q. Ruan, M. F. Xu, Z. K. Wang, J. S. Jie and L. S. Liao, Org. Electron., 2013, 14, 3348 CrossRef CAS.
- Q. Zhang, S. F. Chen, S. Zhang, W. J. Shang, L. H. Liu, M. H. Wang, H. T. Yu, L. L. Deng, G. Q. Qi, L. Y. Wang, S. Y. Han, B. Hu, Q. Kang, Y. J. Liu, M. D. Yi, Y. W. Ma, W. J. Yang, J. Feng, X. G. Liu, H. B. Sun and W. Huang, J. Mater. Chem. C, 2018, 6, 1926 RSC.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS PubMed.
- S. Savagatrup, E. Chan, S. M. Renteria-Garcia, A. D. Printz, A. V. Zaretski, T. F. O'Connor, D. Rodriquez, E. Valle and D. J. Lipomi, Adv. Funct. Mater., 2015, 25, 427 CrossRef CAS.
- X. Crispin, F. L. E. Jakobsson, A. Crispin, P. C. M. Grim, P. Andersson, A. Volodin, C. van Haesendonck, M. Van der Auweraer, W. R. Salaneck and M. Berggren, Chem. Mater., 2006, 18, 4354 CrossRef CAS.
- Y. Wang, R. Song, Y. Li and J. Shen, Surf. Sci., 2003, 530, 136 CrossRef CAS.
- M. Wang, H. T. Yu, X. Q. Ma, Y. Yao, L. Wang, L. H. Liu, K. Cao, S. L. Liu, C. Dong, B. M. Zhao, C. Y. Song, S. F. Chen and W. Huang, Opt. Express, 2018, 26, 769 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02730k |
| This journal is © The Royal Society of Chemistry 2019 |