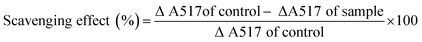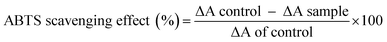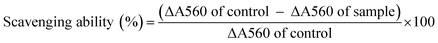Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical structure and biological properties of a polysaccharide isolated from Pleurotus sajor-caju
Palaniappan Seedevi *ab,
Abirami Ramu Ganesan
*ab,
Abirami Ramu Ganesan c,
Kannan Mohane,
Vasantharaja Raguramand,
Murugesan Sivakumara,
Palaniappan Sivasankara,
Sivakumar Loganathana,
Palasundaram Rajamalarb,
Shanmugam Vairamanib and
Annaian Shanmugamb
c,
Kannan Mohane,
Vasantharaja Raguramand,
Murugesan Sivakumara,
Palaniappan Sivasankara,
Sivakumar Loganathana,
Palasundaram Rajamalarb,
Shanmugam Vairamanib and
Annaian Shanmugamb
aDepartment of Environmental Science, Periyar University, Salem, 636011, Tamil Nadu, India. E-mail: seedevisalem@gmail.com; Tel: +91 9629201002
bCentre of Advanced Study in Marine Biology, Faculty of Marine Sciences, Annamalai University, Parangipettai, 608 502, Tamil Nadu, India
cDepartment of Food Science and Home Economics, School of Applied Sciences, College of Engineering, Science and Technology, Fiji National University, Fiji-7222
dCentre for Ocean Research, Sathyabama Institute of Science & Technology, Chennai, 600 119, Tamil Nadu, India
eSri Vasavi College, Erode, Tamilnadu, India
First published on 2nd July 2019
Abstract
Herein, a polysaccharide obtained from Pleurotus sajor-caju was fractionated via anion-exchange column chromatography and purified using gel permeation column chromatography. The chemical characterization of the polysaccharide indicated that it contained 90.16% total carbohydrate, 0% protein, 12.7% ash and 5.2% moisture; on the other hand, the carbon, hydrogen and nitrogen contents were found to be 31.53, 4.28 and 3.01%, respectively. The polysaccharide has the molecular weight of 79 kDa; the chemical structure of the polysaccharide is →6)α-D-Glciv(1→6)α-D-Glciii(1→6)β-D-Glcii(1→6)α-D-Glci(1→units. The polysaccharide exhibited the DPPH radical scavenging activity of 21.67–68.35% at 10–160 μg ml−1, ABTS radical scavenging activity of 16.01–70.09% at 25–125 μg ml−1, superoxide radical scavenging activity of 24.31–73.64% at 50–250 μg ml−1, hydroxyl radical scavenging activity of 16.64–63.51% at 25–125 μg ml−1 and reducing power of 0.366–1.678% at 10–120 μg ml; further evaluation of the polysaccharide revealed its anticancer activity of 18.61–63.21% at 100–500 μg ml−1 concentration against the AGS human gastric carcinoma cell line. The active principle of the polysaccharide may be used in the food and pharmacological industry in the future.
1. Introduction
Mushroom-derived polysaccharides are natural biological macromolecules that have gained widespread attention due to their various biological functions and nontoxic characteristics;1,2 in recent years, a variety of polysaccharides have been obtained from mushrooms, and their chemical structures and bioactivities, such as antioxidant, antitumor, anti-inflammatory and immunological properties, have been elucidated.3,4 In addition, the research findings have demonstrated that mushroom polysaccharides possess potent antioxidant activity.5 These antioxidant properties have been observed for polysaccharides extricated from T. versicolor, L. edodes and Agaricus mushrooms; moreover, these biopolymers have chelating properties that can repress lipid oxidation6 and are closely related to the molecular weight, monosaccharide composition, glycosidic bonds, degree of branching, and polymerization of the biopolymer.7 The structural features of polysaccharides obtained from mushrooms consist of β- or α- or both α- and β-linked glucan backbones displaying different patterns and degrees of branching.8,9In living organisms, oxidation is a fundamental process for the production/conversion of energy for the maintenance of biological processes. However, the uncontrolled production of reactive oxygen species (ROS) is responsible for several diseases such as cancer, rheumatoid arthritis, atherosclerosis, coronary heart disorder, etc.10,11 Antioxidants are substances that can react with ROS and reduce the risk of chronic human diseases; human and other living organisms possess antioxidants that protect them from oxidative damage; however, these antioxidants are not sufficient to prevent the oxidative damage. Synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and propyl gallate (PG), possess strong radical scavenging activities; however, the use of these antioxidants is restricted due to their side effects.12 Hence, extensive interest is being devoted towards the investigation of naturally occurring antioxidants to replace synthetic antioxidants. Currently, the development of effective and safe natural antioxidants is one of the main pursuits in the field of research on antioxidants. From this perspective, the antioxidants present in mushrooms are of great interest as potential protective agents against oxidative damage.13 In addition, the mushroom polysaccharides have anticancer and antidiabetic activities, serve as food and are also used in the pharmaceutical industry;14 recently, several mushroom polysaccharides have been isolated, and their biological activities have been investigated; moreover, they have been chemically and structurally characterized.15 Considering the abovementioned information on mushroom polysaccharides and their biological activities, herein, an attempt was made to isolate a polysaccharide from P. sajor-caju and examine its chemical structure and biological properties.
2. Materials and methods
2.1. Collection and pre-treatment of the mushroom
The mushroom P. sajor-caju was obtained from Krishi Vigyan Kendra (KVK) – ICAR, Salem, Tamil Nadu, India. The mushroom was washed with distilled water and shade dried. The dried mushroom was cut into small pieces and powdered in a mixer grinder for further extraction.2.2. Extraction of the polysaccharide
Extraction of the polysaccharide was carried out following a procedure previously described by Seedevi et al.16 Briefly, 50 g of mushroom powder was dipped in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 volumes (2.5 l) of distilled water and kept at room temperature for 2 h; then, the mixture was homogenized and refluxed at 100 °C for 2 h. After cooling, the mixture was filtered using cheesecloth; further, the supernatant was separated from the mushroom residue by centrifugation at 6000 rpm for 20 min. The residue was further extracted thrice (three times) with double-distilled water at 100 °C for 2 h. All extracts were pooled and concentrated under reduced pressure in a rotary evaporator and dialyzed in a cellulose membrane (the molecular weight cut off 12 kDa) against distilled water for three successive days. The retained fraction was freeze-dried in a lyophilizer (Penguin plus −4 kg, Lark India) and stored in a refrigerator.
50 volumes (2.5 l) of distilled water and kept at room temperature for 2 h; then, the mixture was homogenized and refluxed at 100 °C for 2 h. After cooling, the mixture was filtered using cheesecloth; further, the supernatant was separated from the mushroom residue by centrifugation at 6000 rpm for 20 min. The residue was further extracted thrice (three times) with double-distilled water at 100 °C for 2 h. All extracts were pooled and concentrated under reduced pressure in a rotary evaporator and dialyzed in a cellulose membrane (the molecular weight cut off 12 kDa) against distilled water for three successive days. The retained fraction was freeze-dried in a lyophilizer (Penguin plus −4 kg, Lark India) and stored in a refrigerator.
2.3. Deproteinization of the crude polysaccharide
The crude polysaccharide mixture was dissolved in distilled water and mixed with the Sevag reagent containing chloroform and n-butanol (CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-BuOH) (5
n-BuOH) (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)17 as previously described by Seedevi et al.16
1)17 as previously described by Seedevi et al.16
2.4. Fractionation of the polysaccharide
2.4.2.1 Gel filtration chromatography. The semi-purified polysaccharide was further purified by gel permeation chromatography using the High-Resolution Sepharose 4-LB Fast Flow column (EX 100 mm × 25 cm), equilibrated with distilled water. The column was eluted stepwise with distilled water and then with 0.2 mol l−1 of sodium acetate buffer at the elution rate of 0.33 ml min−1. The carbohydrate content of the obtained fractions was estimated by the phenol-sulphuric acid reaction.18 The fractions showing higher yields were pooled together, dialyzed against distilled water and then freeze-dried.
2.5. Analysis of the biochemical composition
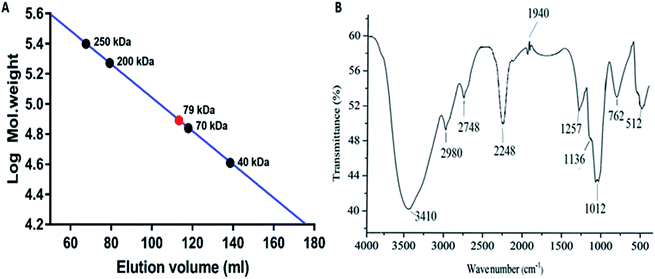
2.5.5.1 FTIR analysis of the polysaccharide. The polysaccharide was analyzed using Bio-Rad FTIR – 40 (USA); typically, 10 mg of the polysaccharide was mixed with 100 mg of dried potassium bromide (KBr) and compressed to prepare a salt disc (10 mm diameter) to obtain its spectrum. Spectra were obtained in the wavenumber range of 4000–500 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) for four times. The organic layer containing the products was obtained and dried. The methylated product was hydrolysed with 90% formic acid (1 ml) at 100 °C for 1 h, and excess formic acid was evaporated by co-distillation with distilled water. After being reduced with sodium borohydride, the methylated and hydrolysed product of the polysaccharide was acetylated with pyridine-acetic anhydride (1
2, v/v) for four times. The organic layer containing the products was obtained and dried. The methylated product was hydrolysed with 90% formic acid (1 ml) at 100 °C for 1 h, and excess formic acid was evaporated by co-distillation with distilled water. After being reduced with sodium borohydride, the methylated and hydrolysed product of the polysaccharide was acetylated with pyridine-acetic anhydride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 45 min at 100 °C. The alditol acetates of the methylated sugars were extracted with chloroform and analysed by GLC-MS. The GLC-MS analysis was performed via the Shimadzu GLC-MS Model QP-2010 Plus automatic system using a ZB-5MS capillary column (30 m × 0.25 mm). The program was isothermal at 150 °C; the hold time was 5 min, with the temperature gradient of 2°C min−1 up to the final temperature of 200 °C.
1) for 45 min at 100 °C. The alditol acetates of the methylated sugars were extracted with chloroform and analysed by GLC-MS. The GLC-MS analysis was performed via the Shimadzu GLC-MS Model QP-2010 Plus automatic system using a ZB-5MS capillary column (30 m × 0.25 mm). The program was isothermal at 150 °C; the hold time was 5 min, with the temperature gradient of 2°C min−1 up to the final temperature of 200 °C.2.6. Antioxidant activity
2.7. Cytotoxicity assay
The cytotoxicity test for the polysaccharide was conducted using MTT according to the method reported by Seedevi et al.20 using Vero cells at different concentrations (100–1400 μg ml−1). The optical density (OD) of each well was measured using an Elisa reader at 620 nm.2.8. Statistical analysis
All experimental data were subjected to one-way analysis of variance (ANOVA), and Dunnett's Multiple Comparison (GraphPad prism version 5.0, GraphPad Software, USA) was used to determine the difference among means at the level of 0.05.3. Results
3.1. Fractionation, purification and yield of the polysaccharide
The polysaccharide was fractionated initially via a Q-Sepharose column using NaCl and eluted stepwise with distilled water, with the linear gradient of 0–3 mol L−1 NaCl. The fractions of 0.1, 0.2 and 0.3 M/NaCl showed higher carbohydrate content and were pooled together and freeze-dried (Fig. 1A). The fractionated polysaccharide was purified through a Sepharose 4-LB column using 0.2 M sodium acetate buffer, and the fraction showed a single peak at higher carbohydrate content and was freeze-dried (Fig. 1B). The yield of the purified polysaccharide was found to be 18.01% (w/w) on a dry weight basis.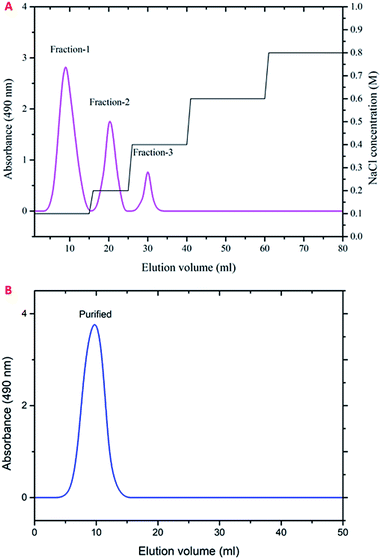 | ||
| Fig. 1 Elution profile of the polysaccharide obtained from P. sajor-caju. (A) Ion exchange chromatography using a Q-Sepharose column. (B) Gel filtration chromatography using a Sepharose 4-LB column. | ||
3.2. Chemical composition of the polysaccharide
The total carbohydrate, protein, ash and moisture contents of the polysaccharide were 90.16, 0, 12.7 and 5.2%, respectively. In the present study, the total carbohydrate content of the purified polysaccharide was higher when compared with that reported in a previous study conducted on the hot water extraction (HWE), ultrasound-assisted extraction (US) and Soxhlet extraction (SE) of a polysaccharide obtained from Ganoderma lucidum, which showed 66.85, 50.24 and 44.47% total carbohydrate content, respectively.313.3. Molecular weight of the polysaccharide
The molecular weight of the polysaccharide obtained from P. sajor-caju was 79 kDa as compared to the standard dextran Mw 250, 200, 70 and 40 kDa (Fig. 2A). In the present study, the molecular weight was found to be higher as compared to that of the Lentinus fusipes mushroom polysaccharide (Mw: 60 kDa).35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
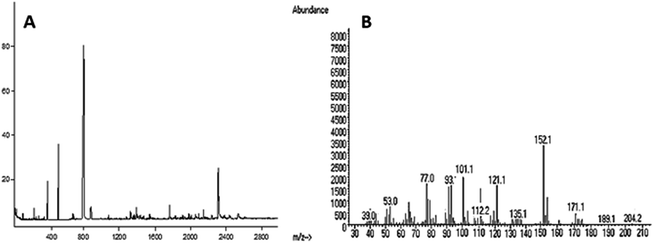
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The total ion and mass spectra are shown in Fig. 3A and B.
1. The total ion and mass spectra are shown in Fig. 3A and B.
| Methylated sugars | Molar ratio | Linkage-type | Major mass fragments (m/z) |
|---|---|---|---|
| 2,3,4-Me3-Glc | 4 | →6)-Glcp-(1→ | 39, 53, 77, 101, 112, 121, 152, 171, 204 |
| 2,3,4,-Me3-Glc | 1 | →6)-Glcp-(1→ | 77, 101, 112, 135, 171, 189, 204 |
| 2,3,4-Me3-Glc | 2 | →6)-Glcp-(1→ | 39, 53, 77, 93, 101, 112, 121, 152 |
| 2,3,4,-Me3-Glc | 1 | →6)-Glcp-(1→ | 39, 53, 77, 101, 121, 135, 161, 171, 189 |
| 2,3,4,6-Me4-Glc | 1 | Glcp-(1→ | 39, 53, 77, 101, 112, 121, 135, 189, 204 |
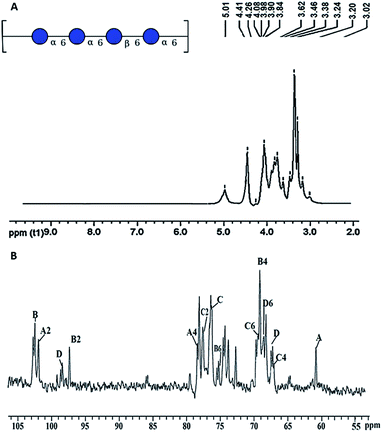
| 1H-NMR (δ) | Assignment |
|---|---|
| 3.62 | α-D-Glci – 2 |
| 3.46 | α-D-Glci – 4 |
| 3.02 | β-D-Glcii – 2 |
| 3.38 | β-D-Glcii – 3 |
| 3.24 | β-D-Glcii – 4 |
| 3.62 | α-D-Glciii – 2 |
| 13C-NMR (δ) | Assignment |
|---|---|
| 101.9 | α-D-Glci – 1 |
| 69.4 | α-D-Glci – 4 |
| 68.8 | α-D-Glci – 6 |
| 102.5 | β-D-Glcii – 1 |
| 78.5 | β-D-Glcii – 3 |
| 69.1 | β-D-Glcii – 4 |
| 77.7 | β-D-Glcii – 5 |
| 67.1 | β-D-Glcii – 6 |
| 97.4 | α-D-Glciii – 1 |
| 75.2 | α-D-Glciii – 3 |
| 60.9 | α-D-Glciii – 6 |
| 98.5 | α-D-Glciv – 1 |
| 76.4 | α-D-Glciv – 3 |
| 67.5 | α-D-Glciv – 6 |
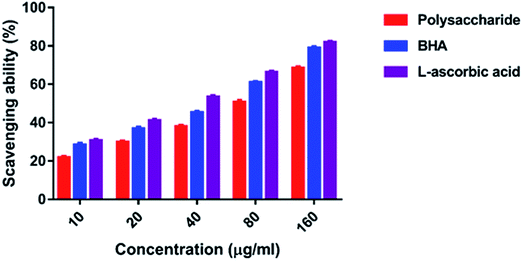
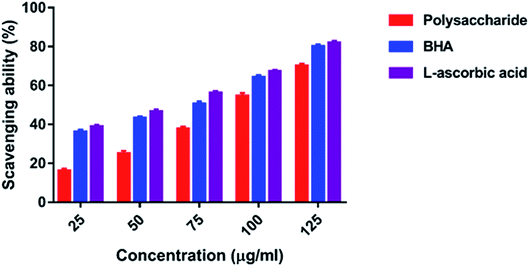
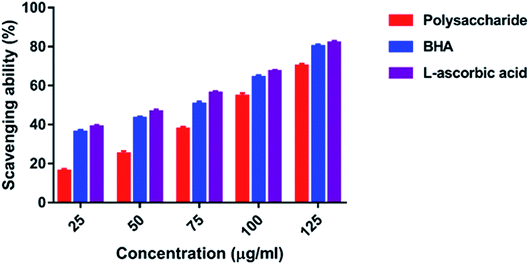
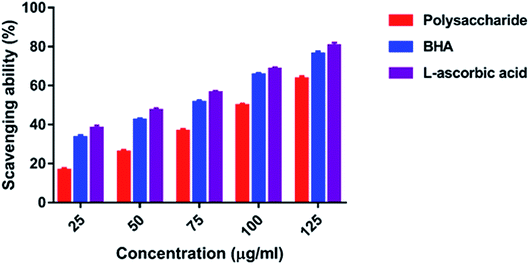
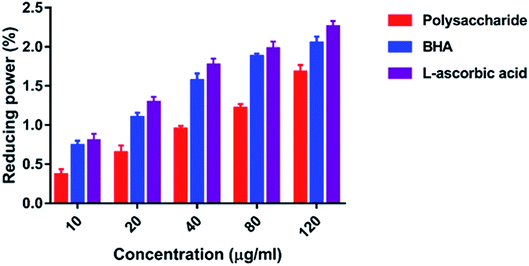
3.4. Antioxidant activity
3.5. Cytotoxicity and anticancer activity of the polysaccharide
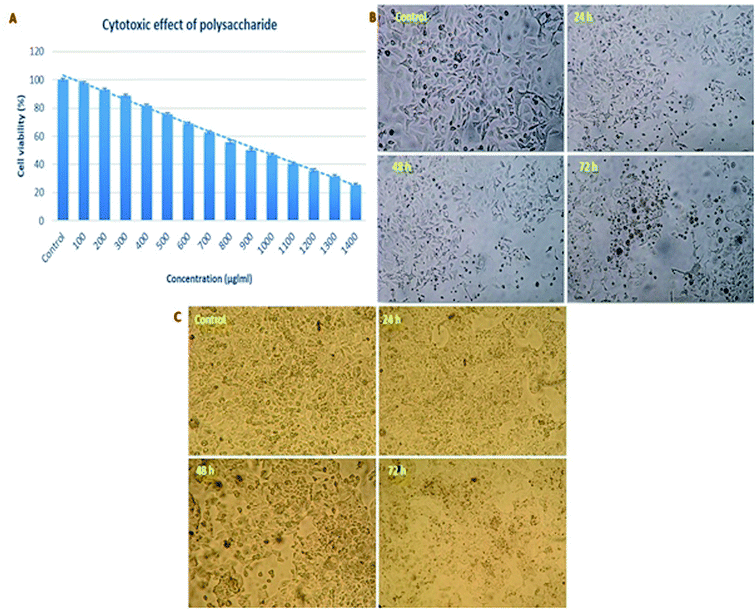
For the development of anticancer drugs, initial screening of the isolated compounds should be carried out before their administration to the cancer cells to investigate the cytotoxicity effect of these compounds on normal cell lines. In the present study, the polysaccharide was initially screened for cytotoxicity effects on a normal cell line (Vero cells). The cytotoxic concentration CC50 value of the polysaccharide was 900 μg ml−1 for a 72 h incubation period (Fig. 10A). On further evaluation, the reported polysaccharide showed the anticancer activity of 18.61, 33.70, 49.65 and 63.21% against the AGS human gastric carcinoma cell line and 15.30, 29.67, 45.01 and 59.70% against the HepG2 cell line at 100, 200, 300, 400 and 500 μg ml−1 concentrations, respectively (Fig. 10B and C).4. Discussion
The yields of the crude and fractionated polysaccharide (F1, F2 and F3) obtained from Agaricus blazei were 14 and 37, 37 and 14%, respectively, on a dry weight basis.27 Similarly, the yields of three fractionated polysaccharides (NTHSP-A, NTHSP-B and NTHSP-C) obtained from Hohenbuehelia serotina were 77.84%, 9.76%, and 12.40% (w/w) on a dry weight basis.28 In addition, the yields of the crude polysaccharide and fractionated polysaccharide (FI and FII) obtained from the edible mushroom Lentinus sajor-caju were 25, 14 and 9 mg, respectively.39 The yields of the hot water-extracted crude and purified polysaccharide from Meripilus giganteus were 25 and 17 mg, respectively.30 The variation in the yield of the polysaccharide may differ from that of the cell wall polysaccharide composition. In addition, the total carbohydrate content of the fractionated polysaccharide (G1) obtained from Fomitiporia punctata was 92.78 ± 2.61%.32 He et al.33 noted the carbohydrate and protein content of the crude and purified polysaccharide obtained from the pent mushroom Pleurotus eryngii as (55.22 ± 1.46% & 98.78 ± 0.12%) and (39.89 ± 1.32% & 1.15 ± 0.014%), respectively. The carbon, hydrogen and nitrogen contents of the present polysaccharide were 31.53, 4.28 and 3.01%, respectively. Similarly, the carbon, hydrogen, nitrogen and sulphur contents of the sulphated polysaccharide obtained from Gracilaria corticata, Acanthophora spicifera and Ulvan were 33.19–38.08%, 5.91–7.04%, 7.21–8.04% and 0.28–3.75%, respectively.20,34,35 Moreover, the molecular weight of glucan obtained from Meripilus giganteus was 1.48 × 105 Da.30 Similarly, the two purified polysaccharides HLP1-1 and HLP2-1 obtained from the Bachu mushroom Helvella leucopus displayed the molecular weights of 21382 Da and 23063 Da, respectively.38 The variation in the molecular weight is due to the influence of the extraction method used with the solvent system.The polysaccharides obtained via microwave-assisted extraction (MAE) and pressurized liquid extraction (PLE) of Pleurotus ostreatus and Ganoderma lucidum had (91.0 & 91.3%) and (89.6 & 86.5%) of glucose and (3.5 & 2.4%) and (7.1 & 9.4%) galactose, respectively. Moreover, it was reported that the mushroom extracts were rich in glucan polysaccharides recovered by the tested methods.33 Thus, it was speculated that the polysaccharide consisted of repeating units of terminal →6)-Glcp-(1→6), Glcp-(1→6), Glcp-(1→6), Glcp-(1→6) and Glcp-(1→ residues. Hay et al.42 reported that the GLC analysis of the alditol acetates of the periodate-oxidized, NaBH4-reduced and hydrolyzed products showed the presence of glucose units only. This good correlation between the terminal and the branched moieties and the linkages were confirmed in the technical study reported by Sims et al.43
In the present study, the DPPH radical scavenging activity of the polysaccharide was higher when compared with that of the previously studied polysaccharide obtained from the wild edible mushroom Lentinus sajor-caju, which was 6.14, 21.88, 30.56, 38.89 and 52.5% at 0.1, 0.5, 0.75, 1.0 and 1.5 mg ml−1 concentrations, respectively.29 He et al.33 reported the DPPH radical scavenging activity of the crude polysaccharide obtained from the pent mushroom Pleurotus eryngii as 85.89% at the concentration of 1.0 mg ml−1. In the present study, the DPPH radical scavenging activity of the polysaccharide may be due to their hydrogen donating ability. Similarly, the ABTS free radical scavenging ability of the sulfated polysaccharide obtained from Monostroma oxyspermum was 25.37–76.81% at various concentrations ranging from 25 to 125 mg ml−1.16 In addition, the ABTS free radical scavenging activity of the polysaccharide obtained from the Jinqian mushroom was 63.96% at the concentration of 5 mg ml−1.47 Similarly, the superoxide scavenging activity of the crude polysaccharide obtained from the pent mushroom Pleurotus eryngii was 85.74% at the concentration of 1.0 mg ml−1.33 Maity et al.30 reported that the superoxide radical scavenging activity of glucan obtained from Meripilus giganteus was 74.60% at 100 μg ml−1 concentration.
The hydroxyl radicals are the most harmful reactive oxygen species that can attack46 and damage almost every bio-macromolecule in living cells and induce severe damage to the adjacent biomolecules.48 Pattanayak et al.29 explained that the hydroxyl radical scavenging activity of the polysaccharide obtained from the wild edible mushroom Lentinus sajor-caju was 18.7, 28.5, 36.5, 43.06 and 53.3% at 0.25, 0.50, 0.75, 1.0 and 1.5 mg ml−1 concentration, respectively. In the present study, the hydrogen ions were released from the hydroxyl groups (–OH) of the polysaccharide and combined with radicals that terminated the radical-initiated chain reactions. In addition, the reducing power of the polysaccharide obtained from the wild edible mushroom Lentinus sajor-caju was 0.328, 0.443 and 0.539% at 1.0, 1.5 and 2.0 mg ml−1 concentrations.29 The reducing power of the polysaccharide may be observed in the presence of the glucopyranosyl chain reaction.
The crude and purified sulfated polysaccharide showed cytotoxicity at 1200 and 1400 μg ml−1 concentration on the normal cell line (HBL-100).50 Similarly, the two polysaccharide fractions HLP1-1 and HLP2-1 obtained from the Bachu mushroom H. leucopus showed the anti-proliferative activities of 26.60 and 62.19% at the concentration of 2000 μg ml−1 against the HepG2 cells, respectively.49 The active principle of the polysaccharide may enhance the level of anti-oxidation and remove the reactive oxygen species in cancer cells; thus, this polysaccharide may inhibit the cell growth.
5. Conclusion
The present study revealed that the polysaccharide obtained from P. sajor-caju had the →6)α-D-Glciv(1→6)α-D-Glciii(1→6)β-D-Glcii(1→6)α-D-Glci(1→structure; glucose linkages, for example, β-D-Glcii(1→6) linkages, in the fundamental chain of the glucan and branch moieties are required for the anticancer action. At the molecular level, the improvement of the anticancer action of polysaccharides and their clinical characteristics by expanding their water solubility and capacity to enter the gastrointestinal wall after administration is regularly conducted; carboxymethylation is one of the principal techniques that the body uses for chemical interactions in anticancer activity; this is clear from this study. Therefore, the active principle of the polysaccharide may be helpful in preventing oxidative damage in human cells and enhancing the level of antioxidants to prevent the formation of reactive oxygen species, which inhibits the cancer cells. Hence, the mushroom polysaccharide might be used as a futuristic food and functional compound in the pharmacological industry.Conflicts of interest
All authors declare that there is no conflict of interest.Acknowledgements
The authors (P. S. PDF/2017/002512) are thankful to the Department of Sciences and Technology, Science and Engineering Research Board (DST-SERB), National Postdoctoral Fellowship (N-PDF), Government of India, for providing financial assistance.References
- M. Friedman, J. Agric. Food Chem., 2015, 63(32), 7108–7123 CrossRef CAS PubMed.
- X. Meng, H. Liang and L. Luo, Carbohydr. Res., 2016, 424, 30–41 CrossRef CAS PubMed.
- A. Nandi, K. I. Sen, S. Samanta, K. Maity, K. S. Devi, S. Mukherjee and S. S. Islam, Carbohydr. Res., 2012, 363, 43–50 CrossRef CAS PubMed.
- A. C. Ruthes, F. R. Smiderle and M. Iacomini, Carbohydr. Polym., 2015, 117, 753–761 CrossRef CAS PubMed.
- X. Sun, Y. Sun, Q. Zhang, H. Zhang, B. Yang, Z. Wang and H. Kuang, Int. J. Biol. Macromol., 2014, 69, 12–19 CrossRef CAS PubMed.
- I. Giavasis, Curr. Opin. Biotechnol., 2014, 26, 162–173 CrossRef CAS PubMed.
- L. Fan, J. Li, K. Deng and L. Ai, Carbohydr. Polym., 2012, 87(2), 1849–1854 CrossRef CAS.
- S. K. Bhanja, D. Rout, P. Patra, C. K. Nandan, M. Behera and S. S. Islam, Carbohydr. Res., 2013, 374, 59–66 CrossRef CAS PubMed.
- A. C. Ruthes, E. R. Carbonero, B. Córdova, A. R. S. Santos, G. L. Sassaki and M. Iacomini, Carbohydr. Polym., 2013, 94(1), 129–136 CrossRef CAS PubMed.
- B. Halliwell and J. Gutteridge, Biochem. J., 1984, 219(1), 1 CrossRef CAS PubMed.
- B. Halliwell, Free Radical Biol. Med., 1989, 7(6), 645–651 CrossRef CAS PubMed.
- A. L. Branen, J. Am. Oil Chem. Soc., 1975, 52(2), 59 CrossRef CAS PubMed.
- J. L. Mau, H. C. Lin and C. C. Chen, J. Agric. Food Chem., 2002, 50(21), 6072–6077 CrossRef CAS PubMed.
- F. Y. Lin, Y. K. Lai, H. C. Yu, N. Y. Chen, L. Chang and H. C. Lo, Food Chem., 2008, 110(2), 446–453 CrossRef CAS PubMed.
- H. Zhang, H. Ma, P. Liu, Z. Wang, H. Zhou and J. Yan, Carbohydr polym, 2014, 113, 380–387 CrossRef CAS PubMed.
- P. Seedevi, M. Moovendhan, S. Sudharsan, S. Vasanthkumar, A. Srinivasan, S. Vairamani and A. Shanmugam, Int. J. Biol. Macromol., 2015, 72, 1459–1465 CrossRef CAS PubMed.
- A. M. Staub, Methods Carbohydr. Chem., 1965, 5, 5–6 CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. T. Rebers and F. Smith, Ann. Chem., 1956, 28(3), 350–356 CrossRef CAS.
- M. M. Bradford, Ann. Chem., 1976, 72(1–2), 248–254 CAS.
- P. Seedevi, M. Moovendhan, S. Viramani and A. Shanmugam, Carbohydr. Polym., 2017, 155, 516–524 CrossRef CAS PubMed.
- P. Maity, I. K. Sen, P. K. Maji, S. Paloi, K. A. Devi, K. Acharya and S. S. Islam, Carbohydr. Polym., 2015, 123, 350–358 CrossRef CAS PubMed.
- Y. Zhang, S. Li, X. Wang, L. Zhang and P. C. Cheung, Food Hydrocolloids, 2011, 25(2), 196–206 CrossRef CAS.
- M. S. Gião, M. L. González-Sanjosé, M. D. Rivero-Pérez, C. I. Pereira, M. E. Pintado and F. X. Malcata, J. Sci. Food Agric., 2007, 87(14), 2638–2647 CrossRef PubMed.
- M. Nishikimi, N. Rao and K. Yagi, Biochem. Biophys. Res. Commun., 1972, 46(2), 849–854 CrossRef CAS PubMed.
- B. Halliwell, J. M. Gutteridge and O. I. Aruoma, Anal. Biochem., 1987, 165(1), 215–219 CrossRef CAS PubMed.
- M. Oyaizu, Jpn. J. Nutr., 1986, 44(6), 307–315 CrossRef CAS.
- H. T. Wang, L. C. Yang, H. C. Yu, M. L. Chen, H. J. Wang and T. J. Lu, J. Food Drug Anal., 2018, 26(2), 678–687 CrossRef CAS PubMed.
- X. Li, L. Wang and Z. Wang, Int. J. Biol. Macromol., 2017, 98, 59–66 CrossRef CAS PubMed.
- M. Pattanayak, P. Maity, S. Samanta, I. K. Sen, D. K. Manna, A. K. Nandi and S. S. Islam, Int. J. Biol. Macromol., 2018, 107, 322–331 CrossRef CAS PubMed.
- P. Maity, A. K. Nandi, D. K. Manna, M. Pattanayak, I. K. Sen, S. K. Bhanja and S. S. Islam, Carbohydr. Polym., 2017, 157, 1237–1245 CrossRef CAS PubMed.
- I. Alzorqi, S. Sudheer, T. J. Lu and S. Manickam, Ultrason. Sonochem., 2017, 35, 531–540 CrossRef CAS PubMed.
- F. Liu, Y. Wang, K. Zhang, Z. Wang, Y. Zeng and T. Ng, Int. J. Biol. Macromol., 2017, 97, 339–347 CrossRef CAS PubMed.
- P. He, F. Li, L. Huang, D. Xue, W. Liu and C. Xu, J. Taiwan Inst. Chem. Eng., 2016, 69, 48–53 CrossRef CAS.
- A. R. Ganesan, M. Shanmugam, S. Palaniappan and G. Rajauria, Food Biosci., 2018, 23, 121–128 CrossRef CAS.
- A. R. Ganesan, M. Shanmugam and R. Bhat, Int. J. Biol. Macromol., 2018, 112, 1164–1170 CrossRef PubMed.
- F. R. Smiderle, D. Morales, A. Gil-Ramírez, A. L. I. de Jesus, B. Gilbert-López, M. Iacomini and C. Soler-Rivas, Carbohydr. Polym., 2017, 156, 165–174 CrossRef CAS PubMed.
- D. K. Manna, P. Maity, A. K. Nandi, M. Pattanayak, B. C. Panda, A. K. Mandal and S. Roy, Carbohydr. Polym., 2017, 157, 1657–1665 CrossRef CAS PubMed.
- D. Zeng and S. Zhu, Int. J. Biol. Macromol., 2018, 107, 1086–1092 CrossRef PubMed.
- Y. Zhang, H. Wang, P. Wang, C. Ma, G. He and M. R. T. Rahman, Int. J. Biol. Macromol., 2016, 92, 1057–1066 CrossRef CAS PubMed.
- Y. D. Sun, Z. H. Wang and Q. S. Ye, Int. J. Biol. Macromol., 2013, 62, 291–295 CrossRef CAS PubMed.
- A. Wiater, R. Paduch, M. Pleszczyńska, K. Próchniak, A. Choma, M. Kandefer-Szerszeń and J. Szczodrak, Biotechnol. Lett., 2011, 33(4), 787–795 CrossRef CAS PubMed.
- G. W. Hay, B. A. Lewis and F. Smith, Methods Carbohydr. Chem., 1965, 5(3), 5 Search PubMed.
- I. M. Sims, I. M., S. M. Carnachan, T. J. Bell and S. F. Hinkley, Carbohydr. Polym., 2018, 188, 1–7 CrossRef CAS PubMed.
- P. K. Agrawal, Phytochemicals, 1992, 31(10), 3307–3330 CrossRef CAS.
- L. P. Leong and G. Shui, Food Chem., 2002, 76(1), 69–75 CrossRef CAS.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 26(9–10), 1231–1237 CrossRef CAS PubMed.
- Y. Liu, J. Zhang, Q. Tang, Y. Yang, Q. Guo, Q. Wang and S. W. Cui, Carbohydr. Polym., 2014, 101, 968–974 CrossRef CAS PubMed.
- R. G. Abirami and S. Kowsalya, J. Herbs, Spices Med. Plants, 2017, 23(1), 9–17 CrossRef CAS.
- R. G. Abirami and S. Kowsalya, Int. J. Res. Phytochem. Pharmacol., 2013, 3(3), 136–141 Search PubMed.
- V. Suresh, N. Senthilkumar, R. Thangam, M. Rajkumar, C. Anbazhagan, R. Rengasamy and P. Palani, Process Biochem., 2013, 48(2), 364–373 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |