DOI:
10.1039/C9RA03331A
(Paper)
RSC Adv., 2019,
9, 25303-25308
Highly efficient recovery of ruthenium from integrated circuit (IC) manufacturing wastewater by Al reduction and cementation
Received
4th May 2019
, Accepted 1st July 2019
First published on 13th August 2019
Abstract
Ruthenium (Ru) is a rare-earth metal, which is employed widely in metal-processing industries. This study recovered Ru from the wastewater of an IC foundry by cementation using metallic aluminum (Al) powder as the sacrificial agent. Ru ions were efficiently reduced to the metal and coagulated with the derived aluminum hydroxide flocs. Experimental parameters included the particle size of Al, molar ratio of Al to Ru, initial Ru concentration and operation temperature. The recovery rate reached 99% under these conditions: particle size Al powder = 88–128 μm, Al/Ru molar ratio = 2.0, initial Ru = 200 mg L−1, temperature = 338.16 K, reaction time = 120 min, stirring speed = 150 rpm. The cemented Ru over Al powder was spherical with a rough surface. Kinetic modelling suggested that the diffusion of Ru through the ash layer of Al powder controlled the reaction rate with an activation energy of 40.75 kJ mol−1. A brief cost analysis demonstrated that the cementation of Ru yielded a profit of $0.180 per 0.1 m3-wastewater.
1. Introduction
Ruthenium (Ru) is a rare-earth metal utilized in chemical and electrical industries. Ru hardens the electroplating and sputtering RuPt and RuPd alloys in electrical contacts.1 Ru oxide is used in thick-film chip resistors, which accounts for approximately 50% of Ru production.2 Besides, Ru is an important element in advanced high-temperature single-crystal superalloys for making jet turbine engines. Ru is also applied in a variety of electrochemical,3 optical,4 medical,5 catalytic,6,7 and analytical chemistries.8 Therefore, the demand of Ru has considerably soared. The average abundance of Ru in the earth's crust is only about 0.001 ppm,9 thus there is a necessity to develop Ru recovery technologies.
Ruthenium is also an unwanted by-product from spent nuclear fuel, electronic materials, and olefin metathesis.10–12 Integrated circuit (IC) foundries or semiconductor fabrication plants require a large quantity of Ru, especially for surface coating. The effluent of IC manufacturing contains a high concentration of Ru and needs to be treated before discharge. So far, the recovery of Ru from IC foundry wastewater has not yet been reported. Al cementation is simple, highly efficient and cost-effective for the recovery of metals from wastewater.13 By contrast, the solvent extraction commonly used in the recovery of metals in solution may have a limiting selectivity and products with low purity.14 Besides, the evaporation consumes more energy than cementation in dealing with the considerable quantity of dilute solution.15,16 In the Al cementation recovery of Ru, metallic Al acts as a reducing agent. The Ru ions which are converted to metal are collected by the derived-Al hydroxide by sweep flocculation as described by the following equations:
| | |
Ru3+ + 3e− ↔ Ru, E0 = +0.704 V
| (1) |
| | |
Al + 4OH− ↔ Al(OH)4− + 3e−, E0 = −1.659 V
| (2) |
The net reaction can be written as:
| | |
Al + 4OH− + Ru3+ → Al(OH)4− + Ru, ΔE0 = 2.363 V
| (3) |
| | |
Al(OH)4− → Al(OH)3(s) + OH− (precipitation)
| (4) |
| | |
Ru + Al(OH)3(s) = Ru-Al(OH)3(s) (coagulation)
| (5) |
During cementation, Ru can be deposited at cationic sites on the surface of Al, whereas the dissolution of Al occurs at anionic sites. The reaction within the Al/Ru system can be deemed irreversible since the electro-motive force between Ru3+ and Al is high.17 This work studied the recovery of Ru from wastewater using aluminum (Al) powder. The effects of particle size of Al powder, Al/Ru molar ratio, initial Ru concentration and temperature on the removal rate of Ru were investigated. The operation cost was analyzed to examine the economic feasibility of Ru recovery.
2. Materials and methods
2.1. Chemicals
Chemicals employed in this study include aluminum powder (purity 99.5%, Qingdao Ocean Import and Export Co., China), sodium hydroxide (Merck KGaA, Germany) and nitric acid (Sigma-Aldrich, U.S.A.). A laboratory-grade RO-ultrapure water system (resistance > 18.0 MΩ) was used for DI water supply. All purchased chemicals were of laboratory grade and used as received. Al powder (purity 99.5%, from Qingdao Ocean Import and Export Co., China) was used as the sacrificial agent for Ru3+ ions removal. In order to study of the effects of different particle sizes of Al powder on the recovery, the powder was sieved into three fractions (as indicated in Table 1) and then employed in recovery experiments.
Table 1 Experimental design for cementation recovery of Ru
| Parameters |
Values |
| Particle sizes of Al powder |
149–177 μm (surface area 0.051 m2 g−1, pHpzc 9.3); 88–125 μm (surface area 0.103 m2 g−1, pHpzc 9.2); 53–88 μm (surface area 0.152 m2 g−1, pHpzc 8.5) |
| Al/Ru molar ratios |
0.5, 1.0, 2.0 |
| Initial Ru concentrations (mg L−1) |
20, 50, 100, 200 |
| Temperature (K) |
308.16, 318.16, 328.16, 338.16 |
2.2. Experimental procedures
Real wastewater collected from a local IC foundry was used for experimental design. Metal composition in the effluent of Ru-plating process under alkaline conditions (pH 8.67) includes 23.5 mg Ru per L, 2 mg Zn per L, 1.3 mg Fe per L, and 0.5 mg Ni per L. The initial concentrations of 20–200 mg L−1 Ru were spiked for cementation experiments. The low concentration of impurity (e.g. Zn, Fe and Ni, which even are absent from EDS analysis) in the wastewater benefits the recovery of Ru. Base metals precipitate more easily than the noble metals and may decrease the purity of Ru.13 The effluent was spiked with standard solution (Sigma-Aldrich Co.) to make up initial certain concentrations of Ru (20–200 mg L−1) for cementation experiments. Duplicate experiments were carried out in 500 mL volumetric flasks at 301.16 K. The pH value of the solution was adjusted using 1 M NaOH. During the experiment, the solutions containing the coagulating agent (Al powder) were added slowly into the Ru wastewater while stirring (150 rpm). Effects of Al/Ru molar ratios, particle sizes of Al powder, initial Ru concentrations and temperature on the cementation efficiency were studied (Table 1). When the chemical reaction had finished, the precipitate was allowed to settle down for 60 min, collected and then dried under vacuum at 313.16 K for 24 h. The precipitate next underwent subsequent solid characterization, while the supernatant was analyzed for residual Ru content.
2.3. Analytical methods
The concentration of Ru and other elements in the solution was determined using an inductively coupled plasma-optical emission spectrometer (ICP-OES, ULTIMA 2000, HORIBA Ltd., Japan). Glassware and plastic sample containers for ICP analysis were soaked in 5% HNO3 and oven-dried before use. The ICP standard solutions were purchased from High-Purity Standards (Charleston, USA). The minimum detection limit (MDL) of 0.0005 mg Ru per L was determined by tripling the standard deviation from the analysis of seven samples with the same concentration. The calibration curve was constructed with a correlation coefficient (R2) of 0.9998. The mid-point checks and spike recoveries ranged from 0.25% to 3.60%, and from 96% to 110%, respectively. The morphology, surface composition and crystallographic structure of Ru precipitate was studied using the scanning electron microscope (SEM, JOEL JXA-840, HITACHI S4100), the energy dispersive spectrometer (EDS, LINKS AN10000/85S), and the X-ray diffraction (XRD, DX III, Rigaku Co., Japan), respectively.
3. Results and discussion
3.1. Recovery of Ru by Al cementation
The Al powder with different particle size range of (53–88, 88–125, and 149–177 μm) were applied to recover Ru in the solution as shown in (Fig. 1a). Accordingly, the cementation efficiency increases from 80.3% to 95.6% with decreasing the particle size of Al powder from 149–177 μm to 88–125 μm, because of the increase in the specific surface area of Al (Table 1). However, as the Al particle size declines to 53–88 μm, the efficiency drops to 77.3%. The high surface area would accelerate the oxidation of fresh metallic Al surface sites, deteriorating the Ru cementation.17 Effect of molar ratio of Al/Ru (0.5, 1 and 2) on the recovery of Ru is shown in (Fig. 1b). The recovery increases substantially from 56.4% to 95.4% when the molar ratio is increased from 0.5 to 1; the ratio increased up to 2 slightly improves the efficiency to 98.8%, which is simply attributed to the greater quantity of active surface sites produced from higher dosage of Al for the cementation reaction.
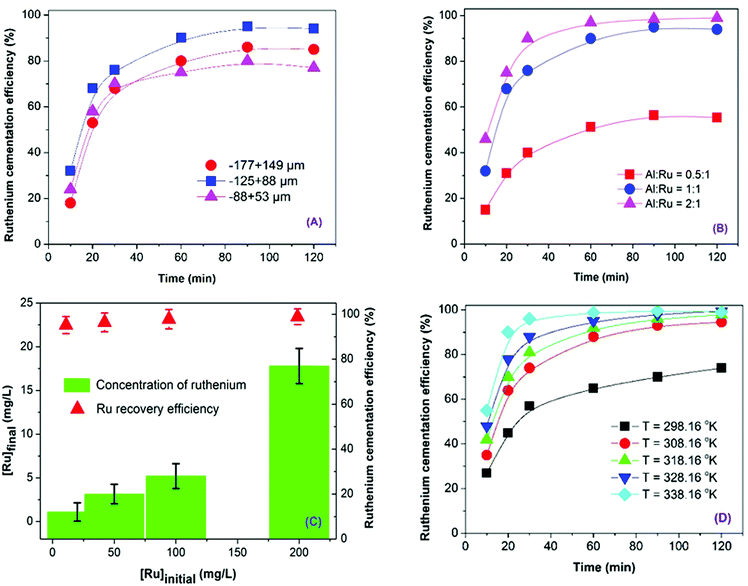 |
| | Fig. 1 Effects of (A) Al powder sizes (Al/Ru molar ratio = 1.5, [Ru] = 200 mg L−1, T = 308.16 K and 150 rpm), (B) Al/Ru molar ratios (Al powder size = 88–128 μm, [Ru] = 200 mg L−1, T = 308.16 K and 150 rpm), (C) initial Ru concentrations (Al/Ru molar ratio = 2.0, Al powder size = 88–128 μm, T = 308.16 K and 150 rpm), (D) temperature (Al/Ru molar ratio = 2.0, Al powder size = 88–128 μm, [Ru] = 200 mg L−1 and 150 rpm) on the cementation of Ru. | |
Fig. 1c presents the effect of initial Ru concentration (20 to 200 mg L−1) on the recovery of Ru using 88–128 μm Al powder under Al/Ru molar ratio of 2.0. The recovery efficiency of Ru increases from 94.5% to 98.4% with increasing the initial Ru concentration from 20 to 200 mg L−1. The cementation rate of Ru was insignificantly influenced by the selected initial Ru concentrations. Whereas, the removal rate of Ru is subject to the Ru adsorption on to the Al precipitates; more Ru left in the solution would be in equilibrium with the adsorbed Ru species over Al as higher initial Ru level was adopted. Effect of temperature on the recovery of Ru are presented in (Fig. 1d). Increasing the cementation temperature increases the recovery efficiency of Ru. Although the adsorption reaction is normally exothermic, the temperature would help breaking up the passive oxide layer over the Al particle, improving the effective contact between Ru and Al. The recovery of Ru is maximized at 98.5% in 120 min at 338.16 K. Consequently, the cementation of Ru from the solution (initial [Ru] = 200 mg L−1) is optimized under conditions: molar ratio of Al/Ru = 2.0, Al powder size = 88–128 μm, temperature = 338.16 K, in 120 min and 150 rpm of stirring speed.
Adsorption and cementation have been employed for the recovery of Ru from wastewater (Table 2). The adsorption can achieve around 90% of Ru recovery, while the cementation shortens duration for attaining the similar rate of Ru recovery. Kwak et al. (2013) and Ohta et al. (2002) synthesized polyethylenimine-modified bacteria,18 and alginate gel polymer,19 as adsorbents to adsorb Ru from acidic solution and achieved a 90% recovery after 24 and 48 hours, respectively. Aktas et al. (2018), however, utilized Ru cementation from electroplating wastewater and; accordingly, increasing temperature from 293.16 to 343.16 K improved the Ru recovery from 75% to 95% after 90 min.20 In this study, with an initial Ru concentration of 200 mg L−1, about 99% of Ru was recovered at 338.16 K after 120 minutes.
Table 2 Summary of recovery technologies for ruthenium
| Methods |
Conditions |
Reclaiming efficiency |
Ref. |
| Cementation reaction |
Batch, a decorative ruthenium plating workshop, 20 and 70 C, 50 mg zinc powder, 90 min |
Ru: <75% at T = 298.16 K, Ru: >95% at T = 338.16 K (add 1000 mg NaCl) |
20 |
| Adsorption |
Batch, acetic acid waste solution containing Ru, polyethylenimine modified bacterial biosorbent, 24 h |
Ru: 90% at T = 293.16 K ([Ru]initial = 61.6 mg L−1) |
18 |
| Adsorption |
Batch, Ru synthetic solution, alginate gel polymers, 2.5 M HNO3, 2 day |
RuNO3+: 90% at T = 298.16 K |
19 |
| Biosorption |
Batch, industrial effluents, Rhodopseudomonas palustris, 298.16 K and 338.16 K, 64 h |
Ru: 42% to 72% (biomass) ([Ru]initial = 1800 mg L−1) |
33 |
| Cementation |
Batch, integrated circuit foundry's wastewater, aluminum powder (88–128 μm), 2 h |
Ru: >99.4% at 338.16 K ([Ru]initial = 200 mg L−1) |
This study |
3.2. Characterization of the cemented Ru
SEM images (Fig. 2a and b) show that the surface cemented Ru on spherical Al powder was roughly aggregated. XRD analysis (Fig. 2c) evidences the Ru on Al is the metallic ruthenium (PDF. # 65-1863), indicating that the cementation was successfully conducted via chemical reduction and deposition of Ru ions from IC foundry's wastewater on to Al surface. Besides, from elemental analysis (Fig. 2d) of EDS, the main constituents of the surface include the cemented Ru, Al2O3 and a small amount of phosphorus (0.35 ± 0.05 mg g−1) and sodium (2.25 ± 0.02 mg g−1). Ru/Al composite has been studied as catalysts. Ru-doped mesoporous Ni–Al oxides catalyst showed high catalytic activity for selective CO methanation.21 Meanwhile, Ru complexation was functionalized into Al metal organic framework (MOF) for catalytic oxidation of alcohols.22 Currently, Ru recovered on Al powder is being tested as a catalyst for the treatment of textile wastewater and the results will be reported in future.
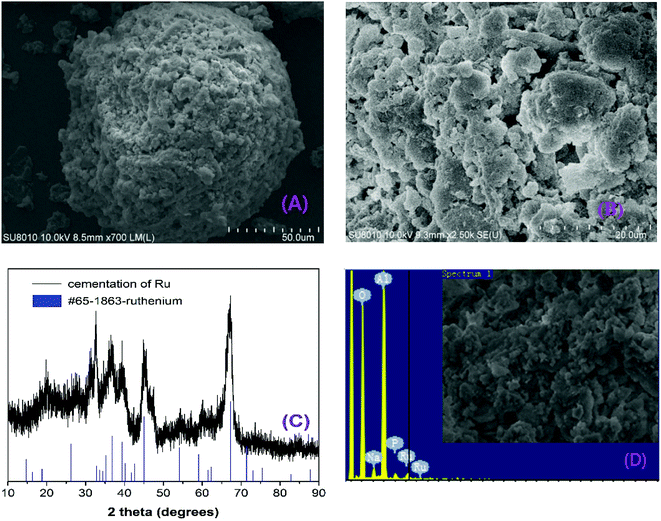 |
| | Fig. 2 Morphology of cemented Ru analyzed by SEM with magnification of (A) ×50, (B) ×20; (C) XRD pattern of cemented Ru; SEM and EDS spectrum of cemented Ru with magnification of (D) ×50. | |
3.3. Kinetics of Ru cementation
The shrinking core model was employed to characterize the cementation of Ru, in which the gas film diffusion, ash diffusion and chemical reactions during Ru cementation were considered. For gas film diffusion, the fraction of Ru (XRu) diffusing into the initial gas film layer of Al powder at time t (min−1) can be described with a rate constant k as follows:23,24
In Ash diffusion, the diffusion of Ru through the ash layer at time t is estimated by:24,25
| | |
1 − 3(1 − XRu)2/3 + 2(1 − XRu) = kt
| (7) |
Further, the chemical reaction model defined the Ru removal as a function of time by:23–27
| | |
1 − (1 − XRu)1/3 = kt
| (8) |
The experimental data in (Fig. 1d) fitted by the models using eqn (6)–(8) was plotted in (Fig. 3). Generally, the data is better fitted with the models at lower temperature according to higher values of correlation coefficient (R2). Cementation of ruthenium is a heterogeneous process which involves the interaction at the solid–liquid interface. Thus, the reactant diffuses through liquid and surface precipitate layers, at which the chemical reaction occurs. This model defines the movement of the reaction zone into the deep unreacted zone. After chemical reaction, the diffuse zone becomes an ash layer which is assumed to be inert.28 The results showed that shrinking core model with diffusion through ash layer controlling process was the most suitable model.29 Similar conclusion was also reported by other researchers.30 As a result, the ash diffusion model better explains the mechanism of Ru cementation over the Al powder than the rest of models.
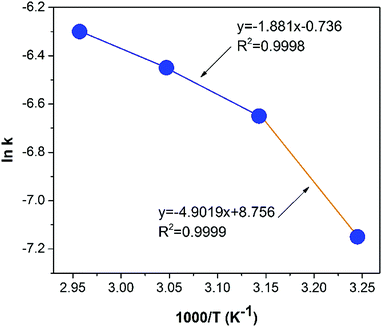 |
| | Fig. 3 Kinetics mechanism plots of the Arrhenius plot for the cementation of Ru by Al powder. | |
The Arrhenius activation energy of cementation can be resulted from the temperature dependence of the reaction rate constant (k) according to:31,32
| |
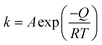 | (9) |
where
A is the frequency factor;
Q (kJ mol
−1) the reaction activation energy;
R the gas constant; and
T the absolute temperature. The values of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k
k from the ash diffusion mechanism against reciprocal of temperature in (
Fig. 3) indicates two slopes in the different temperature ranges of 308.16–318.16 K and 318.16–338.16 K, which yield activation energies of 40.75 kJ mol
−1 and 15.65 kJ mol
−1, respectively. Such a shift in the activation energy is probably due to the change in the reaction mechanisms. In other words, two consecutive processes may control the reaction steps; at low temperature, the diffusion-controlled process (which is strongly dependent on temperature) predominates, while at high temperature the chemically controlled process becomes significant (which is slightly dependent on temperature). Therefore, the reaction-controlling step between 308.16 and 318.16 K is the ash diffusion highly sensitive to temperature. At 318.16–338.16 K, consequently, the activation energy of chemical reaction is 40.75 kJ mol
−1. On the other hand, the profit gained per volume of IC foundry's wastewater was presumed to linearly rise with increasing the chemical concentration (
Table 3). A 0.1 m
3 wastewater of IC foundry typically contains 0.0023 kg Ru. 0.5 kg NaOH and 1.2 kg Al powder are required to recover 98.5% Ru from the wastewater, resulted in a profit of $0.180 per 0.1 m
3-wastewater.
Table 3 Cost-benefit analysis of cementation recovery of Ru
| Cost/revenue |
Used amount |
Cost/benefit (USD) |
Ref. |
| Operational cost: |
| Sodium hydroxide |
0.5 kg |
$0.145 |
($290 per ton)34 |
| Aluminum powder |
1.2 kg |
$2.58 |
($2150 per ton of aluminum powder, Qingdao Ocean Import and Export Co., China) |
| Energy for cementation reaction |
0.035 kW h nm−3 |
$0.016 |
(Calculated as $0.16 per kW per h in Taiwan) |
| Energy for drying the sediment |
|
$0.032 |
(Calculated as $0.16 per kW per h in Taiwan) |
| Cost to treat wastewater effluent |
100 L |
$0.152 |
($1.52 per m−3-wastewater, estimated based on wastewater treatment cost in Taiwan) |
| Total operational cost |
|
$2.925 |
|
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Revenue: |
| Revenue from Ru on aluminum powder precipitation |
0.0023 kg |
$3.105 |
($1.35 per g of ruthenium 5% on aluminum powder, Akshar Noble Pvt Co., India) |
| Total profit gained per 0.1 m3 of integrated circuit (IC) foundry's wastewater |
— |
$0.180 |
This study |
4. Conclusions
This study recovered Ru from the wastewater of IC foundry by cementation using metallic aluminum (Al) powder as the sacrificial agent. Ru ion was efficiently reduced as metal and coagulated with the derived aluminum hydroxide flocs. Experimental parameters included the particle size of Al, molar ratio of Al to Ru, initial Ru concentration and operation temperature. The recovery rate attained 99% under the conditions: particle size Al powder = 88–128 μm, Al/Ru molar ratio = 2.0, initial Ru = 200 mg L−1, temperature = 338.16 K, reaction time = 120 min, stirring speed = 150 rpm. Kinetic modelling suggested that the diffusion of Ru through the ash layer of Al powder controlled the reaction rate with an activation energy of 40.75 kJ mol−1. A brief cost analysis demonstrated that the cementation of Ru yielded a profit of $0.180 per 0.1 m3-wastewater. The method was found to be robust when applied in an industrial scale treatment facility.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors greatly acknowledge the help of Mr Nicolaus Nezha Nunez Mahasti for experimental setup and technical input. We would like to thank Mr I.-Chin Sung, Mr Tsung-Yen Wu and Ms Michelle Chu for their help in SEM, EDS and XRD analyses.
References
- A. M. Weisberg, Met. Finish., 1999, 97, 297 CrossRef.
- J. Emsley, “Ruthenium”. Nature's Building Blocks: An A-Z Guide to the Elements, UK Oxford University Press, 2003, pp. 368–370 Search PubMed.
- F. Cardarelli, Mater. Des., 2008, 237 Search PubMed.
- M. S. Varney, Ocean Sci. Technol., 2000, 322 Search PubMed.
- A. D. Richards and A. Rodger, Chem. Soc. Rev., 2007, 36, 471–483 RSC.
- A. Fürstner, Angew. Chem., Int. Ed., 2000, 39, 3012–3043 CrossRef.
- T. O. R. Noyori and M. Kitamura, J. Am. Chem. Soc., 1987, 109, 5856–5858 CrossRef.
- M. A. Hayat, Language of science, Springer, US, 1993 Search PubMed.
- P. Swain, C. Mallika, R. Srinivasan, U. K. Mudali and R. Natarajan, J. Radioanal. Nucl. Chem., 2013, 298, 781–796 CrossRef CAS.
- K. Naito, T. Matsui, H. Nakahira, M. Kitagawa and H. Okada, J. Nucl. Mater., 1991, 184, 30–38 CrossRef CAS.
- K. Singh, N. L. Sonar, T. P. Valsala, Y. Kulkarni, T. Vincent and A. Kumar, Desalin. Water Treat., 2014, 52, 514–525 CrossRef CAS.
- M. Blicharska, B. Bartoś, S. Krajewski and A. Bilewicz, J. Radioanal. Nucl. Chem., 2013, 298, 1713–1716 CrossRef CAS PubMed.
- S. Aktas, Hydrometallurgy, 2011, 106, 71–75 CrossRef CAS.
- F. L. Bernardis, R. A. Grant and D. C. Sherrington, React. Funct. Polym., 2005, 65, 205–217 CrossRef CAS.
- F. Crundwell, M. Moats, V. Ramachandran, T. Robinson and W. G. Davenport, Extractive metallurgy of nickel, cobalt and platinum group metals, Elsevier, 2011 Search PubMed.
- K. Lillkung and J. Aromaa, Dissolution of Platinum, Palladium and Rhodium in 250 g/l NaCl Solution/TT Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization, 2012, pp. 359–369 Search PubMed.
- F. Farahmand, D. Moradkhani, M. Sadegh Safarzadeh and F. Rashchi, Hydrometallurgy, 2009, 98, 81–85 CrossRef CAS.
- I. S. Kwak, S. W. Won, Y. S. Chung and Y.-S. Yun, Bioresour. Technol., 2013, 128, 30–35 CrossRef CAS PubMed.
- H. Ohta, K. Akiba and Y. Onodera, J. Nucl. Sci. Technol., 2002, 39, 655–660 CrossRef.
- M. H. M. Serdar Aktas, K. Aksu and B. Aksoy, Trans. Indian Inst. Met., 2018, 71, 697–703 CrossRef.
- A. Chen, T. Miyao, K. Higashiyama, H. Yamashita and M. Watanabe, Angew. Chem., Int. Ed., 2010, 49, 9895–9898 CrossRef CAS PubMed.
- F. Carson, S. Agrawal, M. Gustafsson, A. Bartoszewicz, F. Moraga, X. Zou and B. Martín-Matute, Chem.–Eur. J., 2012, 18, 15337–15344 CrossRef CAS PubMed.
- P. K. Gbor and C. Q. Jia, Chem. Eng. Sci., 2004, 59, 1979–1987 CrossRef CAS.
- S. Noorman, F. Gallucci, M. van Sint Annaland and J. A. M. Kuipers, Chem. Eng. J., 2011, 167, 297–307 CrossRef CAS.
- O. Levenspiel, Ind. Eng. Chem. Res., 1999, 38, 4140–4143 CrossRef CAS.
- V. Buscaglia and C. Milanese, Diffusion-Controlled Solid-State Reactions of Spherical Particles, A General Model for Multiphase Binary Systems, J. Phys. Chem. B, 2005, 18475–18482 CrossRef CAS PubMed.
- M. R. Talaghat and E. Zangooei, Journal
of Chemical and Petroleum Engineering, 2017, 51, 9–19 Search PubMed.
- O. Levenspiel, Ind. Eng. Chem. Res., 1999, 38, 4140–4143 CrossRef CAS.
- W. Astuti, T. Hirajima, K. Sasaki and N. Okibe, Trans. Soc. Min., Metall., Explor., 2015, 32, 176–185 Search PubMed.
- V. Safari, G. Arzpeyma, F. Rashchi and N. Mostoufi, Int. J. Miner. Process., 2009, 93, 79–83 CrossRef CAS.
- O. Levenspiel, Ind. Eng. Chem. Res., 1999, 38, 4140–4143 CrossRef CAS.
- L. A. Wall and J. H. Flynn, Polym. Lett., 1966, 4, 323–328 CrossRef.
- G. Colica, S. Caparrotta and R. De Philippis, Appl. Microbiol. Biotechnol., 2012, 95, 381–387 CrossRef CAS PubMed.
- H. Huang, D. Xiao, J. Liu, L. Hou and L. Ding, Sci. Rep., 2015, 5, 10183 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2019 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Chi-Thanh Vub,
Yu-Jen Shihc and
Yao-Hui Huang
a,
Chi-Thanh Vub,
Yu-Jen Shihc and
Yao-Hui Huang *a
*a

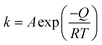
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k from the ash diffusion mechanism against reciprocal of temperature in (Fig. 3) indicates two slopes in the different temperature ranges of 308.16–318.16 K and 318.16–338.16 K, which yield activation energies of 40.75 kJ mol−1 and 15.65 kJ mol−1, respectively. Such a shift in the activation energy is probably due to the change in the reaction mechanisms. In other words, two consecutive processes may control the reaction steps; at low temperature, the diffusion-controlled process (which is strongly dependent on temperature) predominates, while at high temperature the chemically controlled process becomes significant (which is slightly dependent on temperature). Therefore, the reaction-controlling step between 308.16 and 318.16 K is the ash diffusion highly sensitive to temperature. At 318.16–338.16 K, consequently, the activation energy of chemical reaction is 40.75 kJ mol−1. On the other hand, the profit gained per volume of IC foundry's wastewater was presumed to linearly rise with increasing the chemical concentration (Table 3). A 0.1 m3 wastewater of IC foundry typically contains 0.0023 kg Ru. 0.5 kg NaOH and 1.2 kg Al powder are required to recover 98.5% Ru from the wastewater, resulted in a profit of $0.180 per 0.1 m3-wastewater.
k from the ash diffusion mechanism against reciprocal of temperature in (Fig. 3) indicates two slopes in the different temperature ranges of 308.16–318.16 K and 318.16–338.16 K, which yield activation energies of 40.75 kJ mol−1 and 15.65 kJ mol−1, respectively. Such a shift in the activation energy is probably due to the change in the reaction mechanisms. In other words, two consecutive processes may control the reaction steps; at low temperature, the diffusion-controlled process (which is strongly dependent on temperature) predominates, while at high temperature the chemically controlled process becomes significant (which is slightly dependent on temperature). Therefore, the reaction-controlling step between 308.16 and 318.16 K is the ash diffusion highly sensitive to temperature. At 318.16–338.16 K, consequently, the activation energy of chemical reaction is 40.75 kJ mol−1. On the other hand, the profit gained per volume of IC foundry's wastewater was presumed to linearly rise with increasing the chemical concentration (Table 3). A 0.1 m3 wastewater of IC foundry typically contains 0.0023 kg Ru. 0.5 kg NaOH and 1.2 kg Al powder are required to recover 98.5% Ru from the wastewater, resulted in a profit of $0.180 per 0.1 m3-wastewater.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)


