Open Access Article
Open Access ArticlePhoton upconversion for the enhancement of microfluidic photochemical synthesis†
M. Wua,
B. A. Moserb,
T. M. Steevesb,
A. Figueroaa,
B. M. Wallacea,
S. T. Kimc,
A. P. Esser-Kahn b and
R. C. Steinhardt‡
b and
R. C. Steinhardt‡
 *bc
*bc
aUniversity of California, Irvine, Irvine, CA 92697, USA
bUniversity of Chicago, Chicago, IL 60637, USA
cSyracuse University, Syracuse, NY 13244, USA. E-mail: rcsteinh@syr.edu
First published on 21st August 2019
Abstract
Photochemical transformations are greatly improved in yield by fluidic reactor technology. However, the delivery of synthetically-active light to the reactants is a challenge. Here, we use upconversion in a bio-inspired microreactor to augment the flux of critical wavelengths of light. This new technology increased of a model reaction by converting a greater portion of sunlight to photochemically-available photons.
Fluidic systems provide great advantages for use in chemical synthesis due to their size and scalability, limitation of the accumulation of hazardous compounds, reduction of side products, and increase in reaction efficiency. These properties permit pharmaceutical and other fine chemical synthesis to be efficiently miniaturized, with concomitant increase in safety. Processes may then be scaled-up or the optimized reactors may be numbered-up.1,2 Thus, fluidic devices are used as microscale chemical production facilities, enabling multiple drugs to be synthesized with the same reactor, by switching feedstocks. Unfortunately, despite the tremendous potential of fluidic chemistry, many open challenges still remain – one of the most important is reactor design. A “one size fits all” fluidic reactor does not exist. New fluidic reactor designs and technologies are needed to include more reaction conditions and facilitate increased throughput.
One class of reactions critical to fine chemical production that greatly benefits from microfluidic processes is photochemical transformations. This reaction class includes such broadly important methodologies as carbon–carbon bond formations, chlorinations, electrocyclizations, isomerizations, oxidations, and polymerizations.3 In western Europe alone, 6750 kW of power was used for preparative-scale photochemistry in 2012.3 The rapidly-expanding applications of photochemistry benefit and rely on improvements in microfluidic reactor design. This is due to the intrinsic requirements of photochemical reactions – photon transfer and mass transfer – which are highly dependent on the reactor.2
Photochemical transformations require a sufficient flux density of photons throughout the reaction medium. The photon flux density is governed by two prevailing factors: (1) ability for the reactor to transmit light, including losses due to absorption, scattering, and other non-radiative phenomena; and (2) the light absorption of the reactants and solvent itself, which is governed by the Bouger–Lambert–Beer law.
This law relates the absorption of the chemical species is dependent on the molar extinction coefficient (ε) of the light-absorbing molecules, the concentration of the light absorbers, and the path length through which light has to travel. The importance of the interdependence of these factors becomes especially clear in systems that rely on light-absorbing compounds with extremely high ε. These, unfortunately, constitute the bulk of dyes and sensitizers used in photochemical transformations. For example, the specific Bouger–Lambert–Beer dependence of [Ru(bpy)3]2+ – an extremely versatile and popular photochemical reagent employed here – shows that only 20% of incident light is transmitted to a depth of 1 mm in a 0.5 mM [Ru(bpy)3]2+ solution.2,4 Spectral intensity and photon transport are still limiting even on the microscale.
To increase light transmission, we sought a reactor design that would reliably enrich and increase photochemically useful photon delivery. This would permit the use of lower intensity incident light, as well as non-specialized light. Such a reactor would be more efficient, useful with less specialized, expensive, and energy-intensive inputs, require less thermal management, and may be more amenable to known problems with scaling- or numbering-up, due to reduced input light requirements.
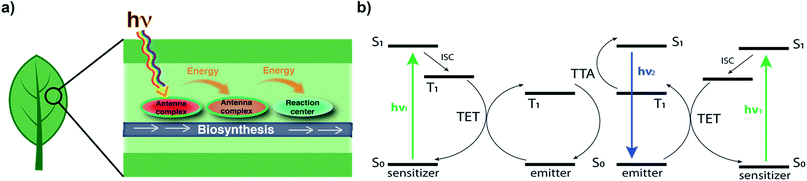
To design our system, we looked to a photochemical reactor optimized by millions of years of evolution: the leaf. The leaf, viewed as a reactor, is an exquisitely optimized fluidic system for using photons to power the chemical conversion of CO2 into carbohydrates and other metabolites. Chlorophyll – a magnesium porphyrin – handles this energy conversion process. Due to the narrow absorbance spectrum of chlorophyll, antenna pigment centres are interspersed throughout the leaf (Fig. 1a). These pigments harvest a larger set of wavelengths of light, and transfer the energy to the photosynthetic reaction centre – expanding the breadth of photosynthetically-active radiation.14,15 We were inspired by this concept, and used it as a model to create a system to convert a larger set of wavelengths of light into synthetically useful energy.
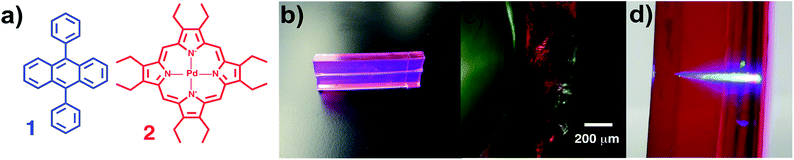
We envisioned a system in which a microfluidic channel was surrounded by a polymeric material that increased the available photochemically useful light. In effect, this would harness light energy through chemical processes and convert it to chemical transformations in a fluidic channel. To replicate the leaf's energy capture process, we used photon upconversion (UC), a process where longer wavelength light is converted to shorter wavelengths (Fig. 1b). Specifically, we chose triplet–triplet annihilation (TTA) UC because it functions with non-coherent, low power light, such as that produced by the sun or artificial lighting.5,9 TTA UC works through a dye-sensitizer combination. In the most common mechanism, the sensitizer molecule is excited from the ground state to the first singlet excited state (1S*), then undergoes intersystem crossing (ISC) to the triplet excited state (3S*), and transfers its energy to the emitter (now 3E*) via triplet–triplet energy transfer (TTET). Triplet–triplet annihilation (TTA) of two 3E* produces a singlet excited emitter 1E* that radiatively decays to the ground state, emitting a photon of light (Fig. 1b).5–8 Of the many dye-sensitizer pairs, we selected diphenylanthracene (1) and 2,3,7,8,12,13,17,18-octaethyl-21H,23H-porphine palladium(II) (2) because of the pair's ability to convert longer-wavelength green light to the strong emission of blue light, which is required for many photochemical transformations.
Solid-state upconverting materials are an area of highly active research.5,8–13 One area of this research is the matrix used to embed the dye-sensitizer pair which must satisfy requirements for optical transmission, dye-sensitizer diffusion, and must exclude O2 to prohibit triplet-state quenching. Clearflex™ clear polyurethane matrix was used to embed 1 and 2, following the work of the Castellano and Kim groups.9 This dye-sensitizer–polymer combination has been shown to generate upconversion with very low power irradiance, with a quantum yield greater than 20%.8,9
We then commenced synthesis of the photochemical reactor. The reactors were fabricated using a number of techniques. Molds were custom milled or 3D printed and tubing was suspended in the molds by securing to the sides of the mold housing with custom-etched brass plates, which allowed us to precisely adjust the height of submerged tubing relative to the surface of the reactor, permitting control of the thickness of the upconverting layer (Fig. 2c). The molds and tubing were coated with mold release and the urethane mixture doped with 1 and 2 was added and the cured in a vacuum oven at 60 °C for 24 h to provide the finished reactor prototypes (Fig. 2b). The prototype readily upconverted green light to blue light, from 532 nm laser (Fig. 2d) or diffuse light from a window (Fig. 2b).
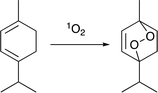
Conversion of α-terpinene to ascaridole.
 | (1) |
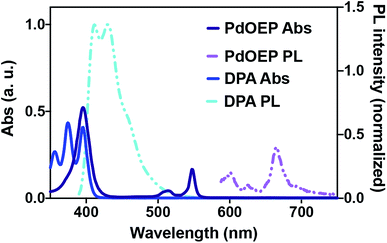
The green light to blue light upconversion effect observed can be explained by analysis of the absorption/photoluminescence spectra of the dye-sensitizer pair, which Fig. 3 shows in toluene. The sensitizer, 2, exhibits photoluminescence peak centered at 668 nm, and absorbs with peaks centered at 398 nm (Soret), and two Q-band peaks in the green wavelengths, centered at 516 and 548 nm.17 In contrast, the lowest energy excitation band for the dye 1 is at ∼397 nm, with emission maxima at approximately 413 and 430 nm. Thus the Q-band absorbtion of 2 drive the photon upconversion process, as green light can only excite the sensitizer at these wavelengths. This energy is then transferred to the emitter molecule 1, which explains the blue light emission observed with green light excitation.17
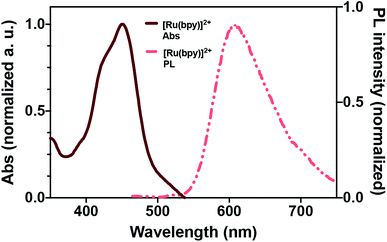
To assay the feasibility of this system, we chose the conversion of α-terpinene to ascaridole, an anti-helminthic drug (eqn (1)).16 This transformation can occur via an ene reaction with singlet oxygen – generated photochemically – to yield the endoperoxide product. This reaction is known to be kinetically fast, and has been examined flow systems due to the spectral requirements of dye-sensitized singlet oxygen generation, and the hazardous nature of oxygen, oxygenated organic solvents, and endoperoxides.2 [Ru(bpy)3]2+ was chosen as the singlet oxygen sensitizer due to its extremely high quantum yield of singlet oxygen and high utility in photochemical transformations. The emission of 2 overlaps well with the blue wavelength excitation (∼450 nm) of [Ru(bpy)3]2+ (Fig. 4). We employed a slug-flow format because of its superior gas–liquid mixing qualities (Fig. 5). We then experimentally validated the ability of photon upconversion reactor to enhance the rate of chemical reactions.
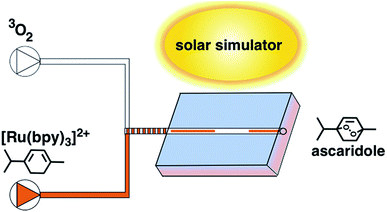 | ||
| Fig. 5 Schematic of photon upconversion reactor. Feedstocks are pumped through the upconverting reactor in slug-flow, reactor is exposed to a solar simulator. | ||
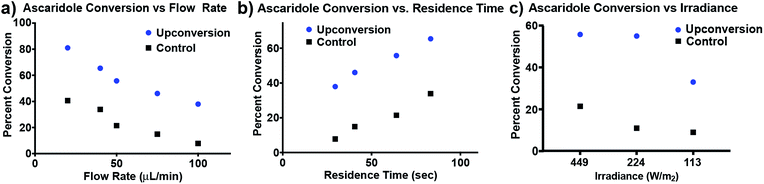
To verify the enhanced production of chemically useful photons, we assayed the production of ascaridole in our reactor using a full-spectrum solar simulator (Fig. 6). We found that our initial flow rate of 40 μL min−1 resulted in a significant increase in yield versus the control reactors that were un-doped, or doped with only 1 or 2 (ESI Fig. S2†). We then assayed percent conversion vs. flow rate and residence time for the doped and un-doped reactor and found a consistent increase in percent conversion for all conditions assayed (Fig. 6a), indicating that light from upconversion phenomena may be used to raise the yield of a photochemical reaction in these limited light conditions. The increase in conversion is most enhanced at higher flow rates and shorter residence times – at a flow rate of 100 μL min−1 there is a 475% increase in yield with the upconversion reactor, corresponding to a residence time of 30 s (Fig. 6b). We then studied yield as a function of irradiance, finding that even at 113 W m−2 (less than 1/5 of a sun) the upconversion system enhances conversion by ∼400%. For a discussion of the chemical scope of this reactor, see ESI Discussion 1.† Overall, these results demonstrate the dramatic increase in production possible with upconversion technology. The improvement observed from upconversion highlights the need for sufficient light transport to the reactants of photochemical transformations, a property that is significantly improved by the material composition and design components of our microreactor.
In conclusion, we designed and developed a microreactor technology that harnesses photon upconversion to significantly enhance the conversion of photochemical reactions. The reactor efficiently upconverts low power non-coherent light, enriching the flux of critical higher energy light, as evidenced by the dramatic increase in reaction efficiency. The device out performed controls at multiple flow rates and illumination conditions. Further, we demonstrated the reactor's utility by synthesizing an endoperoxide-bearing anti-helmenthic drug. Overall, we have broadened the scope of upconversion applications.
We envision this work may lead to new devices that act as adventitious converters of white light to photochemical reactions/organic energy storage molecules. For example, such technologies could be used in conjunction with solar panels, or on the interiors of buildings lit with artificial lighting. By enhancing catalysis of versatile, established organometallics such as [Ru(bpy)]3+, the possibility of synthetic transformations towards targets in fine chemical or organic energy molecule families are now possible with this approach. We anticipate this technology may be employed in reactor scaling- or numbering-up, military field synthesis of rare or unstable drugs, medicine synthesis in disadvantaged countries, and energy storage molecule synthesis.
Conflicts of interest
There are no conflicts to declare.Notes and references
- D. Cambie, C. Bottechia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276 CrossRef CAS PubMed.
- Y. Su, N. J. W. Straathof, V. Hessel and T. Noël, Chem.–Eur. J., 2014, 20, 10562 CrossRef CAS PubMed.
- A. G. Griesbeck, M. Oelgemöller and F. Ghetti, CRC Handbook of Organic Photochemistry and Photobiology, 2012, vol. 1 Search PubMed.
- T. Noël, X. Wang and V. Hessel, Chim. Oggi, 2013, 31, 10 Search PubMed.
- Y. Simon and C. Weder, J. Mater. Chem., 2012, 22, 20817 RSC.
- J. Z. Zhao, S. M. Ji and H. M. Guo, RSC Adv., 2011, 1, 937 RSC.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560 CrossRef CAS.
- A. L. Hagstrom, F. Deng and J.-H. Kim, ACS Photonics, 2017, 4, 127 CrossRef CAS.
- J.-H. Kim, F. Deng, F. N. Castellano and J.-H. Kim, Chem. Mater., 2012, 24, 2250 CrossRef CAS.
- T. N. Singh-Rachford, J. Lott, C. Weder and F. N. Castellano, J. Am. Chem. Soc., 2009, 131, 12007 CrossRef CAS PubMed.
- A. Monguzzi, R. Tubino and F. Meinardi, J. Phys. Chem. A, 2009, 113, 1171 CrossRef CAS PubMed.
- P. B. Merkel and J. P. Dinnocenzo, J. Phys. Chem. A, 2008, 112, 10790 CrossRef CAS PubMed.
- A. Monguzzi, M. Frigoli, C. Larpent, R. Tubino and F. Meinardi, Adv. Funct. Mater., 2012, 22, 139 CrossRef CAS.
- J. F. Allen and J. Forsberg, Trends Plant Sci., 2001, 6, 317 CrossRef CAS PubMed.
- M. Grieco, M. Suorsa, A. Jajoo, M. Tikkanen and A.-M. Aro, Biochim. Biophys. Acta, Bioenerg., 2015, 1847, 607 CrossRef CAS PubMed.
- G. O. Schenck and K. Ziegler, Sci. Nat., 1944, 32, 157 Search PubMed.
- A. Haefele, J. Blumhoff, R. S. Khnayzer and F. N. Castellano, J. Phys. Chem. Lett., 2012, 3, 299 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra03468d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |