Open Access Article
Open Access ArticleEffects and mechanism of riboflavin on the growth of Alcaligenes faecalis under bias conditions†
Miao He a,
Mulan Chena,
Mingxue Liu
a,
Mulan Chena,
Mingxue Liu *ac,
Faqin Dongbc,
Hongfu Weia and
Danni Wanga
*ac,
Faqin Dongbc,
Hongfu Weia and
Danni Wanga
aLife Science and Engineering College, Southwest University of Science and Technology, Mianyang 621010, China. E-mail: liumingxue@swust.edu.cn; Fax: +86-816-6089521; Tel: +86-816-6089521
bKey Laboratory of Solid Waste Treatment and Resource Recycle, Ministry of Education of China, Mianyang 621010, China
cNational Co-innovation Center for Nuclear Waste Disposal and Environmental Safety, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China
First published on 25th July 2019
Abstract
Some microorganisms can utilize photoelectrons and electrode electrons. Exogenous electrons generate enough energy for growth, and electron shuttles may accelerate this process. This research data supported photoelectron-responsive microorganism Alcaligenes faecalis was effected by the growth metabolism due to bias and electron shuttle riboflavin (RF) with an adaptive screening voltage under oligotrophic conditions. A slight change was observed in the redox property of RF. RF played the role of an electron shuttle. Microbial extracellular metabolites could bind additional nicotinamide adenine dinucleotide (NADH) species with RF. The intracellular protein content in the group of RF–Bias was 1.94, 1.93 and 4.02 times higher than those in the RF, bias and control groups, respectively, while the corresponding intracellular contents of humus were 1.10, 0.93 and 1.42 times higher. The content of CoA in RF–Bias, RF and bias increased to 116.0%, 108.5% and 103.8%, respectively. The organic acids of the RF–Bias group in the Krebs cycle are more advanced than those of other groups. Overall, in the Krebs cycle, RF and bias facilitated the growth and metabolism of A. faecalis. Finally, a mechanism was proposed, showing that the electron transfer chain and the Krebs cycle are stimulated by RF and bias.
1 Introduction
Both photosynthetic and non-photosynthetic organisms can use solar energy either directly or indirectly for growth.1 Photosynthetic organisms directly utilize solar energy, while non-photosynthetic organisms can grow and metabolize by utilizing photoelectrons. They are produced by semiconductor minerals under light irradiation. In this process, electronic transitions occur and produce photoelectron–hole pairs.2 Photoelectrons can affect the structure of the microbial community and promote the growth of heterotrophic microorganisms as well as the production of metabolites and energy.4,5 To some certain extent, the photoelectrons generated by semiconductor minerals can reduce nitrates.6 The growth and metabolism of the non-photosynthetic microorganism Alcaligenes faecalis screened from soils is promoted and responsible for photoelectrons.7 The interaction between electrons and microorganisms is mainly based on electrons.Up till now, the efficiency of the microbial utilization of photoelectrons has not been satisfactory.3 To investigate the photoelectron cycle of microbes, external electrons are used to simulate photoelectrons. At a suitable electrode potential, microorganisms can grow by 2.5–3 times.35,36 Even under the photocatalytic electrons, Acidithiobacillus ferrooxidans can grow by 12 times.37
The microbial extracellular electron transfer pathways are discussed below: (1) microorganisms can remain in direct contact with solid surfaces or form agglomerates with electron acceptors; however, only a few microorganisms can follow this mechanism.8–10 (2) Additional energy is required in long-range electron transfer, which is mediated by conductive pili or flagella synthesized by the microorganisms themselves.11 (3) Microorganisms use “wires” as conductive materials for electron transfer.12–14 (4) Electron transfer can be conducted by small molecules with redox properties and electron mediators naturally occurring or manually synthesized. These small molecules are called electron shuttles (ESs). This process reduces a large amount of energy consumption and the limitation of direct contact.8,15,16
ESs are electronic carriers,17,18 and their use is one of the important ways to mediate extracellular electron transfer. Flavin,8 melanin,19 sulfur compounds and humus15 are common ESs. Some ESs interact with cells across membranes,20 while other ESs directly transfer electrons from the structure.21
According to new data, a large number of researchers have applied ESs to control environmental pollution such as that caused by uranium and the reduction of Fe and dichromate.22,24–29 In addition, electron transfer can promote microbial metabolism.30 In anaerobic microbial communities, organic matter can be degraded.31 Hence, the production of methane by microorganisms can play the role of ES to accelerate the degradation process.13
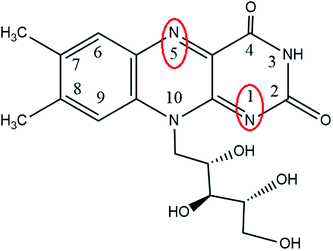
Riboflavin (RF) is one of the isoalloxazines, and its first and fifth nitrogen atoms have a conjugated double bond (Fig. 1). Because of the conjugated double bond, RF can be easily replaced by a hydrogen atom and easily dehydrogenated after reduction; thus, RF has reversible redox characteristics. The addition of RF enables the sulfate-reducing bacteria to increase the pitting depth of 304 stainless steel across the cell membrane.23 First, the extracellular ESs of flavin mononucleotide (FMN) and RF are identified, which are also involved in the metabolism of microbial cells.55 However, the role of ESs in the process where non-photosynthetic microorganisms utilize electrode electrons is not clear.
In this research, A. faecalis was used as a model of non-photosynthetic microorganism treated in oligotrophic conditions with or without an RF medium under bias conditions. The data of the ES role in the process were investigated: A. faecalis utilized electrode electrons through growth and metabolism. Then, the mechanism of RF and electrons in promoting A. faecalis was predicted. At the molecular level, this research provides theoretical possibilities in the interactions among electron shuttle, bias and microorganisms.
2 Materials and methods
2.1 Source of bacteria
Alcaligenes faecalis was isolated and identified by Laboratory of Microbial-Mineral/Nuclear Interactions, College of Life Science and Engineering, Southwest University of Science and Technology.2.2 Materials and reagents
Medium: 1% LB medium, 10 mg L−1 riboflavin (RF, Sigma, 97.5%).Main instruments: field emission scanning electron microscope (SEM, UItra 55 Carl Zeiss), CHI660E Electrochemical Workstation (Shanghai Chenhua Instrument Co., Ltd.), F-7000 Fluorometer (Hitachi, JPN), U-3900 UV-Vis Spectrophotometer (Hitachi, JPN), high-performance liquid chromatograph (HPLC, Jiangsu Hanbang Technology Co., Ltd.), etc.
2.3 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm min−1; slit of 10 nm; step size of 10 nm; and PMT voltage of 700 V.
000 nm min−1; slit of 10 nm; step size of 10 nm; and PMT voltage of 700 V.3 Results and discussion
3.1 Bias condition screening
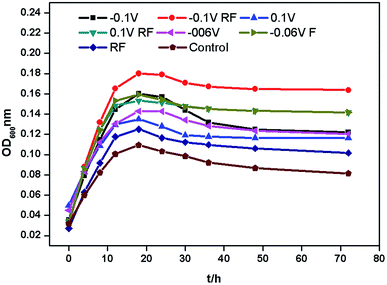
Fig. 2 shows the growth curves of A. faecalis cultured under different conditions. They were almost the same in the growth cycle. The adaptation time period was about 0–4 h, the logarithmic time period was about 4–18 h, and the stable time period was about 18–32 h. The decomposition rate of A. faecalis can be delayed by photoelectrons, while bias and RF have no significant effect on the decomposition rate.6 Both bias and RF promoted the growth of A. faecalis, but the effect of bias was more productive. This is related to the results reported by Wang et al. (2009).38 Moreover, negative bias promoted better growth of A. faecalis than positive bias. When cultured for 18 h, RF showed the lowest effect on the growth of A. faecalis, which was about 1.16 times greater than that of the control group. In contrast, at −0.1 V, RF showed the most potent effect on the growth of A. faecalis, which was about 1.70 times higher than that for the control group. Thereby, −0.1 V bias was used in this research.3.2 Morphological changes
Different electrode potentials can change the morphology and generation time of microorganisms.39,40 A. faecalis was observed using SEM (Fig. 3). Although RF accelerated extracellular electron transfer, A. faecalis exhibited normal size of 0.7–1.0 μm. However, bias caused a significant change, and the size of A. faecalis was longer than 1.0 μm. This is related to the results reported by Yu et al. (2012).41 After the effects of bias, A. faecalis exhibited a slender shape.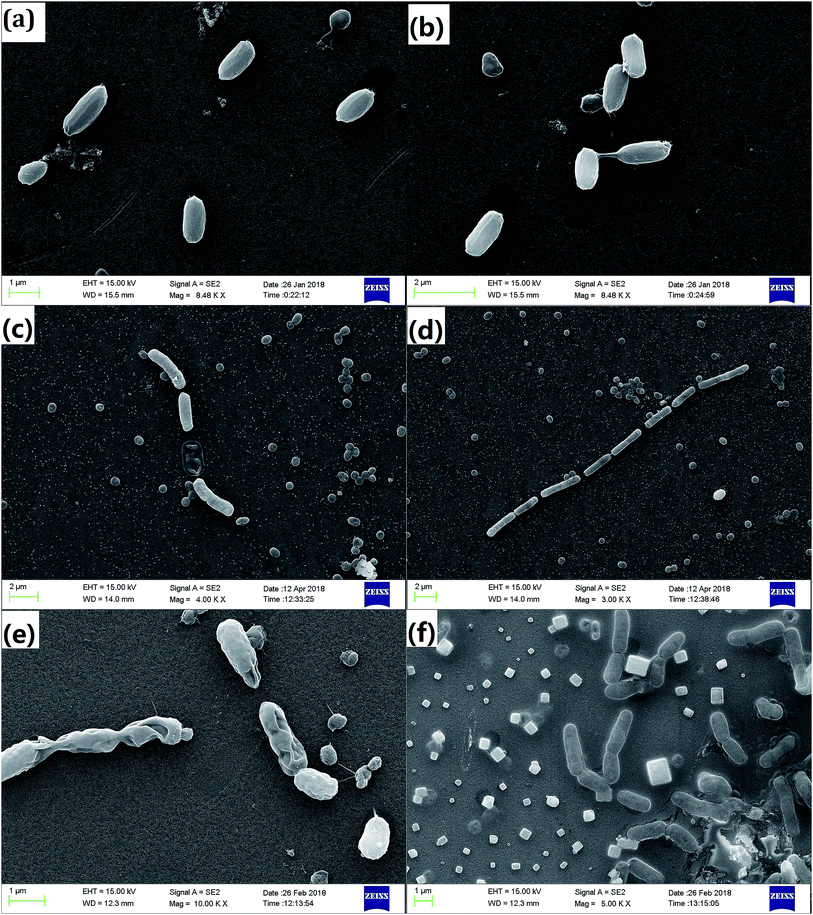 | ||
| Fig. 3 The morphology of A. faecalis incubated in different conditions ((a) control, (b) RF, (c) −0.1 V, (d) −0.06 V, (e) 0.1 V, and (f) RF −0.1 V). | ||
3.3 The redox property of RF analysis
The CV of 5 mg L−1 RF is shown in Fig. 4c with an oxidation peak of approximately −0.4 V and a reduction peak of approximately −0.6 V. The results of CV and DPV measurements are shown in Fig. 4a and b, respectively, before and after incubating A. faecalis. At 0 h, the CVs for the RF groups show redox peaks. The DPV results showed the reduction peak of −0.6 V. According to standard reduction peak, in these conditions, it was indicated that RF could undergo redox reactions. After 18 h of culture, the redox peak positions of the RF groups did not change, but the reduction peak positions of DPVs showed slight shifts. This might be because the microbial metabolites were more vigorous after culturing. Compared with the result at 0 h, the DPVs without RF groups showed reduction peaks in the same position as that of RF, which may be secreted by A. faecalis itself. The amount of RF secreted by A. faecalis was negligible8 compared with the amount of manually added RF. RF was not consumed and played the role of an electron shuttle. FMN and RF secreted by Shewanella can accelerate the microbial reduction of ferrites as an electron shuttle, and RF has the potential to deliver electrons.8,42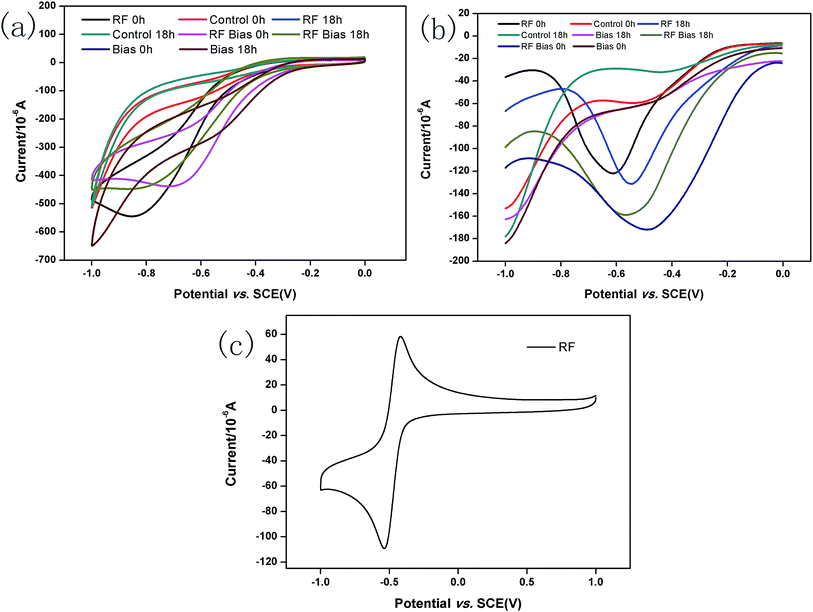 | ||
| Fig. 4 CVs of systems before and after incubating A. faecalis (a); DPVs of systems before and after incubating A. faecalis (b); CV of RF at 10 mg L−1 (c). | ||
3.4 Analysis of three-dimensional fluorescence
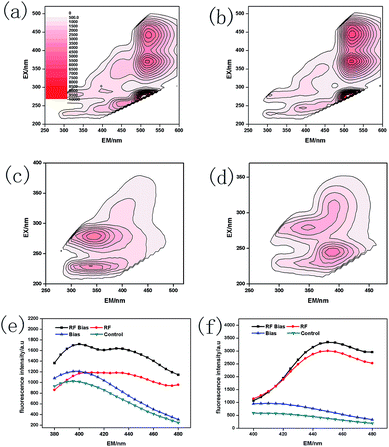
Microorganisms secrete maximum amounts of metabolites such as proteins, humic substances and RF when they are producing or gaining energy.43 The results for the extracellular metabolites of A. faecalis are shown in Fig. 5. Furthermore, RF (λEX/EM = 450/520 nm, 375/520 nm, and 267/520 nm)44 can be shown in Fig. 5a and b. The UV humic-like (λEX/EM = 225 nm/410 nm)46,47 and humic substances (λEX/EM = 320–390 nm/400–480 nm)45 can be represented in Fig. 5. The UV humic-like was mainly high molecular weight humus.48 RF also significantly promoted the formation of nicotinamide adenine dinucleotide (NADH) (λEX/EM = 350 nm/450 nm) (Fig. 5f). The process of glycolysis and the Krebs cycle in NADH could be promoted by RF and bias. NADH is capable of interconversion with nicotinamide adenine dinucleotide (NAD+) through oxidative phosphorylation. NAD+ does not have fluorescence properties and can simultaneously produce ATP.49 However, under anaerobic conditions, the oxidation process is inhibited and NADH will be accumulated.50 Bias directly provides electrons to the oxidation process, while RF transfers them.51 The process of electron transfer accelerated by the conversion of NADH to NAD+ generated more ATP molecules. Then, the processes of glycolysis and oxidative phosphorylation were also promoted. RF and bias could indirectly promote the growth and metabolism of A. faecalis.The results for A. faecalis intracellular metabolites are shown in Fig. 6. The value of RF–Bias was the highest in the intracellular protein, which was 1.94, 1.73 and 4.02 times higher than those of the RF, Bias and control groups, respectively. Compared with the results for other groups, the contents of intracellular humus were 1.10, 0.93 and 1.42 times higher than those of the RF, Bias and control groups, respectively. Microbial intracellular substances mainly include enzymes and genetic materials. The main components are proteins, which help improve the growth and metabolism of bacteria. In addition, A. faecalis will secrete humus itself, which also acts as an electron shuttle.15,52
Bias could promote the redox reaction process and metabolism of microorganisms. The processes of RF might promote the glycolysis of A. faecalis, the Krebs cycle and oxidative phosphorylation electron transfer.
3.5 The content of CoA analysis
In an intracellular cycle, more than seventy enzyme reactions are followed by the CoA cofactor, including the synthesis of amino acids and fatty acids, pyruvate metabolism and decomposition of sugars. CoA provides 90% of energy for life, and it can be acetylated to acetyl-CoA by pyruvate (Fig. 9), which is the promoter of the Krebs cycle. The content of CoA is shown in Fig. 7b. It was found that the CoA activity was enhanced. In RF–Bias, the content of CoA increased by 116%, 108.5% and 103.8%, which boosted the process. Fig. 9 shows the relationship between CoA and the Krebs cycle. The Krebs cycle can produce and consume CoA. Oxaloacetate and acetyl-CoA synthesize citrate under the catalysis of citrate synthase; succinyl-CoA produces succinate under the action of succinyl-CoA synthetase. CoA can be accumulated by both processes. Succinyl-CoA formed by α-ketoglutarate can be regulated by CoA. Therefore, the promotion of microbial metabolism by RF might be related to the Krebs cycle.3.6 Analysis of organic acid content connected with the Krebs cycle
The Krebs cycle is the key of metabolism. Metabolites such as glycolysis and fatty acids enter the Krebs cycle through acetyl-CoA and are decomposed and supplied to the electron transfer chain to form ATP. Electrons are transferred to O2 via the respiratory chain and react with ATP during microbial energy metabolism.11 This process involves the participation of the Krebs cycle, NADH/NAD+ and CoA. Fig. 9 shows the relationship between NADH and the Krebs cycle. The Krebs cycle can produce NADH, which in turn can be involved in the regulation of the Krebs cycle. NADH can be obtained by the conversion of isocitrate to α-ketoglutarate, α-ketoglutarate to succinyl-CoA and malate to oxaloacetate. NADH can be involved in the regulation of the interconversions of pyruvate to lactate, pyruvate to acetyl CoA, oxaloacetate to citric acid, isocitrate to α-ketoglutarate and α-ketoglutarate to succinyl-CoA. NADH accumulated in these processes enters the respiratory chain oxidative phosphorylation process, producing NAD+ and ATP.Fig. 8a shows the analysis of standard pyruvate, malate, lactate, citrate, fumarate and succinate. The six organic acids could be effectively analysed, and there was a good relationship between different concentrations of organic acids (Table 1). The content of intracellular organic acids in A. faecalis is shown in Fig. 8b. Similarly, the contents of CoA and NADH increased in RF–Bias. The content of citrate in each group did not have much difference for consuming. Electrons can participate in the reduction of fumarate to succinate in Geobacter sulfurreducens.53 Hence, RF can promote the conversion of succinate to fumarate. Despite this, NADH can regulate lactate dehydrogenase. Consequently, the content of lactate with RF was significantly higher than that of others. In A. faecalis, the RF relationship in the Krebs cycle due to the increase in CoA, NADH and RF regulates the content of organic acids.
 | ||
| Fig. 8 Standard organic acids (a) ((1) pyruvate; (2) malate; (3) lactate; (4) citrate; (5) succinate; (6) fumarate); the content of intracellular organic acids (b). | ||
| Organic acid | Regression equation | Related parameters |
|---|---|---|
| Malate | y = 0.0246x − 0.0366 | 0.9978 |
| Fumarate | y = 2.7373x − 7.7253 | 0.9902 |
| Citrate | y = 0.0354x − 0.0112 | 0.9970 |
| Succinate | y = 0.0181x − 0.0426 | 0.9915 |
| Lactate | y = 0.0131x − 0.0215 | 0.9994 |
| Pyruvate | y = 248.35x + 0.0187 | 0.9999 |
3.7 The possible mechanism of electron shuttles in microorganisms
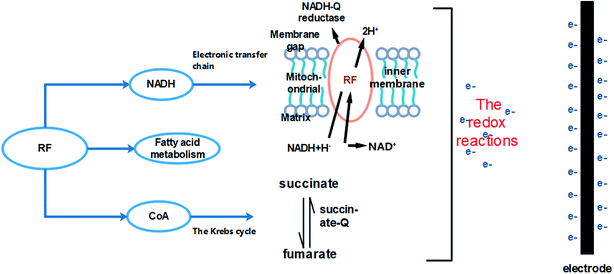
RF and bias affected the growth and metabolism of A. faecalis. The possible mechanisms are as follows (Fig. 10). One way involves promoting and affecting the process of electronic transmission chain. The flavin electron shuttle has two forms: (i) oxidized and (ii) reduced. In microorganisms, the function of flavin electron shuttle is to transfer hydrogen during the redox process. Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) are the main forms of RF. FMN is a prosthetic group of electron transfer chain-enzyme complex I (NADH-Q reductase); FAD is a prosthetic group of enzyme complex II (succinate-Q reductase). NADH-Q reductase promotes electrons across the mitochondrial inner membrane to the mitochondrial space, oxidizing NADH and reducing FMN to deliver electrons.54 Succinate-Q reductase is a protein complex in the mitochondria and succinate dehydrogenase promotes the conversion of succinate to fumarate.54 Electron transfer and intracellular redox reactions were promoted.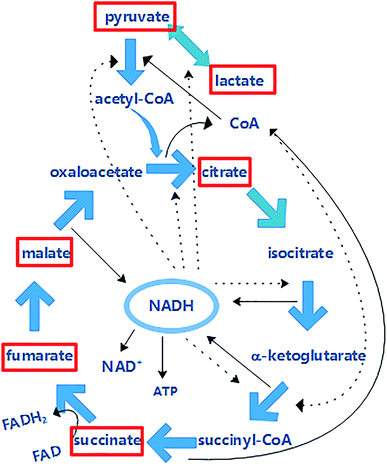 | ||
| Fig. 9 The relationship among NADH, CoA and the Krebs cycle (the solid line represents the generation and the dotted line represents the participation in the regulation). | ||
The promotion of the Krebs cycle is another way. RF and bias increased the content of CoA and related organic acids in A. faecalis. The process of the Krebs cycle should be promoted, and bias can provide electrons to the Krebs cycle.
4 Conclusions
Under oligotrophic conditions, both bias and RF promoted the growth and metabolism of A. faecalis. In terms of accelerating electron transfer, bias provided additional electrons to A. faecalis, while RF acted as a mediator. The growth curves indicated that RF and bias did not affect the growth cycle. At different potential voltages, bias would change the morphology of A. faecalis but not RF would. Through the three-dimensional fluorescence analysis of metabolites, A. faecalis synthesised more humus and NADH with RF, and the latter was closely related to microbial glycolysis and the Krebs cycle. This was confirmed by the content of CoA. Furthermore, these results corresponded to the content of organic acids related to the Krebs cycle. The Krebs cycle ought to be the means by which RF and bias promoted A. faecalis. RF and bias support the promotion collectively. More research needs to be conducted to obtain better results at the molecular level for the growth and metabolism of non-photosynthetic microorganisms, which are directly influenced by electron shuttles.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Key R&D Program of China (2016YFC0502204), National Basic Research Program of China (973 Program: 2014CB846003), National Nature Science Foundation of China (Grant number: 41272371, 41502316), and Longshan Academic Talent Research Supporting Program of SWUST (18LZX507). The authors also thank Dr Toquier Azam for language help.Notes and references
- A. Lu, Y. Yan, X. Xin, H. Ding, C. Zeng, R. Hao and C. Wang, Microbiology, 2013, 40(01), 190–202 CAS.
- K. Sakimoto, A. Wong and P. Yang, Science, 2016, 6268(351), 74–77 CrossRef PubMed.
- A. Lu, Y. Li, X. Wang, H. Ding, Y. Liu and C. Wang, Geosci. Front., 2014, 21(03), 256–264 CAS.
- C. Zeng, A. Lu, Y. Li, J. Wu, X. Wang, H. Ding and Y. Yan, Geol. J. China Univ., 2011, 17(01), 101–106 CAS.
- J. J. Guo, M. Suástegui, K. Sakiomoto, V. Moody, G. Xiao, D. Nocera and N. Joshi, Science, 2018, 6416(362), 813–816 CrossRef PubMed.
- P. Yu, Y. Li, A. Lu, C. Zeng, X. Wang and H. Rui, Acta Petrol. Mineral., 2013, 32(06), 761–766 CAS.
- S. Xiang, M. Liu, G. Zhang, L. Luo, H. Wei and F. Dong, Microbiology, 2017, 33(04), 205–213 Search PubMed.
- H. Canstein, J. Ogawa, S. Shimizu and J. Lloyd, Appl. Environ. Microbiol., 2008, 74(3), 615–623 CrossRef PubMed.
- C. Turick, L. Tisa and F. Caccavo Jr, Appl. Environ. Microbiol., 2002, 68(5), 2436–2444 CrossRef CAS PubMed.
- Z. Summers, H. Fogarty, C. Leang, A. Franks and N. Malvankar, Science, 2010, 330(6009), 1413–1415 CrossRef CAS PubMed.
- S. Kato, K. Hashimoto and K. Watanabe, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(25), 10042–10046 CrossRef CAS PubMed.
- A. Kappler, M. Wuestner and A. Ruecker, Environ. Sci. Technol. Lett., 2014, 1(8), 339–344 CrossRef CAS.
- S. Chen, A. Rotaru, P. Shrestha, N. Malvankar, F. Liu, W. Fan, K. Nevin and D. Lovley, Sci. Rep., 2014, 4, 5019 CrossRef CAS PubMed.
- F. Liu, A. Rotaru, P. Shrestha, N. Malvankar, K. Nevin and D. Lovley, Energy Environ. Sci., 2012, 5(10), 8982 RSC.
- D. Lovley, J. Coates, E. Blunt-Harris, E. Phillips and J. Woodward, Nature, 1996, 382(6590), 445–448 CrossRef CAS.
- K. Nevin and D. Lovley, Appl. Environ. Microbiol., 2002, 68(5), 2294–2299 CrossRef CAS PubMed.
- K. Watanabe, M. Manefield, M. Lee and A. Kouzuma, Curr. Opin. Biotechnol., 2009, 20(6), 633–641 CrossRef CAS PubMed.
- F. Zee and F. Cervantes, Biotechnol. Adv., 2009, 27(3), 256–277 CrossRef PubMed.
- E. Brutinel and J. Gralnick, Appl. Microbiol. Biotechnol., 2012, 93(1), 41–48 CrossRef PubMed.
- X. Li, L. Liu, T. Liu, T. Yuan, W. Zhang, F. Li, S. Zhou and Y. Li, Chemosphere, 2013, 92(2), 218–224 CrossRef CAS PubMed.
- P. Zhang, D. Xu, K. Yang and T. Gu, Bioelectrochemistry, 2015, 101, 14–21 CrossRef CAS PubMed.
- P. Wang, F. Dong, X. Wang, M. Liu, X. Nie, L. Zhou, T. Huo, W. Zhang and H. Wei, RSC Adv., 2018, 8(54), 30692–30700 RSC.
- J. Ma, C. Ma, J. Tang, S. Zhou and L. Li, Prog. Chem., 2015, 27(12), 1833–1840 Search PubMed.
- J. Liu, S. Xie, Y. Wang, Y. Liu, P. Cai, F. Xiong and W. Wang, Trans. Nonferrous Met. Soc. China, 2015, 25(12), 4144–4150 CrossRef CAS.
- S. Xie, Y. Zhang, J. Liu, Y. Liu, S. Li, J. Wang and H. Liu, Trans. Nonferrous Met. Soc. China, 2012, 22(11), 3285–3291 CAS.
- X. Wang, G. Sun, X. Li, T. A. Clarke and Y. Zhu, J. Soils Sediments, 2018, 18(1), 159–168 CrossRef CAS.
- T. M. Flynn, E. J. O'Loughlin, B. Mishra, T. J. DiChristina and K. M. Kemner, Science, 2014, 344(6187), 1039–1042 CrossRef CAS PubMed.
- Y. Hong, P. Wu, W. Li, J. Gu and S. Duan, Appl. Microbiol. Biotechnol., 2011, 93(6), 2661–2668 CrossRef PubMed.
- Y. Meng, Z. Zhao, W. Burgos, Y. Li, B. Zhang, Y. Wang, W. Liu, L. Sun, L. Lin and F. Luan, Sci. Total Environ., 2018, 640, 591–598 CrossRef PubMed.
- Q. Yin, J. Miao, B. Li and G. Wu, Int. Biodeterior. Biodegrad., 2016, S1(119), 104–110 Search PubMed.
- A. J. M. Stams, F. A. M. D. Bok, C. M. Plugge, M. H. A. V. Eekert, J. Dolfing and G. Schraa, Environ. Microbiol., 2006, 8(3), 371–382 CrossRef CAS PubMed.
- J. Ma, Master R446.1, Yanbian university, 2010.
- D. Feng, Y. Zhang, H. Zhang, G. Sun and P. Sun, China Brew., 2009,(03), 157–161 CAS.
- J. Ma, J. Liu, X. Zeng, P. Deng, S. Ru and J. Sun, Mod. Food Sci. Technol., 2011, 27(05), 591–594 CAS.
- M. Fan, P. Liang, X. Cao and X. Huang, Environ. Sci.: Nano, 2008, 29(1), 265–269 Search PubMed.
- H. Richter, K. McCarthy, K. P. Nevin, J. P. Johnson, V. M. Rotello and D. R. Lovley, Langmuir, 2008, 24(8), 4376–4379 CrossRef CAS PubMed.
- Y. Yan, Y. Li, A. Lu, X. Wang, H. Ding, C. Zeng and C. Wang, Acta Petrol. Mineral., 2009, 28(6), 535–540 CAS.
- X. Wang, Y. Li, A. Lu, Y. Yun, C. Zeng and C. Wang, Acta Petrol. Mineral., 2009, 28(06), 527–534 CAS.
- X. Zhang, Master Q932/Q93-3, Southwest University, 2015 Search PubMed.
- J. P. Busalmen and S. R. D. Sanchez, Appl. Environ. Microbiol., 2005, 71(10), 6235–6240 CrossRef CAS PubMed.
- P. Yu and A. Lu, Acta Pet. Sin., 2012,(S1), 200–202 Search PubMed.
- E. Marsili, D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick and D. R. Bond, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(10), 3968–3973 CrossRef CAS PubMed.
- A. Pal and A. K. Paul, Indian J. Microbiol., 2008, 48(1), 49–64 CrossRef CAS PubMed.
- T. Fan, Master O629.4, Hebei Normal University, 2010.
- L. Liu, Chemical Engineering & Equipment, 2010, 2, 144–146 Search PubMed.
- C. A. Stedmon, S. Markager and R. Bro, Mar. Chem., 2003, 82(3–4), 239–254 CrossRef CAS.
- Q. Cheng, B. Zheng, S. Wang, L. Jiao and M. Huang, Spectrosc. Spectral Anal., 2014, 34(03), 698–703 CAS.
- R. M. Cory and D. M. McKnight, Environ. Sci. Technol., 2005, 39(21), 8142–8149 CrossRef CAS PubMed.
- D. E. Harrison and B. Chance, J. Appl. Microbiol., 1970, 19(3), 446–449 CAS.
- G. Farabegoli, C. Hellinga, J. J. Heijnen and M. C. M. V. Loosdrecht, Water Res., 2003, 37(11), 2732–2738 CrossRef CAS PubMed.
- P. Fu, C. Liu and F. Wu, Spectrosc. Spectral Anal., 2005, 25(12), 2024–2028 CAS.
- L. Klüpfel, A. Piepenbrock, A. Kappler and M. Sander, Nat. Geosci., 2014, 7(3), 195–200 CrossRef.
- S. M. Strycharz, R. H. Glaven, M. V. Coppi, S. M. Gannon, L. A. Perpetua, A. Liu, K. P. Nevin and D. R. Lovley, Bioelectrochemistry, 2011, 80(2), 142–150 CrossRef CAS PubMed.
- M. Saraste, Science, 1999, 283(5407), 1488–1493 CrossRef CAS PubMed.
- H. V. Canstein, J. Ogawas, S. Shimizu and J. R. Lloyd, Appl. Environ. Microbiol., 2008, 74(3), 615–623 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra04066h |
| This journal is © The Royal Society of Chemistry 2019 |