 Open Access Article
Open Access ArticleA review of modification of carbon electrode material in capacitive deionization
Yutuo Cheng†
ab,
Zhiqi Hao†ab,
Changrun Haoab,
Yu Dengab,
Xingying Liab,
Kexun Li *ab and
Yubo Zhao*ab
*ab and
Yubo Zhao*ab
aThe College of Environmental Science and Engineering, Nankai University, Tianjin 300071, China. E-mail: likx@nankai.edu.cn; 1244174212@qq.com
bMOE Key Laboratory of Pollution Processes and Environmental Criteria, Tianjin Key Laboratory of Environmental Technology for Complex Trans-Media Pollution, Nankai University, Tianjin 300071, China
First published on 7th August 2019
Abstract
Capacitive deionization (CDI) technology has attracted wide attention since its advent and is considered as one of the most promising technologies in the field of desalination and ion recycling. It is constructed with an electric field by applying a low voltage of direct-current to make ions migrate directionally in solution to achieve the purpose of ion separation and removal. The performance of CDI is heavily dependent on the electrode material. Carbon is widely used as CDI electrode material because of its lower price and better stability. To enhance the adsorption capacity, extensive research efforts have been made for the modification of carbon material. In this review, we enumerate and analyze four modification methods of carbon material including element doping, metal oxide modification, chemical treatment and surface coating. The influence of each modification method on CDI performance is concluded in the perspective mechanism and some constructive advice is put forward on how to effectively enhance the performance of CDI by the decoration of carbon materials.
1. Introduction
Ions dissolved in water are very difficult to remove just by physical methods. Up to now, electrodialysis (ED),1 multi-stage flash (MSF),2 multi-effect distillation (MED)4 and reverse osmosis (RO)3 are the primary physical means for ion removal. However, due to the limitation of cost and scope of application, many existing energy-intensive technologies have not been used in a large scale in industry, e.g. microbial desalination,5 ion concentration polarization,6 capacitive deionization (CDI)7,8 and so on.CDI is an emerging electrochemical desalination technology. When compared with other technologies, it has attracted much more attention because of its unique advantages such as environmental benignity, lower energy consumption, higher efficiency and simple operation. CDI devices work at a low direct-current voltage (usually lower than 2 V), which ensures their safety and lower energy consumption. Actually, in terms of economic efficiency, Welgemoed and Schutte conducted a simulation experiment and ran a 3785 m3 per day CDI desalination unit,9 while the desalination capacity of typical RO plant is 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day. They found that under the circumstances of 2000 parts per million (ppm) feed solution, the desalination cost for CDI was $0.11 per m3 against $0.35 of RO, indicating that economic efficiency of CDI is also applicable in high concentration solutions. By changing the preparation methods and experimental conditions, the adsorption capacity of different ions can also be changed and the goal of specific adsorption can be achieved. Seo et al. studied the adsorption force of CDI on divalent ion by preparing graphene nano-flakes.10 In terms of difficulty of ion removal, AlMarzooqi et al. proved that CDI can remove 85% of divalent ions in water, showing the potential for drinking water treatment.11 CDI has been proven to be available in brackish water desalination.7–12 In addition, CDI can also be used in medicine, chemistry, microbial fuel cells and so on.13–16
000 m3 per day. They found that under the circumstances of 2000 parts per million (ppm) feed solution, the desalination cost for CDI was $0.11 per m3 against $0.35 of RO, indicating that economic efficiency of CDI is also applicable in high concentration solutions. By changing the preparation methods and experimental conditions, the adsorption capacity of different ions can also be changed and the goal of specific adsorption can be achieved. Seo et al. studied the adsorption force of CDI on divalent ion by preparing graphene nano-flakes.10 In terms of difficulty of ion removal, AlMarzooqi et al. proved that CDI can remove 85% of divalent ions in water, showing the potential for drinking water treatment.11 CDI has been proven to be available in brackish water desalination.7–12 In addition, CDI can also be used in medicine, chemistry, microbial fuel cells and so on.13–16
Theories and experimental results both showed that porous carbon materials generally possessed high specific surface areas and excellent capacitive properties, which led to better CDI performance. CDI theory is similar to that of supercapacitors.7 Up to now, the core of CDI technology is to seek the maximum adsorption capacity per unit mass and the minimum energy loss power from the perspective of theory and properties. In terms of experience and theory, the ideal CDI electrode materials require the following properties: (1) large specific surface area for adsorption; (2) the stability and applicability of pH, voltage and ion concentration in different areas; (3) high speed ion mobility, i.e. conductivity; (4) good plasticity and expandability; (5) suitable pore size distribution structure; (6) abundant and readily available natural or artificial low-cost materials; (7) stable and reusable physical and chemical properties.
With the rapid development of CDI electrode materials, research work emerges endlessly. In previous literature, researchers have analyzed their progress in terms of activated carbon,17–19 mesoporous carbon,20–22 carbon aerogels,23,24 carbon nanotubes,25–27 graphene,28,29 etc., and reviewed the various carbon-based materials8,30 as well as graphene materials31 for CDI. However, there is still a review gap in the field of modification of carbon electrode materials. To fill this vacancy, we provide a comprehensive summary of common modification methods in the aspects of element doping, metal oxide doping and polymers, and put forward some prospects. This article summarizes the existing successful modification methods of carbon electrode materials in CDI.
2. History of CDI materials
Compared with other traditional desalination methods, CDI is a new effective technology.8 Studies on CDI can trace back to the 1960s and the conception was first reported by Murphy and Caudle. They designed a flow through mode whose electrode sheets consisted of porous activated carbon electrodes for water desalination. Then in 1970s, Johnson et al. studied the reversible process of CDI and proposed a reversible electrosorption model. In this theory, ions hidden in the electrode sheets could be released by removing electrical field.32 On the other hand, they gave a conclusion that the application of high-surface area electrodes would be beneficial to efficient desalination. However, there are still some limitations in carbon materials, such as poor stability, single composition, and too irregular structure. Scientists have tried abundant modification methods of carbon materials in the course of past few decades to seek more suitable modified materials for practical production. Thus, more and more materials such as carbon nanotubes, carbon aerogel, ordered mesoporous carbons, carbon nanofiber and graphene have been applied to CDI technology, which lead to a great boost in the field.18 Here some typical materials will be introduced with their properties and application examples.Generally, activated carbon (AC) is the most widely used electrode material for CDI electrode because of its higher-capacity of environmental contaminants and cheaper price.33 But lower conductivity and higher electrical transfer resistance limit its further development in CDI.34 AC is usually combined with binders and conductive additives to fabricate CDI electrode. Hou et al. found that ten percent of PVDF (a kind of binder) could achieve a better result in CDI experiment.35 Furthermore, some chemical modification methods by introducing hydrophilic reactive functional group can also greatly improve the electrosorption capacity of activated carbon electrodes.36
Carbon Nanofiber (CNF) is a fibrous one-dimension carbon nanomaterial, which could be obtained by electrospinning. In recent years, more and more people have focused concerns on the application in the field of supercapacitors for its high surface area, high electrical conductivity and unique filamentous structure.37 In 2014, Chen et al. fabricated phenolic resin-based CNF through electrospinning and one-step carbonization and applied the electrode materials to CDI.38
Carbon aerogel (CA) possesses unique strengths such as good conductivity, continuous and favorable pore distribution, as well as high surface area.39 In 1990, Farmer et al. discovered carbon aerogel and applied this material to CDI electrodes. From then on, searching new carbon materials with a high specific surface area has become a focus of concern among scientist.40 Although carbon aerogel has unique good properties, the complex processing technology and high cost limit its development.
Carbon nanotube (CNT), a kind of one-dimensional nanomaterial with hollow structure, is well known for its strong active functional groups and proper pore structure. In 2005, carbon nanotubes were first used for CDI according to Dai and his team's study.41 Arvind et al. found amazing adsorption effect by opening the termination of carbon nanotubes involve oxidation to get more organic functional groups.42
Graphene is a new two-dimensional carbon nanomaterial with single atomic layer, possessing great development potentiality. Graphene was first gained by Andre et al. in 2004 and was used into CDI in 2009. It has some derivatives such as graphene oxide and reduced graphene oxide which are usually loaded on other electrode materials. Yu et al. incorporated graphene oxide with carbon nanofibers. The desalination performance was improved for the enhancement of electrical conductivity and mesoporosity ratio.43
Ordered mesoporous carbons (OMCs), possessing ordered pore size and less ion transfer resistance, are promising candidate for CDI electrode. Zou et al. first adopted OMCs to CDI. They found this electrode showed better ion removal performance and faster desorption speed compared with activated carbon.44 Li et al. prepared mesoporous carbon electrode with larger surface area by adding NiSO4·6H2O, which performed a better electrosorption performance.45
As the research continues, researchers found that excellent CDI electrode materials require the properties of large specific surface area, suitable pore size, high hydrophilicity and high conductivity.31,46 To improve those properties, various modification methods have been developed. The timeline is shown in Table 1.
| Time | Event |
|---|---|
| 1960s | Murphy and Caudle first proposed the concept of CDI and made CDI electrode sheets from activated carbon |
| 1970s | Johnson et al. proposed a reversible electrosorption model |
| 1990 | Farmer et al. discovered carbon aerogel and applied this material to CDI electrodes |
| 2005 | Dai et al. applied carbon nanotubes into CDI for the first time |
| 2006 | Han-jun et al. introduced hydrophilic reactive functional group in activated carbon electrodes to improve the adsorbing capacity |
| 2008 | Zou et al. first adopted OMCs to CDI and found better ion removal performance and faster desorption speed compared with activated carbon |
| 2009 | Li et al. obtained the mesoporous structure with a larger surface area by adding NiSO4·6H2O to ordered mesoporous carbons |
| 2009 | Graphene was first used into CDI |
| 2010 | Li et al. used graphite to produce a GO and applied it into CDI |
| 2014 | Chen et al. fabricated phenolic resin-based CNF and applied the electrode materials to CDI |
| 2014 | Yu et al. incorporated graphene oxide into carbon nanofiber to improve the conductivity and the mesoporosity of electrode materials |
| 2015 | Arvind et al. opened the termination of carbon nanotubes involve oxidation and found amazing adsorption effect |
3. Modification methods
3.1. Heteroatom doping
Carbon materials, with the advantages of low cost, light weight, large specific surface area, corrosion resistance, oxidation resistance, excellent ductility and porous structure, occupy a dominant position in CDI electrodes. However, due to their super high stability and structural framework, carbon materials often have a single element type. That makes it difficult for CDI electrode materials to break through in terms of adsorption capacity, rate and types. Fortunately, the known organic species are far more than inorganic species, and carbon is the most basic element of the organic structure skeleton, that is to say, it has strong plasticity and compatibility. That makes it possible for scientists and researchers to dope other ions or elements into carbon materials to improve their electrosorption performance. Actually, heteroatom doping is one of the most used modification methods for carbon materials. It is worth mentioning that experiments showed that not all elements are suitable for element doping. Non-metallic elements, such as N, P, S and Cl are commonly selected and calcined with carbon materials at high temperatures to prepare element doped electrodes. Several common heteroatom doping methods are as followed.Usually, there are three methods to dope nitrogen into carbon materials. The first one is to use nitrogen-containing monomers, such as melamine, pyrrole, sucrose and polyaniline, as precursors to prepare nitrogen-containing polymers which are then heat-treated. Directly heating nitrogen-containing biomass materials or wastes is the second way. The last one is to use NH3 or CO(NH2)2 as nitrogen source to post-treat the material, so that specific nitrogen-containing groups will be introduced into the carbon material. The effects of different nitrogen doping methods on electrochemical properties and electroadsorption capacities of carbon materials will be described in detail below.
When nitrogen doping is used in capacitive deionization, it can effectively enlarge the surface pore size of carbon materials, i.e. enlarging the specific surface area. High specific surface area is one of the most remarkable features of carbon materials, which represents high adsorption capacity. Hence, nitrogen contend is a major consideration of characterization. Zhao et al.51 used polystyrene (PS) as hard template, and dopamine hydrochloride as carbon and nitrogen sources to prepare nitrogen-doped porous carbon microspheres (N-PHCS). Its uniform, spherical and hollow carbon structure was observed in field emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images (Fig. 1(a)–(c)). The nitrogen content, determined by X-ray photoelectron spectroscopy (XPS), was 2.92% (Fig. 1(d)). The result of the SAC curve and current transient of N-PHCS electrodes in 500 mg L−1 NaCl solution also indicated that its electrochemical performance was fantastic (Fig. 1(e)). A 500 mg L−1 NaCl aqueous solution was treated at a flow rate of 40 mL min−1 at 1.4 V. The specific electrosorption capacity (SAC) was 12.95 mg g−1 and its stability was perfect. Li et al.52 prepared similar nitrogen-doped materials. Nitrogen-doped cluster porous carbon materials (NCPC) with layered hollow nanostructures were fabricated under various temperatures by carbonizing dopamine/nano-ZnO composites. They possessed good wettability interconnection layer structure, enabling rapid ion binarization. Among them, the sample carbonized at 900 °C (NCPC-900) showed the best porous structure. Its specific surface area was 1357 m2 g−1 and the maximum adsorption capacity was 11.98 mg g−1. In terms of nitrogen content, NCPC-700 reached the highest of 9.2%. Besides, Liu et al.53 prepared nitrogen-doped porous carbon microspheres (NPCS) by microblog-assisted rapid preparation method. In this process, sucrose was used as a precursor. Through a series of characterization and experimental measurements, it was found that NCPS treated at 1000 °C possessed the highest electroadsorption capacity of 14.91 mg g−1 in 1000 mg L−1 treated solution.
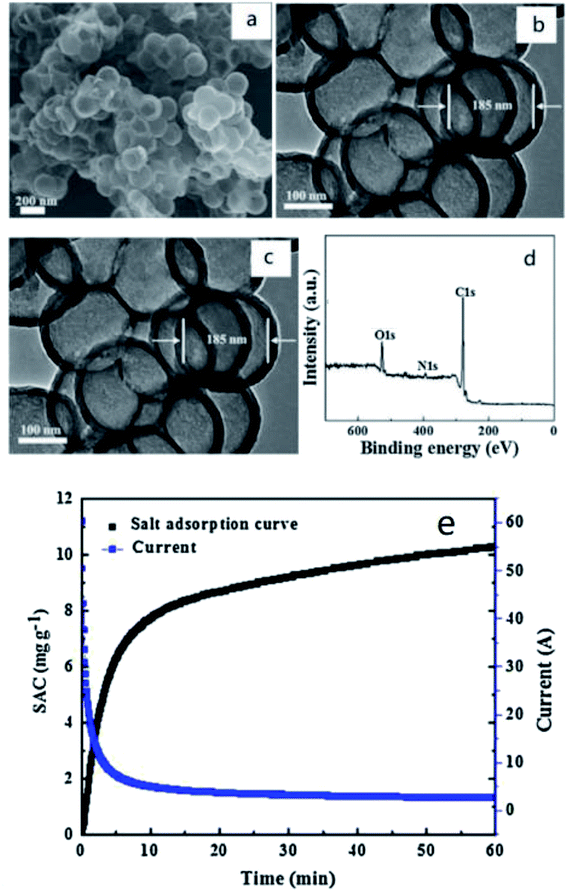 | ||
| Fig. 1 (a) SEM (b) TEM and (c) HRTEM images of the N-PHCS; (d) XPS spectra of the N-PHCS sample; (e) the SAC curve and current transient of N-PHCS electrodes in 500 mg L−1 NaCl solution at the voltage of 1.2 V with a flow rate of 40 mL min−1; reproduced with permission from ref. 51. | ||
Gao et al.54 synthesized graphene polyhedrons directly from pyrolytic imidazolate skeleton (ZIF-8). Characterization results showed that it had close SP-2 skeleton edges. When compared with carbon polyhedrons (CPs) and nitrogen doped carbon polyhedrons (NCPs), besides the benefits of 1134 m2 g−1 remarkable specific surface area and considerable graphitization degree, the conductivity and cycling stability of nitrogen-doped graphitic carbon polyhedrons (NGCP) were also distinguished. In CDI experiment, NGCP exhibited a desalination capacity of 17.73 mg g−1 with 500 mg L−1 NaCl solution at the voltage of 1.4 V. Duan et al.55 and Liu et al.56 also applied ZIF-8 to the preparation of analogous carbon materials and obtained good results (Fig. 2). The above experimental results proved that nitrogen doping was an effective method to improve the electrosorption performance as well as recycling stability of carbon materials.
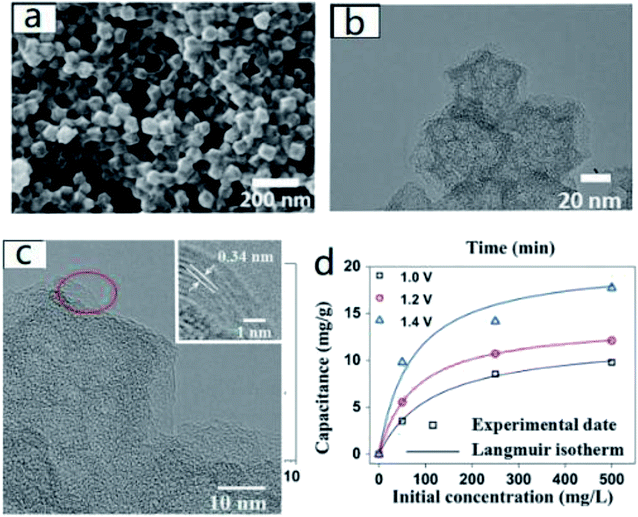 | ||
| Fig. 2 (a) The SEM (b) TEM and (c) HRTEM image of NGCPs; (d) electrosorption capacitance and Langmuir isotherm of NGCPs at various voltage in NaCl solutions with initial concentration ranging from 50 to 500 mg L−1. Reproduced with permission from ref. 54. | ||
In addition, calcination of nitrogen-containing biomass at high temperature under pure nitrogen condition is a common method to prepare carbon materials. It can effectively determine the pore size of carbon materials and the distribution of nitrogen elements in the materials. Classically, Zhao et al.57 fabricated nitrogen-doped porous carbon (NPC) by simple pyrolysis method, using soybean hull as raw material and KHCO3 as catalyst. Then NPC sulfonate group was functionalized in aryl diazonium salt solution, leading to a final specific surface area of 844.0 m2 g−1 and nitrogen content of 1.66 at%. The electrosorption capacity of this material was 15.8 mg g−1, and the adsorption rate was 0.37 mg (g min)−1 due to the high surface negative charge density.
The last kind of nitrogen doping aims at introducing specific nitrogenous groups, such as NH3 and NR4, onto the surface of carbon materials. Yang et al.58 used aryl diazonium salt solution and acetone mixed with APTEs to introduce –SO32− and –NH3+ groups into nanotubes to overcome co-ion effect. Characterizations and electrosorption results indicate that the functionalized-CNTs electrodes enhanced the salt-removing performance. Later, Gao et al.59 introduced ethylenediamine into the surface of microporous spectracarb carbon cloth and used it as the cathode electrode. Meanwhile, the anode carbon material was treated with nitric acid to increase its negative surface charge. The maximum adsorption capacity was 5.3 mg g−1 at 1.1 V working voltage and characterization results showed that the functional groups derived from ethylenediamine increased the microporosity of carbon materials. In the latest research, Ahmed G. et al.60 modified activated graphene electrodes with polyatomic ions (NR4+). Carboxymethyl cellulose (CMC) was negatively charged and quaternary ammonium cellulose (QMC) was used as the disinfectant. The obtained materials asym-QC-3DAPGr (3DPGr: 3D porous graphene) were treated with 300 mg L−1 NaCl solution at 1.4 V cell voltage. And adsorption capacity per unit is up to 18.43 mg g−1. In another aspect, the non-toxic water-soluble adhesive also reduces the interface resistance and improves the flexibility of the electrode.
Nitrogen doping is often combined with graphene. Graphene is a two-dimensional carbon nano-material with hexagonal honeycomb lattice composed of carbon atoms and sp2 hybrid orbital. Its excellent conductivity and pore structure make it a new favorite of electrodes choosing in CDI, and it is often associated with nitrogen doping to form two-dimensional carbon nanomaterials. In fact, as early as 2015, Xu et al.61 have prepared a novel nitrogen-doped graphene sponge (NGS), which has high specific surface area of 526.7 m2 g−1, and its designed structure can be also fabricated. Eventually, when treated with 500 mg L−1 NaCl solution, the adsorption capacity attained 21 mg g−1, which was also the highest at that time. In the same year, Amiri et al.62 published an article to demonstrate that highly-crumpled nitrogen-doped graphene and highly-crumple and few-layered graphene have enormous potentiality in industry desalination. Liu et al.63 then applied this material to the rapid removal of heavy metal ions (Pb2+, Cd2+, Cu2+, Fe2+) in water, obtaining a removal rate of 90% in the range of 0.05–200 ppm and perfect recycling abilities. In the latest research, Zhou et al.64 prepared two-dimensional sandwich graphene/nitrogen-doped carbon nanoparticles composites using graphene/porous SiO2 composite as a template, ethylene diamine and carbon tetrachloride as carbon sources. It exhibits high capacitance up to 189 F g−1 at a current density of 0.1 A g−1. This further proves that nitrogen-doped graphene has broad application prospects in the field of CDI.
Apart from sulfur, phosphorus is another doping element that is often used in carbon modification. Li et al.67 compared the P and none element doped carbon nanofibers aerogels in 1000 mg L−1 NaCl solution, and the adsorption capacity of undoped P was 12.81 mg g−1 while that of (P)-doped carbon nanofiber aerogels (P-CNFA) was 16.20 mg g−1. In addition, the nitrogen and phosphorus dual doped graphene aerogels were prepared to expand the capacitance and cycling stability of the materials. In this way, Li et al.68 greatly improved the performance of carbon anodes used in the construction of electrochemical capacitors. Experiments show that the double doping of nitrogen and phosphorus makes graphene aerogel (NPGA) have high sodium storage capacity and wide application prospect in CDI field, and its cycling stability is excellent. On the level of practical application, Han et al.69 firstly used phytic acid as P source, chitosan as N source, mixed with gallic acid to deal with graphene oxide and succeed in preparing highly hydrophilic N, P co-doped three-dimensional hierarchical carbon architectures. Due to its three-dimensional structure, the material has a high adsorption capacity of 26.8 mg g−1 at an applied voltage of 1.2 V.
To some extent, it seems that the more types of element were used to dope, the more remarkable the effect is. Of course, it is also inseparable from the reasonable element collocation and the selection of appropriate element sources. In 2018, Zhang et al.70 succeeded in making N, P, S co-doped hollow carbon polyhedral. They chose poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) as carbon sources and N, P, S co-doping sources, ZIF-8 as structural templates, which also acted as additional N-doping sources. TEM results showed that ZIF-8@PZS-C (poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) coated zeolitic imidazolate framework-8) owned fabulous pore structure (Fig. 3(a) and (b)). Ultimately, the super-high adsorption capacity of 22.19 mg g−1 was achieved in the treatment of 500 mg L−1 NaCl solution (Fig. 3(c)). It is reasonable to believe that more and more attention will be paid to multielement doping.
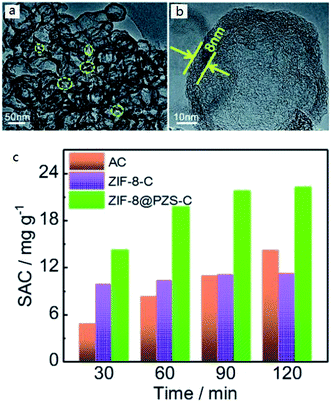 | ||
| Fig. 3 (a) TEM images and (b) HRTEM image of ZIF-8@PZS-C; (c) plots of SAC vs. deionization time of various electrodes in 500 mg L−1 NaCl solution at 1.2 V with a flow rate of 50 mL min−1. Reproduced with permission from ref. 70. | ||
In addition, ion-doped methods can be also associated with polypyrrole (PPy) and CNT. Chloride (Cl) and dodecylbenzene sulfonate (DBS) are both commonly used ion doping reagents. Normally, ion-doped CDI electrodes are affected by ion species, acidity and basicity in solution and experimental conditions. Wang et al.72 investigated the fouling characteristics of ion-doped PPy/CNT composite electrodes in the process of CDI by a half cycle running mode. It was found that the addition of Ca2+ or Fe3+ in the solution would alkalize the regeneration process during desalination, thus affecting the adsorption performance of CDI. In the aspect of organic pollution, the addition of sodium humate significantly reduced the desalination performance of CDI batteries, because the high concentration of sodium humate would destroy the structure of the electrode layer and further destroy the stability of the electrode. Ma et al.73 explored the effects of temperature and pH on ion-doped PPy/CNT composite electrodes. Under the condition of pH = 7, T = 15 °C, the adsorption capacity was the largest, reaching 75% or more. Meanwhile, strong acid and alkali will aggravate the oxidation reaction on the surface of the electrode, and the increase of temperature will lead to the hydrophilic–hydrophobic transition on the surface of the electrode, which results in the decrease of the adsorption capacity. In 2016, Cai et al.74 measured the electrosorption capacity of PPy-DBS/CNT and PPy-Cl/CNT composite electrode. The electrosorption capacity is 40.8–72.36 mg g−1, which directly proves the strong electrosorption of ion-doped composite carbon materials. In another aspect, Cai inferred that the composite electrodes may have the good ability of ion selective adsorption for cations or anions.
3.2. Metal oxide-modified carbon material
For the reason of synergistic effects, the combination of carbon and metal oxides improves the specific capacitance, making them potential materials for capacitive deionization.75 Moreover, the addition of metal oxide can prominently change some physicochemical properties of carbon material, like wettability, surface area and zeta potential, which may contribute to the improvement of capacitive deionization performance.High hydrophilicity of TiO2 is due to abundant hydroxyl groups on its surface. Those Ti–OH groups can act as electrosorption sites on the surface of carbon electrode, which is beneficial to the CDI performance. Kim have et al.77 fabricated one of the TiO2 coated carbon electrodes. As shown in Fig. 4, the wettability of carbon electrode was highly enhanced by coating with TiO2. The TiO2 coated carbon electrode showed an electrosorption capacity of 17 mg g−1 which was nearly twice as high as that of carbon electrode. This improvement was resulted from the easier accessibility of ions and water from solution to the surface of electrode due to enhanced wettability. Recently, Kang et al.79 synthesized TiO2-modified activated carbon fibers (ACF). From the Nyquist impedance plots, it can be calculated that the value of charge transfer resistance decreased with TiO2 doping amount, implying that the introduction of TiO2 promotes ion migration and diffusion on the surface of carbon electrode. Consequently, the highest electrosorption capacity reached up to 10.6 mg g−1, which was 71.9% higher than that of the unmodified ACF electrode.
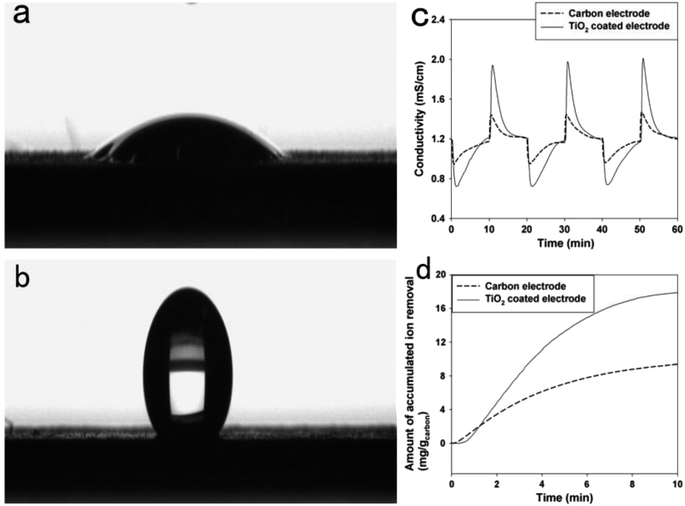 | ||
| Fig. 4 Surface wetting property of (a) the TiO2 coated electrode and (b) carbon electrode. (c) Ion removal performance. (d) Accumulated ion removal amount during the charging step (selected at the third cycle). Reproduced with permission from ref. 77. | ||
The strong hydrophobicity of CNT dramatically limits its CDI performance. With high hydrophilicity, TiO2 is promising for improving this situation. Li et al.80 reported a TiO2/CNTs composite electrode for CDI. This electrode displayed an enhanced electrosorption capacity of 4 mg g−1 which was twice as high as that of pristine CNTs. Feng et al.81 recently fabricated TiO2/CNTs membrane electrodes by atomic layer deposition method. The deposition of TiO2 was reported to transform CNT from strongly hydrophobic to hydrophilic. This composite electrode showed an improved desalination capacity of 5.09 mg g−1 as well as good regeneration.
Cycling stability of CDI is challenged with peroxide formation and rapid oxidation of anodic electrode.82 To overcome this issue, Srimuk et al.83 presented a strategy by hybridizing nano-carbon particles with solgel-derived TiO2. This carbon/metal oxide hybrid showed 90% of the initial electrosorption capacity after 100 cycles, indicating an outstanding cycling stability. TiO2 was confirmed to prevent the formation of local peroxide by modifying redox reactions. In their subsequent research, TiO2-modified activated carbon electrodes were fabricated.84 The electrode loaded with 15 wt% TiO2 displayed a considerable desalination capacity of 17.4 mg g−1 and a better cycling stability, attributed to favorable three-electron transfer reactions.
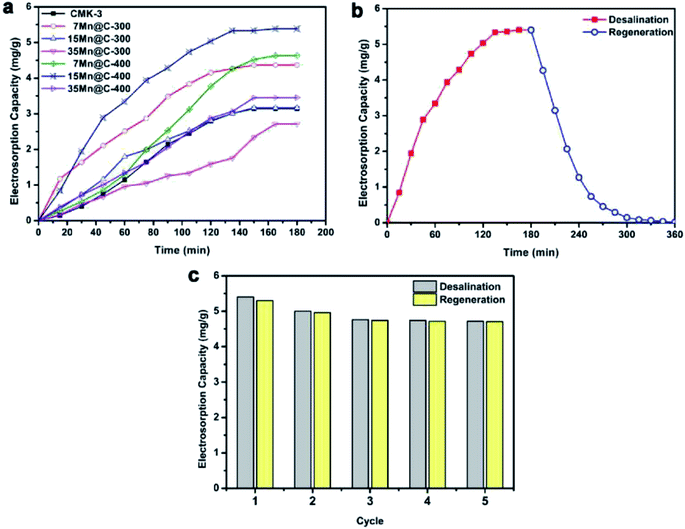 | ||
| Fig. 5 (a) CDI profiles of CMK-3 and xMn@C-T hybrids under 1.2 V; (b) deionization–regeneration profile and (c) deionization–regeneration cycles of the 15Mn@C-400 electrode. Reproduced with permission from ref. 87. | ||
Bryan et al. reported four tunnel crystal structures of manganese oxide nanowires as faradaic electrodes.88 Two of them are square tunnel structured MnO2 (α-MnO2 and todorokite-MnO2), and others have ordered and disordered unions of structural tunnels, which had been shown in SEM and cross-sectional HAADF-STEM images (Fig. 6). It was found that larger tunnels did a great favor to the removal of larger hydrated radii cations compared with the smaller ones. And the surface redox reactions as well as the intercalation of ions to the structural tunnels may be the main reason of ion removal.
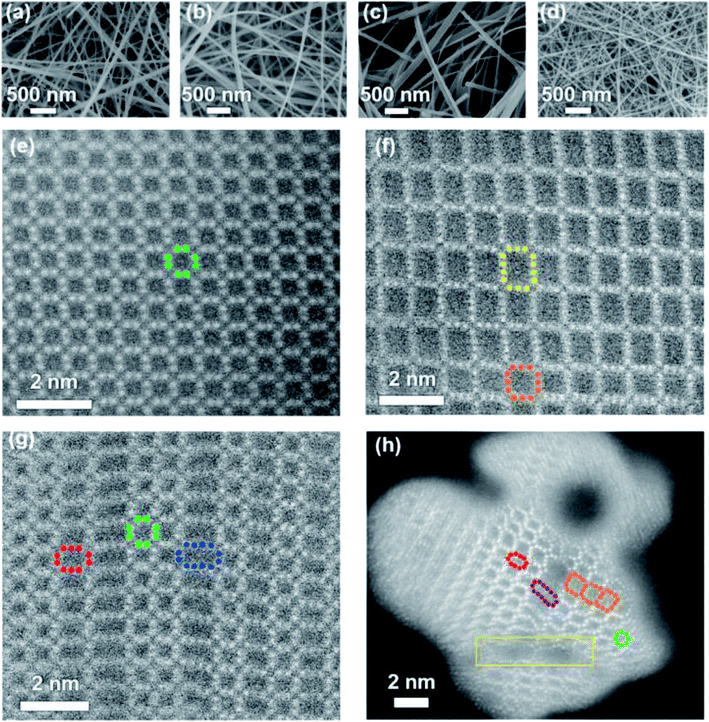 | ||
| Fig. 6 SEM (a–d) and cross-sectional HAADF-STEM (e–h) images of α-MnO2, Tod-MnO2, 2 × n-MnO2, and hybrid-MnO2: (a and e) α-MnO2, (b and f) Tod-MnO2, (c and g) 2 × n-MnO2, and (d and h) hybrid MnO2. Reproduced with permission from ref. 88. | ||
In the study of Steven and Roland,89 electroless deposition (ED) and cyclic voltammetry (CV) were different methods to modify the electrodes and fabricated the MnO2 coated ones. Both techniques increased the electrode capacitance, however, only ED electrodes can improve the desalination performance. MnO2 mass deposition contributed dispersedly and fully in ED modified aerogel and while creating a discrete crust in CV electrodes. Thanks to this, a great specific charge capacitance (77.6 F g−1) was exhibited in a MnO2/AC composite electrode and storage capacity in thin-film sodium manganese oxide (NMO) was increased to 170 times higher than the uncoated electrodes.90 And according to the report of Yang et al.,91 Polystyrene Sodium Sulfonate (PSS) can facilitated the dispersion in composite electrodes and enhance the growth of MnO2, resulting in a great salt removal efficiency (96.8%) and a high ion adsorption capacity (80.4 μmoL g−1) in MnO2/PSS/CNTs electrodes.
Additionally, Liu et al. used polytetrafluoroethylene emulsion to mix AC particles and nano ZnO to prepare ZnO/AC composite electrodes.98 It was reported that −ZnO/AC‖AC capacitor showed a very stable CDI performance with significant electrosorption capacity of 9.4 mg g−1 and charge efficiency of 80.5%, while +ZnO/AC‖AC capacitor showed declining desalination behavior after several charge–discharge cycles, as evident from Fig. 7. The different CDI properties on negative and positive electrodes were due to the changes of pH during charging process, which led to zeta potential variation of ZnO.
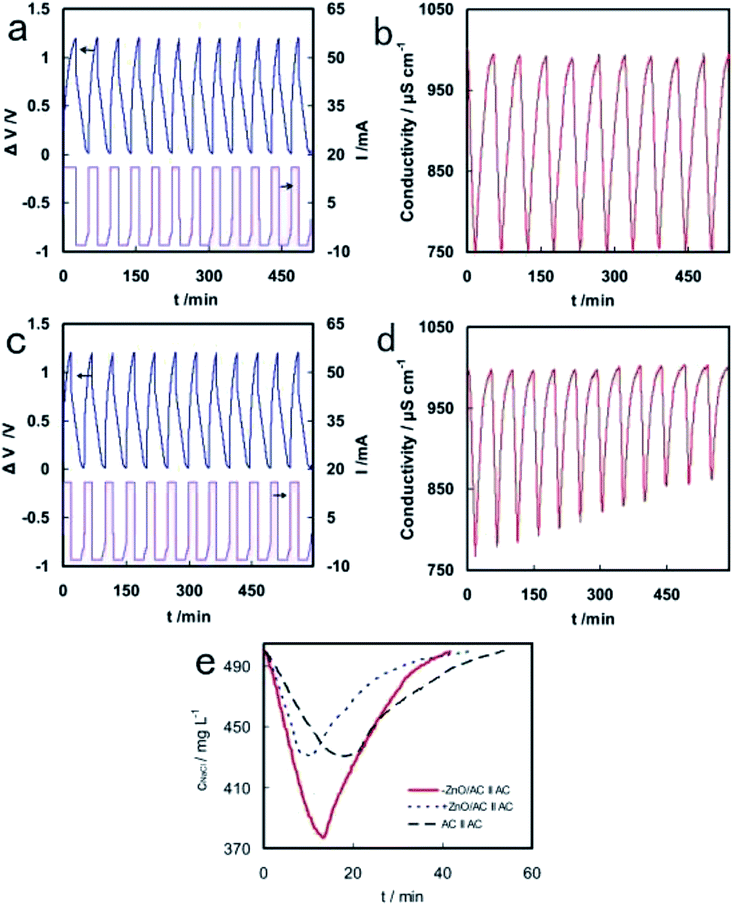 | ||
| Fig. 7 Cell voltage/current curves (a and c) and conductivity variation (b and d) with time on −ZnO/AC‖AC (a and b) and +ZnO/AC‖AC (c and d) in the continuous charge–discharge process. (e) Conductivity variations of −ZnO/AC‖AC, +ZnO/AC‖AC and AC‖AC in CDI experiments. Reproduced with permission from ref. 98. | ||
Additionally, Deen et al.100 reported graphene/tin dioxide nanoparticles composites (Gr/SnO2) fabricated by microwave irradiation. The incorporation of SnO2 led to a high specific capacitance of 323 F g−1, and prevented graphene sheets from aggregating, which brought about a significant increase in surface area. In CDI experiments, electrode with 15 wt% SnO2 showed excellent cycling stability as well as considerable desalination efficiency of 83%.
Fe3O4 is a chemically stable and environmentally friendly material, with high pseudocapacitance. Fe3O4/RGO nanocomposites synthesized via hydrothermal method were reported by Li et al.101 Cyclic voltammetry curve of this composite revealed a typical electrical double layer capacitive behavior and an enhanced specific capacitance, probably attributed to the surface hydroxylation of Fe3O4. The maximum electrosorption capacity of Fe3O4/RGO nanocomposites calculated by Langmuir model was 8.33 mg g−1, which was twice as high as that of RGO (4.63 mg g−1).
3.3. Chemical treatment of carbon material
Acidic oxidizing agents such as concentrated nitric acid and concentrated sulfuric acid have been extensively used for the acid treatment of carbon materials. Huang et al.103 reported nitric acid-modified AC electrodes for capacitive deionization. Attributed to improved capacitance of AC caused by the increase of oxygen-containing functional surface groups, CDI experiments showed that desalination efficiency had increased by 15%. To prepare sulfuric acid functionalized AC(FAC), Niu et al.104 modified activated carbon with 98% sulfuric acid via hydrothermal method. Compared to pure AC, FAC exhibited an improved electrosorption capacity of 3.54 mg g−1. Wu et al.105 treated activated carbon fiber with 6 M nitric acid to reduce co-ion expulsion effect. Electrosorption capacity of this ACF-HNO3 could reach up to 12.8 mg g−1. In this work, carboxylic groups were grafted onto the ACF surface, which positively shifted the potential of zero charge (Epzc) of ACF. It could be observed that ACF-HNO3 is unable to adsorb many anions even at a positive voltage, indicating its excellent cation selectivity. Thus, the co-ion expulsion effect which decreases adsorption capacity was limited.
Though traditional oxidation treatment of carbon materials can promote electrosorption capacity, it is destructive to the surface area and porous structure. In the research of Maroto-Valer et al.,106 nitric acid treatment of activated carbon was reported to decrease the total pore volume and BET surface area by 8.8% and 9.2% respectively. A promising method to improve this status is oxygen plasma treatment, which can oxidize the carbon external surface without modifying the internal structure effectively. Talemi et al.107 reported oxygen plasma modified carbon electrodes. In their study, electrosorption capacity was enhanced but there was no obvious specific surface area change. Also, the SEM images of electrodes at different levels of magnification shows no distinct variation on the surface after oxygen plasma treatment.
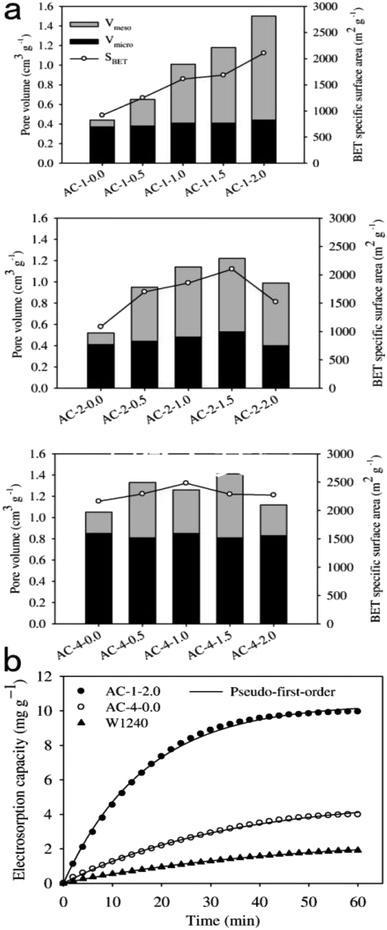 | ||
| Fig. 8 (a) Pore characterization of the activated carbons prepared with different impregnation ratios of KOH. (b) The electrosorption kinetics of AC-1-2.0, AC-4-0.0, and W1240 electrodes. Reproduced with permission from ref. 108. | ||
Dehkhoda et al.109 revealed a novel way to tailor porous activated biochar. By controlling drying conditions and carbonization temperature of KOH activation, biochars with predominantly mesoporous, microporous or combined porous structure could be obtained. Electrochemical analysis showed that electrodes with majorly microporous structure had high total capacitance (222–245 F g−1) but large electrode resistance, while mesopore-dominated electrodes (>90% of total pore volume) possessed ideal capacitive behavior. CDI experiments suggest different ranges of desalination applications, with small hydrated ions (Na+, K+, Cl−, Mg2+, Cu2+) utilizing microporous electrodes and larger ions (Cr3+, Zn2+, Cd3+) utilizing mesoporous electrodes.
Besides optimizing pore structures, KOH also plays an important role in introducing hydrophilic functional groups. Ji et al.110 reported KOH-modified activated carbons with higher surface area and increased surface hydrophilic functional groups, indicating the potential of KOH for improving wettability. Wang et al.111 modified activated carbon with different mass concentrations of KOH solution. The electrode modified with 5% KOH exhibited a maximum specific capacitance of 108.8 F g−1 as well as 23% increase in desalination efficiency comparing to non-modified AC. The contact angle analysis and FTIR spectra image indicated that this promotion resulted from the introduction of hydroxyl functional groups on the electrode surface. Lee et al.112 studied the surface modification of carbon felts by KOH and HNO3. It was observed that KOH increased the BET surface area of the electrode, while HNO3 brought damages on the pore structure. Thus KOH-modified carbon felts displayed higher desalination efficiency of 83.3% compared with HNO3-modified carbon felts (65.4%), although the oxygen-containing functional groups formatted by HNO3 was much greater.
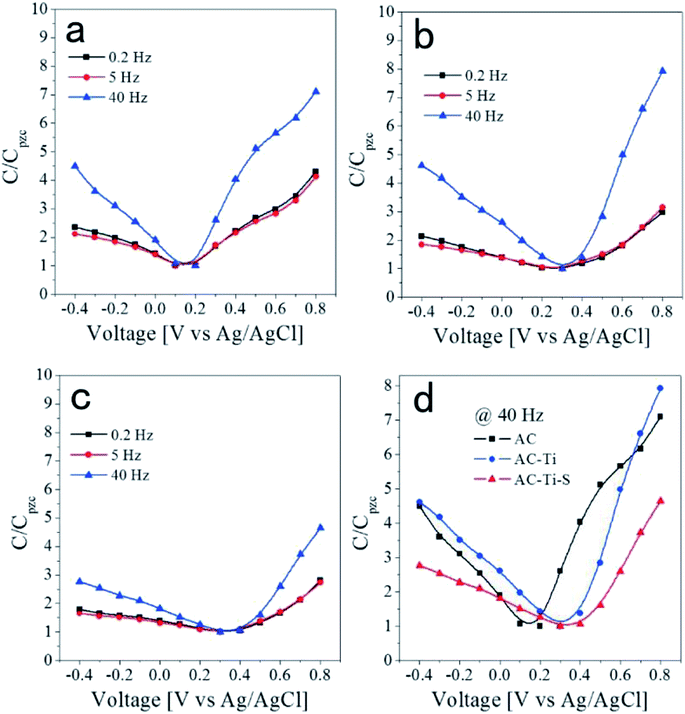 | ||
| Fig. 9 Relative capacitance at various frequencies as a function of the applied voltage for the (a) AC, (b) AC-Ti and (c) AC-Ti-S electrodes. (d) The comparison of the C/CPZC values at 40 Hz. Reproduced with permission from ref. 114. | ||
Additionally, working voltage of CDI is dependent on the potential difference between the Epzc of cathode and anode. More negative Epzc of the cathode and more positive Epzc of the anode are required to enlarge the potential difference. Frequent oxygen-containing functional groups are negatively charged, which can positively shift the Epzc. Therefore, to further enlarge the potential difference, positively charged groups like –NH3+ are of great importance. Gao et al.59 synthesized amine-modified microporous carbon cloths. To attach –NH2 functional groups onto the surface, nitric acid-treated carbon cloth was further modified by ethylenediamine. In neutral solution such as NaCl, –NH2 was protonated to –NH3+, resulting in the increase of surface positive charges which led to promoted desalination capacity of 5.3 mg g−1. Additionally, ion selectivity of –NH3+ was utilized to promote CDI performance, reported by Liu et al.115 They synthesized a novel 3D graphene electrode grafted with amine and sulfonic functional groups. Those charged groups could serve as ion-selective functional coatings to reduce the co-ion effect. As a consequence, this electrode displayed an excellent electrosorption capacity of 13.72 mg g−1 and a high charge efficiency of 85%.
3.4. Electrode coated in CDI
Working voltage of CDI depends on the potential difference between the Epzc of cathode and anode. Cation-exchange polymer and metallic oxide account for a large part of materials used for coating the electrodes. The latter has been introduced in the above article, so we will focus on different cation-exchange polymers coating on electrodes in this part.The cation-exchange polymer coated electrode, widely used in membrane capacitive deionization (MCDI), is found more efficient than most of commercial carbon electrodes.116 Among a large amount of efforts over the years to prolong the service life of a CDI device and decrease the interfacial resistance of the membrane capacitive deionization, coating layers of cation-exchange polymer on the electrodes seems perfectly solving the problems.117
Kim and Choi118 reported a method to fabricate polyvinyl alcohol (PVA) and sulfosuccinic acid (SSA) coated electrodes, which was considered as a milestone in the synthetize of a cation-exchange polymer coated electrode. The salt-removal efficiencies were observed to increase from 50–67% to 75–85%. The similar material, reported by Kim et al.,119 was fabricated by blending PVA with SSA and poly(styrene sulfonic acid-co-maleic acid) (PSSA_MA) (PVA/PSSA_MA/SSA), in which the removal efficiency of these electrodes were different due to disparate crosslinking synthesis temperatures and SSA contents. The PVA/SSA coating showed high adhesion to the electrodes, and the interfacial resistance of membrane capacitive deionization was much lower than the commercial ones, so the removal efficiency increased to 88% in a mixed solution with 300 ppm. According to the images of the surfaces and cross-sections of the electrode and ion exchange polymer-coated electrodes, the carbon electrode surfaces were better coated with both cation and anion exchange polymers.
A new composite, reported by Yan et al., was fabricated in situ polymerization with polymer polyaniline and activated carbon. The combination of materials provided a considerable improvement of conductivity, in the meantime decreasing the number of micropores. And the promotion came from the conducting chains which linked microactivated carbon granules together and blocked a large amount of micropores.
Liu et al. reported m-MCDI electrodes fabricated by leading the anion exchange polymer (dimethyl diallyl ammonium chloride) and cation exchange (polyethyleneimine) polymer into CNT electrodes. What illustrated detailly in the article was that due to the reduction of co-ion expulsion effect while introducing ion exchange polymers and enhancing adhesion between ion exchange polymers and electrodes, it exhibited a relatively high NaCl removal of 93%.120 And the “co-in” effect could also be reduced by coating the sulfonated poly(phenylene oxide) and aminated polysulfone on the surface of traditional carbon electrodes.
With a new electrode, a salt removal efficiency of nearly 100% was obtained, which was illustrated. This electrode was fabricated with synthesized sulfonated poly(phenylene oxide) (SPPO) and aminated polysulfone (APSf), which realized a leap of the performance of membrane capacitive deionization (MCDI). Thanks to its coatings of sufficient thickness at 4.89 μm for SPPO and 5.16 μm for APSf, adequate anchoring of the polymers made sure there wasn't any delamination.121
Moreover, the ratio of selectivity to the degree of resistance enhancement was found to have positive correlation with the degree of promotion in MCDI performance.122 The negative electrode was coated with Ca-alginate in MCDI for hardness control and showed great ion removal efficient for hardness species like calcium ions, which were 44% more than that of the commercial CDI, as shown in Fig. 10.
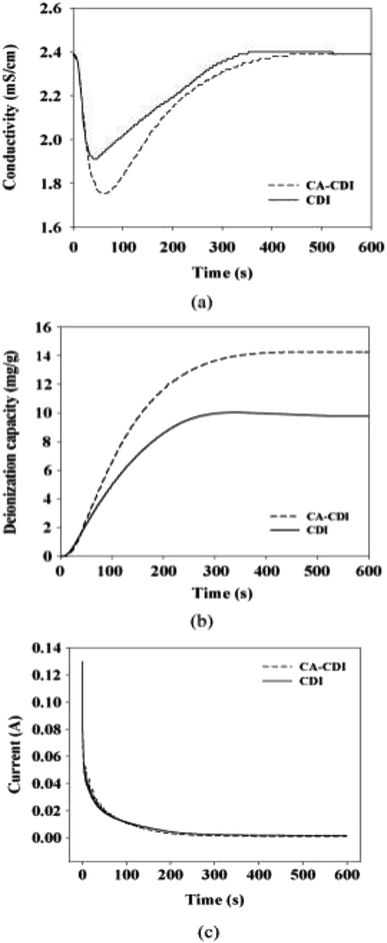 | ||
| Fig. 10 (a) Conductivity profile, (b) deionization capacity and (c) consumed current of Ca-alginate coated CDI (CA-CDI) compared with the control CDI with time. Reproduced with permission from ref. 122. | ||
In recent years, CNTs has been widely used in electrode coated for its special property. Wang et al. synthetized carbon nanotube and polypyrrole (PPy) composite electrode with the method of chemical oxidation and used sodium dodecyl benzene sulfonate as the dopant. Their experiment showed the PPy/CNT composites enhanced the capacitance of electrodes three times than traditional carbon nanotubes and considerably improved their adsorption capacity. Compared to CNT, the TEM image of PPy/CNT composites indicated that PPy/CNT composites were nanotube form with the CNT wrapped by the uniform PPy layer, as shown in Fig. 11.123 Mechanism of the PPy/CNT's satisfying performance was that the uniform and loose PPy layer resulted in the lower charging resistance of the coated electrode than that of commercial CNTs.124
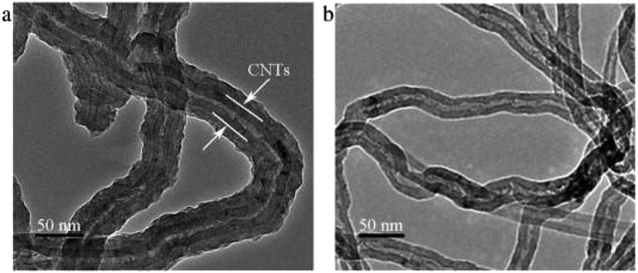 | ||
| Fig. 11 SEM images of (a) PPy/CNT composites and (b) CNT. Reproduced with permission from ref. 123. | ||
Besides the cation-exchange polymer coated electrodes, electrodes coated with other materials were also studied by many researchers. Yoon et al. tested the Ag coated hybrid CDI system.125 The enhanced specific capacity uniting the capacitance in AC electrode with the Ag mediated charge transfer reaction, leading to a significant improvement – the charge efficiency (116%), the CDI deionization capacity (188%) and rate (139%).
4. Summary
As a novel water treatment technology, capacitive deionization has drawn wide attention for the advantages of lower energy consumption and cost, higher efficiency and better regeneration. Selection and modification of electrode materials is one of the most essential factors affecting desalination performance, which has become the focus of CDI research in recent years. In this article, we summarize the modification methods of carbon materials including heteroatom doping, metal oxide modification, chemical treatment and surface coating, and analyze their mechanism for enhancing CDI performance. The electrochemical and electrosorption performances of various carbon electrodes modified by different methods are summarized in Table 2.| Electrode | Specific capacitance (F g−1)/scan rate (mV s−1) | Surface area (m2 g−1) | Voltage (V) | Solution (NaCl mg L−1) | Electrosorption capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|---|
| N-PHCS | 152/5 | 512 | 1.4 | 500 | 12.95 | 51 |
| NCPC-900 | 199.0/1 | 1357 | 1.2 | 100 | 11.98 | 52 |
| NPCS | 290.74/1 | 1640.5 | 1.2 | 1000 | 14.91 | 53 |
| NGCP | 307.4/10 | 1134 | 1.4 | 500 | 17.73 | 54 |
| NPC | 165.2/6 | 1036.2 | 1.28 | 40 | 15.8 | 57 |
| N-SC | — | 1421 | 1.1 | 250 | 5.3 | 59 |
| asym-QC-3DAPGr | 98.9/5 | 2680 | 1.4 | 300 | 13.83 | 60 |
| NGS | 286.86/5 | 526.7 | 1.2 | 500 | 13.83 | 61 |
| NDC-Cs-900 | — | 2830 | 1.2 | — | 15.0 | 66 |
| P-CNFA | 295.1/5 | 728.2 | 1.2 | 1000 | 16.20 | 67 |
| N, P co-doped 3D hierarchical carbon | 221/5 | 451 | 1.2 | 500 | 26.8 | 69 |
| TiO2/AC | 107/2 | 2300 | 1.2 | 585 | 17 | 77 |
| TiO2/ACF | 182/5 | 886.1 | 1.2 | 500 | 10.6 | 79 |
| TiO2/CNTs | 122/1 | — | 1.2 | 500 | 4.3 | 80 |
| TiO2/AC | 94/5 | 1749 | 1.2 | 293 | 17.4 | 84 |
| MnO2 nanoparticles decorated ordered mesoporous carbon | 83.9/10 | 1092 | 1.2 | — | 5.4 | 85 |
| MnO2/nanowires | — | — | 1.2 | 878 | 27.8 | 88 |
| MnO2/PSS/CNTs | 77.2/10 | 191.8 | 1.2 | 128 | — | 91 |
| ZnO/AC | 66/2 | — | 1.2 | 500 | 9.4 | 98 |
| SiO2 and γ-Al2O3 coated electrode | — | 35.3 | 1.2 | 111 (CaCl2) | — | 99 |
| Gr/SnO2 | 323/5 | — | 1.4 | — | 1.49 | 100 |
| Fe3O4/RGO | — | 150.8 | 1.2 | 500 | 8.33 | 101 |
| FAC | 232/1 | 1244.7 | 1.2 | — | 3.54 | 104 |
| HNO3/ACF | — | 1545 | 1.2 | 500 | 12.8 | 105 |
| KOH/AC | 181.8/5 | 2105 | 1.0 | 29 | 9.72 | 108 |
| SG-CNF | 117/2 | — | 1.2 | 400 | 9.54 | 113 |
| AC-Ti-S | 238/20 | — | 1.0 | 500 | 7.6 | 114 |
| 3DNGR-3DSGR | 108.3/10 | 133 | 1.4 | 500 | 13.72 | 115 |
| PVA and SSA coated carbon | 97.4/5 | 1260 | 1.5 | 200 | — | 118 |
| Dimethyl diallyl ammonium chloride and polyethyleneimine coated carbon | 51.51/5 | — | 1.2 | 500 | 9.5 | 120 |
| Ca-alginate coated carbon | — | — | 1.2 | 1110 | 14.2 | 122 |
| PPy coated CNT | 180.61/5 | — | 1.4 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
43.99 | 124 |
| Ag coated carbon | 110.2/2 | — | 0.7 | 584.4 | 23.2 | 125 |
Generally speaking, the purpose of modification is to improve the properties of carbon electrodes, such as the porous structure, specific surface area, wettability and conductivity. Specifically, element doping is mainly used to optimize the pore structure and increase the specific surface area of the carbon materials. Nitrogen doping can also introduce nitrogen-containing functional groups to enhance hydrophilicity and conductivity. Metal oxide modification, represented by TiO2, can considerably improve the wettability of carbon electrode by introducing hydroxyl groups on the surface. Additionally, the combination of carbon and metal oxides is able to increase the specific capacitance for the reason of synergistic effects. Acid treatment generally leads to significant increase in oxygen-containing functional surface groups. Apart from improving wettability and hydrophilicity, these negatively charged functional groups can positively shift the Epzc of carbon materials, thus reducing the co-ion expulsion effect and promoting electrosorption capacity. Alkali treatment is used to tailor porous carbon materials with different ratios of mesoporosity, due to the chemical activation function of alkali. Besides, cation-exchange polymer coating layers can reduce the interfacial resistance of MCDI which leads to the enhancement of electrochemical performance.
Although these methods can lead to an increase in CDI performance, there still exist some shortcomings. Element doping usually needs high temperature, but the specific temperatures are unpredictable thus requiring large numbers of experiment. In addition, acid treatment is destructive to the surface area and porous structure of carbon electrode due to the oxidation process. Talemi et al. proposed a solution of oxygen plasma modification, but the complicated operations limited its further promotion.107 Moreover, the introduction of surface functional groups can accelerate the faradaic reactions which reduce the charging efficiency. By taking such issues, CDI performance will be further improved.
5. Prospects
As a new type of desalination technology developed rapidly in the middle and late 19th century, CDI has attracted the attention of scholars with its high economical value and perfect stability. However, there are still several aspects that limit the development of CDI in practical applications. The biggest problem remains that the electrosorption performance of conventional electrode materials limits the upper limit of CDI material adsorption. High specific area materials such as activated carbon, graphene, and carbon aerogel have good pore structure and electrochemical properties, but for decades, the space for increasing the unit adsorption capacity is still very limited. This is the basic reason for various modification methods proposed to increase the unit adsorption amount. In fact, in addition to the various modification methods discussed in this paper, there is another new idea that can effectively improve the adsorption efficiency of CDI units. That is to replace the traditional electrosorption theory with a reversible chemical reaction. This theory is based on the fact that the Faraday capacitance is much larger than the double layer capacity of the material. Tantalum capacitance refers to the underpotential deposition of electroactive materials in the two-dimensional or quasi-two-dimensional space on the surface or in the bulk phase, resulting in highly reversible chemisorption, desorption or redox reactions, which are related to the charge potential of the electrode capacitance. It can be produced not only on the surface of the electrode but also inside the entire electrode, so its development potential is enormous. For example, a Prussian blue analogue has recently been proposed as an electrode material for high-efficiency capacitive deionization. Its unique open-frame structure of large ion channels makes it an excellent material for Na+ intercalation and deintercalation. It is reported that the unit adsorption capacity can be up to 59.9 mg g−1, this result is difficult to achieve with traditional carbon-based materials, and it indicates that this may be the key to CDI's future use in actual water desalination.Another major factor limiting CDI to the theoretical level is the general adsorption of various types of ions. The simple electrochemical principle of CDI indicates that the highly charged ions with appropriate pore size are more easily absorbed, and the low-charge materials whose pore size does not conform to the pore structure of the material are not easily absorbed. In other words, the selective adsorption capacity of CDI is weak. This is also a major technical vacancy in the field of CDI. Specific adsorption is of great significance for the recycling of various ions and the enrichment of heavy metals. Related studies have shown that from the perspective of pore structure, the unit adsorption capacity of traditional carbon-based materials can be effectively improved, while positive and negative charge modification can achieve the effect of ion selection to some extent. Once a breakthrough is made, it will definitely be a major advancement in the application of CDI to actual water treatment.
In conclusion, capacitive deionization is a promising technology. Through continuous research, CDI will play an important role in sewage disposal and seawater desalination in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National College Students' Innovation and Entrepreneurship Training Program (No. 201810055107) and National Key R&D Program of China (No. 2016YFC 0400704).References
- Z. Amor, B. Bariou, N. Mameri, M. Taky, S. Nicolas and A. Elmidaoui, Desalination, 2001, 133, 215–223 CrossRef CAS.
- Y. Tokui, H. Moriguchi and Y. Nishi, Desalination, 2014, 351, 145–150 CrossRef CAS.
- K. P. Lee, T. C. Arnot and D. Mattia, J. Membr. Sci., 2011, 370, 1–22 CrossRef CAS.
- H. Sayyaadi and A. Saffari, Appl. Energy, 2010, 87, 1122–1133 CrossRef.
- X. X. Cao, X. Huang, P. Liang, K. Xiao, Y. J. Zhou, X. Y. Zhang and B. E. Logan, Environ. Sci. Technol., 2009, 43, 7148–7152 CrossRef CAS PubMed.
- S. J. Kim, S. H. Ko, K. H. Kang and J. Han, Nat. Nanotechnol., 2010, 5, 297–301 CrossRef CAS PubMed.
- T. J. Welgemoed and C. F. Schutte, Desalination, 2005, 183, 327–340 CrossRef CAS.
- S. Porada, R. Zhao, A. van der Wal, V. Presser and P. M. Biesheuvel, Prog. Mater. Sci., 2013, 58, 1388–1442 CrossRef CAS.
- H. B. Li, L. Zou, L. K. Pan and Z. Sun, Sep. Purif. Technol., 2010, 75, 8–14 CrossRef CAS.
- S. J. Seo, H. Jeon, J. K. Lee, G. Y. Kim, D. Park, H. Nojima, J. Lee and S. H. Moon, Water Res., 2010, 44, 2267–2275 CrossRef CAS PubMed.
- F. A. AlMarzooqi, A. A. Al Ghaferi, I. Saadat and N. Hilal, Desalination, 2014, 342, 3–15 CrossRef CAS.
- S. M. Jung, J. H. Choi and J. H. Kim, Sep. Purif. Technol., 2012, 98, 31–35 CrossRef CAS.
- R. Zhao, P. M. Biesheuvel, H. Miedema, H. Bruning and A. van der Wal, J. Phys. Chem. Lett., 2010, 1, 205–210 CrossRef CAS.
- L. L. Yuan, X. F. Yang, P. Liang, L. Wang, Z. H. Huang, J. C. Wei and X. Huang, Bioresour. Technol., 2012, 110, 735–738 CrossRef CAS PubMed.
- C. J. Feng, C. H. Hou, S. H. Chen and C. P. Yu, Chemosphere, 2013, 91, 623–628 CrossRef CAS PubMed.
- M. W. Ryoo and G. Seo, Water Res., 2003, 37, 1527–1534 CrossRef CAS PubMed.
- H. J. Oh, J. H. Lee, H. J. Ahn, Y. Jeong, Y. J. Kim and C. S. Chi, Thin Solid Films, 2006, 515, 220–225 CrossRef CAS.
- G. Wang, B. Q. Qian, Q. Dong, J. Y. Yang, Z. B. Zhao and J. S. Qiu, Sep. Purif. Technol., 2013, 103, 216–221 CrossRef CAS.
- G. X. Zhao, J. X. Li, X. M. Ren, C. L. Chen and X. K. Wang, Environ. Sci. Technol., 2011, 45, 10454–10462 CrossRef CAS PubMed.
- Z. Peng, D. S. Zhang, L. Y. Shi and T. T. Yan, J. Mater. Chem., 2012, 22, 6603–6612 RSC.
- H. Wang, L. Y. Shi, T. T. Yan, J. P. Zhang, Q. D. Zhong and D. S. Zhang, J. Mater. Chem. A, 2014, 2, 4739–4750 RSC.
- P. Xu, J. E. Drewes, D. Heil and G. Wang, Water Res., 2008, 42, 2605–2617 CrossRef CAS PubMed.
- H. H. Jung, S. W. Hwang, S. H. Hyun, L. Kang-Ho and G. T. Kim, Desalination, 2007, 216, 377–385 CrossRef CAS.
- L. Wang, M. Wang, Z. H. Huang, T. X. Cui, X. C. Gui, F. Y. Kang, K. L. Wang and D. H. Wu, J. Mater. Chem., 2011, 21, 18295–18299 RSC.
- C. J. Yan, L. Zou and R. Short, Desalination, 2012, 290, 125–129 CrossRef CAS.
- C. Y. Nie, L. K. Pan, H. B. Li, T. Q. Chen, T. Lu and Z. Sun, J. Electroanal. Chem., 2012, 666, 85–88 CrossRef CAS.
- H. B. Li, L. D. Zou, L. K. Pan and Z. Sun, Environ. Sci. Technol., 2010, 44, 8692–8697 CrossRef CAS PubMed.
- H. B. Li, L. K. Pan, T. Lu, Y. K. Zhan, C. Y. Nie and Z. Sun, J. Electroanal. Chem., 2011, 653, 40–44 CrossRef CAS.
- Y. Liu, C. Y. Nie, X. J. Liu, X. T. Xu, Z. Sun and L. K. Pan, RSC Adv., 2015, 5, 15205–15225 RSC.
- J. Oladunni, J. H. Zain, A. Hai, F. Banat, G. Bharath and E. Alhseinat, Sep. Purif. Technol., 2018, 207, 291–320 CrossRef CAS.
- P. Y. Liu, T. T. Yan, L. Y. Shi, H. S. Park, X. C. Chen, Z. G. Zhao and D. S. Zhang, J. Mater. Chem. A, 2017, 5, 13907–13943 RSC.
- B. P. Jia and W. Zhang, Nanoscale Res. Lett., 2016, 11, 64 CrossRef PubMed.
- P. J. Lu, H. C. Lin, W. T. Yu and J. M. Chern, J. Taiwan Inst. Chem. Eng., 2011, 42, 305–311 CrossRef CAS.
- K. Y. Foo and B. H. Hameed, J. Hazard. Mater., 2009, 170, 552–559 CrossRef CAS PubMed.
- C. H. Hou, J. F. Huang, H. R. Lin and B. Y. Wang, J. Taiwan Inst. Chem. Eng., 2012, 43, 473–479 CrossRef CAS.
- K. K. Park, J. B. Lee, P. Y. Park, S. W. Yoon, J. S. Moon, H. M. Eum and C. W. Lee, Desalination, 2007, 206, 86–91 CrossRef CAS.
- B. H. Kim, K. S. Yang, Y. A. Kim, Y. J. Kim, B. An and K. Oshida, J. Power Sources, 2011, 196, 10496–10501 CrossRef CAS.
- Y. Z. Chen, M. B. Yue, Z. H. Huang and F. Y. Kang, Chem. Eng. J., 2014, 252, 30–37 CrossRef CAS.
- S. S. Wang, Y. L. Xu, M. F. Yan, Z. Z. Zhai, B. Ren, L. H. Zhang and Z. F. Liu, J. Electroanal. Chem., 2018, 809, 111–116 CrossRef CAS.
- D. S. Zhang, L. Y. Shi, J. H. Fang, K. Dai and X. K. Li, Mater. Chem. Phys., 2006, 97, 415–419 CrossRef CAS.
- K. Dai, L. Y. Shi, J. H. Fang, D. S. Zhang and B. K. Yu, Mater. Lett., 2005, 59, 1989–1992 CrossRef CAS.
- A. H. Jadhav, X. T. Mai, F. A. Ofori and H. Kim, Chem. Eng. J., 2015, 259, 348–356 CrossRef CAS.
- Y. Bai, Z. H. Huang, X. L. Yu and F. Y. Kang, Colloids Surf., A, 2014, 444, 153–158 CrossRef CAS.
- L. D. Zou, L. X. Li, H. H. Song and G. Morris, Water Res., 2008, 42, 2340–2348 CrossRef CAS PubMed.
- L. X. Li, L. D. Zou, H. H. Song and G. Morris, Carbon, 2009, 47, 775–781 CrossRef CAS.
- J. N. Coleman, Adv. Funct. Mater., 2009, 19, 3680–3695 CrossRef CAS.
- K. A. Kurak and A. B. Anderson, J. Phys. Chem. C, 2009, 113, 6730–6734 CrossRef CAS.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. M. Zhang, Z. H. Zhu, S. C. Smith, M. Jaroniec, G. Q. Lu and S. Z. Qiao, J. Am. Chem. Soc., 2011, 133, 20116–20119 CrossRef CAS PubMed.
- M. Sevilla, P. Valle-Vigon and A. B. Fuertes, Adv. Funct. Mater., 2011, 21, 2781–2787 CrossRef CAS.
- N. Alam and R. Mokaya, Energy Environ. Sci., 2010, 3, 1773–1781 RSC.
- S. S. Zhao, T. T. Yan, H. Wang, G. R. Chen, L. Huang, J. P. Zhang, L. Y. Shi and D. S. Zhang, Appl. Surf. Sci., 2016, 369, 460–469 CrossRef CAS.
- Y. Li, Y. X. Liu, J. M. Shen, J. W. Qi, J. S. Li, X. Y. Sun, J. Y. Shen, W. Q. Han and L. J. Wang, Desalination, 2018, 430, 45–55 CrossRef CAS.
- Y. Liu, T. Q. Chen, T. Lu, Z. Sun, D. H. C. Chua and L. K. Pan, Electrochim. Acta, 2015, 158, 403–409 CrossRef CAS.
- T. Gao, Y. J. Du and H. B. Li, Sep. Purif. Technol., 2019, 211, 233–241 CrossRef CAS.
- X. Y. Duan, W. Liu and L. M. Chang, J. Taiwan Inst. Chem. Eng., 2016, 62, 132–139 CrossRef CAS.
- N. L. Liu, S. Dutta, R. R. Salunkhe, T. Ahamad, S. M. Alshehri, Y. Yamauchi, C. H. Hou and K. C. W. Wu, Sci. Rep., 2016, 6, 28847 CrossRef CAS PubMed.
- C. J. Zhao, G. Q. Liu, N. Sun, X. Zhang, G. Z. Wang, Y. X. Zhang, H. M. Zhang and H. J. Zhao, Chem. Eng. J., 2018, 334, 1270–1280 CrossRef CAS.
- J. Yang, L. D. Zou and N. R. Choudhury, Electrochim. Acta, 2013, 91, 11–19 CrossRef CAS.
- X. Gao, A. Omosebi, J. Landon and K. L. Liu, Environ. Sci. Technol., 2015, 49, 10920–10926 CrossRef CAS PubMed.
- A. G. El-Deen, R. M. Boom, H. Y. Kim, H. W. Duan, M. B. Chan-Park and J. H. Choi, ACS Appl. Mater. Interfaces, 2016, 8, 25313–25325 CrossRef CAS PubMed.
- X. T. Xu, Z. Sun, D. H. C. Chua and L. K. Pan, Sci. Rep., 2015, 5, 8458 CrossRef CAS PubMed.
- A. Amiri, G. Ahmadi, M. Shanbedi, M. Savari, S. N. Kazi and B. T. Chew, Sci. Rep., 2015, 5, 17503 CrossRef CAS PubMed.
- L. J. Liu, X. R. Guo, R. Tallon, X. K. Huang and J. H. Chen, Chem. Commun., 2017, 53, 881–884 RSC.
- M. Zhou, F. Pu, H. Chen, Z. Wang, H. Y. Zhang and S. Y. Guan, New J. Chem., 2013, 37, 4148–4155 RSC.
- X. L. Ma, X. Y. Song, G. Q. Ning, L. Q. Hou, Y. F. Kan, Z. H. Xiao, W. Li, G. X. Ma, J. S. Gao and Y. F. Li, Ind. Eng. Chem. Res., 2017, 56, 9524–9532 CrossRef CAS.
- S. Porada, F. Schipper, M. Aslan, M. Antonietti, V. Presser and T. P. Fellinger, Chemsuschem, 2015, 8, 1867–1874 CrossRef CAS PubMed.
- Y. J. Li, Y. Liu, M. Wang, X. T. Xu, T. Lu, C. Q. Sun and L. K. Pan, Carbon, 2018, 130, 377–383 CrossRef CAS.
- C. Li, Q. Fu, K. J. Zhao, Y. P. Wang, H. Tang, H. H. Li, H. B. Jiang and L. Chen, Carbon, 2018, 139, 1117–1125 CrossRef CAS.
- J. L. Han, L. Y. Shi, T. T. Yan, J. P. Zhang and D. S. Zhang, Environ. Sci.: Nano, 2018, 5, 2337–2345 RSC.
- J. Zhang, J. H. Fang, J. L. Han, T. T. Yan, L. Y. Shi and D. S. Zhang, J. Mater. Chem. A, 2018, 6, 15245–15252 RSC.
- T. Zhang, H. Zhao, X. X. Huang and G. Wen, Desalination, 2016, 379, 118–125 CrossRef CAS.
- Z. Q. Wang, Y. Wang, D. Y. Ma, S. C. Xu and J. X. Wang, Sep. Purif. Technol., 2018, 192, 15–20 CrossRef CAS.
- D. Y. Ma, Y. Wang, X. Y. Han, S. C. Xu and J. X. Wang, Sep. Purif. Technol., 2018, 201, 167–178 CrossRef CAS.
- Y. M. Cai, Y. Wang, X. Y. Han, L. W. Zhang, S. C. Xu and J. X. Wang, J. Electroanal. Chem., 2016, 768, 72–80 CrossRef CAS.
- Y. P. Zhang, X. W. Sun, L. K. Pan, H. B. Li, Z. Sun, C. P. Sun and B. K. Tay, Solid State Ionics, 2009, 180, 1525–1528 CrossRef CAS.
- M. W. Ryoo, J. H. Kim and G. Seo, J. Colloid Interface Sci., 2003, 264, 414–419 CrossRef CAS PubMed.
- C. Kim, J. Lee, S. Kim and J. Yoon, Desalination, 2014, 342, 70–74 CrossRef CAS.
- L. M. Chang, X. Y. Duan and W. Liu, Desalination, 2011, 270, 285–290 CrossRef CAS.
- D. H. Kang, H. Jo, M. J. Jung, K. L. Kim and Y. S. Lee, Carbon Letters, 2018, 27, 64–71 Search PubMed.
- H. B. Li, Y. L. Ma and R. Niu, Sep. Purif. Technol., 2016, 171, 93–100 CrossRef CAS.
- J. H. Feng, S. Xiong and Y. Wang, Sep. Purif. Technol., 2019, 213, 70–77 CrossRef CAS.
- I. Cohen, E. Avraham, Y. Bouhadana, A. Soffer and D. Aurbach, Electrochim. Acta, 2013, 106, 91–100 CrossRef CAS.
- P. Srimuk, L. Ries, M. Zeiger, S. Fleischmann, N. Jackel, A. Tolosa, B. Kruner, M. Aslan and V. Presser, RSC Adv., 2016, 6, 106081–106089 RSC.
- P. Srimuk, M. Zeiger, N. Jackel, A. Tolosa, B. Kruner, S. Fleischmann, I. Grobelsek, M. Aslan, B. Shvartsev, M. E. Suss and V. Presser, Electrochim. Acta, 2017, 224, 314–328 CrossRef CAS.
- C. X. Zhao, X. Y. Lv, J. S. Li, T. Xie, Y. Y. Qi and W. Chen, J. Electrochem. Soc., 2017, 164, E505–E511 CrossRef CAS.
- J. Yang, L. D. Zou, H. H. Song and Z. P. Hao, Desalination, 2011, 276, 199–206 CrossRef CAS.
- T. T. Wu, G. Wang, S. Y. Wang, F. Zhan, Y. Fu, H. Y. Qiao and J. S. Qiu, Environ. Sci. Technol. Lett., 2018, 5, 98–102 CrossRef CAS.
- B. W. Byles, D. A. Cullen, K. L. More and E. Pomerantseva, Nano Energy, 2018, 44, 476–488 CrossRef CAS.
- S. Hand and R. D. Cusick, Environ. Sci. Technol., 2017, 51, 12027–12034 CrossRef CAS PubMed.
- J. M. Wallas, M. J. Young, H. X. Sun and S. M. George, J. Electrochem. Soc., 2018, 165, A2330–A2339 CrossRef CAS.
- J. Yang, L. D. Zou and H. H. Song, Desalination, 2012, 286, 108–114 CrossRef CAS.
- L. Schmidt-Mende and J. L. MacManus-Driscoll, Mater. Today, 2007, 10, 40–48 CrossRef CAS.
- S. Baruah and J. Dutta, Sci. Technol. Adv. Mater., 2009, 013001 CrossRef PubMed.
- M. T. Z. Myint, S. H. Al-Harthi and J. Dutta, Desalination, 2014, 344, 236–242 CrossRef CAS.
- K. Laxman, M. T. Z. Myint, R. Khan, T. Pervez and J. Dutta, Desalination, 2015, 359, 64–70 CrossRef CAS.
- M. T. Z. Myint and J. Dutta, Desalination, 2012, 305, 24–30 CrossRef CAS.
- K. Laxman, M. T. Z. Myint, H. Bourdoucen and J. Dutta, ACS Appl. Mater. Interfaces, 2014, 6, 10113–10120 CrossRef CAS PubMed.
- J. Y. Liu, M. Lu, J. M. Yang, J. Cheng and W. S. Cai, Electrochim. Acta, 2015, 151, 312–318 CrossRef CAS.
- J. J. Wouters, J. J. Lado, M. I. Tejedor-Tejedor, R. Perez-Roa and M. A. Anderson, Electrochim. Acta, 2013, 112, 763–773 CrossRef CAS.
- A. G. El-Deen, N. A. M. Barakat, K. A. Khalil, M. Motlak and H. Y. Kim, Ceram. Int., 2014, 40, 14627–14634 CrossRef CAS.
- H. B. Li, Z. Y. Leong, W. H. Shi, J. Zhang, T. P. Chen and H. Y. Yang, RSC Adv., 2016, 6, 11967–11972 RSC.
- C. Y. Yin, M. K. Aroua and W. M. A. W. Daud, Sep. Purif. Technol., 2007, 52, 403–415 CrossRef CAS.
- W. Huang, Y. M. Zhang, S. X. Bao, R. Cruz and S. X. Song, Desalination, 2014, 340, 67–72 CrossRef CAS.
- R. Niu, H. B. Li, Y. L. Ma, L. J. He and J. Li, Electrochim. Acta, 2015, 176, 755–762 CrossRef CAS.
- T. T. Wu, G. Wang, Q. Dong, B. Q. Qian, Y. L. Meng and J. S. Qiu, Electrochim. Acta, 2015, 176, 426–433 CrossRef CAS.
- M. M. Maroto-Valer, I. Dranca, T. Lupascu and R. Nastas, Carbon, 2004, 42, 2655–2659 CrossRef CAS.
- P. Hojati-Talemi, L. D. Zou, M. Fabretto and R. D. Short, Electrochim. Acta, 2013, 106, 494–499 CrossRef CAS.
- C. L. Yeh, H. C. Hsi, K. C. Li and C. H. Hou, Desalination, 2015, 367, 60–68 CrossRef CAS.
- A. M. Dehkhoda, E. Gyenge and N. Ellis, Biomass Bioenergy, 2016, 87, 107–121 CrossRef CAS.
- Y. B. Ji, T. H. Li, L. Zhu, X. X. Wang and Q. Lin, Appl. Surf. Sci., 2007, 254, 506–512 CrossRef CAS.
- D. Wang, C. Li, J. Guo and T. Li, Desalin. Water Treat., 2016, 57, 17731–17737 CrossRef CAS.
- J. H. Lee, H. J. Ahn, D. Cho, J. I. Youn, Y. J. Kim and H. J. Oh, Carbon Letters, 2015, 16, 93–100 CrossRef.
- B. Q. Qian, G. Wang, Z. Ling, Q. Dong, T. T. Wu, X. Zhang and J. S. Qiu, Adv. Mater. Interfaces, 2015, 2, 1500372 CrossRef.
- B. H. Min, J. H. Choi and K. Y. Jung, Electrochim. Acta, 2018, 270, 543–551 CrossRef CAS.
- P. Y. Liu, H. Wang, T. T. Yan, J. P. Zhang, L. Y. Shi and D. S. Zhang, J. Mater. Chem. A, 2016, 4, 5303–5313 RSC.
- X. Gao, A. Omosebi, N. Holubowitch, A. Liu, K. Ruh, J. Landon and K. Liu, Desalination, 2016, 399, 16–20 CrossRef CAS.
- J. Y. Lee, S. J. Seo, S. H. Yun and S. H. Moon, Water Res., 2011, 45, 5375–5380 CrossRef CAS PubMed.
- J. S. Kim and J. H. Choi, J. Membr. Sci., 2010, 355, 85–90 CrossRef CAS.
- Y. J. Kim and J. H. Choi, Water Res., 2010, 44, 990–996 CrossRef CAS PubMed.
- Y. Liu, L. K. Pan, X. T. Xu, T. Lu, Z. Sun and D. H. C. Chua, Electrochim. Acta, 2014, 130, 619–624 CrossRef CAS.
- J. S. Kim, C. S. Kim, H. S. Shin and J. W. Rhim, Macromol. Res., 2015, 23, 360–366 CrossRef CAS.
- H. Yoon, J. Lee, S. R. Kim, J. Kang, S. Kim, C. Kim and J. Yoon, Desalination, 2016, 392, 46–53 CrossRef CAS.
- Y. Wang, L. W. Zhang, Y. F. Wu, S. C. Xu and J. X. Wang, Desalination, 2014, 354, 62–67 CrossRef CAS.
- Y. Wang, X. Y. Han, R. G. Wang, S. C. Xu and J. X. Wang, Electrochim. Acta, 2015, 182, 81–88 CrossRef CAS.
- H. Yoon, J. Lee, S. Kim and J. Yoon, Desalination, 2017, 422, 42–48 CrossRef CAS.
Footnote |
| † Author Y. Cheng and Z. Hao contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |
