Open Access Article
Open Access ArticleSuture retention strength of P(LLA-CL) tissue-engineered vascular grafts
Xin Menga,
Xiaofeng Wang *a,
Yongchao Jianga,
Bo Zhanga,
Kun Li*b and
Qian Lia
*a,
Yongchao Jianga,
Bo Zhanga,
Kun Li*b and
Qian Lia
aSchool of Mechanics Science and Engineering, National Center for International Research of Micro-nano Molding Technology, Zhengzhou University, Zhengzhou, China. E-mail: xiaofengwang@zzu.edu.cn
bPeople's Hospital of Henan Province, Zhengzhou University, Zhengzhou, China. E-mail: xinmei1331@163.com
First published on 9th July 2019
Abstract
The suture retention strength of artificial vascular grafts is a key mechanical property that affects the function of the grafts. Different from the conventional method of testing the suture retention strength, this study simulated the actual suturing state of vascular scaffolds approximately and investigated the effect of graft type, graft wall thickness, and number of stitches on the suture retention strength of poly(L-lactide-co-ε-caprolactone) P(LLA-CL) vascular grafts. The results showed that the P(LLA-CL) tissue-engineered vascular scaffolds have excellent suture performance and, when the graft wall thickness is greater than 0.24 mm, the suture retention strength will meet the vascular transplantation standard. Under the same conditions, the greater the number of stitches, the more uniform the force of the anastomosis and then the greater the suture retention strength. This experiment provides a more comprehensive and accurate suture retention strength value for the application of P(LLA-CL) tissue engineered vascular grafts, which helps to guide the further optimization of tissue-engineered vascular grafts to meet specific mechanical performance requirements.
1. Background
Because of atherosclerotic disease, there is a widespread demand for vascular grafts around the world. However, before a tissue-engineered vascular graft is transplanted into the human body, many performance evaluations are needed, including test in vivo and in vitro.1,2 This requires that it must match the mechanical properties of the implant site in order to maintain the stability and integrity of the structure within the biomechanical microenvironment of the human body.3 Therefore, the vascular grafts should be able to resist distortion and compression, and have enough tensile strength and shear strength to ensure that the scaffold can withstand the tensile load of the sutures during the grafting operation, without rupture or scattering,4,5 to defend against wear of the edge and tear of the seams and keep the strength in the circumferential direction to withstand arterial blood pressure.6 So a detailed understanding of the interaction between sutures and the surrounding materials is essential to determine the applicability of vascular scaffolds and avoid local damage caused by excessive loading.7The interaction between the suture sites and the surrounding tissue is usually assessed by using specific mechanical tests designed to quantify the suture retention strength of tissue-engineered vascular grafts.7 Suture retention strength is defined as the peak strength when the surgical wire is pulled out the wall of the tube. It is related to the difficulty of the suturing operation and the firmness of the suture connection, and it is an important biomechanical property. However, for the test of this parameter, there is still a lack of detailed global standards.8 For example, when measuring suture retention strength, the American National Standards Institute/Medical Instrument Promotion Association (ANSI/AAMI) standard does not stipulate the material of suture, the suture diameter, the needle size or the type of grafts,3 which limits the mass production of tissue-engineered vascular grafts and seriously hampers the progress of research on artificial blood vessels and international standardization.9 Moreover, most of the current studies on the suture retention strength of tissue-engineered vascular grafts are also carried out using Smitten's experimental method. Smitten cuts the vascular graft longitudinally into rectangular membranes, then passes the suture through the specimen, fixes the two ends of the suture to one end of the clamp and fixes the specimen to the other end of the clamp for uniaxial tensile test to obtain the suture retention strength.10 The method is seriously inconsistent with the stress state of the graft during the operation, which may lead to a severe measurement error in the experimental results.
In this study, we investigated techniques for accurately measuring anastomotic strength, a key surgical parameter, by simulating the actual suturing state of vascular grafts, and systematically analyzed the effect of fiber direction, graft wall thickness, and number of stitches on the suture retention strength of P(LLA-CL) blood vessel grafts.
2. Materials and methods
2.1. Materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (commercial product, Jinan Biotech Co., Ltd.). The intrinsic viscosity coefficient is 3.6 dL g−1, glass transition temperature (Tg) of 51.4 °C, melting point of 125.1 °C, and elastic modulus of 7.98 ± 0.70 MPa. The copolymer combines the advantages of PLLA and PCL with excellent strength, elasticity, biocompatibility and suitable degradation rate, so it has shown good potential for application in the field of tissue-engineered vascular grafts.11–15
1 (commercial product, Jinan Biotech Co., Ltd.). The intrinsic viscosity coefficient is 3.6 dL g−1, glass transition temperature (Tg) of 51.4 °C, melting point of 125.1 °C, and elastic modulus of 7.98 ± 0.70 MPa. The copolymer combines the advantages of PLLA and PCL with excellent strength, elasticity, biocompatibility and suitable degradation rate, so it has shown good potential for application in the field of tissue-engineered vascular grafts.11–15Dichloromethane (DCM, Analytical Pure) and N,N-dimethylformamide (DMF, Analytical Pure) were supplied by Tianjin Kemiou Chemical Co., Ltd. DCM is a good organic solvent with strong volatility. DMF can be miscible with water and most organic solvents, and can conduct electricity.
2.2. Preparation of tissue-engineered vascular graft
At present, there are many methods for preparing tissue-engineered vascular grafts, such as fiber-bonding, solution casting/particle leaching, gas foaming, phase separation, rapid prototyping, and electrospinning. Among them, the vascular grafts obtained by electrospinning have their unique advantages: (1) the structure is composed of stacked nano/micron-sized fibers, so the binding between fibers is weak. When the cells proliferate, the fibers around the pores can be pushed to increase the permeability of the cells; (2) electrospun grafts with small diameter have high porosity and specific surface area, and these grafts can simulate the topology of the extracellular matrix, which is conducive to cell adhesion and growth; (3) it is possible to meet various requirements for morphological and mechanical properties of the artificial blood vessel by controlling the polymer composition and changing the electrospinning parameters. Therefore, electrospinning technology has broad application prospects in the field of tissue engineering vascular grafts.19First, DCM and DMF were mixed in a volume ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
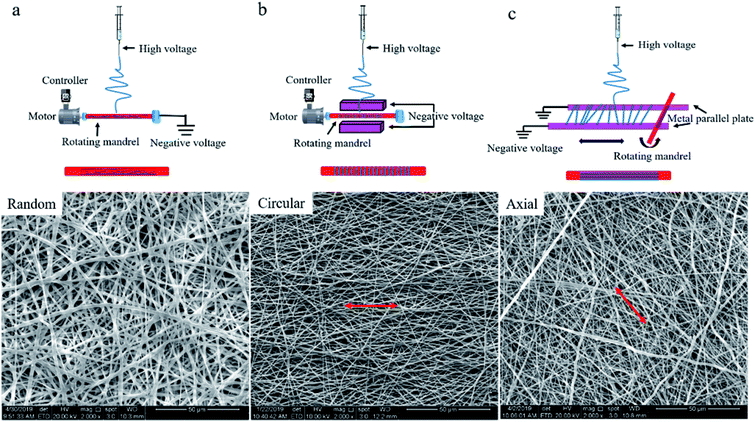
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and P(LLA-CL) was dissolved to prepare a spinning solution with a solution concentration of 0.1 g mL−1. The spinning solution was then filled into a disposable plastic syringe with an 18# spinneret. The spinning voltage was set to 16 kV, the receiving distance between the devices was 18 cm, and the solution injection rate was 2 mL h−1. Finally, three kinds of tissue-engineered blood vessel grafts were prepared by self-made collecting devices, randomly arranged (RA) fiber tubular graft, circumferentially oriented (CO) fiber tubular graft, and axially oriented (AO) fiber tubular graft, respectively shown in Fig. 1, wherein RA fiber tubular grafts were constructed using a metal mandrel at a rotational speed of 800 rpm, as shown in (a). The production speed of the fibers were much higher than the linear velocity of the rotating mandrel surface, resulting in the fibers being randomly stacked. The parallel electrodes with negative charges in (b) caused the fibers to align in the radial direction of the metal mandrel and then fast rotating metal mandrel (2500 rpm) twined it to form a CO fiber tubular graft. The fibers in (c) were first aligned on the surface of the metal parallel plate receiver, and then the metal mandrel which was perpendicular to place by the parallel plate receiver rotated at 30 rpm, and simultaneously reciprocated along the parallel plates at a speed of 26 mm s−1, thereby obtaining an AO fiber tubular graft. The prepared grafts were 40 ± 0.5 mm in length and 2.3 ± 0.1 mm in diameter.
3, and P(LLA-CL) was dissolved to prepare a spinning solution with a solution concentration of 0.1 g mL−1. The spinning solution was then filled into a disposable plastic syringe with an 18# spinneret. The spinning voltage was set to 16 kV, the receiving distance between the devices was 18 cm, and the solution injection rate was 2 mL h−1. Finally, three kinds of tissue-engineered blood vessel grafts were prepared by self-made collecting devices, randomly arranged (RA) fiber tubular graft, circumferentially oriented (CO) fiber tubular graft, and axially oriented (AO) fiber tubular graft, respectively shown in Fig. 1, wherein RA fiber tubular grafts were constructed using a metal mandrel at a rotational speed of 800 rpm, as shown in (a). The production speed of the fibers were much higher than the linear velocity of the rotating mandrel surface, resulting in the fibers being randomly stacked. The parallel electrodes with negative charges in (b) caused the fibers to align in the radial direction of the metal mandrel and then fast rotating metal mandrel (2500 rpm) twined it to form a CO fiber tubular graft. The fibers in (c) were first aligned on the surface of the metal parallel plate receiver, and then the metal mandrel which was perpendicular to place by the parallel plate receiver rotated at 30 rpm, and simultaneously reciprocated along the parallel plates at a speed of 26 mm s−1, thereby obtaining an AO fiber tubular graft. The prepared grafts were 40 ± 0.5 mm in length and 2.3 ± 0.1 mm in diameter.
2.3. Mechanical testing
Common vascular suture methods include interrupted suture, continuous suture, and patch suture. The continuous suturing can reduce the number of tying knots, accelerate the suture speed, and evert the vascular wall, so as to ensure the smoothness of the intima and reduce the possibility of thrombosis. Therefore, continuous suture is applied in most arterial suture surgeries to close the vascular incision. Different stitch distance and margin correspond to different diameter of the graft, thickness of the wall, and blood pressure in the vascular cavity during suturing. The margin is approximately two times the wall thickness of the graft, and the needle distance is approximately two times the margin. However, in addition to large diameter grafts or grafts with particularly thick walls, the general needle distance and margin can be 1–2 mm.16,18 Knots should not be too tight or too loose after suturing, so as to avoid tearing the wall or causing bleeding and affecting blood vessel healing.
Based on the aforementioned requirements, before the suture retention strength test, we first cut each prepared tissue-engineered vessel graft from the middle, then made incisions in the sample at a 45° slant, and pruned the periphery. The graft incision was sutured by continuous suturing with a margin of 1 mm. The needle holes were evenly distributed along the circumference of the tube. Finally, it was knotted three times to avoid silk thread slipping off. The test samples were divided into five groups, including six-needle RA fiber tubular grafts, CO fiber tubular grafts, and AO fiber tubular grafts, as well as five-needle AO fiber tubular grafts and four-needle AO fiber tubular grafts. Five samples were tested in each group.
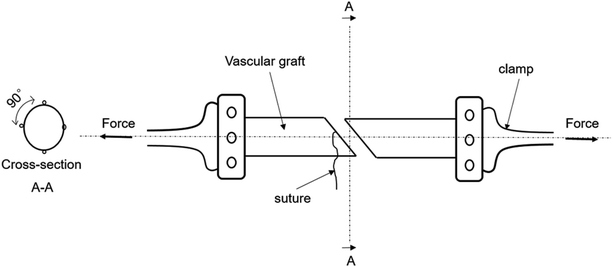
Different from the traditional method for testing the suture retention strength by Smitten et al.,6,21–25 in the experiment, the two vascular grafts were completely sutured together and the grafts were maintained in a tubular shape during uniaxial stretching,23,26–29 as shown in Fig. 2. The burst force is divided by the number of pinholes to obtain the maximum tensile force per needle, which is the suture retention strength of the tissue-engineered vascular graft, and the unit is N per needle.9
3. Results
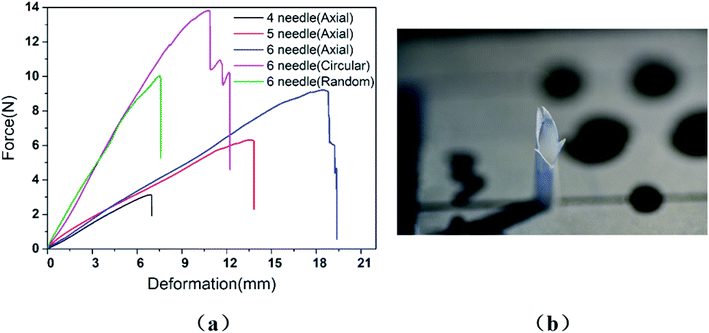
In the study, it was found that when the tensile strength of the graft reached the maximum value, the curve showed a steep downward trend, which indicated that the graft was cut instantly by the suture line, as shown in Fig. 3(a). Fig. 3(b) is the fracture cross-section of the AO fiber tubular graft with four stitches photographed by a Nikon D750 camera in macro mode. It was clear to see that the vascular graft suture had only a sharp incision in the longitudinal direction and there was no tear in the circumferential direction. The fracture cross sections of all specimens were similar, including the test specimens of RA fiber tubular grafts and CO fiber tubular grafts. Therefore, we think that the longitudinal cutting usually caused by a narrow wires.3.1. Graft type
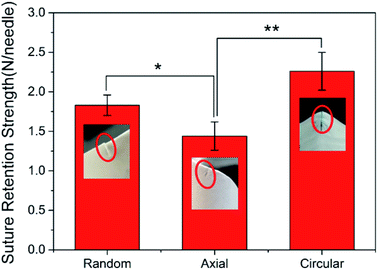
The suture retention strength test was performed on three tissue-engineered grafts with different fiber orientations, which were sutured with six needles to investigate the effect of fibrous direction on the suture retention strength of the vascular graft. The graft wall thickness was 0.26 ± 0.01 mm. The test results was shown in Fig. 4.When the suture was perpendicular to the fiber (the fiber oriented in the circumferential direction), the fiber structure was completely resistant to the movement of the suture, the pinholes on the surface of the graft formed obvious depressions showing a tendency of the circumferential tear, and the suture retention strength was maximized. The random distribution of the fibers to form a net-like microstructure could also hinder the passage of sutures, but not all fibers were completely obstructed, so the suture retention strength was moderate. The fiber direction of the AO fiber tubular graft was consistent with the direction of movement of the suture, so the suture movement was hindered by the least force and the suture retention strength was minimal. The results suggested that the suture retention strength was highly related to the fiber orientation on the wall of vascular grafts.
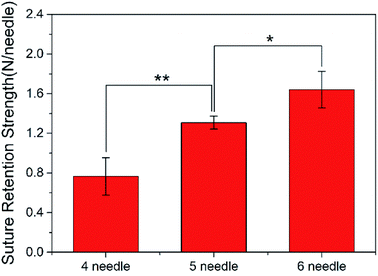
3.2. Stitch numbers
There are many factors that affect the suture retention strength,7,9,23 and stitch distance is also one of the important factors.7 The suture is evenly distributed along the circumferential direction of the graft, making the anastomosis evenly stressed, which is beneficial to the healing of the postoperative wound.According to the clinicians' recommendations, if the suture density is too high, there is a large amount of damage to the wall, and if it is too low, blood leakage is likely to occur. Therefore, in the case of the current tube diameter (2.3 ± 0.1 mm), the suture retention strength was tested by AO fiber tubular grafts with sewing 4 needles, 5 needles, and 6 needles, respectively. The wall thickness of the samples was 0.26 ± 0.01 mm, and the results were shown in Fig. 5.
As the number of sutures increases, the suture retention strength of the vascular grafts gradually increased. The test results were completely complied with the vascular transplantation standard of 0.6 to 1.5 N per needle,30 and when 6 stitches were sutured, the suture retention strength was comparable to that of the fresh saphenous vein suture,31,32 which indicated that the electrospun P(LLA-CL) tissue-engineered grafts have good suturing performance.25
3.3. Wall thickness
Wall thickness is one of the most important factors affecting the biomechanical properties of tissue-engineered grafts.13 In the study, the effect of different wall thickness on the suture retention strength of P(LLA-CL) tissue-engineered grafts was determined by AO fiber tubular grafts with four stitches. The results were shown in Table 1.| Wall thickness (mm) | Suture retention strength (N per needle) |
|---|---|
| 0.20 | 0.3753 ± 0.0649 |
| 0.24 | 0.7438 ± 0.0366 |
| 0.28 | 1.0766 ± 0.1242 |
| 0.32 | 1.3832 ± 0.1673 |
The results showed that the suture retention strength of P(LLA-CL) vascular grafts increased linearly with the increase of tubular wall thickness. And only when the wall thickness of the vascular graft was larger than 0.24 mm, the suture retention strength of the P(LLA-CL) vascular grafts can meet the vascular transplantation standards and the clinical application requirements.
4. Discussion
At present, most of the experimental studies on tissue engineering vascular grafts focus on biological hemodynamics problems,33,34 graft and vascular compliance mismatch34,35 and vascular restenosis,34,36 and so on. However, the data show that most of graft surgery failures are due to rupture of the graft anastomosis.8 When the suture retention strength of the graft is tested by a conventional method, the stress state of the graft anastomosis is completely different from the serving state of the graft when it is transplanted. The existence of the shape stress of the graft itself might have an important impact on the test results. Therefore, we believe that it is necessary to carefully study the strength of the wall of the P(LLA-CL) tissue engineering scaffold when it is in contact with the suture line.Suture retention strength is one of the indexes of graft anastomosis strength established by ANSI/AAMI and is an important indicator to obtain transplant marketing approval.3,8 Manufacturers use their own methods to measure and evaluate the suture retention strength. However, we believe that the suture retention strength is affected by a number of unregulated factors such as suture material and diameter, graft type, and suturing methods. Especially when it comes to electrospun tissue-engineered vascular grafts, the relationship between the fiber arranged direction and the suture moving direction has a more significant effect on the suture retention strength. We used special fiber collection devices to prepare different types of electrospun tubular grafts, and designed an experimental method which reproduced a state similar to that of a graft used in a patient to test suture retention strength.
However, only a single suture on the sample is applied to test the suture retention strength of grafts in most of the research experiments. The test results are likely to increase suddenly due to the local stress concentration at the suture. However, in fact, the suture retention strength of the grafts is insufficient, which causes the grafts to rupture and leads many vascular graft transplantation surgery to failure. The method of cutting a vascular graft longitudinally into a rectangular membrane to test the suture retention strength of the graft also neglects the effect of the shape stress generated by the tubular shape of vascular graft on the suture retention strength, which inevitably leads to experimental errors. For vascular graft transplantation surgery, these errors might lead to a disastrously consequences.
In the experiment, we found that suture retention strength is highly affected by the fiber arranged direction. That is to say the obstruction effect of the fibers in different directions in the vascular graft on suture movement is different. Therefore, it is extremely important to determine the corresponding suture retention strength after determining the fibrous direction of the graft.
The smaller stitch distance makes the graft anastomosis more evenly stressed and thus able to withstand greater tension. However, a large number of pinholes causes greater damage to the vascular wall, and even the wall may be torn. Stimulation of sutures can also cause long-term foreign body reactions at the anastomosis, which can easily lead to intimal hyperplasia, thrombosis and pseudoaneurysm, and so on.36,37 The thicker wall makes the overlap between the fibers more complex and the binding force greater, which causes the suture movement more difficult. However, the wall thickness of the graft is not friendly to porosity and compliance.13,38 The porosity and compliance of vascular grafts have an important effect on cell behavior,39,40 smaller porosity and compliance are not conducive to the proliferation of endothelial cells.31,41,42 Therefore, there should be a balance in achieving high suture retention strength and the functionality of the vascular grafts.
5. Conclusion
Suture retention strength is a key parameter for vascular grafts, which are extensively applied but seldom standardized. In this study, the actual suturing status of the graft was simulated to obtain a more accurate suture retention strength of the P(LLA-CL) tissue-engineered vascular scaffolds. It was showed that the fiber alignment direction, the thickness of the graft wall and the number of stitches seriously affected the suture retention strength. Moreover, the favorable suturing properties of P(LLA-CL) tissue-engineered scaffolds also provides a theoretical reference for the designing of biodegradable vascular grafts to meet the requirements of specific mechanical performance. The study might arise a retrospective attention to the suture retention strength, therefore it might be of significance to clinical application of electrospun vascular grafts.Conflicts of interest
There are no conflicts to declareAcknowledgements
The authors acknowledge financial support from the International Science & Technology Cooperation Program of China (2015DFA30550), the “111” Project of Henan Province and the Key Science & Technology Project for Institutions of Higher Education of Henan Province (19A430003, 162102210159, 172102210489).References
- E. Ercolani, C. Del Gaudio and A. Bianco, J. Tissue Eng. Regener. Med., 2015, 9, 861–888 CrossRef CAS.
- K. A. Rocco, M. W. Maxfield, C. A. Best, E. W. Dean and C. K. Breuer, Tissue Eng., Part B, 2014, 20, 628–640 CrossRef CAS.
- A. Suite, J. Clin. Eng., 2016, 41, 59–60 CrossRef.
- F. E. Chlupac and L. Bacakova, Physiol. Res., 2009, 58, s119–s139 Search PubMed.
- M. W. King, B. S. Gupta and R. Guidoin, Biotextiles as Medical Implants, Woodhead Publishing Ltd, 2013 Search PubMed.
- Y. Xie, Y. Guan, S. H. Kim and M. W. King, J. Mech. Behav. Biomed. Mater., 2016, 61, 410–418 CrossRef CAS.
- M. Pensalfini, S. Meneghello, V. Lintas, K. Bircher, A. E. Ehret and E. Mazza, J. Mech. Behav. Biomed. Mater., 2018, 77, 711–717 CrossRef CAS.
- Y. Mine, H. Mitsui, Y. Oshima, Y. Noishiki, M. Nakai and S. Sano, Acta Med. Okayama, 2010, 64, 121–128 CAS.
- Y. Oshima, Y. Noishiki, M. Nakai, S. Sano, Y. Mine and H. Mitsui, Acta Med. Okayama, 2010, 64, 121–128 Search PubMed.
- K. Billiar, J. Murray, D. Laude, G. Abraham and N. Bachrach, J. Biomed. Mater. Res., 2001, 56, 101–108 CrossRef CAS PubMed.
- W. Fu, Z. L. Liu, B. Feng, R. J. Hu, X. M. He, H. Wang, M. Yin, H. M. Huang, H. B. Zhang and W. Wang, Int. J. Nanomed., 2014, 9, 2335–2344 CrossRef PubMed.
- Y. Dong, T. Yong, S. Liao, C. K. Chan and S. Ramakrishna, J. R. Soc., Interface, 2008, 5, 1109–1118 CrossRef CAS.
- H. Inoguchi, I. K. Kwon, E. Inoue, K. Takamizawa, Y. Maehara and T. Matsuda, Biomaterials, 2006, 27, 1470–1478 CrossRef CAS.
- C. Xu, Biomaterials, 2004, 25, 877–886 CrossRef CAS.
- Y. X. Dong, T. Yong, S. S. Liao, C. K. Chan, M. M. Stevens and S. Ramakrishna, Tissue Eng., Part A, 2010, 16, 283–298 CrossRef CAS.
- J. R. Haller, Facial Plast. Surg., 1996, 12, 3–7 CrossRef CAS.
- Y. M. Fan, China Text. Leader, 2006, 1, 58–62 Search PubMed.
- E. J. Wylie, R. J. Stoney and W. K. Ehrenfeld, Comprehensive Manuals of Surgical Specialties, 1980, pp. 9–48 Search PubMed.
- Q. P. Pham, U. Sharma and A. G. Mikos, Tissue Eng., 2006, 12, 1197–1211 CrossRef CAS.
- H. Y. Mi, X. Jing, E. Yu, X. Wang, Q. Li and L. S. Turng, J. Mech. Behav. Biomed. Mater., 2018, 78, 433–441 CrossRef CAS.
- Z. Asvar, E. Mirzaei, N. Azarpira, B. Geramizadeh and M. Fadaie, J. Mech. Behav. Biomed. Mater., 2017, 75, 369–378 CrossRef CAS.
- M. B. Browning, D. Dempsey, V. Guiza, S. Becerra, J. Rivera, B. Russell, M. Hook, F. Clubb, M. Miller, T. Fossum, J. F. Dong, A. L. Bergeron, M. Hahn and E. Cosgriff-Hernandez, Acta Biomater., 2012, 8, 1010–1021 CrossRef CAS PubMed.
- F. J. Chaparro, M. E. Matusicky, M. J. Allen and J. J. Lannutti, J. Biomed. Mater. Res., Part B, 2016, 104, 1525–1534 CrossRef CAS.
- G. Konig, T. N. McAllister, N. Dusserre, S. A. Garrido, C. Iyican, A. Marini, A. Fiorillo, H. Avila, W. Wystrychowski, K. Zagalski, M. Maruszewski, A. L. Jones, L. Cierpka, L. M. de la Fuente and N. L'Heureux, Biomaterials, 2009, 30, 1542–1550 CrossRef CAS.
- A. Yin, G. L. Bowlin, R. Luo, X. Zhang, Y. Wang and X. Mo, Regener. Biomater., 2016, 3, 239–245 CrossRef CAS.
- H. Bergmeister, C. Schreiber, C. Grasl, I. Walter, R. Plasenzotti, M. Stoiber, D. Bernhard and H. Schima, Acta Biomater., 2013, 9, 6032–6040 CrossRef CAS.
- M. J. McClure, S. A. Sell, D. G. Simpson, B. H. Walpoth and G. L. Bowlin, Acta Biomater., 2010, 6, 2422–2433 CrossRef CAS.
- A. F. Pellegata, M. A. Asnaghi, I. Stefani, A. Maestroni, S. Maestroni, T. Dominioni, S. Zonta, G. Zerbini and S. Mantero, BioMed Res. Int., 2013, 2013, 918753 Search PubMed.
- S. A. Sell, M. J. McClure, C. P. Barnes, D. C. Knapp, B. H. Walpoth, D. G. Simpson and G. L. Bowlin, Biomed. Mater., 2006, 1, 72–80 CrossRef CAS.
- K. Billiar, J. Murray, D. Laude, G. Abraham, N. Bachrach, K. Billiar and J. Murray, J. Biomed. Mater. Res., Part B, 2001, 56, 101–108 CrossRef CAS.
- Y. Elsayed, C. Lekakou and P. Tomlins, Biotechnol. Bioeng., 2019, 116, 1509–1522 CrossRef CAS.
- P. J. Schaner, N. D. Martin, T. N. Tulenko, I. M. Shapiro, N. A. Tarola, R. F. Leichter, R. A. Carabasi and P. J. Dimuzio, J. Vasc. Surg., 2004, 40, 146–153 CrossRef.
- A. T. Yokobori, T. Maeyama and T. Ohkuma, J. Biomech. Eng., 1986, 108(4), 295–300 CrossRef CAS.
- A. Fortier, V. Gullapalli and R. A. Mirshams, IJC Heart Vessels, 2014, 4, 12–18 CrossRef.
- D. Mehigan, B. Fitzpatrick, H. I. Browne and D. J. Bouchier-Hayes, Is compliance mismatch the major cause of anastomotic arterial aneurysms? Analysis of 42 cases, 1985 Search PubMed.
- G. Holzapfel, M. Stadler and T. C. Gasser, J. Biomech. Eng., 2005, 127, 166–180 CrossRef.
- A. Tiwari, K. S. Cheng, H. Salacinski, G. Hamilton and A. M. Seifalian, Eur. J. Vasc. Endovasc. Surg., 2003, 25, 287–295 CrossRef CAS PubMed.
- X. Kong, B. Han, H. Wang, H. Li, W. Xu and W. Liu, J. Biomed. Mater. Res., Part A, 2012, 100, 1938–1945 CrossRef.
- K. Madhavan, W. H. Elliott, W. Bonani, E. Monnet and W. Tan, J. Biomed. Mater. Res., Part B, 2013, 101, 506–519 CrossRef.
- S. Noel, B. Liberelle, A. Yogi, M. J. Moreno, M. N. Bureau, L. Robitaille and G. De Crescenzo, J. Mater. Chem. B, 2013, 1, 230–238 RSC.
- Y. Elsayed, C. Lekakou, F. Labeed and P. Tomlins, Mater. Sci. Eng., C, 2016, 61, 473–483 CrossRef CAS.
- Y. Elsayed, C. Lekakou, F. Labeed and P. Tomlins, J. Biomed. Mater. Res., Part A, 2016, 104, 313–321 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |