Open Access Article
Open Access ArticleSynthesis and discovery of 18β-glycyrrhetinic acid derivatives inhibiting cancer stem cell properties in ovarian cancer cells†
Xiaojing Li *ab,
Yihua Liuc,
Na Wanga,
Yuyu Liud,
Shuai Wanga,
Hongmin Wanga,
Aihua Lie and
Shaoda Ren*c
*ab,
Yihua Liuc,
Na Wanga,
Yuyu Liud,
Shuai Wanga,
Hongmin Wanga,
Aihua Lie and
Shaoda Ren*c
aLaboratory of Drug Discovery and Design, School of Pharmacy, Liaocheng University, Liaocheng 252000, China. E-mail: lixiaojing@lcu.edu.cn
bDepartment of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmacy, Shandong University, Jinan 250012, China
cCentral Laboratory, Liaocheng People's Hospital, Liaocheng 252000, China. E-mail: zslrsd@163.com
dShandong Qidu Pharmaceutical Co., Ltd., Zibo 255400, China
eDepartment of Obstetrics and Gynecology, Liaocheng People's Hospital, Liaocheng 252000, China
First published on 30th August 2019
Abstract
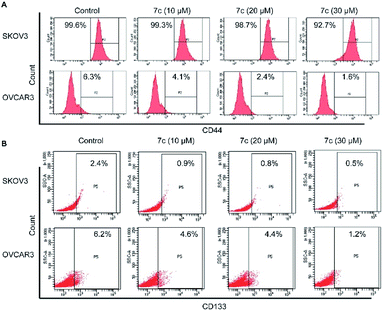
Despite advances in ovarian cancer treatment, the five-year overall survival rate is less than 30% with the presence of cancer stem cells (CSCs). To develop CSC-targeting therapy, a series of 18β-glycyrrhetinic acid (GA) derivatives containing cinnamamide moiety have been designed, synthesized, and screened for their antiproliferative activity in SKOV3 and OVCAR3 cells. Most of the compounds exhibited stronger antiproliferative activity than GA, and compound 7c was the most active one. Further biological studies showed that compound 7c could induce apoptosis and suppress migration. In addition, compound 7c could not only observably decrease the colony formation and sphere formation ability, but also significantly reduce the CD44+, CD133+, and ALDH+ subpopulation in SKOV3 and OVCAR3 cells. In conclusion, these results indicate that compound 7c is a promising anti-CSC agent for further anti-ovarian cancer studies.
1. Introduction
Ovarian cancer is a highly heterogeneous neoplasm in histology, biological properties, and one of the most deadly malignant tumours of the female reproductive organs.1 Over 70% of ovarian cancer patients would experience recurrence, and the five-year overall survival rate is less than 30%.2 The current standard treatment of advanced ovarian cancer is cytoreductive surgery followed the chemotherapy treatment of a combination of cisplatin or carboplatin with paclitaxel. Standard treatment can eliminate the size of primary tumours and the bulk of cancer cells, but complete tumour eradication is not always achieved due to the presence of cancer stem cells (CSCs).3CSCs account for about 0.1% of the cancer cells and have been separated from multiple solid tumours, including ovarian cancer, breast, brain, colon, prostate, and so on.4 Over the years, identification of CSCs depends on the specific markers on the surfaces of cell membranes and intracellular markers, including CD44, CD133, and aldehyde dehydrogenase (ALDH).5 CSCs display differentiation and self-renewal, and can form secondary or tertiary tumours. Accumulating evidence has proved that CSCs are linked to cancer initiation, progression, metastasis, relapse, and resistance to conventional therapies.6 In ovarian cancer, high expression of the CSC transcription factor was independently associated with poor overall survival.3,7 Thus, targeted therapy of CSCs is considered a potential therapeutic strategy against ovarian cancer.8
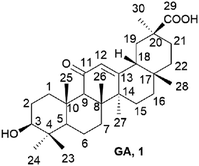
Natural compounds have aroused increasing attention in the field of cancer and CSCs due to their high activity and low side effects.9–11 Currently, about 60% of approved anticancer drugs come from unmodified natural products, their semisynthetic derivatives, or molecules synthesized based on natural products.12,13 18β-glycyrrhetinic acid (GA, 1, Fig. 1), a pentacyclic triterpenoid extracted from the root of Licorice, exerts a wide range of pharmacological activities including anti-inflammatory, antiviral, anti-oxidative and anticancer properties.14 GA has been investigated moderate anti-proliferative activity in ovarian cancer, breast, hepatoma, and stomach cancer cells.15 Although GA was not as active as other triterpenes, it can be easily obtained in large amounts. Thus, using GA as a scaffold to develop new low-toxicity and high-effectivity antitumor agents has aroused the great interest of scientists.16–20 The other natural compound cinnamic acid isolated from cinnamon or blossom, was found to have extensive physiological, including certain inhibitory activity against cancer cells.21,22 The amide bond is an important functional group in medicines as hydrogen bond donors and/or receptors. Therefore, we designed a series of GA derivatives containing cinnamamide moiety to investigate their anti-ovarian cancer activities.
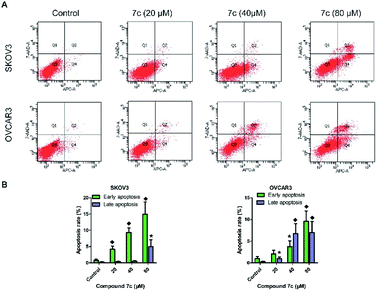
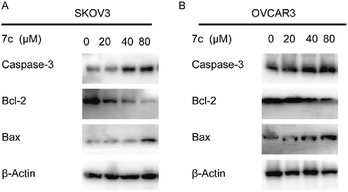
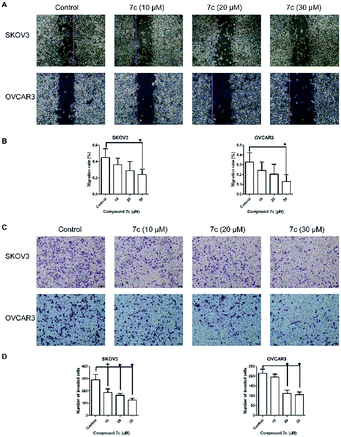
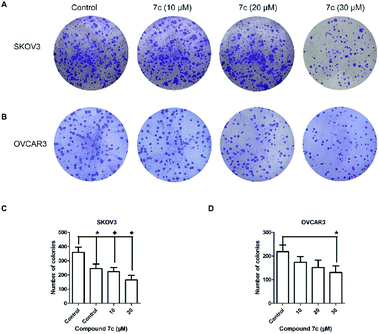
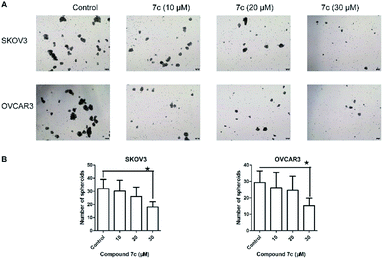
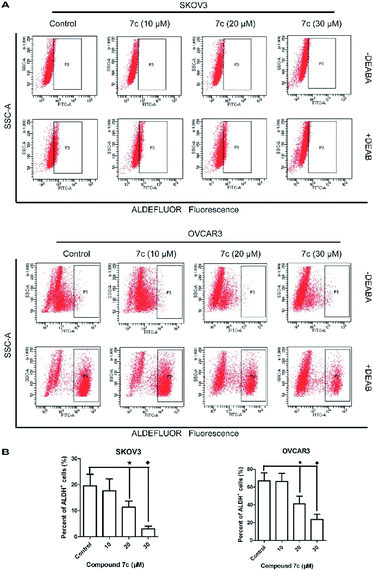
In this study, we designed and synthesized a series of GA derivatives containing CA moiety with piperazine or ethylenediamine (EDA) as linkers. These semisynthetic derivatives were tested for anti-proliferation activities in vitro against ovarian cancer cells. Compound 7c exhibited the strongest growth inhibition activities and the best selectivity among all of the target compounds. Thus 7c was selected for further biological assays, including apoptosis induction, migration inhibition, and the colony and spheres formation ability, the percentage of ovarian CSCs with CD44+ or CD133+ biomarker, and activity of ALDH.
2. Results and discussion
2.1 Chemistry
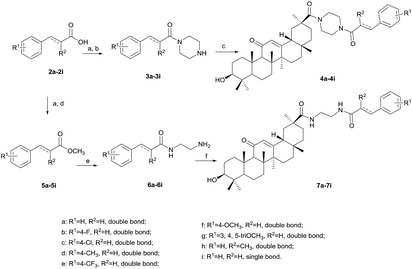
The preparation of the target compounds is outlined in Scheme 1. Compound 2a–2i, as the starting material, was reacted with SOCl2 in dichloromethane to obtain the corresponding acyl chloride, and then dropwise added into the solution of anhydrous piperazine in glacial acetic acid to afford 3a–3i. Compound 4a–4i was prepared in 63–87% yield by condensation between GA and 3a–3i in the presence of HATU/DIPEA at 100 °C for five hours. Methyl cinnamate 5a–5i can be easily obtained with cinnamoyl chloride and methanol in almost quantitative yield. 6a–6i was achieved by the ammonolysis of the ester group of 5a–5i with EDA under refluxing. Compound 7a–7i was synthesized in a similar way to 4a–4i in 50–70% yield, except that the reaction temperature changed to room temperature. The structures of GA derivatives were characterized using spectral methods.2.2 Biology
| Compounds | IC50a (μM) | Compounds | IC50a (μM) | ||
|---|---|---|---|---|---|
| SKOV3 | OVCAR3 | SKOV3 | OVCAR3 | ||
| a IC50: concentration that inhibits 50% of cell growth. The values are presented as the mean ± SD (standard error of the mean) from three separated experiments. | |||||
| 4a | 78.0 ± 0.8 | 71.6 ± 2.4 | 7a | 58.7 ± 1.8 | 53.0 ± 2.0 |
| 4b | 42.1 ± 1.2 | 69.9 ± 1.8 | 7b | 59.0 ± 1.0 | 53.2 ± 2.2 |
| 4c | 59.9 ± 1.7 | 68.4 ± 1.2 | 7c | 27.9 ± 1.4 | 33.9 ± 1.1 |
| 4d | >100 | 84.6 ± 2.0 | 7d | >100 | 56.5 ± 2.8 |
| 4e | 71.3 ± 1.1 | >100 | 7e | 45.4 ± 2.1 | 54.4 ± 1.0 |
| 4f | 84.9 ± 1.7 | 78.0 ± 2.2 | 7f | 58.9 ± 1.1 | 48.2 ± 2.0 |
| 4g | 74.3 ± 1.9 | 63.1 ± 2.6 | 7g | 49.4 ± 3.0 | 44.0 ± 1.8 |
| 4h | >100 | >100 | 7h | 56.7 ± 1.9 | 45.0 ± 1.9 |
| 4i | 95.0 ± 2.4 | 60.7 ± 2.0 | 7i | >100 | 48.2 ± 2.4 |
| Paclitaxel | 0.43 ± 0.03 | 0.38 ± 0.04 | GA, 1 | 98.5 ± 1.6 | 72.4 ± 2.2 |
| Compounds | IC50a (μM) | SIb | |
|---|---|---|---|
| IOSE80 | SKOV3 | OVCAR3 | |
| a IC50: concentration that inhibits 50% of cell growth. The values are presented as the mean ± SD (standard error of the mean) from three separated experiments.b SI (selectivity index): IC50 IOSE80 cell line/IC50 ovarian cancer cell line. | |||
| 7a | 66.8 ± 0.9 | 1.1 | 1.3 |
| 7b | 48.4 ± 0.6 | 0.8 | 0.9 |
| 7c | 86.7 ± 0.9 | 3.1 | 2.6 |
| 7d | 65.7 ± 3.7 | — | 1.2 |
| 7e | 71.8 ± 1.9 | 1.6 | 1.3 |
| 7f | 92.0 ± 1.1 | 1.6 | 1.9 |
| 7g | 53.0 ± 0.8 | 1.1 | 1.2 |
| 7h | 97.6 ± 5.7 | 1.7 | 2.2 |
| 7i | >100 | — | — |
| GA | >100 | — | — |
| Paclitaxel | 1.7 ± 0.1 | 4.0 | 4.5 |
3. Conclusions
In the present study, a series of GA derivatives containing cinnamamide moiety were designed and synthesized. Through evaluation of anticancer activity in ovarian cancer cell lines, the results showed that the antiproliferative activities of GA derivatives were significantly stronger compared with GA. The most potent compound 7c was selected for further biological studies in SKOV3 and OVCAR3 cells. The compound 7c could induce apoptosis and suppress migration. In addition, the compound 7c could not only observably decreased the colony and sphere formation ability, but also significantly reduced the CD44+, CD133+, and ALDH+ subpopulation in SKOV3 and OVCAR3. In conclusion, these results showed that compound 7c exhibited the potent elimination of ovarian CSCs and need to be further studied.4. Experimental section
4.1 Chemistry
Melting points (mp) were determined on an electrically heated X4 digital visual melting point apparatus. 1H NMR and 13C NMR spectra were performed on a Bruker ARX-400 or AVANCE NEO-500 NMR spectrometer in the indicated solvents (TMS as internal standard) with CDCl3 or d6-DMSO as the solvent. Mass spectra (MS) were measured on a Finnigan MAT/USA spectrometer (LC-MS). High-resolution mass spectra (HR-MS) were obtained using a Q-TOF instrument equipped with an ESI source. Most chemicals were commercial suppliers in analytic grade without further purification unless otherwise noted. All of the target compounds were ≥95% purity determined by HPLC analysis.(E)-3-Phenyl-1-(piperazin-1-yl)prop-2-en-1-one (3a). Yellow solid; mp 86.3–87.5 °C; yield 82%; 1H NMR (500 MHz, d6-DMSO) δ 7.73–7.68 (2H, m), 7.47 (1H, d, J = 15.4 Hz), 7.43–7.34 (3H, m), 7.23 (1H, d, J = 15.4 Hz), 3.61 (2H, m), 3.49 (2H, m), 2.69 (4H, s), 1.23 (1H, s); LC-MS: [M + H]+ 217.1.
(E)-3-(4-Fluorophenyl)-1-(piperazin-1-yl)prop-2-en-1-one (3b). Yellow solid; mp 76.7–77.7 °C; yield 71%; 1H NMR (500 MHz, d6-DMSO) δ 7.77 (2H, dd, J = 8.6, 5.7 Hz), 7.45 (1H, d, J = 15.4 Hz), 7.20 (3H, dd, J = 20.1, 12.0 Hz), 3.59 (2H, m), 3.48 (2H, m), 2.67 (4H, m), 1.22 (1H, s); LC-MS: [M + H]+ 234.1.
(E)-3-(4-Chlorophenyl)-1-(piperazin-1-yl)prop-2-en-1-one (3c). Yellow solid; mp 85.4–86.1 °C; yield 73%; 1H NMR (500 MHz, d6-DMSO) δ 7.82–7.74 (2H, m), 7.47 (1H, d, J = 15.4 Hz), 7.25–7.19 (3H, m), 3.63 (2H, m), 3.51 (2H, m), 2.72 (4H, m), 1.23 (1H, s); LC-MS: [M + H]+ 251.2.
(E)-1-(Piperazin-1-yl)-3-(p-tolyl)prop-2-en-1-one (3d). Yellow solid; mp 77.8–78.4 °C; yield 75%; 1H NMR (500 MHz, CDCl3) δ 7.65 (1H, d, J = 15.4 Hz), 7.41 (2H, d, J = 8.0 Hz), 7.17 (2H, d, J = 7.9 Hz), 6.82 (1H, d, J = 15.4 Hz), 3.72–3.66 (4H, m), 3.05–2.85 (4H, m), 2.36 (3H, s), 1.25 (1H, s); LC-MS: [M + H]+ 231.1.
(E)-1-(Piperazin-1-yl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (3e). Yellow solid; mp 83.1–83.3 °C; yield 76%; 1H NMR (500 MHz, d6-DMSO) δ 7.74 (2H, d, J = 8.5 Hz), 7.44 (3H, dd, J = 11.9, 3.3 Hz), 7.26 (1H, d, J = 15.5 Hz), 3.60 (2H, m), 3.49 (2H, m), 2.68 (4H, m), 1.22 (1H, s); LC-MS: [M + H]+ 285.2.
(E)-3-(4-Methoxyphenyl)-1-(piperazin-1-yl)prop-2-en-1-one (3f). Yellow solid; mp 79.2–80.4 °C; yield 83%; 1H NMR (500 MHz, d6-DMSO) δ 7.64 (2H, d, J = 8.7 Hz), 7.42 (1H, d, J = 15.4 Hz), 7.07 (1H, d, J = 15.3 Hz), 6.94 (2H, d, J = 8.7 Hz), 3.78 (3H, s), 3.59 (2H, m), 3.47 (2H, m), 2.68 (4H, m); LC-MS: [M + H]+ 247.1.
(E)-1-(Piperazin-1-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (3g). Yellow solid; mp 81.2–82.5 °C; yield 62%; 1H NMR (500 MHz, d6-DMSO) δ 7.42 (1H, d, J = 15.3 Hz), 7.16 (1H, d, J = 15.3 Hz), 7.02 (2H, s), 3.81 (6H, s), 3.67 (3H, s), 3.58 (4H, m), 2.74 (4H, m), 1.22 (1H, s); LC-MS: [M + H]+ 307.3.
(E)-2-Methyl-3-phenyl-1-(piperazin-1-yl)prop-2-en-1-one (3h). Yellow solid; mp 85.2–86.1 °C; yield 60%; 1H NMR (500 MHz, d6-DMSO) δ 7.38–7.27 (5H, m), 6.44 (1H, s), 3.43–3.32 (4H, m), 2.69–2.67 (4H, m), 1.98 (3H, d, J = 1.4 Hz), 1.22 (1H, s); LC-MS: [M + H]+ 231.2.
3-Phenyl-1-(piperazin-1-yl)propan-1-one (3i). Yellow oil; yield 72%; 1H NMR (500 MHz, d6-DMSO) δ 7.30–7.13 (5H, m), 3.36–3.24 (4H, m), 2.79–2.77 (2H, m), 2.59–2.49 (6H, m), 1.22 (1H, s); LC-MS: [M + H]+ 219.1.
(E)-1-(4-(3β-Hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-3-phenyl-2-propen-1-one (4a). White solid; mp 169.7–172.2 °C; yield 71%; 1H NMR (400 MHz, CDCl3) δ 7.70 (1H, d, J = 15.3 Hz), 7.52 (2H, s), 7.37 (3H, s), 6.86 (1H, d, J = 15.1 Hz), 5.69 (1H, s), 3.69 (8H, m), 3.27–3.15 (1H, m), 2.77 (1H, d, J = 12.8 Hz), 2.33 (2H, m), 2.12–1.92 (3H, m), 1.88–1.19 (1H, m), 1.68–1.58 (5H, m), 1.50–1.39 (5H, m), 1.36 (3H, s), 1.24 (3H, s), 1.22–1.14 (2H, m), 1.12 (3H, s), 1.11 (3H, s), 1.04 (1H, d, J = 1.3 Hz), 1.00 (3H, s), 0.96–0.91 (1H, m), 0.81 (3H, s), 0.80 (3H, s), 0.69 (1H, d, J = 11.2 Hz); 13C NMR (101 MHz, CDCl3) δ 200.06, 174.25, 169.27, 143.53, 134.96, 129.82, 128.81 (3C), 128.60, 127.82 (2C), 116.45, 78.72, 61.77, 54.90, 48.03, 45.25, 43.94 (2C), 43.88 (2C), 43.25, 39.09 (2C), 37.70, 37.05, 33.01, 32.78, 31.76, 29.67, 28.39, 28.06, 27.26, 27.05, 26.67, 26.37, 23.16 (2C), 18.65, 17.45, 16.36, 15.56; HRMS (ESI): m/z calcd for C43H61N2O4 [M + H]+ 669.4631, found 669.4654.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-3-(4-(trifluoromethyl)phenyl)-2-propen-1-one (4b). White solid; mp 179.9–182.1 °C; yield 67%; 1H NMR (400 MHz, CDCl3) δ 7.65 (1H, d, J = 15.3 Hz), 7.51 (2H, dd, J = 8.4, 5.5 Hz), 7.06 (2H, t, J = 8.5 Hz), 6.77 (1H, d, J = 15.4 Hz), 5.67 (1H, s), 3.68 (8H, m), 3.23–3.19 (1H, m), 2.77 (1H, d, J = 13.5 Hz), 2.33–2.28 (2H, m), 2.07–1.96 (3H, m), 1.87–1.80 (1H, m), 1.68–1.57 (5H, m), 1.49–1.39 (5H, m), 1.36 (3H, s), 1.24 (3H, s), 1.18 (2H, d, J = 15.2 Hz), 1.12 (3H, s), 1.11 (3H, s), 1.04 (1H, s), 0.99 (3H, s), 0.96–0.94 (1H, m), 0.81 (3H, s), 0.79 (3H, s), 0.69 (1H, d, J = 11.3 Hz); 13C NMR (101 MHz, CDCl3) δ 200.05, 174.26, 169.27, 165.50, 163.56 (d, J = 251.5 Hz), 142.28, 131.20 (d, J = 4.0 Hz), 129.63 (2C, J = 8.1 Hz), 128.59, 116.14 (d, J = 2.0 Hz), 115.92 (2C, d, J = 21.2 Hz), 78.71, 61.77, 54.91, 48.07, 45.25, 43.94 (2C), 43.84 (2C), 43.26, 39.10 (2C), 37.69, 37.06, 33.06, 32.78, 31.77 (2C), 28.39, 28.07, 27.27, 27.03, 26.68, 26.37, 23.14 (2C), 18.65, 17.46, 16.36, 15.56; HRMS (ESI): m/z calcd for C43H60FN2O4 [M + H]+ 687.4537, found 687.4558.
(E)-1-(4-(3β-Hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-3-(4-(chloro)phenyl)-2-propen-1-one (4c). White solid; mp 168.5–169.6 °C; yield 73%; 1H NMR (400 MHz, CDCl3) δ 7.64 (1H, d, J = 15.5 Hz), 7.46 (2H, d, J = 7.8 Hz), 7.35 (2H, d, J = 8.2 Hz), 6.83 (1H, d, J = 14.9 Hz), 5.68 (1H, s), 3.68 (8H, m), 3.24–3.20 (1H, m), 2.78 (1H, d, J = 12.9 Hz), 2.30 (2H, d, J = 21.1 Hz), 2.09–1.97 (3H, m), 1.87–1.80 (1H, m), 1.69–1.58 (5H, m), 1.49–1.40 (5H, m), 1.36 (3H, s), 1.24 (3H, s), 1.19 (2H, d, J = 12.3 Hz), 1.12 (3H, s), 1.11 (3H, s), 1.05 (1H, s), 1.00 (3H, s), 0.95 (1H, d, J = 9.5 Hz), 0.81 (3H, s), 0.80 (3H, s), 0.69 (1H, d, J = 12.1 Hz); 13C NMR (101 MHz, CDCl3) δ 200.07, 174.27, 169.29, 165.38, 142.14, 135.64, 133.45, 129.06 (2C), 128.99 (2C), 128.58, 116.96, 78.71, 61.77, 54.91, 48.07, 45.25, 43.94 (2C), 43.83 (2C), 43.26, 39.11, 39.10, 37.69, 37.06, 33.05, 32.78, 31.77 (2C), 28.38, 28.07, 27.26, 27.03, 26.68, 26.37, 23.14 (2C), 18.65, 17.45, 16.35, 15.56; HRMS (ESI): m/z calcd for C43H60ClN2O4 [M + H]+ 703.4242, found 703.4258.
(E)-1-(4-(3β-Hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-3-(p-tolyl)-2-propen-1-one (4d). White solid; mp 172.8–173.9 °C; yield 87%; 1H NMR (400 MHz, CDCl3) δ 7.67 (1H, d, J = 15.3 Hz), 7.42 (2H, d, J = 7.8 Hz), 7.18 (2H, d, J = 7.7 Hz), 6.80 (1H, d, J = 15.2 Hz), 5.68 (1H, s), 3.67 (8H, m), 3.23–3.19 (1H, m), 2.77 (1H, d, J = 13.1 Hz), 2.36 (3H, s), 2.31 (2H, d, J = 13.0 Hz), 2.11–1.94 (3H, m), 1.85–1.79 (1H, m), 1.66–1.57 (5H, m), 1.47–1.38 (5H, m), 1.35 (3H, s), 1.23 (3H, s), 1.18 (2H, d, J = 13.5 Hz), 1.12 (3H, s), 1.10 (3H, s), 1.04 (1H, s), 0.99 (3H, s), 0.96–0.94 (1H, m), 0.81 (3H, s), 0.79 (3H, s), 0.69 (1H, d, J = 11.6 Hz); 13C NMR (101 MHz, CDCl3) δ 200.06, 174.27, 169.29, 165.89, 143.55, 140.16, 132.23, 129.54 (2C), 128.61, 127.82 (2C), 115.28, 78.71, 61.78, 54.93, 48.04, 45.27, 43.95 (2C), 43.92 (2C), 43.27, 39.11 (2C), 37.72, 37.07, 32.99, 32.80, 31.87, 31.78, 28.40, 28.09, 27.28, 27.04, 26.70, 26.38, 23.16 (2C), 21.43, 18.67, 17.47, 16.37, 15.59; HRMS (ESI): m/z calcd for C44H63N2O4 [M + H]+ 683.4788, found 683.4810.
(E)-1-(4-(3β-Hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-3-(4-(trifluoromethyl)phenyl)-2-propen-1-one (4e). White solid; mp 172.4–174.3 °C; yield 80%; 1H NMR (400 MHz, CDCl3) δ 7.70 (1H, d, J = 15.5 Hz), 7.63 (4H, s), 6.93 (1H, d, J = 15.4 Hz), 5.67 (1H, s), 3.69 (8H, m), 3.26–3.15 (1H, m), 2.77 (1H, d, J = 13.6 Hz), 2.33 (1H, s), 2.29 (1H, d, J = 12.0 Hz), 2.06–1.94 (3H, m), 1.85 (1H, d, J = 13.1 Hz), 1.68–1.61 (5H, m), 1.47–1.41 (5H, m), 1.36 (3H, s), 1.32 (1H, s), 1.24 (3H, s), 1.19 (1H, d, J = 12.8 Hz), 1.12 (3H, s), 1.11 (3H, s), 1.05 (1H, s), 1.00 (3H, s), 0.97–0.92 (1H, m), 0.81 (3H, s), 0.80 (3H, s), 0.69 (1H, d, J = 11.4 Hz); 13C NMR (101 MHz, CDCl3) δ 200.01, 174.32, 169.22, 165.07, 141.68, 138.49, 128.61, 127.93 (2C), 127.85, 125.78 (2C, q, J = 4.0 Hz), 123.83 (d, J = 272.7 Hz), 119.09, 78.73, 61.81, 54.97, 48.13, 45.28, 43.98 (2C), 43.87 (2C), 43.30, 39.16, 39.12, 37.71, 37.11, 33.13, 32.83, 31.80 (2C), 28.39, 28.09, 27.30, 27.03, 26.74, 26.41, 23.14 (2C), 18.69, 17.49, 16.35, 15.56; HRMS (ESI): m/z calcd for C44H60F3N2O4 [M + H]+ 737.4505, found 737.4526.
(E)-1-(4-(3β-Hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-3-(4-(methoxy)phenyl)-2-propen-1-one (4f). White solid; mp 1172.3–174.2 °C; yield 80%; 1H NMR (400 MHz, CDCl3) δ 7.66 (1H, d, J = 15.3 Hz), 7.48 (2H, d, J = 8.7 Hz), 6.90 (2H, d, J = 8.7 Hz), 6.72 (1H, d, J = 15.4 Hz), 5.69 (1H, s), 3.83 (3H, s), 3.68 (8H, m), 3.26–3.16 (1H, m), 2.78 (1H, d, J = 13.4 Hz), 2.32 (2H, d, J = 13.2 Hz), 2.08–1.98 (3H, m), 1.86–1.81 (1H, m), 1.65–1.57 (5H, m), 1.47–1.42 (4H, m), 1.36 (3H, s), 1.31 (1H, s), 1.24 (3H, s), 1.18 (2H, d, J = 16.1 Hz),1.13 (3H, s), 1.12 (3H, s), 1.04 (1H, s), 1.00 (3H, s), 0.96 (1H, d, J = 14.9 Hz), 0.82 (3H, s), 0.80 (3H, s), 0.69 (1H, d, J = 11.4 Hz); 13C NMR (101 MHz, CDCl3) δ 200.06, 174.27, 169.14, 165.85, 160.92, 143.28, 129.45 (2C), 128.60, 127.72, 114.23 (2C), 113.85, 78.76, 61.79, 55.36, 54.93, 48.05, 45.27, 43.96 (2C), 43.91 (2C), 43.28, 39.12 (2C), 37.73, 37.08, 33.00, 32.81, 31.78 (2C), 28.40, 28.08, 27.29, 27.06, 26.71, 26.39, 23.16 (2C), 18.68, 17.48, 16.37, 15.57; HRMS (ESI): m/z calcd for C44H63N2O5 [M + H]+ 699.4737, found 699.4765.
(E)-1-(4-(3β-Hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-3-(3,4,5-(trimethoxy)phenyl)-2-propen-1-one (4g). White solid; mp 1163.2–164.8 °C; yield 63%; 1H NMR (400 MHz, CDCl3) δ 7.62 (1H, d, J = 15.3 Hz), 6.72 (3H, d, J = 17.7 Hz), 5.68 (1H, s), 3.90 (6H, s), 3.88 (3H, s), 3.70 (8H, m), 3.22 (1H, m), 2.78 (1H, d, J = 13.2 Hz), 2.34 (1H, s), 2.29 (1H, d, J = 16.0 Hz), 2.10–1.98 (3H, m), 1.87–1.82 (1H, m), 1.71–1.60 (5H, m), 1.48–1.41 (5H, m), 1.37 (3H, s), 1.25 (3H, s), 1.20 (2H, d, J = 15.3 Hz), 1.13 (3H, s), 1.12 (3H, s), 1.05 (1H, s), 1.00 (3H, s), 0.96 (1H, d, J = 10.7 Hz), 0.82 (3H, s), 0.81 (3H, s), 0.70 (1H, d, J = 11.1 Hz); 13C NMR (101 MHz, CDCl3) δ 200.06, 174.30, 169.28, 165.68, 153.42 (2C), 143.64, 139.84, 130.54, 128.60, 115.65, 105.15 (2C), 78.73, 61.81, 60.94, 56.23 (2C), 54.96, 48.14, 45.28, 43.98 (2C), 43.85 (2C), 43.30, 39.15, 39.11, 37.71, 37.10, 33.14, 32.82, 31.79 (2C), 28.39, 28.08, 27.29, 27.03, 26.74, 26.41, 23.14 (2C), 18.69, 17.48, 16.35, 15.55; HRMS (ESI): m/z calcd for C46H67N2O7 [M + H]+ 759.4948, found 759.4978.
(E)-1-(4-(3β-Hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-2-methyl-3-phenyl-2-propen-1-one (4h). White solid; mp 1145.3–146.6 °C; yield 80%; 1H NMR (400 MHz, CDCl3) δ; 7.40–7.29 (5H, m), 6.56 (1H, s), 5.68 (1H, s), 3.69–3.62 (8H, m), 3.21 (1H, dd, J = 10.2, 5.1 Hz), 2.78 (1H, d, J = 13.3 Hz), 2.33 (1H, s), 2.29 (1H, d, J = 11.5 Hz), 2.11 (3H, s), 2.05–1.96 (3H, m), 1.87–1.80 (1H, m), 1.69–1.59 (6H, m), 1.48–1.38 (5H, m), 1.36 (3H, s), 1.24 (3H, s), 1.19 (1H, d, J = 13.9 Hz), 1.13 (3H, s), 1.11 (3H, s), 1.05 (1H, s), 1.00 (3H, s), 0.95 (1H, d, J = 14.3 Hz), 0.81 (3H, s), 0.80 (3H, s), 0.69 (1H, d, J = 11.1 Hz); 13C NMR (101 MHz, CDCl3) δ 200.05, 174.28, 172.59, 169.27, 135.57, 132.23, 130.41, 129.02 (2C), 128.57, 128.36 (2C), 127.64, 78.72, 61.77, 54.93, 48.10, 45.26, 43.92 (2C), 43.82 (2C), 43.27, 39.10 (2C), 37.69, 37.06, 33.12, 32.79, 31.77 (2C), 28.37, 28.07, 27.26, 27.03, 26.69, 26.39, 23.12 (2C), 18.66, 17.46, 16.34, 16.20, 15.56; HRMS (ESI): m/z calcd for C44H63N2O4 [M + H]+ 683.4788, found 683.4813.
(E)-1-(4-(3β-Hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)piperazin-1-yl)-3-phenylpropyl-1-one (4i). White solid; mp 134.8–136.1 °C; yield 63%; 1H NMR (400 MHz, CDCl3) δ 7.27 (2H, d, J = 7.0 Hz), 7.20 (3H, d, J = 7.1 Hz), 5.64 (1H, s), 3.60–3.34 (8H, m), 3.22–3.18 (1H, m), 3.10–3.02 (1H, m), 2.96 (2H, t, J = 7.6 Hz), 2.76 (1H, d, J = 13.5 Hz), 2.62 (2H, t, J = 7.6 Hz), 2.31 (1H, s), 2.25 (1H, d, J = 12.2 Hz), 2.07–1.91 (3H, m), 1.86–1.77 (1H, m), 1.64–1.56 (5H, m), 1.46–1.37 (5H, m), 1.34 (3H, s), 1.19 (3H, s), 1.15 (1H, s), 1.11 (3H, s), 1.09 (3H, s), 1.02 (1H, s), 0.98 (3H, s), 0.94 (1H, d, J = 12.5 Hz), 0.78 (6H, s), 0.68 (1H, d, J = 11.5 Hz); 13C NMR (101 MHz, CDCl3) δ 200.00, 174.14, 170.89, 169.25, 140.85, 128.49 (2C), 128.38 (2C), 128.36, 126.24, 78.62, 61.71, 54.86, 47.99, 45.41, 45.20, 43.82 (2C), 43.75 (2C), 43.20, 41.56, 39.05, 37.62, 37.00, 34.85, 32.96, 32.73, 31.69, 31.39, 28.32, 28.03, 27.20, 26.93, 26.62, 26.32, 23.08 (2C), 18.60, 17.40, 16.30, 15.54; HRMS (ESI): m/z calcd for C43H63N2O4 [M + H]+ 671.4788, found 671.4811.
N-(2-Aminoethyl)cinnamamide (6a). Yellow oil; yield 52%; 1H NMR (500 MHz, d6-DMSO) δ 8.12 (1H, s), 7.55 (2H, d, J = 7.1 Hz), 7.42–7.34 (4H, m), 6.63 (1H, d, J = 15.8 Hz), 3.19 (2H, dd, J = 12.2, 6.2 Hz), 2.65 (2H, t, J = 6.4 Hz), 1.22 (1H, s); LC-MS: [M + H]+ 191.1.
(E)-N-(2-Aminoethyl)-3-(4-fluorophenyl)acrylamide (6b). Yellow oil; yield 61%; 1H NMR (500 MHz, d6-DMSO) δ 8.52 (1H, t, J = 5.5 Hz), 7.64 (2H, dd, J = 8.7, 5.6 Hz), 7.47 (1H, d, J = 15.9 Hz), 7.26 (2H, t, J = 8.8 Hz), 6.60 (1H, d, J = 15.9 Hz), 3.42 (2H, dd, J = 12.1, 6.1 Hz), 2.90 (2H, t, J = 6.2 Hz), 1.23 (2H, s); LC-MS: [M + H]+ 209.1.
(E)-N-(2-Aminoethyl)-3-(4-chlorophenyl)acrylamide (6c). Yellow oil; yield 51%; 1H NMR (500 MHz, d6-DMSO) δ 8.16 (1H, t, J = 5.4 Hz), 7.58 (2H, d, J = 8.5 Hz), 7.46 (2H, d, J = 8.5 Hz), 7.40 (1H, d, J = 15.8 Hz), 6.63 (1H, d, J = 15.8 Hz), 3.21 (2H, dd, J = 12.2, 6.2 Hz), 2.67 (2H, t, J = 6.4 Hz), 1.22 (2H, s); LC-MS: [M + H]+ 225.2.
(E)-N-(2-Aminoethyl)-3-(p-tolyl)acrylamide (6d). Yellow oil; yield 56%; 1H NMR (500 MHz, d6-DMSO) δ 8.08 (1H, t, J = 5.3 Hz), 7.43 (2H, d, J = 8.0 Hz), 7.36 (1H, d, J = 15.8 Hz), 7.21 (2H, d, J = 7.9 Hz), 6.57 (1H, d, J = 15.8 Hz), 3.18 (2H, dd, J = 12.2, 6.2 Hz), 2.64 (2H, t, J = 6.4 Hz), 2.31 (3H, s), 1.22 (2H, s); LC-MS: [M + H]+ 205.1.
(E)-N-(2-Aminoethyl)-3-(4-(trifluoromethyl)phenyl)acrylamide (6e). Yellow oil; yield 60%; 1H NMR (500 MHz, d6-DMSO) δ 8.59 (1H, t, J = 5.6 Hz), 7.84–7.76 (4H, m), 7.54 (1H, d, J = 15.9 Hz), 6.78 (1H, d, J = 15.9 Hz), 3.43 (2H, q, J = 6.1 Hz), 2.91 (2H, t, J = 6.3 Hz), 1.23 (2H, s); LC-MS: [M + H]+ 259.2.
(E)-N-(2-Aminoethyl)-3-(4-methoxyphenyl)acrylamide (6f). Yellow oil; yield 68%; 1H NMR (500 MHz, d6-DMSO) δ 8.03 (1H, m), 7.49 (2H, d, J = 8.7 Hz), 7.35 (1H, d, J = 15.8 Hz), 6.96 (2H, d, J = 8.7 Hz), 6.47 (1H, d, J = 15.8 Hz), 3.77 (3H, s), 3.20–3.17 (2H, m), 2.66–2.62 (2H, m), 1.22 (2H, s); LC-MS: [M + H]+ 221.1.
(E)-N-(2-Aminoethyl)-3-(3,4,5-trimethoxyphenyl)acrylamide (6g). Yellow oil; yield 52%; 1H NMR (500 MHz, d6-DMSO) δ 8.04 (1H, t, J = 5.5 Hz), 7.35 (1H, d, J = 15.7 Hz), 6.88 (2H, s), 6.58 (1H, d, J = 15.7 Hz), 3.80 (6H, s), 3.67 (3H, s), 3.21–3.17 (2H, m), 2.65 (2H, m), 1.22 (2H, s); LC-MS: [M + H]+ 281.1.
(E)-N-(2-Aminoethyl)-2-methyl-3-phenylacrylamide (6h). Yellow oil; yield 54%; 1H NMR (500 MHz, d6-DMSO) δ 8.06 (1H, t, J = 5.0 Hz), 7.50–7.27 (5H, m), 7.24 (1H, s), 3.53 (1H, s), 3.23 (1H, dd, J = 12.1, 6.2 Hz), 2.72 (1H, t, J = 6.4 Hz), 2.00 (3H, d, J = 1.2 Hz), 1.22 (2H, s); LC-MS: [M + H]+ 205.1.
N-(2-Aminoethyl)-3-phenylpropanamide (6i). Yellow oil; yield 59%; 1H NMR (500 MHz, d6-DMSO) δ 8.23 (1H, s), 7.30–7.11 (5H, m), 3.27–3.24 (2H, m), 2.82–2.76 (4H, m), 2.41–2.38 (2H, m), 1.22 (2H, s); LC-MS: [M + H]+ 193.1.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-3-phenyl-2-propen-1-one (7a). White solid; mp 168.9–170.1 °C; yield 68%; 1H NMR (400 MHz, CDCl3) δ 7.61 (1H, d, J = 15.8 Hz), 7.50–7.49 (2H, m), 7.34–7.33 (3H, m), 6.68 (1H, s), 6.58 (1H, s), 6.42 (1H, d, J = 14.7 Hz), 5.72 (1H, s), 3.62–3.36 (4H, m), 3.23–3.20 (1H, m), 2.77 (1H, d, J = 13.0 Hz), 2.30 (1H, s), 2.12 (1H, d, J = 10.7 Hz), 2.07–1.95 (1H, m), 1.88 (2H, d, J = 9.1 Hz), 1.83–1.72 (2H, m), 1.67–1.56 (7H, m), 1.45–1.37 (4H, m), 1.35 (3H, s), 1.16 (1H, s), 1.11 (6H, s), 1.01 (3H, s), 0.99 (3H, s), 0.90 (1H, s), 0.80 (3H, s), 0.74 (3H, s), 0.68 (1H, d, J = 9.9 Hz); 13C NMR (101 MHz, CDCl3) δ 200.02, 177.53, 169.18, 167.25, 141.46, 134.65, 129.69, 128.79 (2C), 128.51, 127.89 (2C), 120.30, 78.75, 61.78, 54.94, 48.04, 45.32, 43.64, 43.18, 41.56, 40.67, 40.19, 39.17, 39.12, 37.56, 37.08, 32.74, 31.84, 31.42, 29.48, 28.43, 28.10, 27.28, 26.46, 26.43, 23.36, 18.57, 17.46, 16.35, 15.58; HRMS (ESI): m/z calcd for C41H59N2O4 [M + H]+ 643.4475, found 643.4500.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-3-(4-(fluoro)phenyl)-2-propen-1-one (7b). White solid; mp 178.2–179.3 °C; yield 50%; 1H NMR (400 MHz, CDCl3) δ 7.57 (1H, d, J = 15.5 Hz), 7.53–7.43 (2H, m), 7.03 (2H, t, J = 8.4 Hz), 6.65 (2H, s), 6.34 (1H, d, J = 15.5 Hz), 5.71 (1H, s), 3.65–3.36 (4H, m), 3.21 (1H, dd, J = 10.2, 5.0 Hz), 2.76 (1H, d, J = 13.3 Hz), 2.30 (1H, s), 2.10 (1H, d, J = 12.3 Hz), 2.01 (1H, dd, J = 12.9, 9.1 Hz), 1.88 (2H, d, J = 14.1 Hz), 1.77 (1H, dd, J = 14.3, 10.7 Hz), 1.68–1.60 (6H, m), 1.42–1.38 (5H, m), 1.35 (3H, s), 1.18 (1H, s), 1.11 (6H, s), 0.99 (6H, s), 0.94 (2H, d, J = 8.2 Hz), 0.80 (3H, s), 0.73 (3H, s), 0.67 (1H, d, J = 11.2 Hz); 13C NMR (101 MHz, d6-DMSO) δ 199.11, 175.54, 169.60, 165.19, 162.68 (d, J = 247.4 Hz), 137.65, 131.54 (d, J = 3.0 Hz), 129.71 (2C, d, J = 8.1 Hz), 127.67, 122.05, 115.86 (2C, d, J = 22.2 Hz), 76.66, 61.20, 54.18, 47.54, 44.82, 43.00 (2C), 42.88, 40.80, 38.84, 38.62, 37.31, 36.70, 32.20, 31.45 (2C), 30.43, 28.84, 28.22, 28.20, 27.03, 26.10, 26.03, 23.10, 18.24, 17.21, 16.15, 16.06; HRMS (ESI): m/z calcd for C41H58FN2O4 [M + H]+ 661.4381, found 661.4402.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-3-(4-(chloro)phenyl)-2-propen-1-one (7c). White solid; mp 164.7–166.2 °C; yield 70%; 1H NMR (400 MHz, CDCl3) δ 7.54 (1H, d, J = 15.3 Hz), 7.41 (2H, d, J = 8.0 Hz), 7.30 (2H, d, J = 8.0 Hz), 6.90 (1H, s), 6.81 (1H, s), 6.42 (1H, d, J = 15.5 Hz), 5.69 (1H, s), 3.63–3.36 (4H, m), 3.21 (1H, dd, J = 10.0, 4.6 Hz), 2.74 (1H, d, J = 13.4 Hz), 2.29 (1H, s), 2.08 (1H, d, J = 13.8 Hz), 1.98 (1H, d, J = 14.2 Hz), 1.89 (2H, d, J = 11.9 Hz), 1.81–1.71 (2H, m), 1.66–1.56 (6H, m), 1.43–1.35 (5H, m), 1.34 (3H, s), 1.16 (1H, s), 1.10 (6H, s), 0.99 (3H, s), 0.97 (3H, s), 0.89 (1H, d, J = 18.5 Hz), 0.79 (3H, s), 0.71 (3H, s), 0.66 (1H, d, J = 11.3 Hz); 13C NMR (101 MHz, d6-DMSO) δ 199.06, 175.53, 169.54, 165.02, 137.46, 133.83, 133.80, 129.22 (2C), 128.91 (2C), 127.65, 122.93, 76.64, 61.18, 54.16, 47.51, 44.79, 42.97, 42.86, 40.79, 38.88, 38.81, 38.59, 37.29, 36.68, 32.18, 31.43 (2C), 30.40, 28.82, 28.18 (2C), 27.01, 26.08, 26.02, 23.07, 18.21, 17.19, 16.12, 16.03; HRMS (ESI): m/z calcd for C41H58ClN2O4 [M + H]+ 677.4085, found 677.4112.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-3-(p-tolyl)-2-propen-1-one (7d). White solid; mp 166.8–169.1 °C; yield 52%; 1H NMR (400 MHz, d6-DMSO) δ 8.02 (1H, s), 7.65 (1H, s), 7.41 (2H, d, J = 8.0 Hz), 7.34 (1H, d, J = 15.7 Hz), 7.17 (2H, d, J = 7.8 Hz), 6.51 (1H, d, J = 15.7 Hz), 5.53 (1H, s), 4.31 (1H, d, J = 5.0 Hz), 3.19–3.16 (4H, m), 2.98 (1H, m), 2.58 (1H, d, J = 13.2 Hz), 2.29 (3H, s), 2.27 (1H, s), 2.07–2.00 (2H, m), 1.88–1.86 (1H, m), 1.79–1.75 (1H, m), 1.71–1.63 (1H, m), 1.62–1.55 (2H, m), 1.52–1.46 (2H, m), 1.40–1.37 (1H, m), 1.31 (3H, s), 1.29–1.18 (6H, m), 1.09 (1H, d, J = 8.4 Hz), 1.02 (1H, s), 0.99 (6H, s), 0.89 (3H, s), 0.89 (3H, s), 0.84 (1H, s), 0.67 (6H, s); 13C NMR (101 MHz, d6-DMSO) δ 199.12, 175.57, 169.61, 165.45, 139.12, 138.82, 132.16, 129.53 (2C), 127.69, 127.56 (2C), 121.07, 76.67, 61.23, 54.19, 47.56, 44.84, 43.01, 42.90, 40.82, 38.86, 38.68, 38.64, 37.33, 36.72, 32.21, 31.46 (2C), 30.45, 28.86, 28.27, 28.22, 27.05, 26.12, 26.04, 23.12, 21.03, 18.28, 17.23, 16.18, 16.08; HRMS (ESI): m/z calcd for C42H61N2O4 [M + H]+ 657.4631, found 657.4657.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-3-(4-(trifluoromethyl)phenyl)-2-propen-1-one (7e). White solid; mp 1174.5–176.9 °C; yield 72%; 1H NMR (400 MHz, d6-DMSO) δ 8.13 (1H, s), 7.75–7.69 (4H, m), 7.63 (1H, s), 7.44 (1H, d, J = 15.7 Hz), 6.72 (1H, d, J = 15.9 Hz), 5.52 (1H, s), 4.28 (1H, d, J = 4.8 Hz), 3.26–3.13 (4H, m), 3.04–2.98 (1H, m), 2.56 (1H, d, J = 13.1 Hz), 2.26 (1H, s), 2.05–1.98 (2H, m), 1.88 (1H, d, J = 6.8 Hz), 1.75 (1H, s), 1.68 (1H, d, J = 13.4 Hz), 1.47 (2H, d, J = 9.6 Hz), 1.39 (1H, d, J = 11.7 Hz), 1.30 (3H, s), 1.27–1.22 (5H, m), 1.08 (1H, d, J = 13.5 Hz), 1.02 (1H, s), 0.99 (3H, s), 0.94 (3H, s), 0.91 (1H, s), 0.88 (3H, s), 0.83 (3H, s), 0.72 (1H, s), 0.66 (6H, s); 13C NMR (101 MHz, d6-DMSO) δ 199.09, 175.57, 169.57, 164.75, 138.94, 137.17, 128.95 (d, J = 9.1 Hz), 128.19 (2C), 127.61 (q, J = 10.1 Hz), 125.75 (2C, q, J = 4.0 Hz), 124.92, 124.16 (d, J = 273.7 Hz), 76.65, 61.19, 54.15, 47.51, 44.80, 43.01, 42.86, 40.78, 38.83, 38.61, 38.51, 37.30, 36.71, 36.66, 32.17, 31.45, 30.40, 28.85, 28.19, 28.15, 27.02, 26.09, 26.03, 23.09, 18.17, 17.19, 16.09, 16.03; HRMS (ESI): m/z calcd for C42H58F3N2O4 [M + H]+ 711.4349, found 711.4374.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-3-(4-(methoxy)phenyl)-2-propen-1-one (7f). White solid; mp 162.9–165.3 °C; yield 69%; 1H NMR (400 MHz, d6-DMSO) δ 7.97 (1H, s), 7.65 (1H, s), 7.47 (2H, d, J = 8.6 Hz), 7.33 (1H, d, J = 15.7 Hz), 6.92 (2H, d, J = 8.6 Hz), 6.42 (1H, d, J = 15.7 Hz), 5.53 (1H, s), 4.31 (1H, d, J = 5.0 Hz), 3.76 (3H, s), 3.30–3.04 (4H, m), 3.06–2.96 (1H, m), 2.58 (1H, d, J = 12.8 Hz), 2.28 (1H, s), 2.03–2.00 (2H, m), 1.88–1.86 (1H, m), 1.78–1.75 (1H, m), 1.71–1.68 (1H, m), 1.62–1.57 (2H, m), 1.52–1.49 (2H, m), 1.42–1.38 (1H, m), 1.31 (3H, s), 1.27–1.22 (5H, m), 1.09 (1H, d, J = 10.5 Hz), 1.01 (1H, m), 1.00 (6H, s), 0.91 (3H, s), 0.89 (3H, s), 0.83 (1H, d, J = 9.1 Hz), 0.67 (6H, s); 13C NMR (101 MHz, d6-DMSO) δ 199.52, 175.91, 170.00, 165.97, 160.66, 138.93, 129.51 (2C), 128.04, 127.84, 119.98, 114.73 (2C), 77.03, 61.58, 55.64, 54.53, 47.92, 45.21, 43.36, 43.26, 41.16, 39.21, 39.06, 39.00, 37.68, 37.07, 32.56, 31.82 (2C), 30.78, 29.20, 28.63, 28.57, 27.39, 26.46, 26.40, 23.46, 18.65, 17.57, 16.57, 16.44; HRMS (ESI): m/z calcd for C42H61N2O5 [M + H]+ 673.4580, found 673.4603.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-3-(3,4,5-(trimethoxy)phenyl)-2-propen-1-one (7g). White solid; mp 166.3–167.5 °C; yield 58%; 1H NMR (400 MHz, d6-DMSO) δ 8.02 (1H, s), 7.64 (1H, s), 7.33 (1H, d, J = 15.7 Hz), 6.85 (2H, s), 6.52 (1H, d, J = 15.7 Hz), 5.54 (1H, s), 4.28 (1H, d, J = 5.0 Hz), 3.79 (6H, s), 3.65 (3H, s), 3.26–3.11 (4H, m), 3.00–2.99 (1H, m), 2.57 (1H, d, J = 12.9 Hz), 2.26 (1H, s), 2.03–1.99 (2H, m), 1.88 (1H, m), 1.77–1.74 (1H, m), 1.67–1.62 (1H, m), 1.59–1.56 (2H, m), 1.50–1.46 (2H, m), 1.39 (1H, m), 1.30 (3H, s), 1.29–1.21 (6H, m), 1.09 (1H, d, J = 10.0 Hz), 1.00 (1H, m), 0.99 (3H, s), 0.95 (3H, s), 0.90 (3H, s), 0.88 (3H, s), 0.83 (1H, m), 0.69 (3H, s), 0.65 (3H, s); 13C NMR (101 MHz, d6-DMSO) δ 199.20, 175.47, 169.62, 165.49, 153.09 (2C), 139.13, 138.64, 130.55, 127.81, 121.33, 104.97 (2C), 76.68, 61.25, 60.13, 55.88 (2C), 54.19, 47.53, 44.84, 43.02, 42.91, 40.79, 38.88, 38.81, 38.65, 37.36, 36.71, 32.20, 31.48 (2C), 30.53, 28.89, 28.34, 28.23, 27.05, 26.13, 26.07, 23.15, 18.24, 17.24, 16.23, 16.09; HRMS (ESI): m/z calcd for C44H65N2O7 [M + H]+ 733.4792, found 733.4821.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-2-methyl-3-phenyl-2-propen-1-one(7h). White solid; mp 145.8–147.2 °C; yield 51%; 1H NMR (400 MHz, d6-DMSO) δ 7.96 (1H, s), 7.70 (1H, s), 7.41–7.27 (5H, m), 7.22 (1H, s), 5.51 (1H, s), 4.31 (1H, d, J = 5.0 Hz), 3.28–3.20 (4H, m), 3.05–2.95 (1H, m), 2.58 (1H, d, J = 12.6 Hz), 2.29 (1H, s), 2.10–2.01 (2H, m), 1.99 (3H, s), 1.89–1.88 (1H, m), 1.80–1.77 (1H, m), 1.73–1.67 (1H, m), 1.62–1.57 (2H, m), 1.52–1.49 (2H, m), 1.47–1.37 (1H, m), 1.30–1.19 (6H, m), 1.32 (3H, s), 1.11 (2H, d, J = 14.3 Hz), 1.01 (6H, s), 0.97 (3H, s), 0.92 (1H, m), 0.89 (3H, s), 0.68 (6H, s); 13C NMR (101 MHz, d6-DMSO) δ 199.09, 175.74, 169.66, 168.84, 136.12, 132.49, 132.30, 129.24 (2C), 128.39 (2C), 127.66, 127.61, 76.65, 61.22, 54.18, 47.60, 44.86, 42.97, 42.92, 40.81, 38.83, 38.59 (2C), 37.30, 36.72, 32.21, 31.43 (2C), 30.51, 28.81, 28.30, 28.20, 27.03, 26.10, 26.00, 23.10, 18.35, 17.21, 16.22, 16.05, 14.27; HRMS (ESI): m/z calcd for C42H61N2O4 [M + H]+ 657.4631, found 657.4654.
(E)-1-(4-(3β-hydroxy-11-oxo-18β-olean-12-en-30-carbonyl)ethanediamine-1-yl)-3-phenylpropyl-1-one (7i). White solid; mp 142.2–143.2 °C; yield 67%; 1H NMR (400 MHz, d6-DMSO) δ 7.80 (1H, s), 7.58 (1H, s), 7.24 (2H, t, J = 7.3 Hz), 7.19–7.11 (3H, m), 5.51 (1H, s), 4.25 (1H, s), 3.17–3.03 (4H, m), 3.03–2.96 (1H, m), 2.82–2.74 (2H, m), 2.58 (1H, d, J = 13.5 Hz), 2.38–2.32 (2H, m), 2.31 (1H, s), 2.10–2.01 (2H, m), 1.87–1.85 (1H, m), 1.78–1.73 (2H, m), 1.63–1.57 (2H, m), 1.54–1.46 (2H, m), 1.49–1.36 (2H, m), 1.33 (3H, s), 1.30–1.20 (5H, m), 1.13 (1H, d, J = 10.5 Hz), 1.02 (3H, s), 1.01 (3H, s), 0.99 (3H, s), 0.96–0.92 (1H, m), 0.90 (3H, s), 0.72 (3H, s), 0.70 (1H, s), 0.68 (3H, s); 13C NMR (101 MHz, d6-DMSO) δ 199.09, 175.36, 171.57, 169.66, 141.33, 128.27 (2C), 128.18 (2C), 127.57, 125.87, 76.64, 61.22, 54.17, 47.66, 44.87, 42.93 (2C), 40.81, 38.82, 38.69, 38.58, 37.30, 37.15, 36.71, 32.20, 31.43 (2C), 31.09, 30.48, 28.75, 28.40, 28.18, 27.02, 26.10, 25.99, 23.08, 18.37, 17.20, 16.20, 16.03; HRMS (ESI): m/z calcd for C41H61N2O4 [M + H]+ 645.4631, found 645.4659.
4.2 Pharmacology
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with B-27 (Thermo Fisher Scientific, Grand Island, NY, USA), 10 ng mL−1 fibroblast growth factor-basic (bFGF, R&D, Minneapolis, MN, USA), and 10 ng mL−1 epidermal growth factor (EGF, Gibco, Carlsbad, CA, USA). After 10 days, tumourspheres were harvested, and then equal cells were plated to form secondary generation. The compound was added at indicated concentrations after plating for 24 h. Cells were cultured for a subsequent 10 days. The culture medium with the compound was replaced three times during incubation. The cells were visualized, and the number of spheres (>50 μm) was counted using an inverted microscope (CKX71, Olympus Corporation, Tokyo, Japan)
1) with B-27 (Thermo Fisher Scientific, Grand Island, NY, USA), 10 ng mL−1 fibroblast growth factor-basic (bFGF, R&D, Minneapolis, MN, USA), and 10 ng mL−1 epidermal growth factor (EGF, Gibco, Carlsbad, CA, USA). After 10 days, tumourspheres were harvested, and then equal cells were plated to form secondary generation. The compound was added at indicated concentrations after plating for 24 h. Cells were cultured for a subsequent 10 days. The culture medium with the compound was replaced three times during incubation. The cells were visualized, and the number of spheres (>50 μm) was counted using an inverted microscope (CKX71, Olympus Corporation, Tokyo, Japan)Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2016HB05, ZR2016HB45 and ZR2019PC030), the open project of Shandong Collaborative Innovation Centre for Antibody Drugs (CIC-AD1827), the Postdoctoral Science Foundation of China (2018M632607), and the National Natural Science Foundation of China (81600087).Notes and references
- B. M. Reid, J. B. Permuth and T. A. Sellers, Cancer Biol. Med., 2017, 14, 9–32 CAS.
- L. A. Torre, B. Trabert, C. E. DeSantis, K. D. Miller, G. Samimi, C. D. Runowicz, M. M. Gaudet, A. Jemal and R. L. Siegel, CA A Cancer J. Clin., 2018, 68, 284–296 CrossRef PubMed.
- Z. Pieterse, M. A. Amaya-Padilla, T. Singomat, M. Binju, B. D. Madjid, Y. Yu and P. Kaur, Int. J. Biochem. Cell Biol., 2019, 106, 117–126 CrossRef CAS PubMed.
- J. E. Visvader and G. J. Lindeman, Nat. Rev. Cancer, 2008, 8, 755–768 CrossRef CAS PubMed.
- M. E. Toledo-Guzman, G. D. Bigoni-Ordonez, M. Ibanez Hernandez and E. Ortiz-Sanchez, World J. Stem Cells, 2018, 10, 183–195 CrossRef PubMed.
- B. C. Prager, Q. Xie, S. Bao and J. N. Rich, Cell Stem Cell, 2019, 24, 41–53 CrossRef CAS PubMed.
- M. K. Siu, E. S. Wong, D. S. Kong, H. Y. Chan, L. Jiang, O. G. Wong, E. W. Lam, K. K. Chan, H. Y. Ngan, X. F. Le and A. N. Cheung, Oncogene, 2013, 32, 3500–3509 CrossRef CAS PubMed.
- A. Mihanfar, J. Aghazadeh Attari, I. Mohebbi, M. Majidinia, M. Kaviani, M. Yousefi and B. Yousefi, J. Cell. Physiol., 2019, 234, 3238–3253 CrossRef CAS PubMed.
- W. F. Taylor and E. Jabbarzadeh, Am. J. Cancer, 2017, 7, 1588–1605 CAS.
- F. Pistollato, R. Calderon Iglesias, R. Ruiz, S. Aparicio, J. Crespo, L. Dzul Lopez, F. Giampieri and M. Battino, Cancer Lett., 2017, 411, 191–200 CrossRef CAS PubMed.
- X. Li, H. Wang, J. Ding, S. Nie, L. Wang, L. Zhang and S. Ren, Eur. J. Pharmacol., 2019, 842, 146–156 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2007, 70, 461–477 CrossRef CAS PubMed.
- G. M. Cragg and D. J. Newman, Biochim. Biophys. Acta, 2013, 1830, 3670–3695 CrossRef CAS PubMed.
- H. Hussain, I. R. Green, U. Shamraiz, M. Saleem, A. Badshah, G. Abbas, N. U. Rehman and M. Irshad, Expert Opin. Ther. Pat., 2018, 28, 383–398 CrossRef CAS PubMed.
- X. F. Wang, Q. M. Zhou, Y. Y. Lu, H. Zhang, S. Huang and S. B. Su, Expert Opin. Ther. Targets, 2015, 19, 577–587 CrossRef CAS PubMed.
- F. Zhou, G. R. Wu, D. S. Cai, B. Xu, M. M. Yan, T. Ma, W. B. Guo, W. X. Zhang, X. M. Huang, X. H. Jia, Y. Q. Yang, F. Gao, P. L. Wang and H. M. Lei, Eur. J. Med. Chem., 2019, 178, 623–635 CrossRef CAS PubMed.
- L. Jin, L. Dai, M. Ji and H. Wang, Bioorg. Chem., 2019, 85, 179–190 CrossRef CAS PubMed.
- R. Wang, W. Yang, Y. Fan, W. Dehaen, Y. Li, H. Li, W. Wang, Q. Zheng and Q. Huai, Bioorg. Chem., 2019, 88, 102951 CrossRef CAS PubMed.
- B. Xu, G. R. Wu, X. Y. Zhang, M. M. Yan, R. Zhao, N. N. Xue, K. Fang, H. Wang, M. Chen, W. B. Guo, P. L. Wang and H. M. Lei, Molecules, 2017, 22, 924 CrossRef PubMed.
- Y. Li, L. Feng, Z. F. Song, H. B. Li and Q. Y. Huai, Molecules, 2016, 21, 199 CrossRef PubMed.
- P. Su, Y. Shi, J. Wang, X. Shen and J. Zhang, Anti Cancer Agents Med. Chem., 2015, 15, 980–987 CrossRef CAS PubMed.
- Z. Zhao, H. Song, J. Xie, T. Liu, X. Zhao, X. Chen, X. He, S. Wu, Y. Zhang and X. Zheng, Eur. J. Med. Chem., 2019, 173, 213–227 CrossRef CAS PubMed.
- D. Kim, B. H. Choi, I. G. Ryoo and M. K. Kwak, Cell Death Dis., 2018, 9, 896 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra04961d |
| This journal is © The Royal Society of Chemistry 2019 |