Open Access Article
Open Access ArticleHexachlorobenzene exerts genotoxic effects in a humpback whale cell line under stable exposure conditions†
Jenny Maner‡
 ab,
Michael Burkard‡
ab,
Michael Burkard‡ ac,
Juan Carlos Cassanod,
Susan M. Bengtson Nashc,
Kristin Schirmer
ac,
Juan Carlos Cassanod,
Susan M. Bengtson Nashc,
Kristin Schirmer *abe and
Marc J.-F. Suter
*abe and
Marc J.-F. Suter ab
ab
aDepartment Environmental Toxicology, Eawag, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, Switzerland. E-mail: kristin.schirmer@eawag.ch
bDepartment of Environmental Systems Science, ETH Zürich, 8092 Zürich, Switzerland
cSouthern Ocean Persistent Organic Pollutants Program, Environmental Futures Research Institute, Griffith University, Brisbane, QLD 4108, Australia
dEmpa, Swiss Laboratories for Material Science and Technology, Particle-Biology Interactions Laboratory, 9014 St Gallen, Switzerland
eSchool of Architecture, Civil and Environmental Engineering, EPF Lausanne, 1015 Lausanne, Switzerland
First published on 29th November 2019
Abstract
Humpback whales, like other polar wildlife, accumulate persistent organic pollutants. In Southern hemisphere populations, hexachlorobenzene (HCB) dominates the contaminant profiles. HCB is linked to a variety of health effects and is classified as a group 2B carcinogen, but the mechanism of action is a matter of contention. Potential toxicological effects to humpback whales remain entirely unknown. The recently established humpback whale fibroblast cell line (HuWa) offers an in vitro model for toxicological investigations. We here combine this novel cell line with a passive dosing strategy to investigate whale-specific toxicity of HCB. The relevant partitioning coefficients were determined to produce stable and predictable exposure concentrations in small-scale bioassays. The system was used to assess acute toxicity as well as genotoxicity of HCB to the HuWa cell line. While we found some transient reductions in metabolic activity, measured with the indicator dye alamarBlue, no clear acute toxic effects were discernible. Yet, a significant increase in DNA damage, detected in the alkaline comet assay, was found in HuWa cells exposed to 10 μg L−1 HCB during the sensitive phase of cell attachment. Collectively, this work provides a ready-to-use passive dosing system and delivers evidence that HCB elicits genotoxicity in humpback whale cells.
1. Introduction
Persistent organic pollutants (POPs) deposited in polar regions have been found to bioaccumulate in polar wildlife.1,2 In the Southern hemisphere, hexachlorobenzene (HCB) dominates contaminant profiles,3–7 including those of humpback whales (Megaptera novaeangliae) feeding in Antarctica.8–10HCB exhibits typical legacy POP characteristics such as high stability (>6 years half-life in biota), hydrophobicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow = 5.73, max. water solubility = 5 μg L−1)11,12 and volatility (H = 35 Pa m3 mol−1).13,14 In humans and mice, HCB exposure is associated with low acute toxicity, but chronic exposure has been linked to a variety of health effects, including immunotoxicity, reproductive and developmental toxicity, neurobehavioral impairment, endocrine disruption, hepatic toxicity, kidney damage and cardiotoxicity.15,16 The international agency for research on cancer classified HCB as a group 2B carcinogen interfering with the liver, ovary and the central nervous system.17 However, whether HCB is genotoxic is a matter of debate in the literature.
Kow = 5.73, max. water solubility = 5 μg L−1)11,12 and volatility (H = 35 Pa m3 mol−1).13,14 In humans and mice, HCB exposure is associated with low acute toxicity, but chronic exposure has been linked to a variety of health effects, including immunotoxicity, reproductive and developmental toxicity, neurobehavioral impairment, endocrine disruption, hepatic toxicity, kidney damage and cardiotoxicity.15,16 The international agency for research on cancer classified HCB as a group 2B carcinogen interfering with the liver, ovary and the central nervous system.17 However, whether HCB is genotoxic is a matter of debate in the literature.
Dramatic weight loss has been found to result in the remobilisation of HCB from adipose tissue into the blood stream, and subsequent redistribution to other tissues in mice.18 This may be particularly problematic for humpback whales due to their unique life history traits, involving migration between feeding and breeding grounds, associated with extended periods of fasting and depletion of fat reserves.8,19 To date, however, nothing is known about the species-specific toxicity of HCB to baleen whales. Controlled experimentation on large, free-roaming cetaceans is neither logistically nor ethically feasible, hence novel effect assessment tools have been repeatedly sought by researchers and environmental agencies alike.20,21
Cell lines can be a powerful method to study mechanisms of toxicity and potential species differences.22 Cell lines have been established and successfully applied in toxicity testing for several cetaceans, including a number of Mediterranean dolphin species and Arctic beluga whales.23,24 Recently, we developed a humpback whale fibroblast cell line (HuWa), for the first time opening up the possibility for in vitro effect assessment for this species.25 First toxicological investigations using this cell line highlighted human-whale interspecies differences in sensitivity to typical POP compounds accumulating in the blubber of humpback whales, underscoring the need for species-specific risk assessment.25 HuWa cells have moreover been transfected by exogenous gene transfer with telomerase reverse transcriptase (TERT), which enables long-term preservation of HuWa cell lines.26
While the establishment of the HuWa cell line offers novel opportunities for exploring the toxicity of chemicals to humpback whales, HCB's physico-chemical properties comprise a challenge, especially in small-scale cell bioassay systems, which favour sorption of hydrophobic chemicals and are prone to losses through volatilisation. Most effect evaluation studies to date have been conducted by dissolving HCB in an organic solvent.27–30 However, stable exposure concentrations cannot be reached in this way.31–33 It was, for example, shown that concentrations of a PAH and a chlorobenzene decrease dramatically over time in a solvent-spiked setup: concentrations of benzo(a)pyrene and 1,2,4-trichlorobenzene were reduced to 64% and 11% of the original concentrations, respectively, 48 hours after direct spiking.33 As a consequence, effect concentrations derived from nominal concentrations may grossly underestimate toxicity.33
One approach to achieve well-defined exposure conditions is silicone-based passive dosing. Silicone is chemically inert, biologically compatible, and features high absorption capacities for hydrophobic chemicals; it can thus be ‘loaded’ with hydrophobic chemicals and act as a reservoir. Based on the concentration gradient between the reservoir and the other compartments in the system, the chemical diffuses into the exposure medium where a stable concentration is achieved at the equilibrium level. Thus, losses in the exposure system can be compensated and the need for co-solvents eliminated. Both silicone discs, rods, and O-rings have been used in different toxicity assays and with various classes of organic chemicals, including chlorobenzenes.33–41
In the present study, a ready-to-use passive dosing setup for HCB was established and all the necessary partitioning coefficients were determined. Silicon O-rings were used as a reservoir to achieve stable and predictable HCB concentrations in mammalian cell culture medium. The setup was employed to carry out in vitro cytotoxicity and genotoxicity tests with the novel HuWaTERT cell line. This is the first study focussing on species-specific toxicity tests for humpback whales with HCB.
2. Materials and methods
2.1. Chemicals and materials
All chemicals and solvents were purchased from Sigma-Aldrich (Buchs, Switzerland), except 13C6-hexachlorobenzene (nonane, 100 μg mL−1), which was purchased from Cambridge Isotope Laboratories (Cambridge, UK). Cell culture consumables were purchased from Life Technologies Invitrogen (Basel, Switzerland), except trypsin, which was purchased from Biowest (Nuaill, France), and fetal bovine serum (FBS), which was purchased from Eurobio (Courtaboeuf, France). Cell culture flasks were purchased from TPP (Trasadingen, Switzerland) and cell culture plates from Greiner bio-one (Frickenhausen, Germany). Autosampler vials and crimp caps with silicone/polytetrafluoroethylene (PTFE) septa were purchased from BGB Analytik AG (Böckten, Switzerland), while amber glass vials with PTFE-lined melamine resin screw were purchased from Sigma-Aldrich (Buchs, Switzerland). GERSTEL Twisters® were purchased from GERSTEL GmbH & Co. KG (Mülheim an der Ruhr, Germany), and silicone O-rings (9.92 mm × 2.62 mm) were purchased from Hutchinson Suisse (Langnau am Albis, Switzerland). The OxiSelect Comet Assay Kit was purchased from Cell Biolabs, Inc. (San Diego, CA, USA).2.2. Loading of O-rings with HCB and determination of the partitioning coefficient KLB:sil
All equations relating to the establishment of the passive dosing setup are given in Table S1.† O-rings with an inner and outer diameter of 9.92 and 15.16 mm, respectively, a mass of 258 ± 3 mg (n = 75) (Fig. S1†), and a calculated volume of 0.212 mL were used as passive dosing reservoirs. O-rings were pre-cleaned by Soxhlet extraction using cyclohexane and methanol. Clean O-rings were stored in deionised nanopure H2O (dH2O) at room temperature until use.HCB was loaded onto O-rings by partitioning loading as previously described.33 Briefly, a stock solution of HCB in methanol (100 μg mL−1, Sigma-Aldrich) was diluted to the desired concentration in a loading buffer (LB) of HCB in methanol/H2O 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v) at a volume of 2 mL per O-ring, and the clean O-rings were equilibrated in this LB in amber glass vials (room temperature, shaking at 250 rpm on a benchtop orbital shaker (IKA KS125)). Push loading, a variant of partitioning loading, whereby the proportion of water in the LB is steadily increased, thereby “pushing” the HCB into the silicone, was also tested in the current study, but was found to be less efficient (see ESI Section 1.c. and Table S2†), and was therefore not further used.
40 (v/v) at a volume of 2 mL per O-ring, and the clean O-rings were equilibrated in this LB in amber glass vials (room temperature, shaking at 250 rpm on a benchtop orbital shaker (IKA KS125)). Push loading, a variant of partitioning loading, whereby the proportion of water in the LB is steadily increased, thereby “pushing” the HCB into the silicone, was also tested in the current study, but was found to be less efficient (see ESI Section 1.c. and Table S2†), and was therefore not further used.
The partitioning coefficient between LB and silicone (KLB:sil) is defined by the concentrations of HCB in the two phases at equilibrium. In order to determine KLB:sil, various starting concentrations of HCB in LB were used and the resulting equilibrium concentrations in LB and in silicone analysed. KLB:sil could then be used to predict concentrations on O-rings based on the starting concentration of HCB in LB, or conversely to determine the necessary LB starting concentration to achieve a specified concentration on O-rings.
To identify the maximal loading capacity of silicone, a suspension of solid HCB in methanol/H2O (saturation loading) was used as LB as previously described.34,35,38 For this, the HCB suspension was sonicated for one hour at 4 °C to enhance dissolution and the LB was equilibrated for 1 week. In this setup, suspended HCB crystals served as reservoir to maintain the saturation level in the LB by dissolution, while O-rings were saturated by partitioning from the LB.
2.3. Passive dosing and determination of the partitioning coefficients Ksil:DMEM/F12
Size and density of the O-rings were selected to fit into the wells of a 24-well plate; they float at the surface of the aqueous medium due to the surface tension. Passive dosing was carried out under sterile conditions. For this, loaded O-rings were rinsed first with methanol and then sterile dH2O, dabbed dry and placed into 20 mL amber vials to equilibrate in Dulbecco's Modified Eagle Medium (DMEM)/F12 (2 mL per O-ring, 37 °C, 250 rpm). Partitioning coefficients between silicone and DMEM/F12 (Ksil:DMEM/F12) were calculated from the concentration of HCB in silicone and the equilibrium concentration of HCB in DMEM/F12 for both serum-free (0% FBS) and serum-containing (1% FBS) medium.2.4. Chemical analysis
For quantification of HCB in silicone, each O-ring was extracted by solid–liquid extraction using 10 mL cyclohexane and incubation for 72 hours at 300 rpm in 20 mL amber glass vials. Samples of the LB (1.5 mL) were extracted twice by liquid–liquid extraction with 750 μL cyclohexane and the extracts were combined. Medium samples were extracted by liquid–solid extraction using PDMS-coated magnetic stir bars (GERSTEL Twisters®), which were subsequently extracted three times overnight with 500 μL cyclohexane after which the extracts were combined. 13C6-marked HCB was added as internal standard to all loading buffer and medium samples prior to extraction (nominal final concentration in extract 100 μg L−1) and the extracts were then concentrated to a volume of 100 μL using laminar nitrogen flow. Samples were transferred to glass inserts (100 μL) in autosampler vials (2 mL). All aliquots were analysed using a gas chromatograph coupled to a mass spectrometer (Trace GC Ultra/ITQ900, Thermo Scientific). Mass m/z 284 ([M + 2] isotope) was monitored for the analyte, while mass m/z 294 [M + 6] was monitored for the 13C6-labelled internal standard in order to minimise isotope contribution from the unlabelled analyte (0.36% contribution). For quantification, the chromatographic peak area of m/z 284 was divided by the chromatographic peak area of m/z 294, using 20, 50, 100, 200, 500, 1000, 2000, 5000, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1 HCB for the calibration curve. For O-ring extracts, pure cyclohexane was used as blank, while for loading buffer and medium samples a methanol–water mixture (60
000 μg L−1 HCB for the calibration curve. For O-ring extracts, pure cyclohexane was used as blank, while for loading buffer and medium samples a methanol–water mixture (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) was spiked with 13C6-marked HCB and extracted in the same manner as the samples. The LOD was 5 μg L−1 (signal/noise ≥ 2), and the LOQ 10 μg L−1 (signal/noise ≥ 10).
40) was spiked with 13C6-marked HCB and extracted in the same manner as the samples. The LOD was 5 μg L−1 (signal/noise ≥ 2), and the LOQ 10 μg L−1 (signal/noise ≥ 10).
2.5. Cell culture and exposure setup
In vitro toxicity testing was performed using TERT-transfected cells of the HuWa cell line of humpback whale fibroblasts (HuWaTERT, passages 16–36),26 a cell line established in this research group. Cells were handled under sterile conditions, and all materials used were autoclaved. Cell lines were cultured in DMEM/F12 medium, containing 10% FBS, 0.1 M non-essential amino acids, 1 M sodium pyruvate and 1% penicillin–streptomycin (5.000 U mL−1). Cultures were maintained in 75 cm2 cell culture flasks at 37 °C and 5% CO2. The medium was changed twice a week and at 80–90% confluency, cells were trypsinised, centrifuged (600 × g, 3 min, room temperature) and passaged at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
For toxicity testing, HuWaTERT were either exposed to HCB in a confluent monolayer or during the phase of suspension and attachment to well bottoms. For exposure of cells in confluent monolayer, cells were trypsinised, centrifuged, seeded into the wells of 24 well plates at a density of 1.7 × 104 cells per cm2, and allowed to attach and form confluent monolayers for 48 hours. Exposure medium containing 1% FBS was used in order to allow cells to maintain their normal function while minimising interference of FBS constituents with HCB. The exposure medium was pre-equilibrated with HCB by placing loaded O-rings in 2 mL DMEM/F12 per ring for 20 hours in 20 mL amber glass vials (37 °C, 250 rpm). Then, HCB-loaded O-rings were removed from medium, rinsed with 70% ethanol and sterile dH2O, and air-dried for 30 min, to remove any medium components which may impede the O-rings' staying afloat in the well plates. The culture medium from the wells containing the cell monolayers was exchanged with 700 μL per well of the HCB-pre-equilibrated DMEM/F12 culture medium, and one O-ring per well was carefully placed afloat with sterile metal tweezers. For the negative controls, O-rings were ‘loaded’ in methanol/H2O solution without HCB, and control medium was pre-equilibrated with these blank O-rings. The control medium was then added to wells of cells used as unexposed controls with the blank O-rings placed afloat. Pre-experiments confirmed that the O-rings by themselves did not impact HuWaTERT cell viability; thus, controls without O-rings were subsequently omitted from all tests. Plates were covered with aluminium foil, sealed and incubated under gentle agitation of 30 rpm at 37 °C or, in case of the temperature stress experiment, 30 °C, for up to 24 hours.
For exposure from suspension to attachment, 1.5 × 104 cells per cm2 were seeded in 700 μL of pre-equilibrated DMEM/F12 in 24 well plates, or, for genotoxicity testing, in 1.4 mL pre-equilibrated DMEM/F12 in 12-well plates. One O-ring per well was placed afloat, plates covered with aluminium foil, sealed, and incubated at 37 °C and 30 rpm, for up to 24 hours.
2.6. HCB exposure concentrations
Measured blubber concentrations of HCB in a population of Southern hemisphere humpback whales have been found to range from an average of ∼60 ng glipid−1 post-summer feeding to an average of ∼200 ng glipid−1 about halfway into their seasonal migration.8 No values of blood plasma concentrations of HCB in humpback whales are available; however, a rough estimation may be done based on either the partitioning coefficients for HCB between blubber and plasma derived for bottlenose dolphins,42 or a physiologically-based pharmacokinetic model of HCB distribution in a humpback whale.43 Both approaches yield very similar results of HCB blood plasma concentrations ranging from ∼0.3 to 1.0 μg Lplasma−1 from the beginning to the middle of the fasting period (see ESI Section 1.e. for details†). Hence, to assess the impact of HCB at environmentally relevant as well as elevated concentrations representative of potential peak exposure towards the end of migration, HCB exposure concentrations of 1, 5, and 10 μg L−1 HCB were chosen, assuming that blood plasma concentrations approximately equate exposure concentrations to cells in vivo.2.7. Cytotoxicity testing
Toxicity was evaluated by measuring cell metabolic activity and membrane integrity by means of the fluorescent indicator dyes alamarBlue (AB) and 5-carboxyfluorescein diacetate acetoxymethylester (CFDA-AM), respectively, as previously described.44 AB is based on the cell-permeable, non-fluorescent dye resazurin, which is transformed by mitochondrial, microsomal, or cytoplasmic oxidoreductases to the highly fluorescent resorufin,45 serving as an indicator for cellular metabolism of viable cells.46 CFDA-AM is a non-polar molecule which diffuses into cells, where it is converted into the polar, fluorescent dye, 5-carboxyfluorescein,47 by intracellular esterases. An intact plasma membrane will retain the polar product and thus yield higher fluorescent intensity. Briefly, after exposure for three, six, and 24 hours, the medium was discarded, cells were washed once with phosphate-buffered saline (PBS) and incubated for 25 min with 5% (v/v) AB and 1‰ (v/v) CFDA-AM in PBS at 37 °C. Fluorescence was measured at excitation/emission wavelengths of 530/595 nm and 493/541 nm for AB and CFDA-AM, respectively. The results were expressed as percentage of the unexposed control, which was set to 100%.2.8. Genotoxicity testing
DNA damage was measured by the alkaline comet assay, also called single-cell gel electrophoresis, which detects both single- and double-strand breaks, as well as DNA adducts.48 The principle of this assay is that smaller fractions of DNA migrate faster through agarose gels during electrophoresis. For this purpose, cells are lysed and DNA is denatured prior to a micro-gel electrophoresis. While undamaged DNA migrates in bulk, damaged DNA (single- and double strand breaks) exhibits a characteristic tailing, termed comet, which can be identified visually by DNA staining.Based on the cell viability tests, HuWaTERT cells were more sensitive to HCB during the transition from suspension to attachment (which takes about three hours) compared to established cell monolayers (which takes about 24 hours). Therefore, we decided to assess DNA damage occurring within this sensitive window of suspension to attachment, i.e. after three hours of HCB exposure. The comet assay was performed using the OxiSelect Comet Assay Kit (Cell Biolabs, Inc., San Diego, CA, USA) according to the provided protocol with minor modifications. Briefly, after three hours of HCB exposure during the attachment phase, cells were washed with ice-cold PBS (without Mg2+/Ca2+), trypsinised, and centrifuged. The pellet was washed with ice-cold PBS (without Mg2+/Ca2+) and centrifuged again. Cells were then re-suspended in ice-cold PBS (without Mg2+/Ca2+) at 1 × 105 cells per mL, mixed with preheated agarose (37 °C) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v), and pipetted onto the provided comet slides (75 μL per well). Control and HCB-treated cells were placed in different wells on the same slide. Slides were incubated for 15 minutes at 4 °C in the dark, and subsequently immersed in lysis buffer and incubated overnight at 4 °C in the dark. Next, slides were incubated in alkaline solution for 30 minutes at 4 °C in the dark. Thereafter, electrophoresis was performed in alkaline electrophoresis solution in a small chamber (15 cm diameter, Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 20 V (∼300 mA) for 30 minutes. Following electrophoresis, slides were washed twice with dH2O and once with ethanol and left to dry. Finally, cells were stained with Vista Green DNA dye and analysed by fluorescence microscopy (Leica DMI 6000B, Leica Microsystems, Wetzlar, Germany) at excitation/emission wavelengths of 494/521 nm.
10 (v/v), and pipetted onto the provided comet slides (75 μL per well). Control and HCB-treated cells were placed in different wells on the same slide. Slides were incubated for 15 minutes at 4 °C in the dark, and subsequently immersed in lysis buffer and incubated overnight at 4 °C in the dark. Next, slides were incubated in alkaline solution for 30 minutes at 4 °C in the dark. Thereafter, electrophoresis was performed in alkaline electrophoresis solution in a small chamber (15 cm diameter, Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 20 V (∼300 mA) for 30 minutes. Following electrophoresis, slides were washed twice with dH2O and once with ethanol and left to dry. Finally, cells were stained with Vista Green DNA dye and analysed by fluorescence microscopy (Leica DMI 6000B, Leica Microsystems, Wetzlar, Germany) at excitation/emission wavelengths of 494/521 nm.
2.9. Data evaluation
With regard to the passive dosing set-up, linear regression and the coefficient of determination (R2) were assessed between HCB concentrations in loading buffer and resulting HCB concentrations in silicone, as well as between HCB concentrations in silicone, and resulting HCB concentrations in DMEM/F12. Time to equilibrium was determined by fitting a one-compartment model using MATLAB. The partitioning coefficients were calculated according to the formulae given in Table S1.† Unless otherwise stated, shown data represent the mean of three technical replicates measured in a single experiment, and error bars represent the standard deviation (SD).In case of cell-based experiments, data represent the mean of at least three biological replicates and error bars indicate the 95% confidence interval (CI). Statistical difference was assessed for metabolic activity and membrane integrity of cells exposed to HCB in monolayer or suspension at different time-points. For this, cell viability was compared between HCB concentrations (1, 5, and 10 μg L−1) and the unexposed control. Data were log-transformed and analysed by one-way ANOVA with Dunnett's post hoc test, and by two-tailed unpaired t-test in case of the temperature–stress experiments, for which only 10 μg L−1 HCB concentration was tested. Any value for p < 0.05 was considered significant and marked with an asterisk. The analysis was done using GraphPad Prism Version 5 (La Jolla, CA, USA).
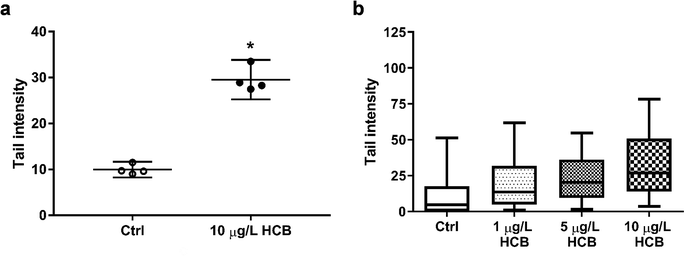
Comet assay images were analysed with Comet Assay IV software (Perceptive Instruments, Bury St Edmunds, UK). Tail intensity was chosen as descriptor and a total of 100 cells each for HCB-treated and control samples were analysed. While the data generated in the comet assay are never normally distributed, the mean of several replicate comet assays will be approximately normally distributed.49 Thus, mean tail intensities of cells exposed to 10 μg L−1 HCB and the unexposed controls were compared by means of a two-tailed unpaired t-test from four biological replicates. Any value for p < 0.05 was considered as significant and marked with an asterisk. The comet assay performed with all three HCB concentrations (1, 5, and 10 μg L−1) is based on technical replicates (one biological replicate) and thus no statistical analysis was performed.
3. Results and discussion
3.1. Characterisation of the passive dosing setup for HCB
Silicone O-rings were chosen as passive dosing reservoir because they are commercially available in various standardised sizes,34 which is important to minimize variance in exposure concentration. They were loaded with HCB by partitioning loading, whereby the chemical partitions from a methanol-based LB into silicone by diffusion until equilibrium between the two phases is reached.33–35,38 Hydrophobic chemicals preferentially partition from this polar solution into the silicone. Excess LB can be washed off with water to avoid contact of the solvent or crystallised chemical with the cells.35 In this study, a loading efficiency of 84 ± 10% was achieved using an LB of methanol/water 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
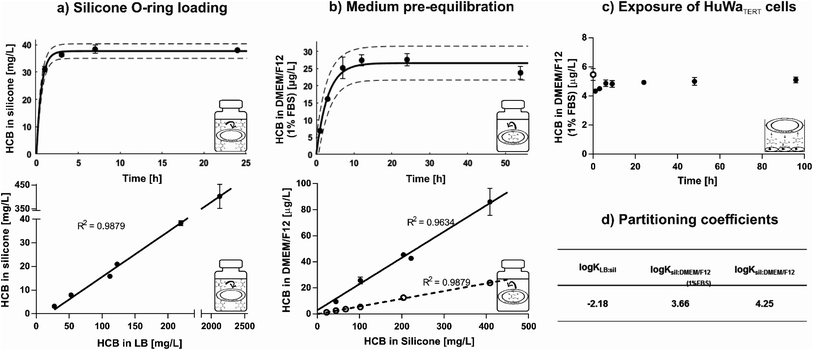
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40% (v/v). In contrast, a loading efficiency of only ∼70% was achieved in a study where hexane was used as LB and evaporated off to force absorption of HCB into the reservoir.50 Equilibrium distribution of HCB between LB and silicone was reached within six hours (Fig. 1a, top) and remained stable for at least five days (Fig. S2†). HCB equilibrium was established from different concentrations in the LB, with a linear relationship between HCB concentrations in LB and on O-rings at equilibrium (Fig. 1a, bottom). The saturation concentration of HCB in silicone was ∼610 mg L−1.
40% (v/v). In contrast, a loading efficiency of only ∼70% was achieved in a study where hexane was used as LB and evaporated off to force absorption of HCB into the reservoir.50 Equilibrium distribution of HCB between LB and silicone was reached within six hours (Fig. 1a, top) and remained stable for at least five days (Fig. S2†). HCB equilibrium was established from different concentrations in the LB, with a linear relationship between HCB concentrations in LB and on O-rings at equilibrium (Fig. 1a, bottom). The saturation concentration of HCB in silicone was ∼610 mg L−1.
Loaded O-rings placed into DMEM/F12 exposure medium revealed rapid release of HCB in the first hours of pre-equilibration. Equilibrium was established within nine hours and remained constant for at least 54 hours in closed glass vials with submerged O-rings (Fig. 1b, top). Release kinetics in serum-free DMEM/F12 were similar (Fig. S3†), but as the standard setup was with 1% FBS, time until equilibrium was determined only for medium with FBS. HCB concentration in exposure medium showed a linear relationship with HCB concentration in the silicone O-ring (Fig. 1b, bottom). While HCB solubility in water is only 5 μg L−1,12 concentrations in serum-free DMEM/F12 of up to 25 μg L−1 have been achieved in this study using passive dosing. In DMEM/F12 with 1% FBS, approximately four times higher HCB concentrations were reached compared with serum-free DMEM/F12 (Fig. 1b, bottom).
Upon transfer of the pre-equilibrated DMEM/F12 into 24-well-plates containing confluent monolayers of HuWaTERT cells, covered with aluminium foil, a new equilibrium established itself within 6 hours, and HCB concentration remained constant for at least 96 hours (Fig. 1c). The same was true if plates were covered with adhesive plastic foil, but due to the plastic foil acting as an additional sink for HCB, a new equilibrium concentration in DMEM/F12 established itself at a slightly lower concentration (Fig. S4†).
The partitioning coefficients for distribution of HCB between LB, silicone, and DMEM/F12 were calculated based on the equilibrium concentration of HCB ([μg L−1]) in these phases (Fig. 1d). For comparison purposes, partitioning coefficients based on the HCB concentration in silicone, defined by mass of silicone ([μg kg−1]), were also calculated, with only slight variations in the resulting values (Table S3†). The determined HCB partitioning coefficient between silicone and serum-free DMEM/F12 (0% FBS), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ksil:DMEM/F12(0% FBS) = 4.25, is slightly lower than the previously published partitioning coefficients for HCB between silicone and water (Table S4†), indicating that the medium components in DMEM/F12 (e.g. glucose, amino acids, salts and vitamins) influence the distribution of HCB slightly in favour of partitioning to the medium, even without the addition of FBS. The partitioning coefficient for HCB in medium with serum (1% FBS), log
Ksil:DMEM/F12(0% FBS) = 4.25, is slightly lower than the previously published partitioning coefficients for HCB between silicone and water (Table S4†), indicating that the medium components in DMEM/F12 (e.g. glucose, amino acids, salts and vitamins) influence the distribution of HCB slightly in favour of partitioning to the medium, even without the addition of FBS. The partitioning coefficient for HCB in medium with serum (1% FBS), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ksil:DMEM/F12(1% FBS) = 3.66, is in line with other findings for chemicals with similar physico-chemical properties (Table S5†). It is lower than the partitioning coefficient for serum-free medium, indicating that serum increases the solubility of HCB and thus drives its distribution into the medium. Serum constituents such as lipids and proteins are known to significantly bind certain chemicals; for example, bovine serum albumin is reported to bind chlorinated substances such as hexachlorobiphenyl.51
Ksil:DMEM/F12(1% FBS) = 3.66, is in line with other findings for chemicals with similar physico-chemical properties (Table S5†). It is lower than the partitioning coefficient for serum-free medium, indicating that serum increases the solubility of HCB and thus drives its distribution into the medium. Serum constituents such as lipids and proteins are known to significantly bind certain chemicals; for example, bovine serum albumin is reported to bind chlorinated substances such as hexachlorobiphenyl.51
An experiment was performed to determine whether loaded O-rings could be re-used for passive dosing. However, it was found that a slightly reduced loading concentration occurs with each usage cycle (Fig. S5†). Thus, new O-rings were loaded for each experiment in our study. However, it is conceivable to re-use the loaded O-rings in order to save starting materials and produce less toxic waste if the decrease in concentration in the silicone, and consequently in the dosed medium, is accounted for.
In summary, we found that equilibrium loading is an efficient method to load HCB onto O-rings, and concentrations in silicone rings can be well predicted from defined LB concentrations. Silicone O-rings present a large enough reservoir and suitable partitioning properties to allow the establishment of precise exposure concentrations for HCB over a wide concentration range, even above the presumed level of water solubility. The determined partitioning coefficients are in agreement with previously published values. Thus, the characterised passive dosing setup is a suitable and convenient method for toxicity testing with HCB.
3.2. Toxicity of HCB to humpback whale fibroblasts
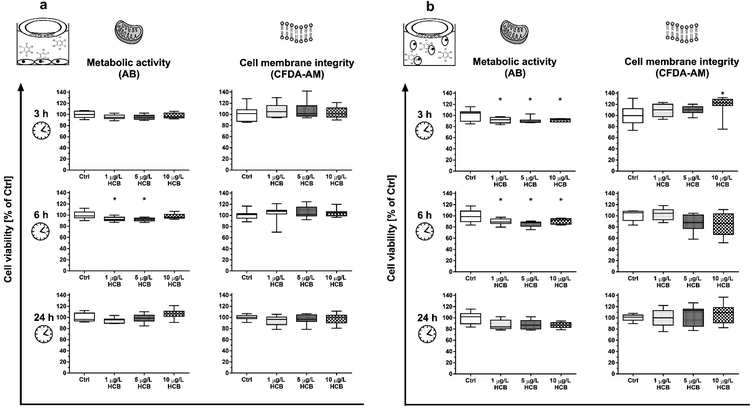
Combinations of HCB with other stress factors did not reveal any clear trends: cells exposed to HCB in a confluent monolayer at a reduced temperature of 30 °C instead of the optimal 37 °C showed slightly reduced metabolic activity after three and 24 hours, but not after six hours (Fig. S6†), while cells exposed to HCB in a confluent monolayer in medium without FBS showed no reduction in cell viability (Fig. S7†).
To our knowledge, no prior study has explored the impact of HCB on vertebrate cell viability under stable exposure conditions. However, other studies have assessed the impact of HCB on different vertebrate cells by means of solvent-mediated dosing. The findings vary for different cell types and concentration ranges, with some studies having dosed HCB at concentrations several orders of magnitude above its maximum water solubility.27,28 Resulting concentration instability may be one of the reasons for divergence in reported concentration-responses as outlined below.
Mouse embryonic fibroblast cells (NIH 3T3), similarly to our own findings, showed a transient decrease in cell viability and a decrease in total cell number within 24 hours at concentrations of 0.1 and 1 μg L−1 HCB.29 As cell-cycle progression was unaffected, the authors attributed the reduction in cell number to apoptosis. In the same study, human embryonic fibroblasts (WS1) exposed to 1 μg L−1 HCB also decreased in cell numbers but without any reduction of cell viability. In this case, cell-cycle arrest and reduced proliferation as a consequence of HCB exposure was held to be the cause of the reduced cell number. A study with rat thyroid cells (FRTL-5) found a significant reduction in cell viability after eight hours exposure to HCB concentrations of 142 and 1424 μg L−1, i.e. one to two orders of magnitude higher than what we used in our study, while, at the more comparable concentration ranges of 1.4 and 14 μg L−1, no effect was observed.28 In contrast, human colon adenocarcinoma cells (Caco-2), exposed to HCB in a confluent monolayer, showed a reduction of viability already at 0.01 μg L−1 HCB, i.e. one order of magnitude lower than our lowest concentration, with a dose-dependent increase in the observed effect up to the maximum tested concentration of 570 μg L−1, but this effect was only observed after 14 days of exposure.27
Membrane integrity in our study appeared to be unaffected by HCB, with the exception of a transient increase in CFDA-AM fluorescence in cells exposed in suspension (Fig. 2b). Being a highly lipophilic compound, it appears likely that HCB affects plasma and organelle membranes. However, literature is controversial about the impact of HCB on cellular membranes. Human embryonic fibroblasts (WS1) were reported to become hyperpermeable from one to three hours after treatment with 0.1 and 1 μg L−1 HCB, while mouse embryonic fibroblast cells (NIH 3T3) only showed membrane damage three hours after exposure to 1 μg L−1 HCB.29 Conversely, a study conducted with the crustacean Squilla mantis found that HCB caused an increase in rigidity of plasma membranes of muscular and gonadic primary cells at a concentration of 14 μg L−1.53 An increase in rigidity – which leads to a decrease in permeability – may also be the cause for the transient increase in CFDA-AM fluorescence we observed. A potential explanation is described in a study conducted with the prokaryote Pseudomonas putida, where a cis–trans-isomerisation of membrane lipids was found to be the cause of an increase in rigidity following exposure to a concentration range of 0.01 to 1 μg L−1 HCB.54 Thus, the transient effect in CFDA-AM fluorescence in HuWaTERT upon exposure to HCB may be attributed to the cells' sensitivity window immediately after seeding.
Overall, while sensitivity of cells to HCB appears to be tissue and species specific,55 HCB does not appear to exert strong acute toxicity at environmentally relevant concentrations in humpback whale cells. This is in line with the fact that HCB is not generally classified as an acutely toxic chemical.56
The International Agency for Research on Cancer (IARC) classifies HCB as a possible carcinogen, but there is some debate as to whether it is genotoxic. A variety of genotoxicity studies has produced predominantly negative findings.17,57–61 However, there have also been some contradictory results. While one study reported negative results for chromosomal aberrations of HCB in human lymphocytes at concentrations of 2.8–22.8 g L−1 and 44 hours exposure,62 a different study found that HCB caused both an increase in the frequency of micronuclei formation and of DNA breaks in primary human lymphocytes at concentrations of 30 to 160 mg L−1 and 20 hours exposure.30 The same study found that, using the same exposure conditions, there was also an increase in micronuclei formation in primary rat hepatocytes, but without concurrent increase in DNA breaks, indicating an aneugenic mechanism of action. The authors concluded that HCB is a weak genotoxic carcinogen. Using concentrations better comparable to ours, Salmon et al. (2002)29 found that both mouse and human embryonic fibroblasts showed a significant increase in DNA breaks following four hours of exposure to 0.1 and 1 μg L−1 HCB. Finally, a significant increase in DNA breaks was also found in human colon carcinoma cells exposed to 0.1 and 114 μg L−1 HCB for 14 days.27
It is difficult to compare the results of different genotoxicity studies not only due to different species and endpoints assessed, but also due to the different exposure setups and vast differences in HCB concentrations applied. Nonetheless, there is previous evidence in the literature that HCB has the potential to cause DNA damage. Our results add to this body of evidence. In the absence of significant cytotoxic effects as seen in this study, the observed DNA fragmentation is unlikely to be a result of apoptotic processes of DNA disintegration, suggesting that HCB causes DNA single and/or double strand breaks at environmentally relevant concentrations in humpback whale fibroblasts. In the future, it would be interesting to investigate the apparent concentration dependence and time course of DNA damage, as well as to explore the underlying mechanism of action not only for DNA damage but as well the transient effects seen on cell viability.
Taken together, we established a ready-to-use passive dosing setup for exposure experiments with HCB, providing all the necessary partitioning coefficients, and thus offering a stable system and controlled conditions applicable to a broad variety of in vitro assays. By applying the unique HuWaTERT cell line in this exposure set-up, we have shown that HCB has the potential to reduce cell viability, and to clearly cause DNA damage. These findings are of particular relevance for the toxicity assessment of HCB in humpback whales because highest organism-internal exposures occur when females are pregnant and nursing, thus coinciding with vulnerable developmental stages. Our combined passive dosing-HuWaTERT system therefore offers a wealth of opportunities to study the action of Antarctic priority pollutants on humpback whale cells in a species-specific manner.
Abbreviations
| AB | AlamarBlue |
| CFDA-AM | 5-Carboxyfluorescein diacetate acetoxymethylester |
| CI | Confidence interval |
| DMEM | Dulbecco's modified Eagle medium |
| FBS | Fetal bovine serum |
| HCB | Hexachlorobenzene |
| IARC | International Agency for Research on Cancer |
| LB | Loading buffer |
| PBS | Phosphate-buffered saline |
| POPs | Persistent organic pollutants |
| PTFE | Polytetrafluoroethylene |
| SD | Standard deviation |
| TERT | Telomerase reverse transcriptase |
| Caco-2 | Human colon adenocarcinoma cell line |
| CHL | Chinese hamster lung cell line |
| FRTL-5 | Rat thyroid cell line |
| HuWa | Humpback whale fibroblast cell line |
| NIH 3T3 | Mouse embryonic fibroblast cell line |
| WS1 | Human embryonic fibroblast cell line |
Author contributions
All authors contributed to the design of this study. JM and MB carried out the experiments and evaluated all the data, with the exception of the comet assay, which was analysed by JCC. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Funding sources
This work was supported by Eawag internal funds. MB acknowledges initial support by a PhD scholarship from Griffith University. JCC acknowledges funding from the NanoScreen Materials Challenge co-funded by the Competence Centre for Materials Science and Technology (CCMX).Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to René Schönenberger for support in chemical analysis, as well as Melanie Fischer and Nadine Bramaz for support in cell culture and cytotoxicity testing.References
- R. J. Letcher, J. O. Bustnes, R. Dietz, B. M. Jenssen, E. H. Jørgensen, C. Sonne, J. Verreault, M. M. Vijayan and G. W. Gabrielsen, Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish, Sci. Total Environ., 2010, 408, 2995–3043 CrossRef CAS PubMed.
- R. Kallenborn, H. Hung and E. Brorström-Lundén, Atmospheric long-range transport of persistent organic pollutants (POPs) into polar regions, Compr. Anal. Chem., 2015, 67, 411–432 CAS.
- A. L. Chiuchiolo, R. M. Dickhut, M. A. Cochran and H. W. Ducklow, Persistent organic pollutants at the base of the Antarctic marine food web, Environ. Sci. Technol., 2004, 38, 3551–3557 CrossRef CAS PubMed.
- S. M. Bengtson Nash, A. H. Poulsen, S. Kawaguchi, W. Vetter and M. Schlabach, Persistent organohalogen contaminant burdens in Antarctic krill (Euphausia superba) from the eastern Antarctic sector: a baseline study, Sci. Total Environ., 2008, 407, 304–314 CrossRef CAS PubMed.
- S. M. Bengtson Nash, S. J. Wild, D. W. Hawker, R. A. Cropp, H. Hung, F. Wania, H. Xiao, P. Bohlin-Nizzetto, A. Bignert and S. Broomhall, Persistent organic pollutants in the East Antarctic atmosphere: inter-annual observations from 2010 to 2015 using high-flow-through passive sampling, Environ. Sci. Technol., 2017, 51, 13929–13937 CrossRef CAS PubMed.
- S. M. Bengtson Nash, A. H. Poulsen, S. Kawaguchi and M. Schlabach, Hexachlorobenzene in a Southern Ocean food web; contaminant accumulation & global comparisons, Organohalogen Compd., 2007, 69, 1685–1688 Search PubMed.
- R. Kallenborn, K. Breivik, S. Eckhardt, C. R. Lunder, S. Manø, M. Schlabach and A. Stohl, Long-term monitoring of persistent organic pollutants (POPs) at the Norwegian Troll station in Dronning Maud Land, Antarctica, Atmos. Chem. Phys., 2013, 13, 6983–6992 CrossRef.
- S. M. Bengtson Nash, C. A. Waugh and M. Schlabach, Metabolic concentration of lipid soluble organochlorine burdens in the blubber of southern hemisphere humpback whales through migration and fasting, Environ. Sci. Technol., 2013, 47, 9404–9413 CrossRef CAS PubMed.
- P. R. Dorneles, J. Lailson-Brito, E. R. Secchi, A. C. Dirtu, L. Weijs, L. Dalla Rosa, M. Bassoi, H. A. Cunha, A. F. Azevedo and A. Covaci, Levels and profiles of chlorinated and brominated contaminants in Southern Hemisphere humpback whales, Megaptera novaeangliae, Environ. Res., 2015, 138, 49–57 CrossRef CAS PubMed.
- K. Das, G. Malarvannan, A. Dirtu, V. Dulau, M. Dumont, G. Lepoint, P. Mongin and A. Covaci, Linking pollutant exposure of humpback whales breeding in the Indian Ocean to their feeding habits and feeding areas off Antarctica, Environ. Pollut., 2017, 220, 1090–1099 CrossRef CAS PubMed.
- J. De Bruijn, F. Busser, W. Seinen and J. Hermens, Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the “slow-stirring” method, Environ. Toxicol. Chem., 1989, 8, 499–512 CrossRef CAS.
- W. Y. Shiu, F. Wania, H. Hung and D. Mackay, Temperature dependence of aqueous solubility of selected chlorobenzenes, polychlorinated biphenyls, and dibenzofuran, J. Chem. Eng. Data, 1997, 42, 293–297 CrossRef CAS.
- J. L. Barber, A. J. Sweetman, D. Van Wijk and K. C. Jones, Hexachlorobenzene in the global environment: emissions, levels, distribution, trends and processes, Sci. Total Environ., 2005, 349, 1–44 CrossRef CAS PubMed.
- L. M. Jantunen and T. F. Bidleman, Henry's law constants for hexachlorobenzene, p,p′-DDE and components of technical chlordane and estimates of gas exchange for Lake Ontario, Chemosphere, 2006, 62, 1689–1696 CrossRef CAS PubMed.
- ATSDR, Toxicological Profile for Hexachlorobenzene, Atlanta, GA, 2013, vol. 2013 Search PubMed.
- USEPA, Provisional Peer-Reviewed Toxicity Values for Hexachlorobenzene, Cincinnati, OH, 2010 Search PubMed.
- IARC, Hexachlorobenzene, IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, 2001, vol. 79 Search PubMed.
- R. J. Jandacek, Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene, Am. J. Physiol.: Gastrointest. Liver Physiol., 2005, 288, G292–G299 CrossRef CAS PubMed.
- J. Acevedo, K. Rasmussen, F. Félix, C. Castro, M. Llano, E. Secchi, M. T. Saborío, A. Aguayo-Lobo, B. Haase, M. Scheidat, L. Dalla-Rosa, C. Olavarría, P. Forestell, P. Acuña, G. Kaufman and L. A. Pastene, Migratory destinations of humpback whales from the Magellan Strait feeding ground, Southeast Pacific, Mar. Mammal Sci., 2007, 23, 453–463 CrossRef.
- S. M. Bengtson Nash, et al., Report of the IWC Pollution 2000+ Phase II Workshop, J. Cetacean Res. Manage., 2011, 12(supp.), 421–436 Search PubMed.
- L. Weijs and A. Zaccaroni, Toxicology of marine mammals: new developments and opportunities, Arch. Environ. Contam. Toxicol., 2016, 70, 1–8 CrossRef CAS PubMed.
- B. J. Blaauboer, The long and winding road of progress in the use of in vitro data for risk assessment purposes: from ‘carnation test’ to integrated testing strategies, Toxicology, 2015, 332, 4–7 CrossRef CAS PubMed.
- M. C. Fossi, L. Marsili, S. Casini and D. Bucalossi, Development of new-tools to investigate toxicological hazard due to endocrine disruptor organochlorines and emerging contaminants in Mediterranean cetaceans, Mar. Environ. Res., 2006, 62, 200–204 CrossRef PubMed.
- J. M. Gauthier, H. Dubeau and E. Rassart, Induction of micronuclei in vitro by organochlorine compounds in beluga whale skin fibroblasts, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 1999, 439, 87–95 CrossRef CAS.
- M. Burkard, D. Whitworth, K. Schirmer and S. M. Bengtson Nash, Establishment of the first humpback whale fibroblast cell lines and their application in chemical risk assessment, Aquat. Toxicol., 2015, 167, 240–247 CrossRef CAS PubMed.
- M. Burkard, S. Bengtson Nash, G. Gambaro, D. Whitworth and K. Schirmer, Lifetime extension of humpback whale skin fibroblasts and their response to lipopolysaccharide (LPS) and a mixture of polychlorinated biphenyls (Aroclor), Cell Biol. Toxicol., 2019, 35, 387–398 CrossRef CAS PubMed.
- H. Chalouati, E. Boutet, B. Metais, E. Fouche, M. M. Ben Sâad and L. Gamet-Payrastre, DNA damage and oxidative stress induced at low doses by the fungicide hexachlorobenzene in human intestinal Caco-2 cells, Toxicol. Mech. Methods, 2015, 25, 448–458 CAS.
- F. Chiappini, C. Pontillo, A. S. Randi, L. Alvarez and D. L. K. De Pisarev, Reactive oxygen species and extracellular signal-regulated kinase 1/2 mediate hexachlorobenzene-induced cell death in frtl-5 rat thyroid cells, Toxicol. Sci., 2013, 134, 276–290 CrossRef CAS PubMed.
- M. L. Salmon, S. G. Madanagopal, R. Blando, J. Berner and K. Williams, Effects of hexachlorobenzene on embryonic mammalian cells, Toxicol. In Vitro, 2002, 16, 539–548 CrossRef CAS PubMed.
- R. Canonero, G. B. Campart, F. Mattioli, L. Robbiano and A. Martelli, Testing of p-dichlorobenzene and hexachlorobenzene for their ability to induce DNA damage and micronucleus formation in primary cultures of rat and human hepatocytes, Mutagenesis, 1997, 12, 35–39 CrossRef CAS PubMed.
- R. Schreiber, R. Altenburger, A. Paschke and E. Küster, How to deal with lipophilic and volatile organic substances in microtiter plate assays, Environ. Toxicol. Chem., 2008, 27, 1676 CrossRef CAS PubMed.
- K. Tanneberger, A. Rico-Rico, N. I. Kramer, F. J. M. Busser, J. L. M. Hermens and K. Schirmer, Effects of solvents and dosing procedure on chemical toxicity in cell-based in vitro assays, Environ. Sci. Technol., 2010, 44, 4775–4781 CrossRef CAS PubMed.
- N. I. Kramer, F. J. M. Busser, M. T. T. Oosterwijk, K. Schirmer, B. I. Escher and J. L. M. Hermens, Development of a partition-controlled dosing system for cell assays, Chem. Res. Toxicol., 2010, 23, 1806–1814 Search PubMed.
- K. E. C. Smith, G. J. Oostingh and P. Mayer, Passive dosing for defined & constant exposure of hydrophobic organic compounds during in Vitro toxicity tests, Chem. Res. Toxicol., 2010, 23, 55–65 Search PubMed.
- K. E. C. Smith, N. Dom, R. Blust and P. Mayer, Controlling and maintaining exposure of hydrophobic organic compounds in aquatic toxicity tests by passive dosing, Aquat. Toxicol., 2010, 98, 15–24 CrossRef CAS PubMed.
- K. E. C. Smith, M. B. Heringa, M. Uytewaal and P. Mayer, The dosing determines mutagenicity of hydrophobic compounds in the Ames II assay with metabolic transformation: passive dosing versus solvent spiking, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2013, 750, 12–18 CrossRef CAS PubMed.
- T. B. Seiler, N. Best, M. M. Fernqvist, H. Hercht, K. E. C. Smith, T. Braunbeck, P. Mayer and H. Hollert, PAH toxicity at aqueous solubility in the fish embryo test with Danio rerio using passive dosing, Chemosphere, 2014, 112, 77–84 CrossRef CAS PubMed.
- G. J. Oostingh, K. E. C. Smith, U. Tischler, I. Radauer-Preiml and P. Mayer, Differential immunomodulatory responses to nine polycyclic aromatic hydrocarbons applied by passive dosing, Toxicol. In Vitro, 2015, 29, 345–351 CrossRef CAS PubMed.
- D. Gilbert, P. Mayer, M. Pedersen and A. M. Vinggaard, Endocrine activity of persistent organic pollutants accumulated in human silicone implants – Dosing in vitro assays by partitioning from silicone, Environ. Int., 2015, 84, 107–114 CrossRef CAS PubMed.
- F. Fischer, L. Böhm, S. Höss, C. Möhlenkamp, E. Claus, R.-A. Düring and S. Schäfer, Passive dosing in chronic toxicity tests with the Nematode Caenorhabditis elegans, Environ. Sci. Technol., 2016, 50, 9708–9716 CrossRef CAS PubMed.
- H. Schug, F. Begnaud, C. Debonneville, F. Berthaud, S. Gimeno and K. Schirmer, TransFEr: a new device to measure the transfer of volatile and hydrophobic organic chemicals across an in vitro intestinal fish cell barrier, Anal. Methods, 2018, 10, 4394–4403 RSC.
- J. E. Yordy, R. S. Wells, B. C. Balmer, L. H. Schwacke, T. K. Rowles and J. R. Kucklick, Partitioning of persistent organic pollutants between blubber and blood of wild bottlenose dolphins: implications for biomonitoring and health, Environ. Sci. Technol., 2010, 44, 4789–4795 CrossRef CAS PubMed.
- R. Cropp, S. M. Bengtson Nash and D. Hawker, A model to resolve organochlorine pharmacokinetics in migrating humpback whales, Environ. Toxicol. Chem., 2014, 33, 1638–1649 CrossRef CAS PubMed.
- K. Schirmer, A. G. J. Chan, B. M. Greenberg, D. G. Dixon and N. C. Bols, Methodology for demonstrating and measuring the photocytotoxicity of fluoranthene to fish cells in culture, Toxicol. In Vitro, 1997, 11, 107–119 CrossRef CAS PubMed.
- J. O'Brien, I. Wilson, T. Orton and F. Pognan, Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity, Eur. J. Biochem., 2000, 267, 5421–5426 CrossRef PubMed.
- R. C. Ganassin, K. Schirmer and N. C. Bols, in The Laboratory Fish, Elsevier, 2000, pp. 631–651 Search PubMed.
- V. R. Dayeh, N. C. Bols, K. Schirmer and L. E. J. Lee, The use of fish-derived cell lines for investigation of environmental contaminants, Curr. Protoc. Toxicol., 2004, 15, 1.5.1–1.5.17 CrossRef PubMed.
- P. L. Olive and J. P. Banáth, The comet assay: a method to measure DNA damage in individual cells, Nat. Protoc., 2006, 1, 23–29 CrossRef CAS PubMed.
- D. P. Lovell and T. Omori, Statistical issues in the use of the comet assay, Mutagenesis, 2008, 23, 171–182 CrossRef CAS PubMed.
- P. Mayer, J. Wernsing, J. Tolls, P. G. J. De Maagd and D. T. H. M. Sijm, Establishing and controlling dissolved concentrations of hydrophobic organics by partitioning from a solid phase, Environ. Sci. Technol., 1999, 33, 2284–2290 CrossRef CAS.
- M. M. Becker and W. Gamble, Determination of the binding of 2,4,5,2′,4′,5′-hexachlorobiphenyl by low density lipoprotein and bovine serum albumin, J. Toxicol. Environ. Health, 1982, 9, 225–234 CrossRef CAS PubMed.
- N. A. Dorfman, C. I. Civin and J. R. Wunderlich, Susceptibility of adherent versus suspension target cells derived from adherent tissue culture lines to cell-mediated cytotoxicity in rapid 51Cr-release assays, J. Immunol. Methods, 1980, 32, 127–139 CrossRef CAS PubMed.
- A. Dell'Anno, F. Raffaelli, R. Danovaro, L. Nanetti, A. Vignini, C. Moroni and L. Mazzanti, Cytotoxic effects induced by hexachlorobenzene in Squilla mantis (L.) (Crustacea, Stomatopoda), Environ. Toxicol., 2008, 23, 9–14 CrossRef PubMed.
- M. Vodovnik, M. Bistan, M. Zorec and R. M. Logar, Membrane changes associated with exposure of pseudomonas putida to selected environmental pollutants and their possible roles in toxicity, Acta Chim. Slov., 2012, 59, 83–88 CAS.
- A. Delisle, E. Ferraris and I. Plante, Chronic exposure to hexachlorobenzene results in down-regulation of connexin43 in the breast, Environ. Res., 2015, 143, 229–240 CrossRef CAS PubMed.
- B. Starek-Świechowicz, B. Budziszewska and A. Starek, Hexachlorobenzene as a persistent organic pollutant: toxicity and molecular mechanism of action, Pharmacol. Rep., 2017, 69, 1232–1239 CrossRef PubMed.
- S. Haworth, T. Lawlor, K. Mortelmans, W. Speck and E. Zeiger, Salmonella mutagenicity test results for 250 chemicals, Environ. Mutagen., 1983, 5(suppl. 1), 1–142 Search PubMed.
- T. Górski, E. Górska, D. Górecka and M. Sikora, Hexachlorobenzene is non-genotoxic in short-term tests, IARC Sci. Publ., 1986, 77, 399–401 Search PubMed.
- P. Siekel, I. Chalupa, J. Beno, M. Blasko, J. Novotný and J. Burian, A genotoxicological study of hexachlorobenzene and pentachloroanisole, Teratog. Carcinog. Mutagen., 1991, 11, 55–60 CrossRef CAS PubMed.
- D. J. Brusick, Genotoxicity of hexachlorobenzene and other chlorinated benzenes, IARC Sci. Publ., 1986, 77, 393–397 CAS.
- M. Ishidate, Data book of chromosomal aberration test in vitro, Elsevier Science & Technology, Oxford UK, 2nd revise, 1988 Search PubMed.
- S. Ennaceur, D. Ridha and R. Marcos, Genotoxicity of the organochlorine pesticides 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and hexachlorobenzene (HCB) in cultured human lymphocytes, Chemosphere, 2008, 71, 1335–1339 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05352b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |