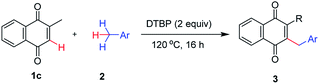Open Access Article
Open Access ArticleMetal-free oxidative cross-dehydrogenative coupling of quinones with benzylic C(sp3)–H bonds†
Yu Dongab,
Jian Yangab,
Shuai Hec,
Zhi-Chuan Shic,
Yu Wangd,
Xiao-Mei Zhang a and
Ji-Yu Wang
a and
Ji-Yu Wang *a
*a
aChengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu 610041, P. R. China. E-mail: Jiyuwang@cioc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cSouthwest Minzu University, Chengdu 610041, P. R. China
dGuizhou Education University, Guiyang 550018, P. R. China
First published on 2nd September 2019
Abstract
A metal-free cross-dehydrogenative coupling of quinones with toluene derivatives has been established. A series of quinones were subjected to reaction with toluene derivatives in the presence of di-tertbutyl peroxide (DTBP) for direct synthesis of benzylquinones. The method exhibits good functional group tolerance, and desired products were obtained in moderate to good yields. Meanwhile, a radical pathway was proposed to describe the cross-dehydrogenative coupling of quinones with toluene derivatives.
Introduction
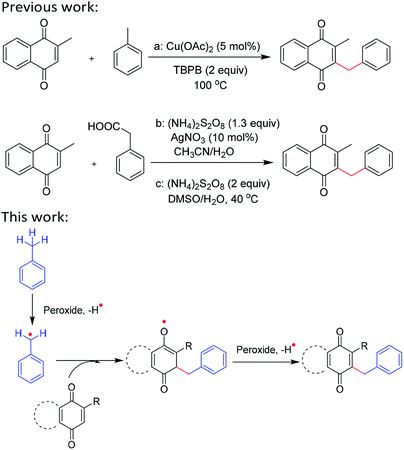
Cross-dehydrogenative coupling reactions (CDC) via transformations of C–H bond into other bonds are emerging as a subclass of C–H functionalizations which have been established as effective and robust methods for the preparation of valuable fragments of pharmaceuticals and intermediates of natural products.1 CDC reactions possess the atom- and step-economical nature avoiding the preparation of the prerequisite functional groups (necessary for classical cross-coupling reactions) which have the perspectives of new chemistry development in developing green chemistry and sustainable procedures.2 Transition-metal (TM)-catalyzed CDC is the most common reaction.3 However, it suffers from various drawbacks, such as expensive TM catalysts, and heavy metal residues in pharmaceutical products. Thus, TM-free CDC reactions have recently come of age and they offer greener approaches, avoid expensive TM catalysts and the challenges involved in the removal of toxic metal residues.4 Hence, CDC reactions have attracted the attention of organic chemists for the preparation of C–C bonds under metal-free conditions in academic as well as industrial research.The quinone is an important type of scaffold found in a number of bioactive natural products and pharmaceutical molecules,5 which show a wide range of biological activities including anticancer, antifungal, antibacterial, antiprotozoal, antiviral, anti-inflammatory, neurological, antiplasmodial, and trypanocidal activities.6 Among the derivatives of quinone, 3-benzylmenadiones represent an extremely important type of bioactive compound which the antimalarial activity of 3-benzylmenadiones results from a subtle interplay between bioactivation, fine-tuned redox properties, and interactions with crucial targets of P. falciparum.7 Additionally, 2-benzyl-1,4-naphthoquinone is also important as an intermediate for the synthesis of biologically active compounds. On the other hand, with the rapid development of free radical chemistry, radical oxidative coupling reaction have emerged as a powerful tool in organic synthesis.8 Hence, the application of the radical oxidative coupling strategy in constructing of quinones derivatives has been attracted much attention by organic chemists. For example, Duan's group reported an efficient and elegant synthesis of 3-benzylcoumarins using a TBPB/Cu(OAc)2-catalyzed reaction via an intermolecular hydrogen abstraction reaction between the coumarins and toluene (Scheme 1a) in 2014.9 Nonetheless, only one reaction of 2-methyl-1,4-naphthoquinone with toluene proceeded to afford 2-benzyl-3-methyl-1,4-naphthoquinone in 56% yield. Zhang's group developed the radical alkylation of 1,4-naphthoquinone with various fatty acids in the presence of (NH4)2S2O8 and AgNO3 (Scheme 1b).10 In 2018, Lee's group discovered a novel route to C–H alkylation of N-heteroarenes and quinones with alkylcarboxylic acids employing a (NH4)2S2O8 catalyst under mild conditions (Scheme 1c).11 Despite the undisputable advance in these papers, there is no mention that organic oxidants oxidized cross-dehydrogenative coupling of quinones with benzylic C(sp3)–H bonds under metal-, photocatalyst-, and light-free conditions.
As we known, benzylic radicals are common intermediates which have been extensively utilized in synthetic organic chemistry.12 In these processes, a variety of reactions initiated with benzylic radicals were reported.13 Among the generation of the reactive benzylic radicals, the oxidative activation of sp3 C–H bonds of alkylarenes by hydrogen atom abstraction has been considered as one of the most popular methods to access such species. On the basis of the above and previous work,14 we envisioned that rich electron benzylic radicals15 could nucleophilic attack the highly electron-deficient double bond of quinones16 to synthesize 2-benzyl-quinones derivatives without any catalyst (Scheme 1). Herein we reported metal-free oxidative cross-dehydrogenative coupling of quinones with benzylic C(sp3)–H bonds.
Results and discussion
Following our effort for the construction of a broad range of 2-benzyl-quinones as potential antimalarial agents. Herein we decided to investigate the use of oxidizing reagents for the direct benzylation of quinones with alkylbenzenes. Initially, we evaluated various reaction conditions for the direct coupling of 2-methyl-1,4-naphthoquinone (1c) with toluene (2a). To our delight, we conducted our investigation by reacting 2-methyl-1,4-naphthoquinone 1c (0.4 mmol) with toluene 2a (2 mL) in the presence of 2.0 equiv. of DTBP at 120 °C for 16 h. The reaction proceeded and afforded the expected product of 2-benzyl-3-methylnaphthalene-1,4-dione 3ca in a good yield (86%, Table 1, entry 1). The reaction could not take place at all if H2O2 (30% aqueous solution), PIFA, DDQ, BQ, K2S2O8, or oxone was employed as the oxidant (Table 1, entries 2–7). And the use of other oxidants such as BPO, PhI(OAc)2, IBX, TBHP (70% aqueous solution), TBPB, or DCP did not provide better results (Table 1, entries 8–13). Almost all of the starting material 1c remained in the reactions with these examined oxidants (entries 2–13). When the amount of DTBP was reduced to 1.0 equiv., a decrease in the yield of product 3ca to 55% was observed (Table 1, entry 14). Further increases in the loading of the oxidant did not result in any improvement (Table 1, entry 15). When the reaction was performed at 100 °C, almost no product was obtained (Table 1, entry 16). No significant effect on the yield of 3ca was found when the reaction was conducted at 140 °C (Table 1, entry 17). Changing the reaction time didn't led to the improvement of the product yield (Table 1, entries 18–19). When the reaction was performed under a N2 atmosphere, it did not result in any improvement of the yield (81%, Table 1, entry 20). Notably, the addition of a metal catalyst, Cu(OAc)2 (10 mol%), suppressed the transformation slightly and the yield reduced (Table 1, entry 21). As per our expectation, no product was detected in the absence of DTBP or other oxidants which the oxidants played a pivotal role in obtaining the desired product (Table 1, entry 22).| Entry | Oxidantb (equiv.) | T (°C) | t (h) | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: unless otherwise noted, the reaction was carried out with 1c (0.4 mmol), 2a (2 mL), oxidant (0.8 mmol) under sealed tube.b DTBP: di-tertbutyl peroxide. H2O2: 30% aqueous solution. PIFA = [Bis(trifluoroacetoxy)iodo] benzene. DDQ: 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. BQ: 1,4-benzoquinone. BPO: benzoyl peroxide. IBX = 2-iodoxybenzoic acid. TBHP: 70% aqueous solution. TBPB: tert-butyl peroxybenzoate. DCP: dicumyl peroxide.c Isolated yields.d Under a N2 atmosphere.e Cu(OAc)2 (0.04 mmol) was added.f NR = no reaction.g Without oxidant. | ||||
| 1 | DTBP (2) | 120 | 16 | 86 |
| 2 | H2O2 (2) | 120 | 16 | NR |
| 3 | PIFA (2) | 120 | 16 | NR |
| 4 | DDQ (2) | 120 | 16 | NR |
| 5 | BQ (2) | 120 | 16 | NR |
| 6 | K2S2O8 (2) | 120 | 16 | NR |
| 7 | Oxone (2) | 120 | 16 | NR |
| 8 | BPO (2) | 120 | 16 | 29 |
| 9 | PhI(OAc)2 (2) | 120 | 16 | 46 |
| 10 | IBX (2) | 120 | 16 | 17 |
| 11 | TBHP (2) | 120 | 16 | 15 |
| 12 | TBPB (2) | 120 | 16 | 21 |
| 13 | DCP (2) | 120 | 16 | 19 |
| 14 | DTBP (1) | 120 | 16 | 55 |
| 15 | DTBP (4) | 120 | 16 | 85 |
| 16 | DTBP (2) | 100 | 16 | Trace |
| 17 | DTBP (2) | 140 | 16 | 83 |
| 18 | DTBP (2) | 120 | 8 | 67 |
| 19 | DTBP (2) | 120 | 24 | 80 |
| 20 | DTBP (2) | 120 | 16 | 81d |
| 21 | DTBP (2) | 120 | 16 | 70e |
| 22 | Nog | 120 | 16 | NRf |
On the basis of the above results, the best oxidative coupling conditions involved 0.4 mmol of 2-methyl-1,4-naphthoquinone (1c), 2 mL of toluene (2a), and 2 equiv. of DTBP at 120 °C.
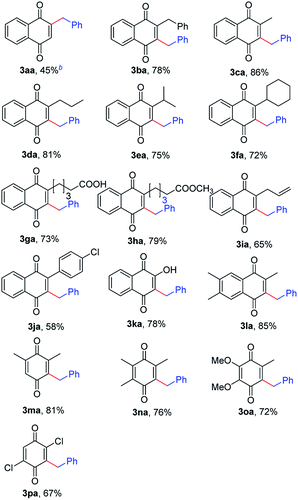
With the optimized reaction conditions in hand, a series of quinones and alkylbenzenes as the substrates were investigated to demonstrate the substrate scope. First, the variation in the quinones part of the reaction was studied (Table 2). The naphthoquinone reacted smoothly to give monobenzylnaphthoquinone (3aa, 45%) and dibenzylnaphthoquinone (3ba, 45%), respectively. Alkyl-substituted naphthoquinones with functional groups including benzyl, methyl, n-propyl, isopropyl, cyclohexyl and long-chain alkyl groups were proceeded smoothly to afford the corresponding products in moderate to good yields (72–86%, 3ba–3ha). Pleasingly, the sensitive carboxyl and ester groups at the β-position of naphthoquinone was compatible with the reaction conditions. Notably, naphthoquinone bearing allyl substituent also worked well under this protocol and afforded the desired product 3ia in moderate yield (65%). Due to steric hindrance caused by the phenyl ring present at the C-2 position, electron-deficient arene substituted naphthoquinone only produced a 58% yield of 3ja. Although the substrate scope did not explicate decent chemoselectivity, the results with hydroxyl substituted naphthoquinone 1k was encouraging while considering the radical quenching nature of hydroxy group. The reaction was found to work well with 2,6,7-trimethylnaphthoquinone in the system giving the corresponding products 3la in good yield. The reaction with 2,6-dimethyl benzoquinone 1m can be directly to monobenzylation (3ma, 81%). Benzoquinones bearing methyl and methoxy groups were also employed, affording corresponding benzylation products 3na and 3oa in moderate yield, respectively. The reaction conditions are well-tolerated by chloro (1p) substituted benzoquinone which would be vulnerable in Pd catalyzed transformations.
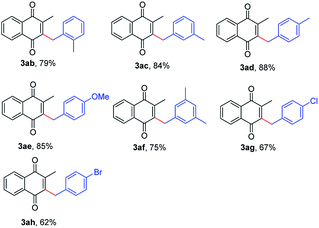
In addition to toluene, we also examined other benzylic hydrocarbons for this transformation. The reaction tolerated a series of toluene derivatives bearing electron-donating or -withdrawing groups, leading to the desired products in moderate to good yields (Table 3, 3ab–3ah). Remarkably, several functional groups such as chlorine and bromine group were tolerated well under the reaction conditions (3ag and 3ah). More importantly, when substrates with multiple methyl groups were used, such as xylenes and mesitylene, only monobenzylation products were obtained in good yields with high selectivities (3ab, 3ac, 3ad and 3af).
| a Reaction conditions: 1c (0.4 mmol), 2 (2 mL), DTBP (2.0 equiv.), 120 °C, 16 h under sealed tube. Isolated yield based on 1c. |
|---|
 |
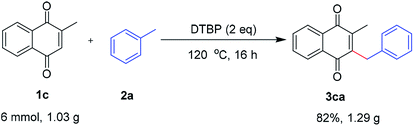
In addition, the scalability of this reaction was carried out by using 1c (6 mmol, 1.03 g) with 2a under the developed optimal reaction conditions. As expected, the desired product 3ca was obtained in 82% yields. These results suggested that the methodology is an economic and practical process for the preparation of benzylquinones (Scheme 2).
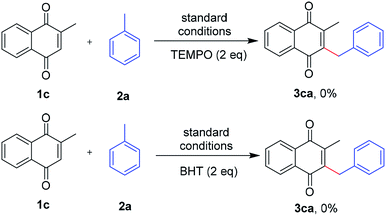
To elucidate the mechanism of the coupling reaction, several control experiments were carried out. Initially, when a radical inhibitor, TEMPO (2,2,6,6-tetra-methyl-piperidine-N-oxyl) or BHT (2,6-di-tert-butyl-4-methylphenol) were added into the reaction, the formation of the desired product was completely suppressed (Scheme 3). These results indicated that this oxidative coupling reaction involved a radical pathway.
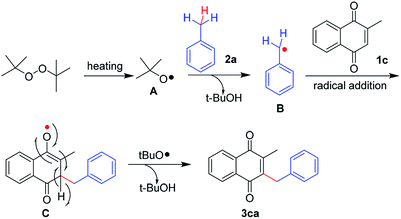
On the basis of the literature reports and observations above, a plausible mechanism for the oxidative radical process is illustrated in Scheme 4. At the beginning, homolysis of DTBP gives tert-butoxy radical intermediate A under the conditions of heating,17 which could abstract hydrogen from toluene 2a to afford a benzyl radical B.18 Due to nucleophilic nature of the benzyl radical, it would be attracted to the electrophilic C-2 or C-3 positions of naphthoquinone, and subsequently, addition of the radical intermediate B to the double bond of 2-methyl-1,4-naphthoquinone 1c afforded the radical intermediate C. Product 3ca can be formed from H radical abstraction by tert-butoxy radical.19
Conclusions
In summary, we have descried a cross-dehydrogenative coupling reaction of quinones with toluene derivatives using DTBP as the oxidant without any catalyst. The reactions of substrates containing various functional groups proceeded smoothly to give the desired products in moderate to good yields. This protocol provides an efficient and green way for the preparation of compounds containing the benzylquinones structural unit. These compounds have potential in biological and pharmaceutical applications. Further investigations to study the synthetic utility of this reaction are currently in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from CAS “Light of West China” Program, Technological Innovation Program of Chengdu, Sichuan province, China (No. 2018-YF05-00244-SN).Notes and references
- (a) K. Yuan, X. Wang and S.-N. Wang, Org. Lett., 2018, 20, 1617 CrossRef CAS PubMed; (b) C. Zhang, C. B. Santiago, J. M. Crawford and M. S. Sigman, J. Am. Chem. Soc., 2015, 137, 15668 CrossRef CAS; (c) Y. Ai, Y. Hu, F.-H. Kang, Y.-S. Lai, Y.-J. Jia, Z.-J. Huang, S.-X. Peng, H. Ji, J.-D. Tian and Y.-H. Zhang, J. Org. Chem., 2015, 58, 4506 CAS; (d) R. Zhang, S. Jin, Q. Liu, S. Lin and Z. Yan, J. Org. Chem., 2018, 83, 13030 CrossRef CAS.
- (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS; (b) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS; (c) R. P. Pandit and Y. R. Lee, Adv. Synth. Catal., 2014, 356, 3171 CrossRef CAS; (d) P. T. Parvatkar, R. Manetsch and B. K. Banik, Chem. Asian J, 2019, 14, 6 CrossRef CAS PubMed.
- (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem. Int. Ed, 2014, 53, 74 (Angew. Chem., 2014, 126, 76) CrossRef CAS PubMed; (c) A. Batra, P. Singh and K. N. Singh, Eur. J. Org. Chem., 2016, 4927 CrossRef CAS; (d) C. J. Scheuermann, Chem.–Asian J., 2010, 5, 436 CrossRef CAS; (e) L. Lv and Z. Li, Top. Curr. Chem., 2016, 374, 38 CrossRef PubMed.
- (a) X. F. Wu, J. L. Gong and X. Qi, Org. Biomol. Chem., 2014, 12, 5807 RSC; (b) C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS PubMed; (c) S. Roscales and A. G. Csaky, Chem. Soc. Rev., 2014, 43, 8215 RSC; (d) F. Jafarpour and M. Abbasnia, J. Org. Chem., 2016, 81, 11982 CrossRef CAS.
- (a) R. H. Thomson, Naturally Occurring Quinones IV, Blackie Academic, London, 1997 Search PubMed; (b) C. Puder, K. Wagner, R. Vettermann, R. Hauptmann and O. Potterat, J. Nat. Prod., 2005, 68, 323 CrossRef CAS; (c) C. Asche, Mini-Rev. Med. Chem., 2005, 5, 449 CrossRef CAS; (d) J.-K. Liu, Chem. Rev., 2006, 106, 2209 CrossRef CAS PubMed.
- (a) A. Mäntylä, T. Garnier, J. Rautio, T. Nevalainen, J. Vepsaelainen, A. Koskinen, S. L. Croft and T. Järvinen, J. Med. Chem., 2004, 47, 188 CrossRef PubMed; (b) J. Grolig and R. Wagner, “Naphthoquinones”, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed; (c) A. Baramee, A. Coppin, M. Mortuaire, L. Pelinski, S. Tomavo and J. Brocard, Bioorg. Med. Chem., 2006, 14, 1294 CrossRef CAS; (d) J. M. Finefield, D. H. Sherman, M. Kreitman and R. M. Williams, Angew. Chem. Int. Ed, 2012, 51, 4802 (Angew. Chem., 2012, 124, 4886) CrossRef CAS PubMed; (e) C. Huo, X. Xu, J. An, X. Jia, X. Wang and C. Wang, J. Org. Chem., 2012, 77, 8310 CrossRef CAS PubMed.
- (a) P. Sidorov, I. Desta, M. Chessé, D. Horvath, G. Marcou, A. Varnek, E. Davioud-Charvet and M. Elhabiri, ChemMedChem, 2016, 11, 1339 CrossRef CAS PubMed; (b) T. Müller, L. Johann, B. Jannack, M. Brückner, D. A. Lanfranchi, H. Bauer, C. Sanchez, V. Yardley, C. Deregnaucourt, J. Schrével, M. Lanzer, R. H. Schirmer and E. Davioud-Charvet, J. Am. Chem. Soc., 2011, 133, 11557 CrossRef PubMed.
- (a) S. Guo, P. S. Kumar and M. Yang, Adv. Synth. Catal., 2017, 359, 2 CrossRef CAS; (b) J. Sun, Y. Zhang, S. Mathan, Y. Wang and Y. Pan, J. Org. Chem., 2016, 81, 3380 CrossRef CAS PubMed; (c) N. Okugawa, K. Moriyama and H. Togo, J. Org. Chem., 2017, 82, 170 CrossRef CAS PubMed; (d) S. Liu, A. Liu, Y. Zhang and W. Wang, Chem. Sci., 2017, 8, 4044 RSC; (e) L. Wang, J. Cao, Q. Chen and M. He, J. Org. Chem., 2015, 80, 4743 CrossRef CAS PubMed; (f) Q. Xia, W. Chen and H. Qiu, J. Org. Chem., 2011, 76, 7577 CrossRef CAS PubMed; (g) Q. Yang, P. Y. Choy, W. C. Fu, B. Fan and F. Y. Kwong, J. Org. Chem., 2015, 80, 11193 CrossRef CAS PubMed; (h) M. K. Singh, H. K. Akula, S. Satishkumar, L. Stahl and M. K. Lakshman, ACS Catal., 2016, 6, 1921 CrossRef CAS PubMed; (i) Z. Luo, Z. Jiang, W. Jiang and D. Lin, J. Org. Chem., 2018, 83, 3710 CrossRef CAS PubMed; (j) L.-Y. Xie, L.-L. Jiang, J.-X. Tan, Y. Wang, X.-Q. Xu, B. Zhang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 14153 CrossRef CAS; (k) L.-Y. Xie, S. Peng, T.-G. Fan, Y.-F. Liu, M. Sun, L.-L. Jiang, X.-X. Wang, Z. Cao and W.-M. He, Sci. China. Chem, 2019, 62, 460 CrossRef CAS.
- S.-L. Zhou, L.-N. Guo and X.-H. Duan, Eur. J. Org. Chem., 2014, 2014, 8094 CrossRef CAS.
- B. Liu, L. Gu and J. Zhang, Recl. Trav. Chim. Pays-Bas, 1991, 110, 99 CrossRef CAS.
- D. R. Sutherland, M. Veguillas, C. L. Oates and A.-L. Lee, Org. Lett., 2018, 20, 6863 CrossRef CAS PubMed.
- (a) H. Togo, in Advanced Free Radical Reactions for Organic Synthesis, Elsevier, Amsterdam, 1st edn, 2004 Search PubMed; (b) H. Yi, G. Zhang, H. Wang, Z. Huang, J. Wang, A. K. Singh and A. Lei, Chem. Rev., 2017, 117, 9016 CrossRef CAS PubMed; (c) W. Liu and J. T. Groves, Acc. Chem. Res., 2015, 48, 1727 CrossRef CAS PubMed; (d) H. Yu, B. Hu and H. Huang, Chem.–Asian J., 2018, 24, 7114 CAS.
- For selected examples: (a) G. Qin, X. Chen, L. Yang and H. Huang, ACS Catal., 2015, 5, 2882 CrossRef CAS; (b) K. Li, Q. Wu, J. Lan and J. You, Nat. Commun., 2015, 6, 8404 CrossRef CAS PubMed; (c) X. Huang, T. Bergsten and J. T. Groves, J. Am. Chem. Soc., 2015, 137, 5300 CrossRef CAS PubMed; (d) W. Zhang, F. Wang, S. D. McCann, D. Wang, P. Chen, S. S. Stahl and G. Liu, Science, 2016, 353, 1014 CrossRef CAS PubMed; (e) W. Zhang, P. Chen and G. Liu, J. Am. Chem. Soc., 2017, 139, 7709 CrossRef CAS PubMed; (f) G. Qin, Y. Wang and H. Huang, Org. Lett., 2017, 19, 6352 CrossRef CAS PubMed; (g) W. Liu, M.-J. Cheng, R. J. Nielsen, W. A. Goddard III and J. T. Groves, ACS Catal., 2017, 7, 4182 CrossRef CAS; (h) M. Rafiee, F. Wang, D. P. Hruszkewycz and S. S. Stahl, J. Am. Chem. Soc., 2018, 140, 22 CrossRef CAS PubMed; (i) S.-h. Hao, L.-X. Li, D.-Q. Dong, Z.-L. Wang and X.-Y. Yu, Tetrahedron Lett., 2018, 59, 4073 CrossRef CAS.
- (a) B. R. Ai, X. L. Chen, Y. Dong, L. Tang and J. Y. Wang, Synthesis, 2017, 49, 4017 CrossRef CAS; (b) X. L. Chen, Y. Dong, S. He, R. Zhang and J. Y. Wang, Synlett, 2019, 30, 615 CrossRef CAS; (c) L.-Y. Xie, T.-G. Fang, J.-X. Tan, B. Zhang, Z. Cao, L.-H. Yang and W.-M. He, Green Chem., 2019, 21, 3858 RSC; (d) F.-L. Zeng, X.-L. Chen, S.-Q. He, K. Sun, Y. Liu, R. Fu, L.-B. Qu, Y.-F. Zhao and B. Yu, Org. Chem. Front., 2019, 6, 1476 RSC; (e) Z. Li, W. Wang, H. Jian, W. Li, B. Dai and L. He, Chin. Chem. Lett., 2019, 30, 386 CrossRef CAS; (f) L.-Y. Xie, Y. Duan, L.-H. Lu, Y.-J. Li, S. Peng, C. Wu, K.-J. Liu, Z. Wang and W.-M. He, ACS Sustainable Chem. Eng., 2017, 5, 10407 CrossRef CAS; (g) L.-Y. Xie, S. Peng, J.-X. Tan, R.-X. Sun, X. Yu, N.-N. Dai, Z.-L. Tang, X. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2018, 6, 16976 CrossRef CAS; (h) C. Wu, X. Xin, Z.-M. Fu, L.-Y. Xie, K.-J. Liu, Z. Wang, W. Li, Z.-H. Yuan and W.-M. He, Green Chem., 2017, 19, 1983 RSC.
- V. F. De, S. V. Van, M. Waroquier, P. Geerlings and P. F. De, Org. Lett., 2007, 9, 2721 CrossRef PubMed.
- (a) J. Aleman, B. Richter and K. A. Jørgensen, Angew. Chem., Int. Ed., 2007, 46, 5515 CrossRef CAS PubMed; (b) J. Aleman, S. Cabrera, E. Maerten, J. Overgaard and K. A. Jørgensen, Angew. Chem., Int. Ed., 2007, 46, 5520 CrossRef CAS PubMed; (c) J. Aleman, C. B. Jacobsen, K. Frisch, J. Overgaard and K. A. Jørgensen, Chem. Commun., 2008, 632 RSC; (d) Z. He, T. Liu, H. Tao and C.-J. Wang, Org. Lett., 2012, 14, 6230 CrossRef CAS PubMed; (e) Ł. Albrecht, C. V. Gomez, C. B. Jacobsen and K. A. Jørgensen, Org. Lett., 2013, 15, 3010 CrossRef PubMed; (f) T. K. Johansen, C. V. Gomez, J. R. Bak, R. L. Davis and K. A. Jørgensen, Chem. - Eur. J, 2013, 19, 16518 CrossRef CAS PubMed; (g) C. Martín-Santos, C. Jarava-Barrera, S. delPozo, A. Parra, S. Díaz-Tendero, R. MasBalleste, S. Cabrera and J. Alema, Angew. Chem., Int. Ed, 2014, 53, 8184 CrossRef PubMed; (h) J. Blom, T. K. Johansen, F. Jensen and K. A. Jørgensen, Chem. Commun., 2016, 52 Search PubMed; (i) A. Skrzyńska, M. Romaniszyn, D. Pomikło and Ł. Albrecht, J. Org. Chem., 2018, 83, 5019 CrossRef PubMed.
- (a) Z.-Q. Xu, C. Wang, L. Li, L. Duan and Y.-M. Li, J. Org. Chem., 2018, 83, 9718 CrossRef CAS PubMed; (b) X.-H. Ouyang, R.-J. Song, B. Liu and J.-H. Li, Adv. Synth. Catal., 2016, 358, 1903 CrossRef CAS; (c) J. Zhao, H. Fang, P. Qian, J. Han and Y. Pan, Org. Lett., 2014, 16, 5342 CrossRef CAS PubMed.
- (a) H. Yang, H. Yan, P. Sun, Y. Zhu, L. Lu, D. Liu, G. Rong and J. Mao, Green Chem., 2013, 15, 976 RSC; (b) S.-L. Zhou, L.-N. Guo, S. Wang and X.-H. Duan, Chem. Commun., 2014, 50, 3589 RSC; (c) Z. Li, L. Cao and C.-J. Li, Angew. Chem. Int. Ed, 2007, 46, 6505 CrossRef CAS.
- (a) P. Patil, A. Nimonkar and K. G. Akamanchi, J. Org. Chem., 2014, 79, 2331 CrossRef CAS PubMed; (b) A. Ilangovan, S. Saravanakumar and S. Malayappasamy, Org. Lett., 2013, 15, 4968 CrossRef CAS PubMed; (c) See ref. 11..
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05678e |
| This journal is © The Royal Society of Chemistry 2019 |