Open Access Article
Open Access ArticleBoth the mono- and di-anions of ellagic acid are effective inhibitors of the serine β-lactamase CTX-M-15†
Nathan
Talbot
a,
Nicholas T.
Powles
b and
Michael I.
Page
 *a
*a
aDepartment of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh EH9 3FJ, UK. E-mail: mike.page@ed.ac.uk
bAnalytical Innovations, Shaw Park, Huddersfield HD5 9AG, UK
First published on 27th September 2019
Abstract
Ellagic acid, a δ-lactone with ionisable phenolic residues, is an efficient time-dependent inhibitor of the serine β-lactamase enzyme CTX-M-15. The pH-dependence of the rate of inhibition shows that both the mono- and di-anionic species of ellagic acid are effective inhibitors, both with second order rate constants of ∼1.5 × 104 M−1 s−1. The structurally similar δ-lactone urolithin A, which lacks the geometrically appropriate phenolic residue, shows only modest inhibitory activity against CTX-M-15. It is proposed that this inhibition by ellagic acid anions involves acylation of the active site serine and that the negative charge on the inhibitor is required for binding to the active site.
Introduction
After over 70 years of clinical use,1 β-lactam antibiotics (penicillins, cephalosporins, and carbapenems) remain the major class of antimicrobial agents,2 but the use of these life-saving therapeutics is increasingly compromised by bacterial resistance.3 The most important resistance mechanism is the bacterial production of a group of enzymes, the β-lactamases, which catalyse the hydrolysis of the β-lactam thus inactivating the antibiotic. These enzymes are grouped into four classes A–D; A, C, and D contain an active site serine residue which catalyses hydrolysis of the β-lactam by nucleophilic attack on the carbonyl carbon leading to the intermediate acylation of the serine residue.4 There have been many reports of inactivating serine β-lactamases (SBLs) by mechanism based inhibitors involving modification of this acylation process.5By contrast the class B enzymes are metallo-β-lactamases (MBLs)6 which employ 1 or 2 Zn ions as part of their catalytic machinery and are distinct mechanistically from the SBLs.7,8
Approved SBL inhibitors are usually paired with a β-lactam antibiotic (BLICs),9 such as amoxicillin/clavulanate, ampicillin/sulbactam, and piperacillin/tazobactam.10 These combinations work well against class A β-lactamases, but are less effective against class C and D enzymes.11 The inhibitors react with the active site serine nucleophile forming an acyl–enzyme complex that is subject to low turnover rates, resulting in inhibition of the enzyme.12 More recently, there has been a development of non-β-lactam inhibitors such as avibactam and related bicyclic ureas13 and the cyclic boronic vaborbactam.14
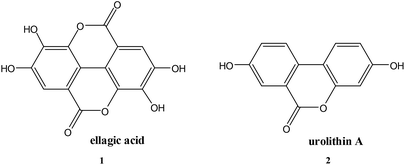
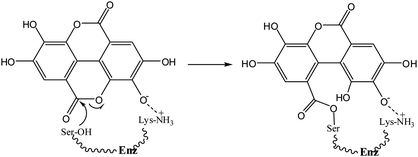
Ellagic acid (1) is a polyphenolic phytochemical15 present in various fruits and vegetables16 and of biological and pharmaceutical interest due to its antioxidant properties.17 Polyphenols play an essential role in plant physiology and also assist in the prevention of infection from plant pathogens.18 Ellagic acid has anti-inflammatory effects19 and has also been investigated for its antibacterial properties15,20. Ellagic acid (1) contains a δ-lactone, which could act as an acylating agent, as well as acidic phenolic groups capable, by ionisation, of acting as the anionic binding to the active site positively charged residue, thus it possesses the two fundamental requirements of an inhibitor of serine β-lactamases4 (Scheme 1). The reverse reaction to regenerate the enzyme is not favourable because of the low nucleophilicity of the phenol.
Results and discussion
The inhibition of SBLs by ellagic acid (1) was investigated using the rate of the enzyme catalysed hydrolysis of the antibiotic substrate cephalothin at 260 nm to measure the changing concentration of the active SBL during its time dependent inhibition. There are more than 100 CTX-M SBLs described and they are the most rapidly spreading family of β-lactamases in Europe and North America.21 The CTX-M-15 catalysed hydrolysis of cephalothin has a low Km and a broad pH-rate profile making it suitable to determine the rate of inhibition by ellagic acid and its pH dependence. Appropriate blanks containing the same reaction conditions but with no ellagic acid present were undertaken to ensure that enzyme activity was maintained during the time scales of the inhibition studies. The rate of SBL (10 nM) inactivation at pH 7.0 was exponential with time (Fig. 1) to generate pseudo-first-order rate constants kiobs which in turn showed a first order dependence on the concentration of ellagic acid. The corresponding second-order rate constant for inactivation ki = 1.16 × 104 M−1 s−1 demonstrates that ellagic acid (1) is a very effective inhibitor of the SBL.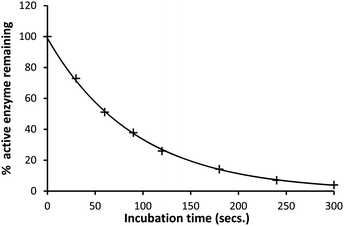 | ||
| Fig. 1 Percentage active CTX-M-15 (10 nM) remaining after incubation with ellagic acid (1 μM) in pH 7.0 HEPES buffer (0.10 M) at 30 °C and I = 1.0 M (KCl). | ||
This time dependence is indicative of a covalent reaction between ellagic acid and the enzyme. A UV scan of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CTX-M-15 or TEM-1 SBL enzymes (70 μM) and ellagic acid (70 μM) showed a decrease in absorbance from 2.7 to 0.8 at 275 nm compatible with a reaction taking place between the enzyme and the ellagic acid.
1 mixture of CTX-M-15 or TEM-1 SBL enzymes (70 μM) and ellagic acid (70 μM) showed a decrease in absorbance from 2.7 to 0.8 at 275 nm compatible with a reaction taking place between the enzyme and the ellagic acid.
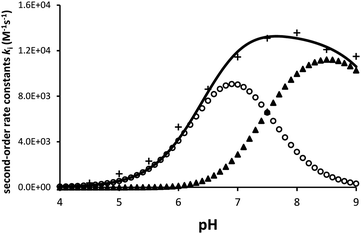
Ellagic acid has four ionisable phenolic groups and which of the ions was responsible for enzyme inactivation was established by measuring the pH dependence of the rate of inactivation. The four pKa's of ellagic acid in water at 25 °C are 6.45, 7.45, 9.61 and 11.50 and a speciation plot (ESI†) showed how the fraction of each ionic form varies with pH, so, for example, at pH 7.0 the fractions of EH4, EH3− and EH22− are 0.16, 0.62 and 0.22, respectively. The pH-dependence of the rate of hydrolysis of the substrate cephalothin by the SBL CTX-M-15 shows a typical broad bell-shaped profile corresponding to pKa1 and pKa2 = 4.75 and 8.95, respectively. Consequently, there is more than 93% active enzyme between pH 6 to 8 and therefore the changes in the second order rate constants ki for the inactivation of CTX-M-15 by ellagic acid at various pH levels (Fig. 2) reflect the activity of the ionic species of the inhibitor. It shows that the unionised species is inactive but both the mono- and di-anions are active. The values of the second-order rate constants for the two anionic species can be estimated from eqn (1), where kitot is the observed second-order rate constant for inhibition, α and β are the fractions of the mono-anion and di-anion of ellagic acid present at different pHs, respectively, and 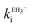 and
and 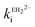 are the calculated second-order rate constants for inhibition by the mono-anion and di-anion of ellagic acid, respectively.
are the calculated second-order rate constants for inhibition by the mono-anion and di-anion of ellagic acid, respectively.
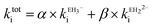 | (1) |
The estimated second-order rate constant for inactivation of CTX-M-15 by the mono-anion 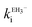 = 1.31 × 104 M−1 s−1 and by the di-anion
= 1.31 × 104 M−1 s−1 and by the di-anion 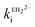 = 1.42 × 104 M−1 s−1. The requirement for a negative charge on the inhibitor is compatible with that for substrates of SBLs4 and reflects its interaction with a positively charged residue (Lys-234) on the enzyme (Scheme 1). Of course, future work on the potential viability of using ellagic acid as an active ingredient for combatting antibiotic resistance would require pharmacological and toxicological studies.
= 1.42 × 104 M−1 s−1. The requirement for a negative charge on the inhibitor is compatible with that for substrates of SBLs4 and reflects its interaction with a positively charged residue (Lys-234) on the enzyme (Scheme 1). Of course, future work on the potential viability of using ellagic acid as an active ingredient for combatting antibiotic resistance would require pharmacological and toxicological studies.
Another indication of the importance of the acidic phenol in ellagic acid providing the anionic binding site is that the structurally similar δ-lactone urolithin A (2) (pKa 7.5), but which lacks the geometrically appropriate phenolic residue, shows only modest inhibitory activity against CTX-M-15. Complete inactivation does not occur with 10 mM urolithin A and CTX-M-15 (10 nM) at pH 7.0 and 30 °C with a ki < 50M−1 s−1.
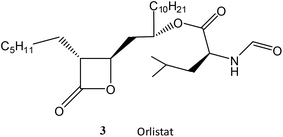
Finally, the chemically more reactive β-lactone, but lipophilic, orlistat (3) which is one of the most effective inhibitors of lipase,22 was tested as a SBL inhibitor. Orlistat (10 mM) is only a weak inhibitor of the SBL CTX-M-15 in a 10% solution of acetonitrile at pH 7.0 and 30 °C with a ki = 65 M−1 s−1. Orlistat (3) is ca. 200-fold less effective as an inhibitor of CTX-M-15 than the chemically less reactive ellagic acid (1)23 highlighting the importance of intermolecular binding interactions between the inhibitor and the enzyme.
The class B β-lactamases are zinc-ion dependent and although ellagic acid can act as a chelating agent for metal ions, it showed no inhibitory activity against NDM-1 MBL up to a concentration of 10 mM.
Conclusion
Both the mono- and di-anions of ellagic acid are effective inhibitors of the SBL CTX-M-15 presumably by acylation of the active site serine with the phenolate anions providing binding to the active site Lys-234. The structurally similar δ-lactone urolithin A but which lacks the geometrically appropriate phenolic residue, shows only modest inhibitory activity against CTX-M-15.Experimental
The methods for detection of any inhibition of the β-lactamases were based on measuring the activity of the enzyme in catalysing the hydrolysis of cephalothin. Stock solutions of ellagic acid in MeOH and in acetonitrile (ACN) for orlistat and urolithin A were prepared. A 1 mM ellagic acid (3.02 mg) in 1.0 cm3 MeOH required sonicating for 20 minutes. The inhibition experiments for ellagic acid were conducted in buffers for the respective pH with 10% v/v MeOH to aid solubility of (1). For example, at pH 8.5, 20 cm3 pH 8.5 TAPS buffer (0.1 M) with 10% MeOH with ionic strength maintained to 1 M with KCl. For orlistat (4.96 mg) was dissolved in 1 cm3 ACN to give a 10 mM stock solution. For urolithin A, (2.28 mg) was dissolved in 1 cm3 ACN to give a 10 mM stock solution. For both of these inhibitors, the inhibition experiments were conducted in pH 7.0 HEPES buffer (0.1 M) with 10% ACN (18 cm3, pH 7.0 HEPES buffer and 2 cm3 ACN) maintained to 1 M ionic strength with KCl.Appropriate controls containing the same concentrations of solvent (10% MeOH/ACN) were used to ensure there was no effect on the activity of the enzyme. The hydrolysis of the antibiotic cephalothin was used to measure the concentration of the enzyme through inhibition/hydrolysis rates of the enzyme.
The enzyme was thawed slowly in melting ice and was added to the stock solution of the potential inhibitors. Samples were taken at intermittent timed intervals after incubation with various β-lactamases and pipetted into a 1 cm3 cuvette at 30 °C and cephalothin solution was added and the absorbance change at 260 nm recorded with time. The rates of hydrolysis of the cephalothin were determined from the initial slopes of absorbance against time. These initial slopes gave the percentage of active enzyme remaining and decayed exponentially with time indicative of a first-order kinetic process (Fig. 1).
The stability of the enzyme over the time periods used for the inhibition studies was determined from observing the initial slopes of the change in absorbance in the hydrolysis of cephalothin. This ensured that any change in the rate of hydrolysis reflects inhibition of the enzyme and not any other loss of activity of the enzyme.
The following buffers were used depending upon the pH required: pH 4–5: acetic acid, pH 5.5–6.5: 2-(N-morpholino)ethanesulfonic acid, pH 7.0–8.0: HEPES, pH 8.5–9.0 TAPS.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Schofield group, University of Oxford for the gift of CTX-M-15.Notes and references
- M. S. Wilke, A. L. Lovering and N. C. J. Strynadka, Curr. Opin. Microbiol., 2005, 8, 525–533 CrossRef CAS
.
- D. M. Livermore and N. Woodford, Trends Microbiol., 2006, 14, 413–420 CrossRef CAS
.
- J. Davies and D. Davies, Microbiol. Mol. Biol. Rev., 2010, 74, 417–433 CrossRef CAS
; Y.-Y. Liu, Y. Wang, T. R. Walsh, L.-X. Yi, R. Zhang, J. Spencer and J. Shen, Lancet Infect. Dis., 2016, 16, 161–168 CrossRef
; R. J. Fair and Y. Tor, Perspect. Med. Chem., 2014, 6, 25–26 Search PubMed
.
-
A. L. Fink and M. I. Page, in β-Lactamases, ed. J.-M.Frère, Nova, 2012, ch. 7, pp. 161–198 Search PubMed
.
- M. I. Page and A. P. Laws, Chem. Commun., 1998, 1609–1617 RSC
.
- M. J. Crowder, J. Spencer and A. J. Vila, Acc. Chem. Res., 2006, 39, 721–728 CrossRef CAS
; Z. Wang, W. Fast, A. M. Valentine and S. J. Benkovic, Curr. Opin. Chem. Biol., 1999, 3, 614–622 CrossRef
.
- J. Brem, R. Cain, S. Cahill, M. A. McDonough, I. J. Clifton, J. C. Jiménez-Castellanos, M. B. Avison, J. Spencer, C. W. G. Fishwick and C. J. Schofield, Nat. Commun., 2016, 7, 12406 CrossRef CAS
.
- M. I. Abboud, M. Kosmopoulou, A. Krismanich, J. W. Johnson, P. Hinchliffe, J. Brem, T. D. W. Claridge, J. Spencer, C. Schofield and G. I. Dmitrienko, Chem. - Eur. J., 2018, 24, 5734–5737 CrossRef CAS
.
- D. T. King, S. Sobhanifar and N. C. J. Strynadka, The Protein Soc, 2016, 25, 787–803 CrossRef CAS
.
- K. A. Toussaint and J. C. Gallagher, Ann. Pharmacother., 2015, 49, 86–89 CrossRef
.
- C. T. Lohans, E. van Groesen, K. Kumar, C. L. Tooke, J. Spencer, R. S. Paton, J. Brem and C. J. Schofield, Angew. Chem., Int. Ed., 2018, 57, 1282–1285 CrossRef CAS
.
- R. P. A. Brown, R. T. Aplin and C. J. Schofield, Biochemistry, 1996, 35, 12421–12432 CrossRef CAS
; C. Thierren and R. C. Levesque, FEMS Microbiol. Rev., 2006, 24, 251–262 CrossRef
.
- D. E. Ehmann, H. Jahic, P. L. Ross, R. F. Gu, J. Hu, G. Kern and S. L. Fisher, Proc. Natl. Acad. Sci., 2012, 109, 11663–11668 CrossRef CAS
; T. A. Blizzard, H. Chen, S. Kim, J. Wu, R. Bodner, C. Gude and M. L. Hammond, Bioorg. Med. Chem. Lett., 2014, 24, 750–785 CrossRef
; M. L. Monogue, S. Giavagnoli, C. Bissantz, C. Zampaloni and D. P. Nicolau, Antimicrob. Agents Chemother., 2018, 17, 2596–2617 Search PubMed
.
- O. Lomovskaya, D. Rubio-Aparicio, K. Nelson, R. Tsivkosvski, D. C. Griffith, M. N. Dudley and D. Sun, Antimicrob. Agents Chemother., 2017, 61, 443–517 CrossRef
.
- D. A. Vettem and K. Shetty, J. Food Biochem., 2005, 29, 234–266 CrossRef
.
- S. Corbett, J. Daniel, R. Drayton, M. Field, R. Steinhardt and N. Garrett, J. PeriAnesth. Nurs., 2010, 25, 214–220 CrossRef CAS
; C. Usta, S. Ozdemir, M. Schiarity and P. E. Puddu, Int. J. Food Sci. Nutr., 2013, 64, 907–913 CrossRef
.
- S. U. M. Talcott, S. T. Talcott and S. S. Percival, J. Nutr., 2003, 133, 2669–2674 CrossRef
.
- M. Daglia, Curr. Opin. Biotechnol., 2012, 23, 174–181 CrossRef CAS PubMed
.
- D. C. Favarin, M. M. Teixeira, E. L. D. Andrade, C. D. F. Alves, J. E. L. Chica, C. A. Sorgi and A. P. Rogerio, Mediators Inflammation, 2013, 1–13 Search PubMed
.
- E. Coppo and A. Marchese, Curr. Pharm. Biotechnol., 2014, 15, 380–390 Search PubMed
; L. Sanhueza, M. Wil, R. Montero, K. Maisey, L. Mendoza and M. Wilkens, PLoS One, 2017, 12, 1–15 CrossRef CAS
.
- R. Bonnet, Antimicrob. Agents Chemother., 2004, 48, 1–14 CrossRef CAS
.
- A. I. M. Gonzalez, E. A. Parrilla, A. G. D. Sanchez, L. A. de la Rosa, J. A. N. Gastelum, A. A. V. Flores and G. A. G. Aguilar, Food Technol. Biotechnol., 2017, 55, 519–530 Search PubMed
.
- M. I. Page, Adv. Phys. Org. Chem., 1987, 23, 165–270 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: pH-rate profile for CTX-M-15 catalysed hydrolysis of cephalothin, pH speciation plot of ellagic acid. See DOI: 10.1039/c9ra05835d |
| This journal is © The Royal Society of Chemistry 2019 |