Open Access Article
Open Access ArticleA dual-mode nanoparticle based on natural biomaterials for photoacoustic and magnetic resonance imaging of bone mesenchymal stem cells in vivo
Hua Zhang†
a,
Zhen-Jun Wang†bc,
Ling-Jie Wang† a,
Ting-Ting Lib,
Sheng Hea,
Li-Ping Lib,
Xiao-Yan Lia,
Shi-Jie Liua,
Jian-Ding Lia,
Si-Jin Li*d and
Rui-Ping Zhang*bc
a,
Ting-Ting Lib,
Sheng Hea,
Li-Ping Lib,
Xiao-Yan Lia,
Shi-Jie Liua,
Jian-Ding Lia,
Si-Jin Li*d and
Rui-Ping Zhang*bc
aDepartment of Medical Imaging, First Hospital of Shanxi Medical University, Taiyuan, Shanxi Province 030001, China
bDepartment of Biochemistry and Molecular Biology, Shanxi Medical University, Taiyuan, Shanxi Province 030001, China
cThe Affiliated Da Yi Hospital of Shanxi Medical University, Taiyuan, Shanxi Province 030032, China. E-mail: zrp_7142@163.com; Tel: +86-351-4639324
dDepartment of Nuclear Medicine, First Hospital of Shanxi Medical University, Taiyuan, Shanxi Province 030001, China. E-mail: lisjnm123@163.com; Tel: +86-351-4639278
First published on 31st October 2019
Abstract
Stem cell imaging in vivo is critical to elucidate the homing, distribution, survival, and repair mechanisms and to evaluate the therapeutic effects of engrafted stem cells. Unfortunately, unimodal imaging of stem cells does not simultaneously satisfy all current requirements owing to their intrinsic limitations. Obviously, bimodal or multimodal imaging of stem cells is a promising strategy for circumventing this issue. This study aimed to design and synthesize a novel dual-modal polyethylene glycol-modified magnetic nanoparticle (Fe3+-PEG-MNP) based on natural biomaterials including melanin and Fe ions for photoacoustic (PA) and magnetic resonance (MR) imaging of stem cells in vivo. The Fe3+-PEG-MNPs were characterized and their PA/MR imaging capability and cytotoxicity were evaluated. Bone marrow mesenchymal stem cells (BM-MSCs) labeled with Fe3+-PEG-MNPs were subjected to PA and MR imaging in vitro and in vivo. Consequently, Fe3+-PEG-MNPs displayed many superior properties, including ultra-small particle size, higher stability, water solubility, easy labeling of cells, lower cytotoxicity, high biosafety, excellent capability of PA/MR imaging, high sensitivity and long-term monitoring in vitro and in vivo. In particular, PA and MR signals of labeled BM-MSCs were maintained for at least 35 and 28 d, respectively, in vivo. Therefore, Fe3+-PEG-MNPs are ideal dual-modal PA/MR nanoparticles for non-invasive and effective monitoring of engrafted stem cells in vivo.
1. Introduction
Stem cell therapy (SCT) has been an effective and promising regenerative therapeutic strategy for many diseases owing to its potential for multipotent differentiation and self-renewal.1 Among the numerous stem cells available, bone marrow mesenchymal stem cells (BM-MSCs) are preferred for SCT owing to minimum ethical constraints, ease of harvesting, low immunogenicity, multi-differential potentialities, and self-renewal capacity. Previous studies have reported that BM-MSC therapy is an effective alternative in various diseases, such as heart disease, cerebral infarction, renal ischemia-reperfusion injury, spinal cord injury, and hepatic failure.2–6 However, numerous issues regarding engrafted BM-MSCs warrant clarification, such as their migratory path in vivo, biodistribution, differentiation capacity, long-term fate, and beneficial effects. Therefore, non-invasive continuous monitoring of engrafted BM-MSCs in vivo is necessary to address the aforementioned issues.Currently, various stem cell imaging methods in vivo have been applied for cell tracking, such as optical imaging (including fluorescence and bioluminescence imaging), magnetic resonance imaging (MRI), radionuclide imaging (including positron emission tomography (PET) and single-photon emission computed tomography (SPECT)), and photoacoustic imaging (PAI). Unfortunately, these methods have intrinsic limitations and cannot satisfy all requirements. Optical imaging methods for tracking stem cells in vivo are generally limited by their low penetration depth in tissue and phototoxicity.7 Although MRI has been widely used for cell tracking because of high tissue resolution and long-term monitoring of engrafted cells in vivo, it has relatively lower sensitivity than other methods, longer scan duration, and difficulty quantifying cell division.8,9 Despite considerable sensitivity, radionuclide imaging is restricted to the imaging of labeled stem cells in vivo owing to poor spatial resolution, a short half-life of radiotracers, and high cost.8,9 PAI not only provides high spatial resolution and sensitivity but also has a relatively greater depth of tissue penetration, and it is a compound imaging method of optical and ultrasound (US) imaging based on ultrasound waves induced by thermoelastic expansion of tissue after photon absorption.10 Consequently, dual-modal or multimodal cell imaging methods are important for efficient monitoring of the homing, number, and fate of engrafted stem cells in vivo.
This study aimed to develop a dual-modal photoacoustic and MR nanoprobe for stem cell imaging in vivo, comprising a nano-chelate of polyethylene glycol-modified melanin and iron ions nanoparticles (Fe3+-PEG-MNPs). We characterized Fe3+-PEG-MNPs and evaluated their cellular internalization and cytotoxicity in vitro. In particular, the feasibility of PAI/MRI dual-modal long-term monitoring of Fe3+-PEG-MNPs labeled BM-MSCs in vivo was verified.
2. Materials and methods
2.1 Synthesis of Fe3+-PEG-MNPs
PEG-MNPs were obtained as described previously.11,12 Briefly, 150 μL of 16 mg mL−1 FeCl3·6H2O aqueous solution was added into 3 mL of 3 mg mL−1 MNPs aqueous solution and then stirred gently for 1 h at 37 °C. Free Fe3+ and Cl− ions were filtered out using a PD-10 column (GE Healthcare, Chicago, IL, USA). The chelation products thus obtained were washed with ultrapure water and centrifuged at room temperature (3500 rpm, 15 min) twice to further eliminate free Fe3+ and Cl− ions and then filtered through a 0.22 μm membrane (Acrodisc Syringe Filters, PALL, New York, USA). The final products (Fe3+-PEG-MNPs) were stored in sterile EP tubes for subsequent analysis.2.2 Characterization of Fe3+-PEG-MNPs
The ultraviolet-visible-near infrared (UV-vis-NIR) absorption spectra of the Fe3+-PEG-MNPs were detected via ultraviolet-visible-near infrared spectroscopy (UV-vis-NIR; UV-6100; MAPADA Instruments, Shanghai, China), and their morphology was observed using a transmission electron microscope (TEM; JEM-2100F; JEOL, Tokyo, Japan). The hydrodynamic diameters and zeta potential were measured via dynamic light scattering (DLS; Zetasizer Nano ZS90; Malvern Instruments, Malvern, UK).The Fe3+ concentration in 14 mL of 71.55 μg mL−1 Fe3+-PEG-MNPs aqueous solution was measured via inductively coupled plasma mass spectrometry (ICP-MS; ICPMS-2030; SHIMADAZU Instruments, Kyoto, Japan) to determine the concentration of chelated Fe3+ ions.
Furthermore, the stability of Fe3+-PEG-MNPs was analyzed by dissolving them in phosphate-buffered saline (PBS; pH = 7.4) and stirring gently at 37 °C. Free Fe3+ concentration was measured via ICP-MS at 0.5 h, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h, and 48 h.
2.3 Dual-modal PA/MR imaging of an aqueous solution of Fe3+-PEG-MNPs
Aqueous solutions of Fe3+-PEG-MNPs and PEG-MNPs of different concentrations (0, 12.5, 25, 50, 100, 200, 400, and 800 μg mL−1) were prepared for PA imaging, using a multispectral optoacoustic tomographic imaging system (MSOT) (inSight 128, iThera Medical GmbH, Munich, Germany). Briefly, laboratory-prepared white thin plastic tubes (inner diameter of 3 mm) filled with the test solution of different concentrations were placed in the phantom. After excitation with tunable pulses at 680–980 nm, photoacoustic signals of aqueous solutions of Fe3+-PEG-MNPs and PEG-MNPs of different concentrations were recorded. The PA imaging parameters were as follows: temperature, 25 °C; selected positions of the phantom, 3; frames per wavelength, 4; repetitions per slice, 1; run, 1; slice thickness, 800 μm.Aqueous solutions of Fe3+-PEG-MNPs and Omniscan (a contrast-enhancing agent commonly used in MRI) were imaged using a 3.0-T clinical MRI equipment (MAGNETOM Skyra; Siemens, Munich, Germany) with a wrist surface coil. The samples of Fe3+-PEG-MNPs and Omniscan aqueous solution were placed in a 96-well plate, each well containing samples of different Fe3+ and Gd3+ concentrations (0.0125, 0.025, 0.05, 0.1, 0.2, 0.4, and 0.8 mmol L−1), and deionized water was used as a negative control. The T1 value was measured using the T1-mapping sequence images. T1WI parameters were as follows: field of view (FOV), 100 × 90 mm2; slice thickness, 1.5 mm; spacing, 0.5 mm; base matrix resolution, 256 × 256; and effective echo time (TE), 20 ms. The T1 relaxation efficient (r1) values of Fe3+-PEG-MNPs and Omniscan were obtained by calculating the ratio of 1/T1 (s−1) to the Fe3+ and Gd3+ concentrations (mmol L−1).
2.4 Labeling and assessment of the cytotoxicity of Fe3+-PEG-MNPs in BM-MSCs in vitro
Ten milliliters of a BM-MSC suspension containing 6 × 105 cells per mL was seeded in 75 cm2 culture flasks. After 24 h of incubation at 37 °C in a 5% CO2 incubator, the medium was replenished with fresh serum-free culture medium containing Fe3+-PEG-MNPs at different concentrations (0, 100, 200, 400, and 800 μg mL−1), and BM-MSCs were cultured for 6 h at 37 °C in a 5% CO2 incubator. Thereafter, the medium was discarded and cells were carefully washed thrice with PBS to eliminate the uninternalized Fe3+-PEG-MNPs and dead cells. After trypsinization, the cell suspension was centrifuged at room temperature (1000 rpm, 5 min) and labeled stem cells were resuspended in PBS for subsequent analysis. The intracellular distribution of Fe3+-PEG-MNPs was observed via TEM and the Fe3+-PEG-MNPs labeling rate of BM-MSCs was analyzed via flow cytometry (FACS-Calibur, BD, Bergen County, New Jersey, USA).Cell Counting Kit-8 (CCK-8) assays were performed to evaluate the cytotoxicity of Fe3+-PEG-MNPs. BM-MSCs were seeded on 96-well plates (5 × 103 cells per well), and co-incubated with Fe3+-PEG-MNPs at different concentrations (0, 12.5, 25, 50, 100, 200, 400, 800, and 1600 μg mL−1) for 24 h at 37 °C in a 5% CO2 incubator. The medium of each well was subsequently replenished with 100 μL fresh serum-free culture medium containing 10 μL CCK-8 reagent (Dojindo, Lot.KR675) and incubated for 2 h at 37 °C in a 5% CO2 incubator. Finally, the absorbance of each well was measured at 450 nm and recorded using a microplate reader (SpectraMax Plus384, MD, San Francisco, California, USA).
2.5 Dual-modal PA/MR imaging of BM-MSCs labeled with Fe3+-PEG-MNPs in vitro
The Fe3+-PEG-MNP-labeled BM-MSC suspension was centrifuged (1000 rpm, 5 min) at room temperature and fixed with 4% paraformaldehyde for 30 min at 4 °C. After paraformaldehyde was removed, the fixed cells were resuspended in 100 μL PBS and pipetted into 96-well PCR plates at 2 × 106 cells per well. Then the obtained cell suspension was imaged using MSOT, followed by 3.0-T MRI. The parameters for PAI were the same as those of the above-mentioned aqueous solution. The parameters for MRI were as follows: FOV, 90 × 60 mm2; slice thickness, 1.5 mm; spacing, 0.5 mm; base resolution matrix, 256 × 180; TR, 500 ms; and TE, 20 ms.2.6 Dual-modal PA/MR imaging in of engrafted BM-MSCs in vivo
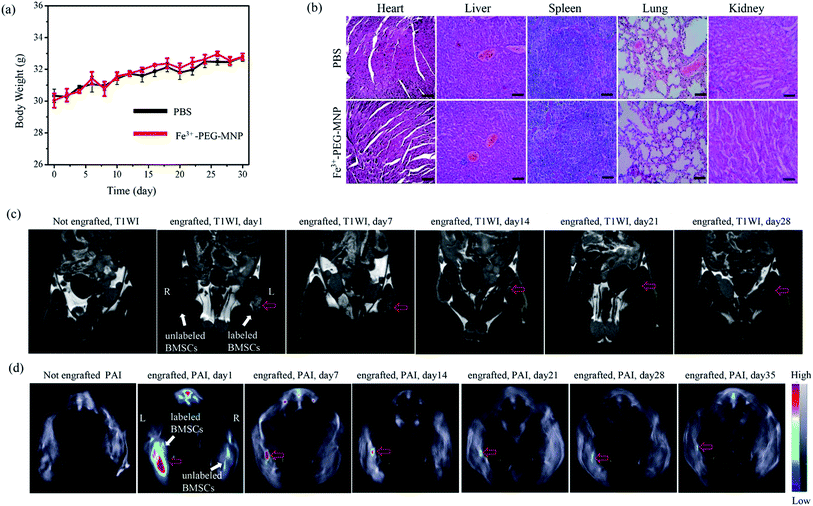
Animal experiments were approved by the Institutional Animal Care and Use Committee of Shanxi Medical University (approval no. SYDL2019002) and carried out in accordance with the U.S. Guide for the Care and Use of Laboratory Animals 8th Edition 2011.13 ICR male mice (Animal Center of Shanxi Medical University, Taiyuan, China; SCXK2015-0001; 8 weeks of age, weighing 30–40 g each) were used herein. All injections were administered under anesthesia with sodium pentobarbital or isoflurane, and efforts were made to minimize suffering. All animals were euthanized via cervical dislocation.Six ICR mice were randomly assigned to the following two groups (n = 3/group): (1) Fe3+-PEG-MNPs injection group (Fe3+-PEG-MNP); (2) PBS injection group (PBS). Two-hundred microliters of 8 mg mL−1 Fe3+-PEG-MNPs solution and PBS were injected into the tail vein of mice in the Fe3+-PEG-MNP and PBS groups, respectively. Body weights in each group were recorded and compared before injection and every 2 d after injection. On day 30 after injection, mice were euthanized via inhalation of an overdose of isoflurane. Thereafter, their organs including the heart, lung, liver, spleen, and kidney were dissected and assessed via HE staining.
Three mice were anesthetized via intraperitoneal administration of 1% pentobarbital sodium (40 mg kg−1), a 50 μL suspension containing 2 × 106 BM-MSCs labeled and unlabeled with Fe3+-PEG-MNPs was intramuscularly injected into the left and right thigh, respectively. PA/MR imaging of these mice were performed using MSOT, followed by 3.0-T MRI, before injection and on days 1, 7, 14, 21, 28, and 35 after BM-MSCs injection. The PA imaging parameters were as follows: temperature, 34 °C; imaging wavelengths, 700, 705, 710, 730, 760, 780, 800 (used as background wavelength), 850, and 875 nm; frames per wavelength, 10; repetitions per slice, 1; run, 1; transverse slices thickness, 800 μm; step size, 0.5 mm. The MR imaging sequence parameters were as follows: FOV, 90 × 60 mm2; slice thickness, 1.5 mm; spacing, 0.5 mm; base resolution matrix, 256 × 256; TR, 500 ms; and TE, 20 ms.
3. Results
3.1 Characterization of Fe3+-PEG-MNPs
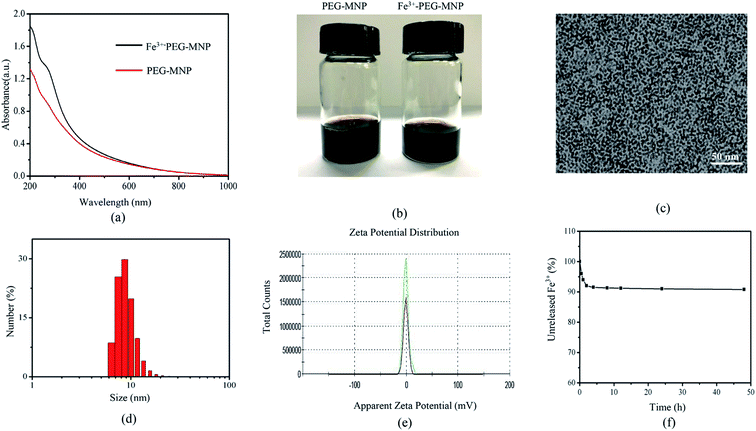
Fe3+-PEG-MNPs and PEG-MNPs displayed different UV-vis-NIR absorption spectra (Fig. 1(a)), indicating the successful synthesis of Fe3+-PEG-MNPs. As shown in (Fig. 1(b)), Fe3+-PEG-MNPs were highly hydrophilic. TEM images (Fig. 1(c)) of Fe3+-PEG-MNPs displayed that they had uniform size, spherical morphology, adequate monodispersion, and an even distribution. DLS analysis (Fig. 1(d) and (e)) revealed ultra-small particles with diameters of 9.2 ± 2.2 nm and a neutral zeta potential of −0.79 ± 4.55 mV in PBS (pH = 7.4). A stability assay (Fig. 1(f)) revealed that 8% iron ions were released from Fe3+-PEG-MNPs during the initial 2 h and none until 48 h, thus indicating the high stability of these particles. ICP-MS analysis revealed that the maximum quantity of Fe3+ chelated to one PEG-MNP was approximately 66.3.2 Dual-modal PA/MR imaging of the aqueous solution of Fe3+-PEG-MNPs
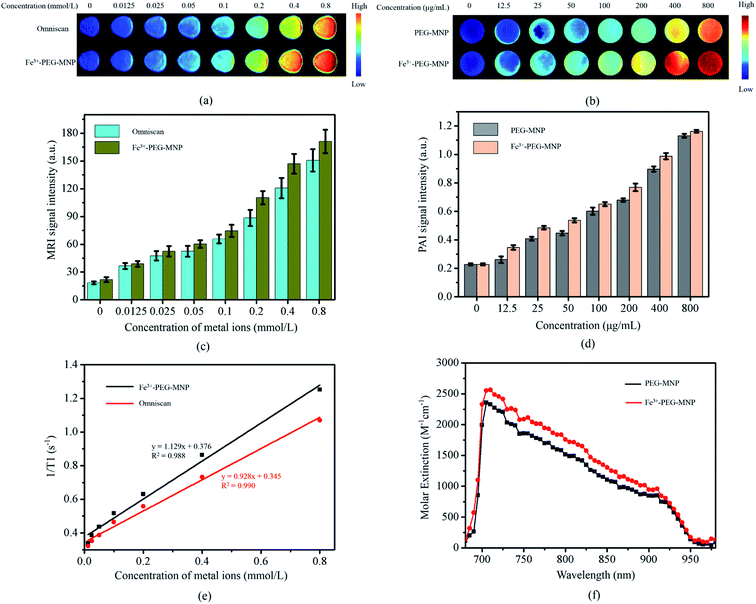
T1WI of aqueous solutions of Fe3+-PEG-MNPs and Omniscan of 0, 0.0125, 0.025, 0.05, 0.1, 0.2, 0.4, and 0.8 mmol L−1 were obtained (Fig. 2(a)) and a T1WI signal intensity (SI) histogram was also plotted (Fig. 2(c)). At the same concentration, the T1WI SI of Fe3+-PEG-MNPs was higher than that of Omniscan. The r1 values represent the T1 relaxation efficacy of Fe3+-PEG-MNPs and Omniscan, obtained by calculating the slopes of metal ions concentration with the reciprocal of the T1 relaxation time. As shown in (Fig. 2(e)), the r1 value of Fe3+-PEG-MNPs was higher than that of Omniscan.The PA absorbance spectra (Fig. 2(f)) revealed that the sensitivity of MNPs was not affected upon chelation of the Fe ions but rather increased by a certain degree. PA images (Fig. 2(b)) and the signal intensity (SI) histogram (Fig. 2(d)) of the aqueous solutions of Fe3+-PEG-MNPs and PEG-MNPs of 0, 12.5, 25, 50, 100, 200, 400, and 800 μg mL−1 also showed that the PA SI of Fe3+-PEG-MNPs was higher than that of PEG-MNPs at the same concentration.
3.3 Successful labeling of BM-MSCs and low cytotoxicity
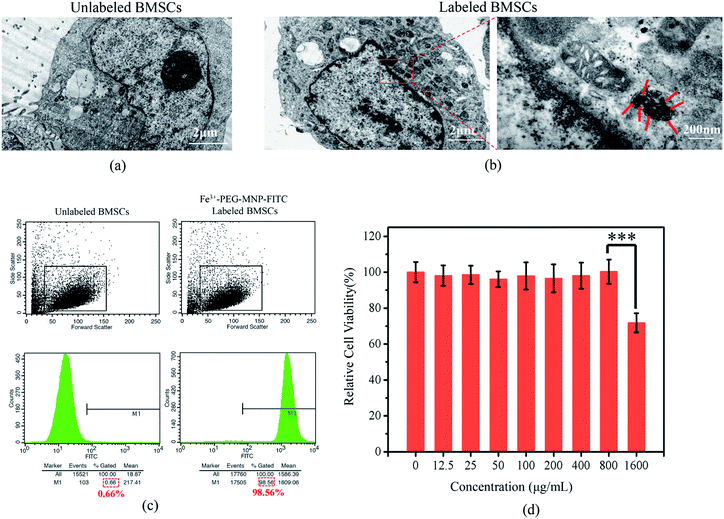
As shown in TEM images (Fig. 3(a) and (b)), numerous black particles were observed in the cytoplasm of labeled rather than unlabeled BM-MSCs. Flow cytometry analysis (Fig. 3(c)) revealed that 98.56% of BM-MSCs were labeled successfully with Fe3+-PEG-MNP-FITC, indicating that Fe3+-PEG-MNPs were easily and efficiently internalized by BM-MSCs via endocytosis without the aid of a transfection agent.On further assessing the cytotoxicity, the CCK8 assay (Fig. 3(d)) revealed that BM-MSCs exhibited higher viability of approximately 100% upon labeling with Fe3+-PEG-MNPs at ≤800 μg mL−1. Although the viability of BM-MSCs labeled with 1600 μg mL−1 Fe3+-PEG-MNPs decreased, they retained a viability of 71.8%. Therefore, Fe3+-PEG-MNPs were considered safe for labeling BM-MSCs at ≤800 μg mL−1.
3.4 Successful dual-modal PA/MR imaging of BM-MSCs in vitro and in vivo
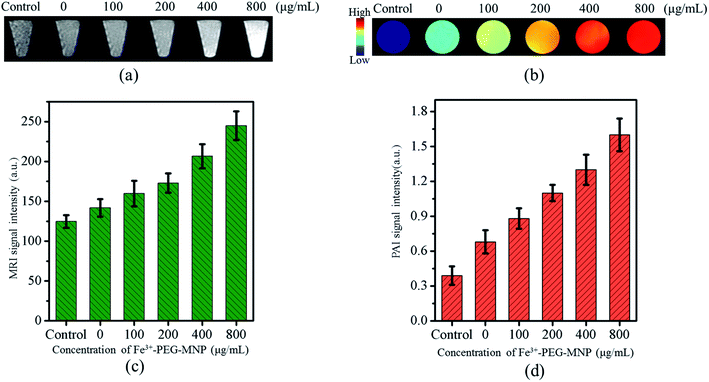
To evaluate the PA and MR imaging capabilities and sensitivities of BM-MSCs labeled with Fe3+-PEG-MNPs, BM-MSCs were labeled with Fe3+-PEG-MNPs at 0, 100, 200, 400, and 800 μg mL−1 and subjected to PA/MR imaging. PBS constituted the control group. As shown in Fig. 4(a)–(d), the labeled BM-MSCs generated a positive signal on PAI and MR T1WI simultaneously and the signal intensity (SI) increased gradually with an increase in the concentration of Fe3+-PEG-MNPs. BM-MSCs labeled with 800 μg mL−1 Fe3+-PEG-MNPs exhibited the highest SI upon PA and MR T1WI at all concentrations.To investigate the biological safety of Fe3+-PEG-MNPs in vivo, solutions of the nanoparticles and PBS were injected into the tail vein in the Fe3+-PEG-MNP and PBS groups, respectively. No significant difference in body weight was observed between the two groups in 1 month (Fig. 5(a)), and no discernible adverse effect was observed in the primary organs of the mice in the Fe3+-PEG-MNP group, including the heart, liver, spleen, lung, and kidney (Fig. 5(b)).
PA and MR imaging of labeled BM-MSCs in vivo were further performed upon their intramuscular administration in the thigh muscle. As shown in Fig. 5(c) and (d), positive signals generated by Fe3+-PEG-MNPs-labeled BM-MSCs in the left thigh were observed, and no positive signals were generated by unlabeled BM-MSCs in the right thigh on PAI and MR T1WI; furthermore, PA and MR signals were retained for at least 35 and 28 d, respectively. On day 35 and 28 after injection, the PA and MR signals were relatively weaker but sufficient to facilitate monitoring of engrafted BM-MSCs.
4. Discussion
Stem cell therapy (especially with BM-MSCs) has beneficial effects and is potentially applicable for numerous diseases and disorders.14–16 In vivo cell imaging is essential to ensure appropriate delivery of stem cells, monitoring of the homing, survival, and distribution of stem cells, and understanding the mechanism underlying stem cell-mediated functional recovery.17–19 Various imaging methods have been widely used for cell tracking, primarily including optical imaging, MRI, PET, and PAI. However, these imaging methods have intrinsic and inevitable limitations; hence, none of them satisfy all clinical or experimental requirements of stem cell tracking. To circumvent these limitations and achieve synergistic advantages, dual-modal or multimodal imaging of cells has been proposed in this study as an alternative and promising strategy for cells tracking.17Currently, MRI is not only a prevalent tool for tracking noninvasive cells in vivo but also a common diagnostic tool for medical imaging in the clinical setting.8,20,21 MRI has high-spatial resolution (up to 100 μm) and is capable of long-term cell tracking (up to 6 weeks).22,23 However, the clinical application of stem cell imaging is limited by its intrinsic deficiencies, including relative low sensitivity, long imaging duration, and the contraindications after the implantation of metal devices.8,24 To circumvent these limitations and improve cell-imaging modalities, this study used a dual-modal imaging strategy involving photoacoustic and magnetic resonance imaging for monitoring stem cells in vivo. PAI is an excellent and rapidly evolving biomedical imaging method. Briefly, a ultrasound wave can be detected by US detector, which is generated by an instant thermoelastic expansion of tissue after photon absorption by tissues.25 PAI is a promising stem cell imaging modality with high sensitivity of optical imaging, outstanding resolution of micrometers equivalent to the size of an organelle and a favorable tissue penetration depth of several centimeters.10,26,27 Furthermore, dual-modal PA/MR imaging compensates for the limitations of each individual modality; hence, the sensitivity and specificity of cell tracking in vivo can be improved.
Successful and effective cell tracking in vivo requires cell tracers with outstanding properties, including high stability, effective cell labeling, low cytotoxicity, high biosafety, excellent imaging capability.28 Nanoparticles have been widely used as stem cell trackers in vivo. In this study, we designed and synthesized novel dual-modal PA/MR nanoparticles (Fe3+-PEG-MNPs) containing melanin and iron ions.
Melanin is a ubiquitous pigment in nature and is widespread in the tissues of many organisms, including human skin, mucous membranes, retina, cerebral pia mater, and ovaries.11,29,30 In addition, it actively chelates various metal ions such as 64Cu2+, Fe3+, Gd3+, and Mn2+.11 Iron is an essential trace element for organismal growth. In the human body, iron is stored as ferritin in the liver, spleen, small intestine mucosa, bone marrow, and other organs or tissues and is primarily excreted through the intestines. Therefore, the novel nanoparticle (Fe3+-PEG-MNP) synthesized herein on the basis of these two biomaterials is expected to display adequate biological safety. As expected, this study shows that the viability of labeled BM-MSCs can be maintained at high levels even at 1600 μg mL−1 of Fe3+-PEG-MNPs (Fig. 3(d)). Moreover, the body weights of the mice were not affected, and no discernible adverse effects were observed in the heart, lung, liver, spleen, and kidney after the injection of Fe3+-PEG-MNPs (Fig. 5(a) and (b)). These results indicate the high biological safety of Fe3+-PEG-MNPs.
PEG-MNPs were previously used as a nanoplatform for multimodal imaging and imaging-guided nanoscale systems for drug delivery.11,31 Herein, Fe3+-PEG-MNPs were easily obtained by stirring at 37 °C for 1 h. BM-MSCs were easily and efficiently labeled with Fe3+-PEG-MNPs via co-incubation without the aid of any transfection agent (Fig. 3(a)–(c)) owing to the ultra-small size of approximately 9.2 nm and surface neutral charge of Fe3+-PEG-MNPs (Fig. 1(d) and (e)). After redissolution in PBS, only approximately 8% of Fe3+ was released from Fe3+-PEG-MNPs during the initial 2 h and the release ratio of Fe3+ was subsequently maintained at this relatively low level, thus indicating the high stability of Fe3+-PEG-MNPs (Fig. 1(f)). As melanin and Fe3+ could yielded PA and MR signals, respectively,11,32–34 both PAI and MR T1WI of BM-MSCs were successfully accomplished using Fe3+-PEG-MNPs in vitro and in vivo (Fig. 4(a) and (b) and 5(c) and (d)). In particular, approximately 1 month after cell transplantation, the PA and MR SI of BM-MSCs labeled with Fe3+-PEG-MNPs in vivo were maintained at a relatively stable level to adequately facilitate the monitoring of engrafted cells (Fig. 5(c) and (d)). In the future studies, Fe3+-PEG-MNPs will be used for monitoring BM-MSCs in animal model of human disease to understand some repair mechanisms underlying BM-MSC-mediated.
5. Conclusions
This study describes a novel dual-modal PA/MR nanoparticle (Fe3+-PEG-MNP) probe based on natural biomaterials for monitoring engrafted BM-MSCs. Briefly, Fe3+-PEG-MNPs are advantageous for tracking engrafted BM-MSCs owing to their simple synthesis, high stability and hydrophilicity, ease of labeling cells and low cytotoxicity, high biological safety, excellent capability of dual-modal PA/MR imaging, high sensitivity and long-term monitoring in vitro and in vivo. Based on these desired properties, Fe3+-PEG-MNPs are an ideal dual-modal PA/MR nanoparticle probe for future clinical SCT, thus contributing to advancements in basic studies using stem cells.Author contributions
Hua Zhang conceived and designed the experiment, prepared the samples, performed the synthesis experiments and revised the manuscript. Zhen-Jun Wang and Ling-Jie Wang analyzed the data and wrote the manuscript. Ting-Ting Li performed the characterization of Fe3+-PEG-MNP. Sheng He contributed to the extracting and culturing BM-MSCs. Li-Ping Li, Xiao-Yan Li and Shi-Jie Liu performed the experiments in vitro and in vivo. Jian-Ding Li contributed to the experimental instruments. Si-Jin Li and Rui-Ping Zhang supervised the entire project and integrated the different parts of the work together. All authors approved the final manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 81571747 and 81771907), Key Research and Development Project of Shanxi Province (no. 201703D321015-3, 201803D31004 and 201803D31109), Engineering Technology Research Center of Shanxi Province (no. 201805D121008), Science and Technology Innovation Team Project of Shanxi Province (no. 201705D131026), Scientific and Technological Achievements Transformation Project of Shanxi Province (no. 201704D131006), Laboratory Construction Project of Shanxi Province, and the Projects for Local Science and Technology Development Guided by the Central Committee (no. YDZX20191400002537).References
- G. I. Im, Eur. Cells Mater., 2017, 33, 183–196 CrossRef.
- B. Lv, F. Li, J. Han, J. Fang, L. Xu, C. Sun, T. Hua, Z. Zhang, Z. Feng and X. Jiang, Front. Mol. Neurosci., 2017, 10, 80 Search PubMed.
- D. Shi, J. Zhang, Q. Zhou, J. Xin, J. Jiang, L. Jiang, T. Wu, J. Li, W. Ding, J. Li, S. Sun, J. Li, N. Zhou, L. Zhang, L. Jin, S. Hao, P. Chen, H. Cao, M. Li, L. Li, X. Chen and J. Li, Gut, 2017, 66, 955–964 CrossRef CAS.
- X. Yuan, X. Wang, C. Chen, J. Zhou and M. Han, Stem Cell Res. Ther., 2017, 8, 146 CrossRef.
- W. Liu, Y. Wang, F. Gong, Y. Rong, Y. Luo, P. Tang, Z. Zhou, Z. Zhou, T. Xu, T. Jiang, S. Yang, G. Yin, J. Chen, J. Fan and W. Cai, J. Neurotrauma, 2019, 36, 469–484 CrossRef.
- V. Jeevanantham, M. Butler, A. Saad, A. Abdel-Latif, E. K. Zuba-Surma and B. Dawn, Circulation, 2012, 126, 551–568 CrossRef.
- T. Schroeder, Nature, 2008, 453, 345–351 CrossRef CAS.
- S. J. Zhang and J. C. Wu, J. Nucl. Med., 2007, 48, 1916–1919 CrossRef CAS.
- J. E. Lemaster, C. Fang, T. Kim, A. Hariri and J. V. Jokerst, ACS Appl. Nano Mater., 2018, 1, 1321–1331 CrossRef CAS.
- L. V. Wang and S. Hu, Science, 2012, 335, 1458–1462 CrossRef CAS.
- Q. Fan, K. Cheng, X. Hu, X. Ma, R. Zhang, M. Yang, X. Lu, L. Xing, W. Huang, S. S. Gambhir and Z. Cheng, J. Am. Chem. Soc., 2014, 136, 15185–15194 CrossRef CAS.
- W. W. Cai, L. J. Wang, S. J. Li, X. P. Zhang, T. T. Li, Y. H. Wang, X. Yang, J. Xie, J. D. Li and S. J. Liu, J. Biomed. Mater. Res., Part A, 2017, 105, 131–137 CrossRef CAS.
- National Research Council, Guide for the care and use of laboratory animals, National Academies Press, Washington, DC, 8th edn, 2011 Search PubMed.
- R. C.-J. Chiu, Heart Failure Rev., 2003, 8, 247–251 CrossRef.
- X. Chenjie, M. N. David, J. A. Ankrum, M. Mads Emil, J. A. Phillips, R. Isaac, G. R. Wojtkiewicz, J. Vikram, K. Jens Roat and Z. Weian, Nano Lett., 2012, 12, 4131–4139 CrossRef.
- R. C.-J. Chiu, Heart Failure Rev., 2003, 8, 247–251 CrossRef.
- N. Sugiyama, A. Y. Sonay, R. Tussiwand, B. E. Cohen and P. Pantazis, Small, 2018, 14, 1703386 CrossRef.
- J. V. Jokerst, M. Thangaraj, P. J. Kempen, R. Sinclair and S. S. Gambhir, ACS Nano, 2012, 6, 5920–5930 CrossRef CAS.
- J. E. Lemaster, F. Chen, T. Kim, A. Hariri and J. V. Jokerst, ACS Appl. Nano Mater., 2018, 1, 1321–1331 CrossRef CAS.
- S. J. Liu, L. J. Wang, Y. Qiao, H. Zhang, L. P. Li, J. H. Sun, S. He, W. Xu, X. Yang, W. W. Cai, J. D. Li, B. Q. Wang and R. P. Zhang, Int. J. Nanomed., 2018, 13, 1749–1759 CrossRef CAS.
- M. E. Kupfer and B. M. Ogle, Biotechnol. J., 2015, 10, 1515–1528 CrossRef CAS.
- K. Daehong, H. Kwan Soo and S. Jihwan, Mol. Cells, 2007, 23, 132 Search PubMed.
- C. Xu, D. Miranda-Nieves, J. A. Ankrum, M. E. Matthiesen, J. A. Phillips, I. Roes, G. R. Wojtkiewicz, V. Juneja, J. R. Kultima, W. Zhao, P. K. Vemula, C. P. Lin, M. Nahrendorf and J. M. Karp, Nano Lett., 2012, 12, 4131–4139 CrossRef CAS.
- J. E. Lemaster, Z. Wang, A. Hariri, F. Chen, Z. Hu, Y. Huang, C. V. Barback, R. Cochran, N. C. Gianneschi and J. V. Jokerst, Chem. Mater., 2018, 31, 251–259 CrossRef.
- Q. Fu, R. Zhu, J. Song, H. Yang and X. Chen, Adv. Mater., 2019, 31, e1805875 Search PubMed.
- X. Qin, H. Chen, H. Yang, H. Wu, X. Zhao, H. Wang, T. Chour, E. Neofytou, D. Ding, H. Daldrup-Link, S. C. Heilshorn, K. Li and J. C. Wu, Adv. Funct. Mater., 2018, 28, 1704939 CrossRef.
- J. Weber, P. C. Beard and S. E. Bohndiek, Nat. Methods, 2016, 13, 639–650 CrossRef CAS.
- J. Wang and J. V. Jokerst, Stem Cells Int., 2016, 2016, 9240652 Search PubMed.
- P. A. Riley, Int. J. Biochem. Cell Biol., 1997, 29, 1235–1239 CrossRef CAS.
- D. L. Longo, R. Stefania, C. Callari, F. De Rose, R. Rolle, L. Conti, L. Consolino, F. Arena and S. Aime, Adv. Healthcare Mater., 2017, 6, 1600550 CrossRef.
- R. Zhang, Q. Fan, M. Yang, K. Cheng, X. Lu, L. Zhang, W. Huang and Z. Cheng, Adv. Mater., 2015, 27, 5063–5069 CrossRef CAS.
- K. Y. Ju, J. Kang, J. Pyo, J. Lim, J. H. Chang and J. K. Lee, Nanoscale, 2016, 8, 14448–14456 RSC.
- H. T. Ta, Z. Li, C. E. Hagemeyer, G. Cowin, S. Zhang, J. Palasubramaniam, K. Alt, X. Wang, K. Peter and A. K. Whittaker, Biomaterials, 2017, 134, 31–42 CrossRef CAS PubMed.
- N. Kuźnik, M. M. Tomczyk, M. Wyskocka, Ł. Przypis, A. P. Herman, R. Jędrysiak, K. K. Koziol and S. Boncel, Int. J. Nanomed., 2015, 10, 3581 Search PubMed.
Footnote |
| † Hua Zhang, Zhen-Jun Wang and Ling-Jie Wang are co-first authors. |
| This journal is © The Royal Society of Chemistry 2019 |