Open Access Article
Open Access ArticleDurable, acid-resistant copolymers from industrial by-product sulfur and microbially-produced tyrosine†
Timmy
Thiounn
a,
Andrew G.
Tennyson
 ab and
Rhett C.
Smith
ab and
Rhett C.
Smith
 *a
*a
aDepartment of Chemistry and Center for Optical Materials Science and Engineering Technologies, Clemson University, Clemson, South Carolina, USA. E-mail: rhett@clemson.edu
bDepartment of Materials Science and Engineering, Clemson University, Clemson, South Carolina, USA
First published on 3rd October 2019
Abstract
The search for alternative feedstocks to replace petrochemical polymers has centered on plant-derived monomer feedstocks. Alternatives to agricultural feedstock production should also be pursued, especially considering the ecological damage caused by modern agricultural practices. Herein, L-tyrosine produced on an industrial scale by E. coli was derivatized with olefins to give tetraallyltyrosine. Tetraallyltyrosine was subsequently copolymerized via its inverse vulcanization with industrial by-product elemental sulfur in two different comonomer ratios to afford highly-crosslinked network copolymers TTSx (x = wt% sulfur in monomer feed). TTSx copolymers were characterized by infrared spectroscopy, elemental analysis, thermogravimetric analysis, differential scanning calorimetry, and dynamic mechanical analysis (DMA). DMA was employed to assess the viscoelastic properties of TTSx through the temperature dependence of the storage modulus, loss modulus and energy damping ability. Stress–strain analysis revealed that the flexural strength of TTSx copolymers (>6.8 MPa) is more than 3 MPa higher than flexural strengths for previously-tested inverse vulcanized biopolymer derivatives, and more than twice the flexural strength of some Portland cement compositions (which range from 3–5 MPa). Despite the high tyrosine content (50–70 wt%) in TTSx, the materials show no water-induced swelling or water uptake after being submerged for 24 h. More impressively, TTSx copolymers are highly resistant to oxidizing acid, with no deterioration of mechanical properties even after soaking in 0.5 M sulfuric acid for 24 h. The demonstration that these durable, chemically-resistant TTSx copolymers can be prepared from industrial by-product and microbially-produced monomers via a 100% atom-economical inverse vulcanization process portends their potential utility as sustainable surrogates for less ecofriendly materials.
Introduction
Polymers are ubiquitous in nearly every aspect of modern society to the extent that each person consumes 50 kg of polymers per year in Europe and 68 kg per year in the United States.1 The vast majority of these polymers are made from petrochemicals and are relatively inert to typical biological degradation processes over any reasonable timeframe.2 As a result, anthropogenic polymers accumulate in the environment in landfills, as floating masses and microplastic in the oceans,3–5 and they have even begun to assimilate into landmasses through the formation of “plasticrust” around island shores.6 Plant-derived monomers have been widely pursued as replacements for petrochemicals in the quest to find sustainable and degradable alternatives to polymers of the petrochemical century.7,8 Agricultural production of crops for chemical feedstocks, however, comes with its own set of ecological problems, as modern agricultural practices wreak havoc on global phosphorus and nitrogen cycles and contaminate water resources through fertilizer and pesticide-laced runoff. Thus, whereas it is certainly imperative that agricultural waste products be leveraged for environmentally and economically advantageous uses, technological advances that rely on increased agricultural activities for the sole purpose of providing a new chemical feedstock should be weighed cautiously.Some attractive alternatives to agricultural production of chemical feedstocks are to employ high production-density aquaculture of algae9,10 or engineered bacteria11–16 to produce target feedstocks. Such processes can be carried out in a closed system often operating at or slightly above ambient temperatures, thus limiting environmental impact and energy consumption.17
Regardless of how they are produced, chemically-resistant polymers often pose an environmental threat in terms of degradability, yet chemical resistance is required for many applications. New advances in production of chemically-resistant polymers should therefore centre on materials that are recyclable, and/or which degrade to environmentally benign compounds. High sulfur-content materials produced by inverse vulcanization of sustainable olefins have emerged recently as promising candidates in this regard.18,19
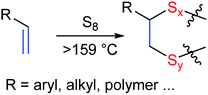
In the inverse vulcanization process,20 sulfur, itself an industrial by-product of fossil fuel refining,21–23 is simply heated with the requisite olefin to form crosslinked network solids with 100% atom economy (Scheme 1).24–27 Many high sulfur-content materials can be remelted and recast over multiple cycles without loss of mechanical strength, and the environmental degradation products can be so environmentally benign that inverse vulcanization products have been used as fertilizers to improve the growth of food crops.28,29 High sulfur-content composites can also exhibit remarkably high mechanical strength. Sulfur composites with lignin30 or cellulose,31 for example, have shown flexural strengths on par with that of Portland cement.
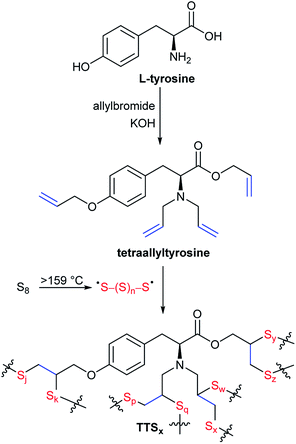
For the current work, tyrosine was an attractive initial feedstock because it is produced on an industrial scale by engineered strains of E. coli.32–35 Furthermore, tyrosine is readily functionalized with four allyl groups, providing up to eight sites for the formation of C–S crosslinks upon inverse vulcanization (Scheme 2). The mechanical strength of high sulfur-content materials produced by inverse vulcanization increases with increased crosslink density,30,31,36 so it was hypothesized that the high crosslink density provided by tetraallyltyrosine would likewise lead to durable copolymers. Inverse vulcanization of tetraallyltyrosine with sulfur was thus undertaken to produce crosslinked network copolymers TTSx (x = wt% sulfur in the monomer feed). Characterization by infrared spectroscopy, thermogravimetric analysis, differential scanning calorimetry, and dynamic mechanical analysis are discussed for TTSx having two different monomer feed compositions. The resistance of TTSx to water uptake and degradation by sulfuric acid are also assessed.
Results and discussion
Synthesis of TTSx
A commercial sample of L-tyrosine produced by an engineered strain of E. coli was selected as the initial starting material for crosslinking with industrial by-product sulfur for the current study. Crosslinking of sulfur by inverse vulcanization requires an olefin-derivatized comonomer, so L-tyrosine was first converted to tetraallyltyrosine by the reported method (Scheme 2; NMR spectra provided in Fig. S1–S5 of the ESI†).37 At STP, elemental sulfur exists primarily as S8 rings in an orthorhombic crystal morphology. Upon heating, S8 first melts at 118 °C and then forms polymeric sulfur radicals at temperatures > 159 °C (Scheme 2).38 These polymeric sulfur radicals are thought to be the active species for reaction with olefins to form crosslinks. In the current case, reaction between sulfur radicals and the four olefin moieties of tetraallyltyrosine affords up to eight S–C crosslinks per molecule. Upon heating a sample of tetraallyltyrosine and sulfur above 159 °C, the solution initially was the dark red colour characteristic of polymeric sulfur radicals and then transformed to a deep brown-red liquid within 5 min. When the sulfur: tetraallyltyrosine mass ratio was >1, the reaction mixture did not homogenize even after extended stirring and heating. Instead, a dark-coloured solid ball of material precipitated from the liquid phase. The separated solid was comprised of sulfur-crosslinked tetraallyltyrosine, whereas the cooled liquid phase was unreacted S8. For reactions in which the sulfur: tetraallyltyrosine mass ratio was ≤1, dark red-brown, homogeneous reaction solutions were observed after 30–45 minutes of heating. Two sulfur: tetraallyltyrosine monomer mixtures, comprised of 50 wt% and 30 wt% sulfur, were thus selected for further investigation to produce TTS50 and TTS30, respectively.In contrast to many reported high sulfur-content materials,30,31,36,39,40 once TTS50 or TTS30 were set as solids they could not be remelted or reshaped. To prepare materials of desired shapes, it was necessary to pour the homogenized reaction solutions into a mould for subsequent heating to complete the crosslinking process. Moulded samples of TTSx prepared in this manner were hard, dark red-brown, semi-transparent solids with smooth, shiny surfaces (Fig. 1). The densities of the materials so produced (1.2–1.3 g cm−3) are somewhat higher than that of a typical organic polymer by merit of the high sulfur content, given that the density of pure sulfur is 2 g cm−3. Infrared spectra (Fig. S6, ESI†) of TTSx reveals near complete loss of the monosubstituted alkene C–H bending mode (920 and 995 cm−1) from the allyl group in tetraallyltyrosine upon reaction with sulfur, demonstrating the efficiency of the crosslinking process.
Some high sulfur-content materials prepared by inverse vulcanization are homogeneous copolymer networks, while others are composites wherein free sulfur occupies voids in a sulfur-crosslinked network polymer.28,30,31 Free sulfur is quite soluble in CS2, whereas the crosslinked network is insoluble, so recursive rinsing of TTSx with CS2 was undertaken to extract any free sulfur. A minimal amount of the material was extractable from TTSx by this method (15 and 11% for TTS30 and TTS50, respectively), and the elemental analysis of the soluble fraction matched that of the bulk material, rather than being free sulfur. Differential scanning calorimetry (DSC, vide infra) data likewise did not show thermal transitions associated with free sulfur. These data support the characterization of TTSx as consisting primarily of sulfur-crosslinked networks without appreciable free sulfur present, as they are depicted in Scheme 2. Considering near complete consumption of alkene units and the limited amount of free sulfur in each material, the average crosslink was calculated to consist of about 6 or 3 sulfur atoms in TTS30 and TTS50, respectively. The short average crosslink length provides an explanation for the observation that TTSx cannot be remelted. The ability to re-melt high sulfur-content materials generally relies on the thermal reversibility of S–S bond formation. With an average crosslink length of only 3–6 sulfur atoms, a significant fraction of crosslinks will comprise a single sulfur atom, and thus lack the S–S bond needed for reversible bond-formation. The high crosslink density in these materials thus unfortunately precludes TTSx from the re-melting exhibited by materials having longer average oligosulfur crosslink lengths.
Thermal properties of TTSx
The thermogravimetric analysis (TGA) data (Table 1 and Fig. 2) reveal that the onset of decomposition for the starting materials, tetraallyltyrosine and sulfur are 182 and 282 °C respectively, and both have char yields of <1%. The decomposition temperatures (Td) for TTS30 (209 °C) and TTS50 (211 °C) lie between those for tetraallyltyrosine and sulfur. The char yields for TTSx materials also show a significant increase from the initial monomers, with an increase to 23–26%.| TTS30 | TTS50 | |
|---|---|---|
a Determined from the onset of the major mass loss peak.
b Determined from the onset of transition from the second heating cycle of the DSC curve.
c Determined from the onset of the storage modulus.
d Determined from the peak of the loss modulus curve.
e Determined from the peak of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve.
f No measurable water uptake. δ curve.
f No measurable water uptake.
|
||
| T d (°C)a | 209 | 211 |
| Char yield | 26% | 23% |
| T g b (°C) | 68 | 58 |
| E′Tgc (°C) | 48.4 | 47.0 |
| E′′Tgd (°C) | 60.8 | 58.2 |
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ Tge (°C) δ Tge (°C) |
77.2 | 70.5 |
| Density (g cm−3) | 1.3 ± 0.1 | 1.2 ± 0.1 |
| H2O uptake | <0.5%f | <0.5%f |
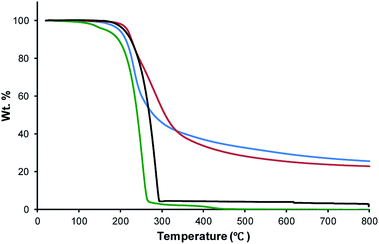 | ||
| Fig. 2 Thermogravimetric analysis (TGA) traces for elemental sulfur (black trace), tetraallyltyrosine (green trace), TTS30 (blue trace) and TTS50 (red trace). | ||
Differential scanning calorimetry was also used to assess the thermal and morphological properties of TTSx (Fig. S7†). The glass transition temperatures (Tg) of TTS30 (68 °C) and TTS50 (58 °C) are both well above room temperature, while the higher Tg of TTS30 reflects fewer degrees of freedom in the more-crosslinked material. Notably, the DSC traces for TTSx do not exhibit a melt peak for orthorhombic sulfur, in agreement with the observation of very little CS2-extractable free sulfur. In contrast, the DSC traces for high sulfur-content composites from which significant quantities of free sulfur are extractable exhibit prominent peaks at 116–120 °C attributable to orthorhombic sulfur melting transitions.30,31,36 Peaks attributable to polymeric sulfur domains are also notably absent from the DSC traces of TTSx. Polymeric sulfur domains generally produce endothermic transitions at around −37 °C and sometimes also cold crystallization peaks over a broad temperature range of −20 to +40 °C.30,31 the absence of such features in the DSC analysis of TTSx further confirms the short average crosslink length.
Mechanical properties and chemical resistance of TTSx
The high crosslink density in TTSx was hypothesized to endow them with high strength and durability. To test this hypothesis, TTS30 and TTS50 were subjected to dynamic mechanical analysis (DMA). The temperature-dependences of storage modulus (E′), loss modulus (E′′) and energy damping factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) were initially assessed (Fig. 3 and Table 1). Expectedly, the E′ is higher for TTS30 whereas the tan
δ) were initially assessed (Fig. 3 and Table 1). Expectedly, the E′ is higher for TTS30 whereas the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ is higher for the less-densely-crosslinked TTS50. The storage and loss moduli for both copolymers are significantly higher than those of uncrosslinked elemental sulfur, which is too brittle to even be clamped in the DMA instrument at the minimum clamping force.
δ is higher for the less-densely-crosslinked TTS50. The storage and loss moduli for both copolymers are significantly higher than those of uncrosslinked elemental sulfur, which is too brittle to even be clamped in the DMA instrument at the minimum clamping force.
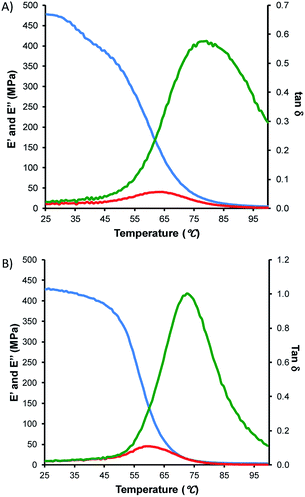 | ||
Fig. 3 Storage modulus (E′, blue trace), loss modulus (E′′, red trace) and damping factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, green trace) for TTS30 (A) and TTS50 (B). δ, green trace) for TTS30 (A) and TTS50 (B). | ||
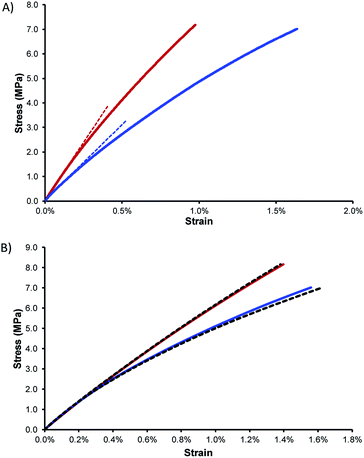
Stress–strain analysis of TTS30 and TTS50 provides the flexural modulus and minimum flexural strength of TTSx copolymers at room temperature (Fig. 4A and Table 2). The flexural storage modulus of TTS30 is greater than that of TTS50 (860 vs. 580 MPa), indicative of the higher crosslink density of the TTS30 sample. Additionally, the flexural strength for TTS30 is slightly greater than TTS50 sample. Note that because the samples did not break at the maximum force applicable by the instrument (10 N), these already high flexural strengths represent a minimum flexural strength for the materials. The flexural strengths of TTSx exceed those of other sulfur-crosslinked materials for which stress–strain data have been reported, including cellulose/sulfur (up to 3.8 MPa) and lignin/sulfur composites (up to 2.1 MPa). For comparison to a more familiar material, the flexural strength of Portland cement ranges from 3–5 MPa. Other materials utilizing sulfur with dicyclopentadiene, linseed oil, and canola oil led to materials with flexural strengths of up to 6 MPa.41 The TTSx materials exhibit flexural strengths in excess of 7.0 MPa; this is a lower limit because samples do not break at maximum instrument force. The high strength may be attributable to higher crosslink density afforded by tetraallyltyrosine, which features eight potential sites for crosslinking per molecule.
| Material | Flexural storage modulus (MPa) | Flexural strength (MPa) |
|---|---|---|
| a Values determined from the average of three runs. b Percentage of initial metric retained after soaking the sample in 0.5 M H2SO4 (aq.) for 24 h. | ||
| TTS30 | 860 ± 110 | >7.0 ± 1 |
| After H2SO4b | 100% | 100% |
| TTS50 | 580 ± 140 | >6.8 ± 0.4 |
| After H2SO4b | 100% | 100% |
With such a high content of tyrosine-derived monomer, water uptake and attendant swelling or loss of mechanical integrity could be a concern for TTSx materials. On the other hand, elemental sulfur is highly hydrophobic, with a critical surface energy of 27 mN m−1, which is between the values for Teflon (24 mN m−1) and polyethylene (32.6 mN m−1).42 Samples of TTSx were thus weighed, submerged in water for 24 h, and reweighed after this soaking period. No water uptake or change in dimensions was measurable for either TTSx sample, confirming exclusion of water by the hydrophobic sulfur. Water exclusion in polymers comprising polar functional groups is an important property given that uptake of even small quantities of water can significantly impact the mechanical properties of a material. The Tg of nylon-6,6, for example, falls from 100 °C when dry to 43 °C after 3.5 wt% water uptake – occurring at a relative humidity of just 55% – and the tensile strength at yield falls concomitantly from 80 MPa to 43 MPa.43 The mechanical strength of TTSx, however, is unchanged even after being submerged in water for 24 h.
Whereas the ready decomposition of high sulfur-content materials by soil bacteria can be an asset from a sustainability standpoint, polymers having some targeted chemical resistance may be advantageous or required for a given application. Sulfur-infused masonry and cementitious materials, for example, have proven especially effective in applications requiring resistance to corrosion by acid.44,45 To assess the extent to which TTSx copolymers could maintain their structural integrity after challenge by acid exposure, a brick of each copolymer was soaked in 0.5 M H2SO4 (aq.) for 24 h and then the stress–strain curve was re-measured. Sulfuric acid was selected because it is a strong and oxidizing acid that reacts with many common organic functional groups. Impressively, neither TTS30 nor TTS50 showed any change in dimensions or flexural strength/modulus after challenge by sulfuric acid (Fig. 4B). This is an especially important finding because it demonstrates that at least 50 wt% tyrosine can be protected by sulfur crosslinking, whereas chemical susceptibility is a common criticism of bioderived polymers comprising polar functional groups as compared to many common petrochemical polymers.
Conclusions
Durable, chemically-resistant TTSx copolymers have been prepared from industrial by-product sulfur and a derivative of microbially-produced L-tyrosine via the 100% atom-economical inverse vulcanization process. The copolymers are highly-crosslinked network copolymers comprised primarily of sulfur-crosslinked tetraallyltyrosine wherein the average crosslinks are 3–6 sulfur atoms in length. The flexural strengths of TTSx copolymers are significantly higher than that of Portland cement. Despite the high tyrosine content (50–70 wt%), the copolymers exhibit no swelling, water uptake or deterioration of mechanical properties even after soaking in 0.5 M aqueous sulfuric acid for 24 h.Experimental
General considerations
All NMR spectra were recorded on a Bruker Avance spectrometer operating at 300 MHz for proton nuclei. Fourier transform infrared spectra were obtained using a Shimadzu IR Affinity-1S instrument operating over the range of 400–4000 cm−1 at ambient temperature. Thermogravimetric analysis (TGA) was recorded on a Mettler Toledo TGA 2 STARe System over the range of 25 to 800 °C with a heating rate of 5 °C min−1 under a flow of N2 (20 mL min−1). Differential Scanning Calorimetry (DSC) was acquired using a Mettler Toledo DSC 3 STARe System over the range of 0 to 140 °C, with a heating rate of 5 °C min−1 under a flow of N2 (200 mL min−1). Each DSC measurement was carried out over 3 heat-cool cycles to get rid of any thermal history. The data reported were taken from the second cycle of the experiment. Dynamic Mechanical Analysis (DMA) was performed using a Mettler Toledo DMA 1 STARe System in single cantilever mode. DMA samples were cast from two stainless steel moulds with sample dimensions of approximately 2 × 11 × 40 mm and 2 × 11 × 19 mm. Temperature scans were taken with the smaller DMA sample while stress strain measurements were taken with the larger samples. The clamping force for both temperature scan and stress strain measurements was set to 1 cN m. Temperature scans were measured from 25–100 °C and the motor displacement was set to 5 μm and a free length of 5 mm. Static stress–strain measurements were taken at 25 °C in single cantilever mode with a force range of 0–10 N, a ramp rate of 0.1 N min−1, and with a free length of 10 mm.Materials and methods
L-Tyrosine (99%, Alfa Aesar), allyl bromide (Oakwood Chemical), elemental sulfur (99.5%, Alfa Aesar) and potassium hydroxide (Fluka Analytical) were used without further purification. Tetraallyltyrosine was prepared as previously reported.37Water uptake measurements were taken after soaking a sample of TTSx in deionized water for 24 h. A sample of TTSx was taken after the initial stress strain measurement, was re-run, followed by soaking in 0.5 M H2SO4 (aq.) solution for 24 h. After 24 h the sample was dried and subjected stress strain measurements.
General synthesis of TTSx
CAUTION: Heating elemental sulfur with organics can result in the formation of H2S gas. H2S is toxic, foul-smelling, and corrosive. Elemental sulfur and tetraallyltyrosine was weighed directly into a 20 mL scintillation vial. The vessel was placed in a thermostat-controlled oil bath set to 150 °C (the internal temperature rises to between 160 and 180 °C). After the sulfur began to melt, two visible layers were formed. The reaction media was vigorously stirred with a Teflon coated stir bar until the reaction mixture became homogenous (30–45 min). Once the reaction mixture became homogenous, the reaction solution was transferred to stainless steel moulds in a 150 °C oven to cure for 4 h. Reagent masses and results of elemental combustion microanalysis are provided below.Synthesis of TTS30
The general synthesis above was used to synthesize TTS30 (30 wt% sulfur) where 3.01 g of elemental sulfur and 7.00 g of tetraallyltyrosine were used in the reaction, with a recovered yield of 95%. Elemental analysis calculated: C, 51.7%; S, 30.0%; H, 5.6%; N, 2.9%. Found: C, 49.6%; S, 33.4%; H, 5.4%; N, 2.9%.Synthesis of TTS50
The general synthesis above was used to synthesize TTS50 (50 wt% sulfur) where 5.02 g of elemental sulfur and 5.02 g of tetraallyltyrosine were used in the reaction, with a recovered yield of 95%. Elemental analysis calculated: C, 36.9%; S, 50.0%; H, 4.0%; N, 2.1%. Found: C, 38.44%; S, 51.39%; H, 3.59%; N, 2.28%.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation (CHE-1708844).Notes and references
- https://www.plasticsinsight.com/world-per-capita-consumption-pe-pp-pvc-resins-2014/, accessed 7-4-19.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef
.
- J. Jeong and J. Choi, Chemosphere, 2019, 231, 249–255 CrossRef CAS
.
- M. Cole, P. Lindeque, C. Halsband and T. S. Galloway, Mar. Pollut. Bull., 2011, 62, 2588–2597 CrossRef CAS
.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Environ. Sci. Technol., 2012, 46, 3060–3075 CrossRef CAS PubMed
.
- I. Gestoso, E. Cacabelos, P. Ramalhosa and J. Canning-Clode, Sci. Total Environ., 2019, 687, 413–415 CrossRef CAS
.
-
L. Lanni, Infinity Line, LMNO Press, Chapin, SC, USA, 2018 Search PubMed
.
- S. L. Kristufek, K. T. Wacker, Y.-Y. Timothy Tsao, L. Su and K. L. Wooley, Nat. Prod. Rep., 2017, 34, 433–459 RSC
.
- N. Wei, J. Quarterman and Y.-S. Jin, Trends Biotechnol., 2013, 31, 70–77 CrossRef CAS
.
-
R. V. Durvasula, D. V. S. Rao and V. S. Rao, Biotechnological Applications of Microalgae, 2013, pp. 201–227 Search PubMed
.
- Y. Zhang, A. Kumar, P. R. Hardwidge, T. Tanaka, A. Kondo and P. V. Vadlani, Biotechnol. Prog., 2016, 32, 271–278 CrossRef CAS
.
- P. Nawabi, S. Bauer, N. Kyrpides and A. Lykidis, Appl. Environ. Microbiol., 2011, 77, 8052–8061 CrossRef CAS PubMed
.
- S. Magdouli, S. Yan, R. D. Tyagi and R. Y. Surampalli, Crit. Rev. Environ. Sci. Technol., 2014, 44, 416–453 CrossRef CAS
.
- C. E. d. F. Silva and A. Bertucco, Process Biochem., 2016, 51, 1833–1842 CrossRef CAS
.
- M.-H. Liang and J.-G. Jiang, Prog. Lipid Res., 2013, 52, 395–408 CrossRef CAS
.
- P. Majidian, M. Tabatabaei, M. Zeinolabedini, M. P. Naghshbandi and Y. Chisti, Renewable Sustainable Energy Rev., 2018, 82, 3863–3885 CrossRef CAS
.
- B. Erickson, J. E. Nelson and P. Winters, Biotechnol. J., 2012, 7, 176–185 CrossRef CAS PubMed
.
- D. J. Parker, S. T. Chong and T. Hasell, RSC Adv., 2018, 8, 27892–27899 RSC
.
- M. J. H. Worthington, R. L. Kucera and J. M. Chalker, Green Chem., 2017, 19, 2748–2761 RSC
.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS
.
- X. Zhang, Y. Tang, S. Qu, J. Da and Z. Hao, ACS Catal., 2015, 5, 1053–1067 CrossRef CAS
.
- A. Demirbas, H. Alidrisi and M. A. Balubaid, Pet. Sci. Technol., 2015, 33, 93–101 CrossRef CAS
.
-
G. Kutney, Sulfur. History, Technology, Applications & Industry, ChemTec, Toronto, 2007 Search PubMed
.
- Y. Zhang, R. S. Glass, K. Char and J. Pyun, Polym. Chem., 2019, 10, 4078–4105 RSC
.
- J. M. Chalker, M. J. H. Worthington, N. A. Lundquist and L. J. Esdaile, Top. Curr. Chem., 2019, 377, 1–27 CrossRef CAS
.
- M. K. Salman, B. Karabay, L. C. Karabay and A. Cihaner, J. Appl. Polym. Sci., 2016, 133, 43655 CrossRef
.
- J. J. Griebel, R. S. Glass, K. Char and J. Pyun, Prog. Polym. Sci., 2016, 58, 90–125 CrossRef CAS
.
- M. Mann, J. E. Kruger, F. Andari, J. McErlean, J. R. Gascooke, J. A. Smith, M. J. H. Worthington, C. C. C. McKinley, J. A. Campbell, D. A. Lewis, T. Hasell, M. V. Perkins and J. M. Chalker, Org. Biomol. Chem., 2019, 17, 1929–1936 RSC
.
- S. F. Valle, A. S. Giroto, R. Klaic, G. G. F. Guimaraes and C. Ribeiro, Polym. Degrad. Stab., 2019, 162, 102–105 CrossRef CAS
.
- M. Karunarathna, M. K. Lauer, T. Thiounn, R. C. Smith and A. G. Tennyson, J. Mater. Chem. A, 2019, 7, 15683–15690 RSC
.
- M. K. Lauer, T. A. Estrada-Mendoza, C. D. McMillen, G. Chumanov, A. G. Tennyson and R. C. Smith, Adv. Sustainable Syst., 2019, 3, 1900062 CrossRef
.
- M. Ikeda, Appl. Microbiol. Biotechnol., 2006, 69, 615–626 CrossRef CAS
.
- D. Na, S. M. Yoo, H. Chung, H. Park, J. H. Park and S. Y. Lee, Nat. Biotechnol., 2013, 31, 170–174 CrossRef CAS
.
- T. Luetke-Eversloh, C. N. S. Santos and G. Stephanopoulos, Appl. Microbiol. Biotechnol., 2007, 77, 751–762 CrossRef CAS
.
- M. I. Chavez-Bejar, J. L. Baez-Viveros, A. Martinez, F. Bolivar and G. Gosset, Process Biochem., 2012, 47, 1017–1026 CrossRef CAS
.
- T. Thiounn, M. K. Lauer, M. S. Bedford, R. C. Smith and A. G. Tennyson, RSC Adv., 2018, 8, 39074–39082 RSC
.
- S. Aoyagi, T. Shimasaki, N. Teramoto and M. Shibata, Eur. Polym. J., 2018, 101, 151–158 CrossRef CAS
.
- B. Meyer, Chem. Rev., 1976, 76, 367–388 CrossRef CAS
.
- J. J. Griebel, N. A. Nguyen, S. Namnabat, L. E. Anderson, R. S. Glass, R. A. Norwood, M. E. MacKay, K. Char and J. Pyun, ACS Macro Lett., 2015, 4, 862–866 CrossRef CAS
.
- A. D. Smith, T. Thiounn, E. W. Lyles, E. K. Kibler, R. C. Smith and A. G. Tennyson, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 1704–1710 CrossRef CAS
.
- J. A. Smith, S. J. Green, S. Petcher, D. J. Parker, B. Zhang, M. J. H. Worthington, X. Wu, C. A. Kelly, T. Baker, C. T. Gibson, J. A. Campbell, D. A. Lewis, M. J. Jenkins, H. Willcock, J. M. Chalker and T. Hasell, Chem.–Eur. J., 2019, 25, 10433–10440 CrossRef CAS
.
- S. Kelebek, J. Colloid Interface Sci., 1988, 124, 504–514 CrossRef CAS
.
- C. C. Pai, R. J. Jeng, S. J. Grossman and J.
C. Huang, Adv. Polym. Technol., 1989, 9, 157–163 CrossRef CAS
.
- H. H. Weber Jr, ACI Symposium Publication, 1993, SP-137, pp. 49–72 Search PubMed.
-
H. H. Weber and W. C. McBee, New market opportunities for sulphur asphalt, Washington, D.C., 2000 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Infrared spectra of TTS30, TTS50, and tetraallyltyrosine; DSC curves of TTS30 and TTS50. Trials 1–3 of stress strain measurements of TTSx. See DOI: 10.1039/c9ra06213k |
| This journal is © The Royal Society of Chemistry 2019 |