Open Access Article
Open Access ArticleIntrinsic poorly-crystallized Fe5O7(OH)·4H2O: a highly efficient oxygen evolution reaction electrocatalyst under alkaline conditions†
Xiaoteng Ding‡
a,
Wei Cui‡b,
Xiaohua Zhuc,
Jianwei Zhang*d and
Yusheng Niu *a
*a
aSchool of Tourism and Geography Sciences, College of Life Sciences, Qingdao University, Qingdao 266071, China. E-mail: nys@qdu.edu.cn
bHuman Resources Department, Qingdao University, Qingdao 266071, China
cKey Laboratory of Eco-geochemistry, Ministry of Natural Resources, National Research Center for Geoanalysis, Beijing 100037, China
dSchool of Environmental Science and Engineering, Qingdao University, Qingdao 266071, China. E-mail: sdqdzjw@qdu.edu.cn
First published on 20th December 2019
Abstract
As the bottleneck of electrochemical overall water splitting, the oxygen evolution reaction (OER) needs efficient catalysts to lower the required overpotential. Electrocatalysts with an amorphous form are highly active but suffer with low structural stability. Poorly crystallized materials with activity like amorphous forms, while maintaining the mechanical robustness of crystalline forms, are expected to be ideal materials. Towards this direction, we, for the first time, developed low-crystalline Fe5O7(OH)·4H2O as an excellent OER electrocatalyst with an overpotential of 269 mV, in order to drive a current density of 100 mA cm−2 in a 1.0 M KOH environment, and this outperforms most of the reported Fe-based electrocatalysts. Notably, its activity can be maintained for at least 100 hours. A one-pot synthesis for the poorly-crystallized material using one of the most abundant metal elements to obtain effective OER catalysis will provide great convenience in practical applications.
Introduction
The ever-increasing depletion of fossil fuels and worsening environmental contamination stimulate extensive research into clean and efficient energy generation systems.1–3 Among these, electrochemical water splitting has attracted significant attention due to hydrogen production utilizing electricity from renewable but intermittent sources such as solar energy, wind, wave power, etc.4,5 However, the associated energy conversion efficiency is greatly limited by the sluggish oxygen evolution reaction (OER), which is a complex four-electron redox process.6–9 IrO2 and RuO2, as the most efficient electrocatalysts, exhibit high OER activity, yet their low-abundance and high cost dramatically hinder commercial utilization.10 Alternatives based on earth-abundant and inexpensive transition-metal based materials are actively being pursued.As non-precious-metal compounds, Fe-based oxides can serve as active OER electrocatalysts, but they are restricted by their low conductivity and chemical stability.11–16 Highly crystallized Fe nitrides,17–20 phosphides,21–23 sulfides,24 etc. have better electronic conductive properties and are more thermodynamically/chemically stable, exhibiting progressive OER activity. However, their electrocatalytic performances are still far from the requirements of practical applications. A challenging route, the topological transformation strategy towards an amorphous structure, has been developed to improve OER dynamics by increasing the intrinsic activity and exposing more active sites. However, the resulting non-crystallized materials are generally susceptible to unavoidable collapse to some degree, exhibiting unsatisfactory robustness.25–27 Designing poorly-crystallized materials with excellent catalytic activity similar to the amorphous form, and with structural stabilization of crystallization will provide a fresh and efficient perspective towards an ideal model.
On the basis of the above consideration, low-crystalline Fe5O7(OH)·4H2O was proposed and was developed by a one-pot hydrothermal method in the presence of Ni foam (NF). Such material anchoring on NF (Fe5O7(OH)·4H2O/NF) demonstrates excellent OER activity with an overpotential of 269 mV to drive 100 mA cm−2 current density in a 1.0 M KOH environment, outperforming most of the reported Fe-based electrocatalysts. More remarkably, its activity can be maintained for at least 100 h. It is shown that the highly efficient electrocatalytic OER performance lies in the unrivaled structure superiority, stemming from the poor-crystallization state that is caused by the existence of massive oxygen-deficiencies.
Results and discussion
Fig. 1a shows the X-ray diffraction (XRD) pattern of the solvothermally obtained products. The diffraction peaks at 36.08°, 40.54°, 46.43°, 53.15°, 61.32°, and 63.02° are indexed to the (110), (200), (113), (114), (115), and (106) crystal planes of Fe5O7(OH)·4H2O, respectively (JCPDS No. 29-0712).28 Moreover, X-ray photoelectron spectroscopy (XPS) characterization was performed on the resulting Fe5O7(OH)·4H2O. The corresponding XPS survey spectrum (Fig. 1b) provides evidence for the presence of Fe and O elements. The N element should be derived from the adsorbed ferric nitrate impurity.29 In the Fe 2p region (Fig. 1c), the binding energy (BE) of 709.2 eV can be attributed to the ferric nitrate adsorbed on the Fe5O7(OH)·4H2O nanocrystallites. The peak at 710.2 eV is assigned to the BE of Fe 2p3/2 in Fe5O7(OH)·4H2O.29 Moreover, the peaks centered at 530.4 eV, 531.2 eV, and 533.1 eV in the O 1s region (Fig. 1d) are ascribed to the Fe–O bond, the oxygen vacancies and the hydroxyl group, respectively, in the Fe5O7(OH)·4H2O.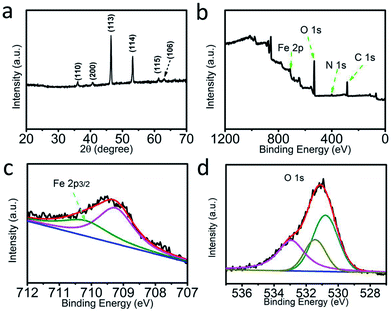 | ||
| Fig. 1 (a) XRD pattern of Fe5O7(OH)·4H2O. (b) XPS survey spectrum of Fe5O7(OH)·4H2O. XPS spectra of Fe5O7(OH)·4H2O in the (c) Fe 2p and (d) O 1s regions. | ||
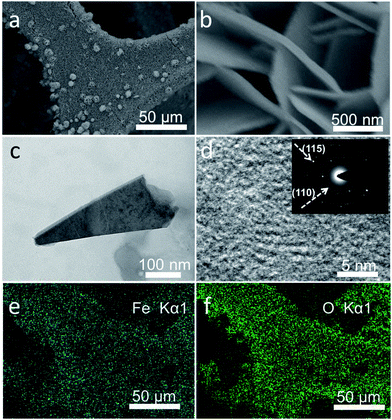
Fig. 2a and b show the scanning electron microscopy (SEM) images of Fe5O7(OH)·4H2O/NF. It can be seen that the Fe5O7(OH)·4H2O nanosheets grow tightly on the NF and form arrays on the NF. The Brunauer–Emmett–Teller (BET) tests revealed that the surface area of Fe5O7(OH)·4H2O/NF is 50.2 cm2. The associating transmission electron microscopy (TEM) image of Fe5O7(OH)·4H2O further confirms its nanosheet structure (Fig. 2c), and the high-resolution TEM (HRTEM) image demonstrates that the lattice is not well-defined (Fig. 2d), due to its intrinsic poorly-crystallized state. The electron spin resonance (ESR) spectroscopy determination unveils the presence of a surface oxygen vacancy. The obtained samples show two distinct symmetrical ESR signal peaks at g = 2.002 (Fig. S1†). The corresponding selected area electron diffraction (SAED) pattern shows rings indexed to the (115) and (110) planes of Fe5O7(OH)·4H2O. Energy-dispersive X-ray (EDX) elemental mapping images of Fe5O7(OH)·4H2O/NF (Fig. 2e and f) also show that Fe and O elements are uniformly distributed in the Fe5O7(OH)·4H2O product.
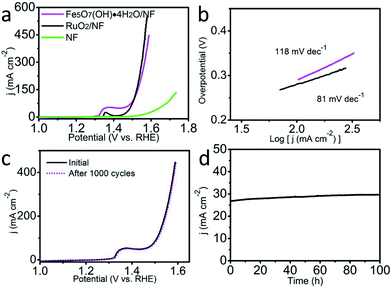
The electrochemical OER performance was examined in 1.0 M KOH using a typical three-electrode configuration. Fe5O7(OH)·4H2O/NF (Fe5O7(OH)·4H2O loading: 0.6 mg cm−2) was used as the working electrode, while Hg/HgO (filled with 6 M KOH solution) and a graphite plate were utilized as the reference and counter electrodes, respectively. For comparison, NF and RuO2 deposited on NF (RuO2/NF, RuO2 loading: 0.6 mg cm−2) were also tested under the same conditions. Considering ohmic potential drop (iR) losses, experimental data were all corrected for further analysis, thus directly reflecting the intrinsic behavior of the catalysts. The overpotentials were converted and reported as values vs. the reversible hydrogen electrode (RHE). As observed from the linear sweep voltammetry (LSV) curves in Fig. 3a, Fe5O7(OH)·4H2O/NF demonstrates an outstanding catalytic activity with an overpotential of 269 mV to drive 100 mA cm−2 in 1.0 M KOH, outperforming most of the reported Fe-based electrocatalysts such as Ni–Fe LDH (269 mV – 5 mA cm−2),30 Ni–Fe LDH/CNT (269 mV – 40 mA cm−2),31 FeOOH/Au (450 mV – 9 mA cm−2),32 etc. (Table S1†). More impressively, such an electrocatalytic performance even surpasses that of RuO2/NF (279 mV, 100 mA cm−2). As for the Tafel plots shown in Fig. 3b, RuO2/NF gives a Tafel slope of 81 mV dec−1. The corresponding Tafel slope for Fe5O7(OH)·4H2O/NF (118 mV dec−1) is relatively larger than that for RuO2/NF, implying the further engineering requirement towards more favorable catalytic kinetics. The high surface area of the array structure, affirmed by the BET measurements, and the existence of abundant oxygen-defects verified by the electron spin resonance spectrum, both contribute to the outstanding catalytic activity. The intrinsic oxygen-defects of Fe5O7(OH)·4H2O result in its poorly-crystallized state. We deliberately treated the sample under high-temperature and the degraded catalytic activity was observed, related to the overpotentials of 280 mV and 480 mV to deliver 15 mA cm−2 and 100 mA cm−2, respectively (Fig. S2†). However, such a performance of the sample after high-temperature treatment is still very attractive for scientific researchers. To further gain insight into the excellent OER activity of Fe5O7(OH)·4H2O, we examined the electrochemical double layer capacitances (Cdl), proportional to the electrochemical surface area (ECSA), for Fe5O7(OH)·4H2O/NF. Fig. S3† presents the corresponding cyclic voltammograms. The slope for the linear plot of the non-faradaic capacitance currents as a function of scan rates is the value of Cdl. The Cdl for Fe5O7(OH)·4H2O/NF is 18 mF cm−2, and ECSA was determined to be 45 cm2 on the basis of the equation: ECSA = Cdl/0.4. A series of cyclic voltammograms for Fe5O7(OH)·4H2O/NF and Fe5O7(OH)·4H2O/NF after heat treatment were collected, and are shown in Fig. S4.† A linear plot related to the oxidation currents for the redox species as a function of the scan rates can be obtained from the cyclic voltammograms, and the turnover frequency (TOF) was determined to be 0.017 mol of O2 s−1 at an overpotential of 269 mV for Fe5O7(OH)·4H2O/NF, and this is superior to those of samples after heat treatment (0.00468 mol O2 s−1 for overpotential of 300 mV). The long-term stability of Fe5O7(OH)·4H2O/NF was also evaluated by continuous cyclic voltammetry scanning at a scan rate of 100 mV s−1. After 1000 cycles in 1.0 M KOH, the obtained LSV curve almost coincides with the initial one (Fig. 3c). The superb durability of Fe5O7(OH)·4H2O/NF was also recorded by OER electrolysis at a fixed overpotential, preserving its catalytic activity for at least 100 h (Fig. 3d).
The experimentally emerging gas species was qualitatively determined by gas chromatography analysis and was further qualified using a calibrated pressure sensor, monitoring the pressure change in the anodic compartment of the H-type electrolytic cell. The faradaic efficiency (FE) of Fe5O7(OH)·4H2O/NF was then calculated to be 100% by comparing the molar number of the practically evolved oxygen with the theoretically calculated number (Fig. 4).
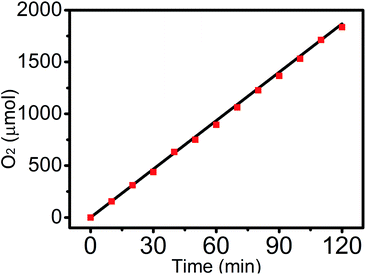 | ||
| Fig. 4 The amount of oxygen theoretically calculated and experimentally measured versus time for Fe5O7(OH)·4H2O/NF in 1.0 M KOH. | ||
Conclusions
In summary, poorly-crystallized Fe5O7(OH)·4H2O was designed and exploited by a one-pot hydrothermal method as a high-efficiency water-oxidation electrocatalyst. Profiting from its low-crystallization state, such a material exhibits a low overpotential of 269 mV at 100 mA cm−2 current density, exceeding most of the reported Fe-based electrocatalysts. More encouragingly, the activity can be maintained for at least 100 h. This current work not only provides an outstanding OER electrocatalyst, but also demonstrates a new avenue to construct highly efficient poorly-crystallized catalysts, by a relatively quick method using one of the most abundant metal elements, for other electrocatalytic systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21575137).References
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474–6502 CrossRef CAS PubMed.
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2016, 16, 16–22 CrossRef PubMed.
- F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu, L. Liardet and X. Hu, J. Am. Chem. Soc., 2018, 140, 7748–7759 CrossRef CAS PubMed.
- D. Chen, M. Qiao, Y. R. Lu, L. Hao, D. Liu, C.-L. Dong, Y. Li and S. Wang, Angew. Chem., Int. Ed., 2018, 57, 8691–8696 CrossRef CAS PubMed.
- M. Goerlin, J. Ferreira de Araújo, H. Schmies, D. Bernsmeier, S. Dresp, M. Gliech, Z. Jusys, P. Chernev, R. Kraehnert, H. Dau and P. Strasser, J. Am. Chem. Soc., 2017, 139, 2070–2082 CrossRef CAS PubMed.
- M. Tahir, L. Pan, F. Idrees, X. Zhang, L. Wang, J. Zou and Z. Wang, Nano Energy, 2017, 37, 136–157 CrossRef CAS.
- B. M. Hunter, H. B. Gray and A. M. Muller, Chem. Rev., 2016, 116, 14120–14136 CrossRef CAS PubMed.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. Xu and H. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- F. Dionigi and P. Strasser, Adv. Energy Mater., 2016, 6, 1600621 CrossRef.
- L. Han, S. Dong and E. Wang, Adv. Mater., 2016, 28, 9266–9291 CrossRef CAS PubMed.
- A. Indra, P. W. Menezes, N. R. Sahraie, A. Bergmann, C. Das, M. Tallarida, D. Schmeiber, P. Strasser and M. Driess, J. Am. Chem. Soc., 2014, 136, 17530–17536 CrossRef CAS PubMed.
- H. Yang, Y. Liu, S. Luo, Z. Zhao, X. Wang, Y. Luo, Z. Wang, J. Jin and J. Ma, ACS Catal., 2017, 7, 5557–5567 CrossRef CAS.
- T. Li, Y. Lv, J. Su, Y. Wang, Q. Yang, Y. Zhang, J. Zhou, L. Xu, D. Sun and Y. Tang, Adv. Sci., 2017, 4, 1700226 CrossRef PubMed.
- X.-F. Lu, L.-F. Gu, J.-W. Wang, J.-X. Wu, P.-Q. Liao and G.-R. Li, Adv. Mater., 2017, 29, 1604437 CrossRef PubMed.
- B. K. Kang, M. H. Woo, J. Lee, Y. H. Song, Z. Wang, Y. Guo, Y. Yamauchi, J. H. Kim, B. Lim and D. H. Yoon, J. Mater. Chem. A, 2017, 5, 4320–4324 RSC.
- F. Yu, H. Zhou, Z. Zhu, J. Sun, R. He, J. Bao, S. Chen and Z. Ren, ACS Catal., 2017, 7, 2052–2057 CrossRef CAS.
- B. Zhang, C. Xiao, S. Xie, J. Liang, X. Chen and Y. Tang, Chem. Mater., 2016, 28, 6934–6941 CrossRef CAS.
- Y. Fan, S. Ida, A. Staykov, T. Akbay, H. Hagiwara, J. Matsuda, K. Kaneko and T. Ishihara, Small, 2017, 13, 1700099 CrossRef PubMed.
- Y. Zhang, K. Rui, Z. Ma, W. Sun, Q. Wang, P. Wu, Q. Zhang, D. Li, M. Du, W. Zhang, H. Lin and J. Zhu, Chem. Mater., 2018, 30, 4762–4769 CrossRef CAS.
- D. Li, H. Baydoun, B. Kulikowski and S. L. Brock, Chem. Mater., 2017, 29, 3048–3054 CrossRef CAS.
- J. Xu, J. Li, D. Xiong, B. Zhang, Y. Liu, K.-H. Wu, I. Amorim, W. Li and L. Liu, Chem. Sci., 2018, 9, 3470–3476 RSC.
- Y. Tian, H. Wang, P. Liu, Y. Shen, C. Cheng, A. Hirata, T. Fujita, Z. Tang and M. Chen, Energy Environ. Sci., 2016, 9, 2257–2261 RSC.
- X. Zou, Y. Wu, Y. Liu, D. Liu, W. Li, L. Gu, H. Liu, P. Wang, L. Sun and Y. Zhang, Chem, 2018, 4, 1139–1152 CAS.
- L. Xie, R. Zhang, L. Cui, D. Liu, S. Hao, Y. Ma, G. Du, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2017, 56, 1064–1068 CrossRef CAS PubMed.
- M. Ma, F. Qu, X. Ji, D. Liu, S. Hao, G. Du, A. M. Asiri, Y. Yao, L. Chen and X. Sun, Small, 2017, 13, 1700394 CrossRef PubMed.
- K. Xu, H. Cheng, L. Liu, H. Lv, X. Wu, C. Wu and Y. Xie, Nano Lett., 2017, 17, 578–583 CrossRef CAS PubMed.
- X. Gong, J. Li, Y. Lin, X. Liu, L. Chen, J. Li and D. Li, Chin. Sci. Bull., 2014, 59, 3904–3911 CrossRef CAS.
- L. Lin, J. Li, Y. Lin, X. Liu, L. Chen, J. Fu and D. Li, Mater. Res. Innovations, 2015, 19, 118–124 CrossRef CAS.
- D. Tang, J. Liu, X. Wu, R. Liu, X. Han, Y. Han, H. Huang, Y. Liu and Z. Kang, ACS Appl. Mater. Interfaces, 2014, 6, 7918–7926 CrossRef CAS PubMed.
- M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2013, 135, 8452–8455 CrossRef CAS PubMed.
- S. Zou, M. S. Burke, M. G. Kast, J. Fan, N. Danilovic and S. W. Boettcher, Chem. Mater., 2015, 27, 8011–8020 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06374a |
| ‡ Xiaoteng Ding and Wei Cui contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |