Open Access Article
Open Access ArticleRecent advances in metallopolymer-based drug delivery systems
Gulzhian I. Dzhardimalieva
ab,
Lev N. Rabinskiy
b,
Kamila A. Kydralieva
b and
Igor E. Uflyand
 c
c
aLaboratory of Metallopolymers, The Institute of Problems of Chemical Physics RAS, Academician Semenov Avenue 1, Chernogolovka, Moscow Region, 142432 Russian Federation. E-mail: dzhardim@icp.ac.ru
bMoscow Aviation Institute (National Research University), Volokolamskoe Shosse, 4, Moscow 125993, Russia. E-mail: f9_dec@mai.ru; kamila.kydralieva@gmail.com
cDepartment of Chemistry, Southern Federal University, B. Sadovaya Str. 105/42, Rostov-on-Don, 344006 Russian Federation. E-mail: ieuflyand@sfedu.ru
First published on 13th November 2019
Abstract
Metallopolymers (MPs) or metal-containing polymers have shown great potential as new drug delivery systems (DDSs) due to their unique properties, including universal architectures, composition, properties and surface chemistry. Over the past few decades, the exponential growth of many new classes of MPs that deal with these issues has been demonstrated. This review presents and assesses the recent advances and challenges associated with using MPs as DDSs. Among the most widely used MPs for these purposes, metal complexes based on synthetic and natural polymers, coordination polymers, metal–organic frameworks, and metallodendrimers are distinguished. Particular attention is paid to the stimulus- and multistimuli-responsive metallopolymer-based DDSs. Of considerable interest is the use of MPs for combination therapy and multimodal systems. Finally, the problems and future prospects of using metallopolymer-based DDSs are outlined. The bibliography includes articles published over the past five years.
1. Introduction
The administration of drugs is one of the main methods of pharmaceutical and medicinal use.1 However, traditional direct drug administration to patients is limited due to poor solubility, undesirable side effects, limited cancer targeting, poor pharmacokinetics and biodistribution, and rapid metabolization and excretion.2,3 Despite the wide arsenal of drugs used in medicine, one of the most important problems remains their targeted delivery in order to increase the effectiveness of treatment. Drug delivery is the most promising approach to solving the problem of the safe transportation of a drug through the body to achieve its target.4,5 Targeted drug delivery will solve several problems: protect drugs from degradation and unwanted interactions with biological molecules, increase the selective absorption of drugs by tumor cells, monitor pharmacokinetics, and increase the bioavailability of drugs inside tumor cells. Targeted delivery combined with controlled drug release plays a key role in the future of personalized medicine. Key features of the drug delivery system (DDSs) include the following three indicators: effective drug encapsulation, successful delivery of these drugs to the target area of the body, and successful drug release there. Drugs equipped with a delivery system have several advantages compared to free drugs: they increase the solubility of hydrophobic drugs, improve their penetration into cells, improve pharmacokinetics, and many drugs have the ability to overcome membrane and blood–brain barriers.DDS includes two main components: a carrier and a drug, and its optimal properties are controlled by careful selection of these components.6 To date, numerous DDSs have been created that can reduce side effects and increase therapeutic efficacy.7–9 Thus, a variety of inorganic materials were used, including carbon (nanotube, nanodiamond, graphene),10,11 magnetic nanoparticles (NPs) of iron oxide,12,13 mesoporous silica nanoparticles (MSNPs),14,15 gold (gold nanorod, nanocage, and NP),16,17 and rare earth upconversion nanoparticles.18,19 Among DDSs based on organic materials, we note liposomes20,21 and lipid particles.22,23 Although some of these DDSs showed promising results and a number of formulations were approved for use in the clinic, the problem remains unresolved. In addition, all DDSs have their own limitations in bio-application, for example, liposomes usually suffer from low loads, and inorganic porous materials have undesirable toxicity and high degradability.
In recent years, researchers' attention to polymers-based DDSs has grown significantly due to their flexibility, the diversity of polymers in terms of composition and properties, their easy functionalization, and the possibility of smart behavior in response to environmental stimuli.24,25 Due to the difference of several orders of magnitude between the size of polymeric materials and the size of, for example, cancer cells, polymeric materials are ideally suited as in vivo DDSs.26–33 Three different methods for producing polymer-based DDSs have been developed: (1) physical encapsulation, (2) complexation of drugs with donor atoms of the polymer chains, and (3) conjugation of drugs with a polymeric carrier via a linker (polymeric prodrug). The undoubted advantage of the latter method is the delay in the release and even the trigger of the release when the DDS reaches its target.
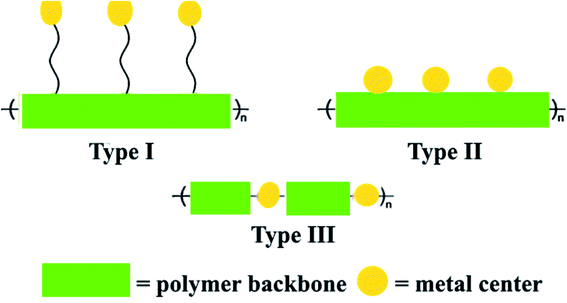
Metallopolymers (MPs) or metal-containing polymers are one of the main classes of polymers discovered in the 20th century.34–41 Various locations of metal centers from main group metals to transition metals and lanthanides relative to the polymer backbone were described by Wolf as Type I, Type II or Type III (Fig. 1), in which the metal center is either tethered to the polymer backbone by an organic linker, either covalently coupled to the backbone, or directly included in the polymer backbone, respectively.42,43 As a further subdivision, MPs are linear, highly branched, or dendritic. The bonds of metals with the polymer are covalent, which leads to essentially irreversible or ‘static’ binding, or noncovalent, which could allow potentially reversible ‘dynamic’ binding.
Characteristic features, allowing reliably identify MPs as particular class of chemical compounds, is the presence of the polymeric chain (organic, inorganic, hybrid or biological nature) and metal centers. Their properties can be adjusted by changing the interaction strength of metal centers with the polymer backbone.44–48 MPs are highly interesting materials with properties combining the technological advantages of polymers with the functionality of metal centers, therefore, these compounds have outstanding potential for use in a broad range of advanced sustainable applications including functional materials,49–55 catalysis and biocatalysis,56–63 electroactive,64 photoactive,65–70 conducting71 and magnetic45 materials, as well as environment72 and nanomaterials science.73–80
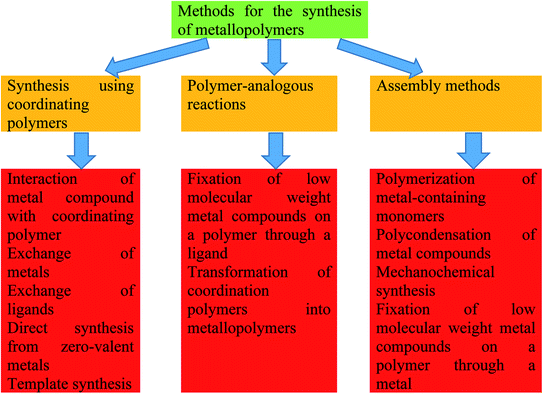
MPs have been known for a long time but have recently become the subject of large development. Over the years, the development of MP chemistry has been hampered by the lack of readily available synthesis techniques, difficulties in purifying the end products and convincing methods of their identification. In the early 1990s, a significant breakthrough was made in the development of new synthetic approaches (for example, polycondensation, ring-opening polymerization, electropolymerization, living ionic polymerization, controlled radical polymerization) and expanded access to characterization tools (for example, MALDI-TOF, ESI-MS, XPS, electron microscopy, spatially resolved optical spectroscopy), which allowed the production of metal-containing polymer materials with a well-defined spatial architectures.81 A variety of protocols for the synthesis of MPs of Type I and II were established by developing new synthetic approaches that are compatible with the presence of metal centers (Fig. 2).34,82–94
Coordination polymers (CPs) relate to MPs of Type III containing metal in the main chain, whose special feature is breaking of a polymer chain after a metal is removed. Because they contain large voids in the crystal structure, they were called porous coordination polymers or metal–organic frameworks (MOFs). Over the last decade, MOFs have received a great deal of interest in materials science due to their excellent material properties such as high surface area, porosity, high chemical and thermal stability, luminescence, high sorptive capacity, and potential use in a wide range of applications.95–106 To date, around 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MOFs have been produced for a range of applications with different combinations of metal nodes and organic linkers. To date, a wide variety of strategies and approaches to the design of coordination polymers have been developed (Fig. 3).107–113
000 MOFs have been produced for a range of applications with different combinations of metal nodes and organic linkers. To date, a wide variety of strategies and approaches to the design of coordination polymers have been developed (Fig. 3).107–113
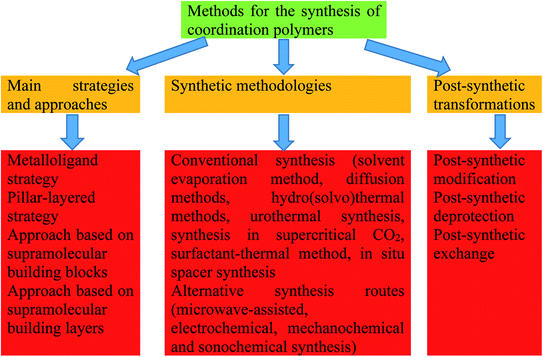 | ||
| Fig. 3 The main strategies and approaches, synthetic methodologies and post-synthetic transformations of coordination polymers. | ||
Already early pioneer work showed a high potential of MPs in biomedical applications. The construction of MPs is a new strategy for significantly increase the effectiveness of biomedical materials, in particular, to address current issues of traditional methods of treatment and prophylactics.48,114–132 Research on MPs in the biomedical field is impressive. Materials scientists are designing MPs as innovative solutions to improve the effectiveness of DDSs. Combined with the a rich and ever-growing array of ligands and synthesis methods for varieties of the metals, these compounds have outstanding potential for use in a wide variety of DDSs.
To the best of our knowledge, there is no general information on the use of metallopolymers as DDSs. The present review aims to describe the latest advances (over the last 5 years) in the field of metallopolymers-based DDSs. The review will mainly discuss the main types of metallopolymers-based DDSs, as well as use MPs as stimuli- and multistimuli-responsive DDSs, for combination therapy and as multimodal systems (Fig. 4). We are not trying to give an exhaustive analysis of the entire array of experimental data to date. The main focus will be paid to the main directions of modern research in this interesting field.
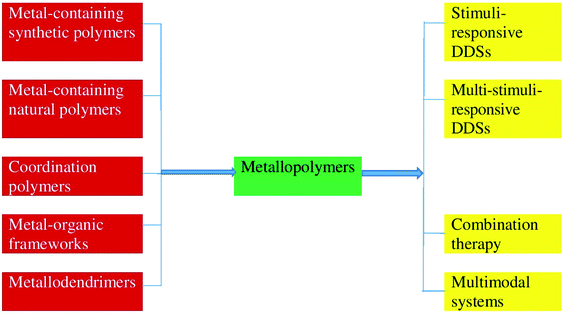 | ||
| Fig. 4 The main types of metallopolymers-based DDSs, as well as use MPs as stimuli- and multistimuli-responsive DDSs, for combination therapy and as multimodal systems. | ||
2. The main types of metallopolymers-based drug delivery systems
The use of MPs as DDSs attracts considerable attention due to their unique tailorable structures and physicochemical properties, namely, given spatial organization, in many cases nanoscale size, abundant exterior or interior functionalities and universal structural control. In this section, the discussed main types of metallopolymers-based DDSs involve metal complexes based on synthetic and natural polymers, coordination polymers, MOFs and metallodendrimers.2.1 Metal-containing synthetic polymers
In recent years, more and more attention has been paid to the use of synthetic polymers for the creation of metallopolymers-based DDSs. These DDSs include linear or branched polymer chain that works either as the bioactive molecule, e.g., polymeric drug or as the inert carrier to which a drug can be covalently linked. A wide variety of synthetic polymers have been used as carriers for metallodrugs, especially biodegradable synthetic polymers, which mainly include polyesters, polypeptides and recombinant protein-based polymers. Typically, two different approaches are used to attach these metallodrugs to polymers: using polymers as a stable ligand for metallodrugs or the use of polymers as a leaving group of a metallodrug. At the same time, due to the lack of sufficient hydrophobicity or hydrophilicity, MPs are usually not good candidates for encapsulating organic drug.As a typical example, we note the use of polymeric “ruthenium-cyclopentadienyl” conjugates bearing an organometallic fragment and polylactide chains as potential anti-cancer agents with proven cytotoxic activity133,134 and adequate aqueous stability.134 In another interesting example, a poly(L-glutamic acid)-graft-methoxy poly(ethylene glycol) (PGA-g-mPEG) has been proposed as a has been for inclusion of cisplatin.135,136 The resulting complex showed prolonged blood circulation time and excellent tumor accumulation. In particular, its plasma area-under-curve (AUC) was 31 times higher than that of free cisplatin, and its AUC in Lewis lung carcinoma tumors was 9.4 times higher.
Promising DDSs are metallopolymers based on polymer micelles.137,138 In particular, covalent attachment of a drug directly to a micelle is of interest to avoid its premature release in the circulation. So, poly(ethylene glycol)-block-PGA (PEG-b-PGA) was used as leaving group of the Pt(II) drugs of cisplatin and oxaliplatin.139 These MPs showed especially high in vivo micellar stability, reduced nephrotoxicity, increased accumulation in neoplastic tissues and reached a level of efficacy comparable to cisplatin. It should also be noted the use of polymer micelles for the delivery of Pt(II) and Ru(II) complexes to cancer cells.140
Palladium catalysts were used to catalyze intracellular reactions by uncaging various model prodrugs, including the doxorubicin (DOX) prodrug (pro-DOX), for the effective tumor treatment in vivo.141 The Pd(II) catalysts were encapsulated in the micellar core of diblock amphiphilic copolymers poly(lactic acid-co-glycolic acid) (PLGA)-PEG, which were intravenously delivered to various tumor cell models using the enhanced permeability and retention (EPR) effect. In tumor cells, delivered Pd(II) into micelles can catalyze the cleavage of the alloc group to uncage the pro-DOX to initiate regression of tumor growth and apoptosis (Fig. 5).
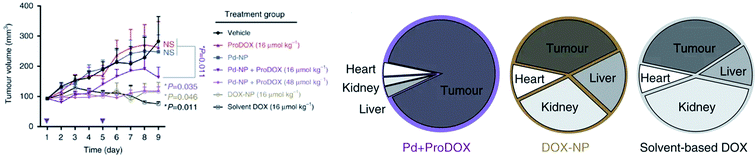 | ||
| Fig. 5 (left) HT1080 tumor growth profile during treatment; (right) pie chart showing the distribution of nuclear DOX accumulation in orthotopic ovarian tumors and key organs associated with toxicity [reprinted from ref. 141 under a Creative Commons Attribution 4.0 International License]. | ||
Self-assembling cyclic peptides were conjugated to a biocompatible polymer poly(2-hydroxypropyl methacrylamide) and functionalized using highly potent candidate organoiridium anticancer complexes.142 The antiproliferative activity of the three compounds (free drug, loaded onto the polymer, and the conjugate) was determined against human ovarian fibroblasts (HOF), a model for healthy, non-cancerous cells, and compared to that against A2780 ovarian cancer cells (Fig. 6). It turned out that drug-loaded nanotubes showed comparable or increased toxicity towards human ovarian cancer cells compared to free drug. It is important that the drug-bearing conjugates do not increase the uptake of iridium, but rather demonstrate a more effective mode of action.
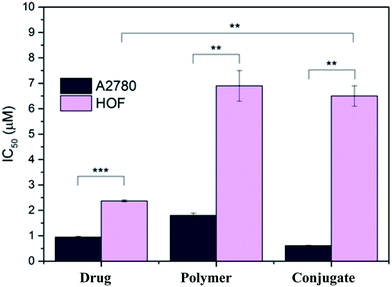 | ||
| Fig. 6 Antiproliferative activity of free organoiridium complex, drug-bearing polymer and drug-bearing conjugate in A2780 (cancer, dark) and HOF (healthy, light) ovarian cells. **p < 0.01, ***p < 0.001 [reprinted with permission from ref. 142. Copyright 2018 American Chemical Society]. | ||
The acid-responsive design of the Pt(IV) prodrug conjugates and the hydrophobic segment of the mPEG-b-PLA diblock copolymer, where PLA is a poly(lactic acid), helps to stabilize blood circulation, while also triggering the release of the drug into acidic endolysomal compartments (Fig. 7).143
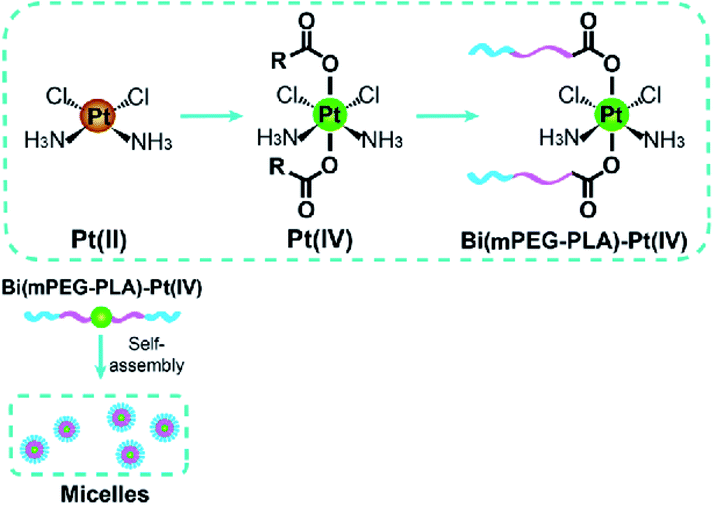 | ||
| Fig. 7 Pt(IV) drugs as crosslinking agents of NPs. Self-assembly of bis(mPEG-b-PLA)-Pt(IV) conjugates in micelles [reprinted with permission from ref. 143. Copyright 2015, American Chemical Society]. | ||
A localized dual-drug delivery device was developed by simultaneously incorporating the Pt(IV) prodrug backboned micelles and dichloroacetate into the poly(vinyl alcohol) nanofibers.144 It should also be noted the type of metallodrug, which is based on the Pt(IV) prodrug self-cross-linked nanostructure with extremely high cisplatin loading efficiency.145
It should be noted that metal complexes based on synthetic polymers have several advantages as DDS, such as easier synthesis of large quantities, insignificant batch-to-batch differences, and countless possibilities for post-synthetic modifications to achieve the desired properties. In addition, they focus on: increasing the solubility and stability of the drug, optimizing the dosage of the drug, protecting the drug from physical or chemical degradation, increasing the bioavailability, improving the pharmacokinetic behavior of the drug, reducing the duration of treatment, ensuring controlled release of the drug, reducing toxicity to tissue health and transporting the drug to the specific target sites.
However, when using DDS based on metal complexes with synthetic polymers, there are a number of serious drawbacks. For example, all types of synthetic polymers have some level of heterogeneity, since each MP molecule differs depending on molecular weight, drug loading and subsequent conformation. In addition, the difficulties in synthesis and characterization increase as MPs become more complex (i.e., multifunctional nanomedicines). Proven methods for physico-chemical characterization should also be established to certify the quality of the reproducible product. There is still an under-assessed problem with the possible toxicity and side effects of the metallopolymers-based DDSs. Although they can be biodegradable, their degradation rate must be analyzed and toxicity profiles evaluated in various in vitro, ex vivo and in vivo models. The development and application of strict safety rules is necessary to create an effective and safe dosage form.
2.2 Metal-containing natural polymers
Natural biomacromolecules, such as proteins and peptides, are promising carriers for small metallodrug molecules due to their inherent biodegradability, biocompatibility, low toxicity and high solubility in water. It is also important that biological metallodrug carriers can recognize tumor markers overexpressed by cancer cells, which is easily used in targeted treatment methods. Finally, peptides and proteins are easy to manufacture and stable, which is often difficult for synthetic NPs.146For example, the SV40 large T antigen-derived PKKKRKV peptide known as the nuclear localization sequence (NLS) peptide was used as a Pt(II) drug delivery vehicle (Fig. 8A).147 The construct efficiently translocated across the cellular membrane (Fig. 8B) and delivers platinum to the nucleus. The hybrid showed high biological activity in ovarian cancer cell lines, overcoming resistance to platinum, which may be associated with a higher Pt(II) content in the resistant cells. Quantitative determination of Pt(II) in the nucleus from two isogenic A2780 (Pt sensitive) and CP70 (Pt resistant) cells showed that in the first cells, one Pt(II) was attached to every 20th turn of DNA, while in the second cells every 10th turn.
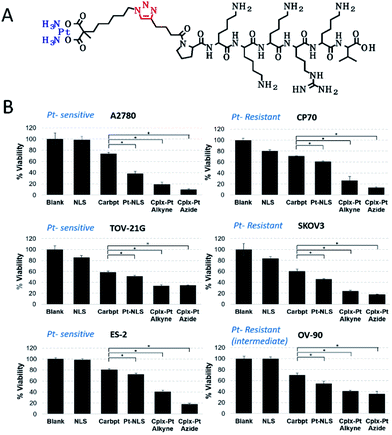 | ||
| Fig. 8 (A) The chemical structure of the Pt(II)-NLS hybrid. (B) Viability of platinum-sensitive (left) and resistant (right) cells after 72 h incubation with Pt(II)-NLS hybrid and controls. *Each column represents the mean and standard deviation of N = 3 and p < 0.005 [reprinted with permission from ref. 147. Copyright 2018, American Chemical Society]. | ||
Of interest are antibody drug conjugates (ADCs) with metallodrugs in which a Pt(II)-based linker can re-bridge interchain cysteines in an antibody (Fig. 9, left).148 ADC-Pt(II) constructs are more stable than conventional ADCs connected via maleimide-based chemistry, which reduces drug metabolism with blood serum albumin and off-target toxicity. ADC-Pt(II) is highly toxic in vitro, and in vivo studies using the A549 non-small cell lung cancer model showed marked tumor growth inhibition (TGI) with ∼80% of apoptotic cells in tumor sections (Fig. 9, right).
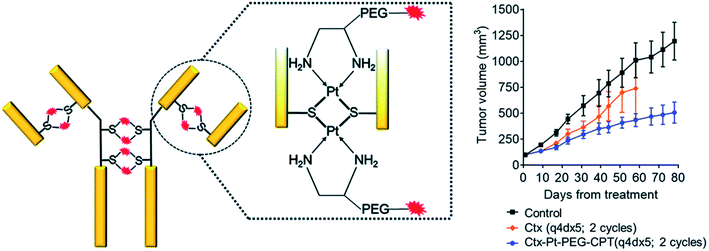 | ||
| Fig. 9 (left) Pt(II)-linker re-bridges the antibody chains with a strong Pt–S interaction, giving stability, homogeneity and site-specificity. (right) In vivo efficacy in animals treated with Ctx–Pt–PEG–CPT (Ctx is cetuximab and CPT is camptothecin) showed significant differences in tumor volume compared with the control group (P < 0.016), with TGI of 55% [reprinted from ref. 148 with permission from The Royal Society of Chemistry]. | ||
Natural polymers used in DDSs also include polysaccharides (agarose, hyaluronic acid (HA),149 alginate, carrageenan, etc.) and protein-based polymers (collagen, albumin, gelatin, etc.). In particular, HA is a polyanionic polysaccharide that can be used both as DDS and as a target motif for drug delivery.150 Thus, the HA-Pt(IV) conjugate was obtained by attaching Pt(IV) prodrugs to ethylenediamine (EDA)-modified HA (Fig. 10) via a succinamide bond (HA-EDA-Pt(IV)). This complex was additionally labeled with fluorescent dyes for bio-imaging. The nanoconjugates accumulated in the tumor tissue and were selectively up taken by the tumor cells, which led to a decrease in systemic toxicity and prolongation of animal survival.151
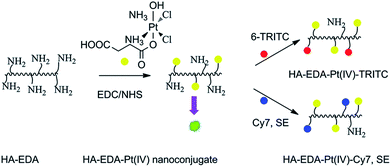 | ||
| Fig. 10 Synthetic pathways to the HA-EDA–Pt(IV) nanoconjugate, HA-EDA–Pt(IV)–TRITC, and HA-EDA–Pt(IV)–Cy7, SE [reprinted from ref. 151 with permission from The Royal Society of Chemistry]. | ||
A cisplatin templated hyaluronan-nanogel with targeting ability has been developed.152 In the same way, cisplatin-bearing chondroitin sulfate-nanogels were obtained, which were then incorporated into pH- and temperature-responsive bioresorbable PEG-poly(β-aminoester urethane) hydrogels for the cancer cell-specific delivery of cisplatin.153
DDSs based on metal complexes with natural polymers have several advantages that allow you to control the release of drugs, avoid the use of hazardous organic solvents in the manufacture of samples, improve the pharmacokinetics and increase the biodistribution of therapeutic agents in target organs, which leads to increased efficiency, including a non-invasive drug delivery method. In addition, they have a wide molecular weight distribution, a variety of viscoelastic properties, biocompatibility, phase transition characteristics and an acceptable taste. Apart from advantages of DDSs based on metal complexes with natural polymers they have some disadvantages, for example, microbial contamination during production due to their natural sources, heavy metal contamination which is often associated with plant polymers, differences from batch to batch as a result of difference of resources and resource regions, slow production process, etc. These deficiencies depend on the environment and many other factors, and potential impurities can lead to unwanted immune reactions.
2.3 Coordination polymers
A significant number of studies are devoted to the use of coordination polymers as DDSs. As an example, we note long-circulating core–shell CPs, which effectively deliver multiple drugs with various mechanisms of action to enhance the synergistic therapeutic effects (Fig. 11).154 These CP particles contain high payloads of drugs cisplatin or cisplatin plus gemcitabine (GEM) in the core and pooled siRNAs that target multidrug resistant (MDR) genes in the shell. Even at low doses, intraperitoneal injections of NPs led to effective and prolonged tumor regression/eradication in subcutaneous and intraperitoneal xenograft mouse models of cisplatin-resistant ovarian cancer (OCa).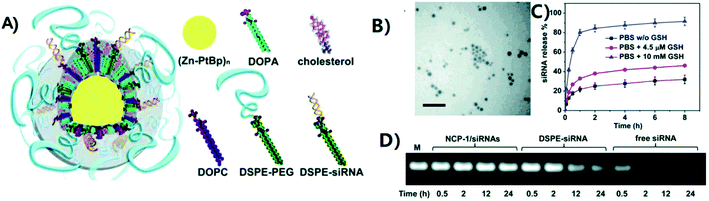 | ||
| Fig. 11 Preparation and characterization of CP-1/siRNAs. (A) Schematic representation of CP-1/siRNAs carrying cisplatin in the solid core and siRNAs in the lipid shell. (B) Transmission electron microscopy (TEM) image of CP-1/siRNAs showing the spherical and monodispersed nanostructure. Bar = 100 nm. (C) siRNA release from CP-1/siRNAs in the presence or absence of reducing agents. (D) Improved siRNA stability in serum of CP-1/siRNAs compared to free siRNA and DSPE-siRNA conjugate as evaluated by electrophoresis (2% agarose gel). “M” stands for untreated siRNA marker [reprinted with permission from ref. 154. Copyright 2016, American Chemical Society]. | ||
The use of a core–shell nanoscale metallopolymer CP-Carbo/GMP, where Carbo is carboplatin and GMP is gemcitabine monophosphate, allows the delivery of high loads of Carbo (28.0 ± 2.6 wt%) and GMP (8.6 ± 1.5 wt%) (Fig. 12).155 A strong synergistic effect was observed between Carbo and GMP in relation to platinum-resistant OCa cells, SKOV-3 and A2780/CDPP, in vitro. In addition, the use of this DDS led to an increase in the blood circulation half-life (11.8 ± 4.8 h) and improved tumor uptake of drugs (10.2 ± 4.4% ID g−1 at 24 h), resulting in 71% regression and 80% TGI of SKOV-3 and A2780/CDDP tumors, respectively.
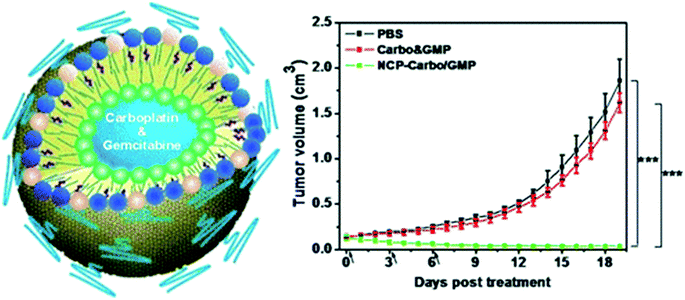 | ||
| Fig. 12 (left) Schematic of CP-Carbo/GMP. (right) TGI in vivo. Carbo (dose, 10 mg kg−1) and GMP (4.6 mg kg−1) and CP-Carbo/GMP (doses, 10 mg kg−1 and 4.6 mg kg−1) were administered on days 0, 3, and 6 [reprinted with permission from ref. 155. Copyright 2016, American Chemical Society]. | ||
PEG-modified CPs obtained by adding a polyhistidine (pHis)-PEG copolymer to a mixture of metal ions and organic ligands exhibit effective passive accumulation in the tumor, in which a reduced pH (about 6.5) will trigger charge conversion and size expansion to enhance their tumor retention and cell internationalization (Fig. 13).156 After cellular uptake of CPs in cell endo-/lysosomes, a further decrease in pH leads to the decomposition of these CPs and, consequently, to the release of the drug. Chemotherapy using CPs in an animal tumor model showed great efficacy at low doses of the drug and is especially effective against solid tumors with a reduced pH.
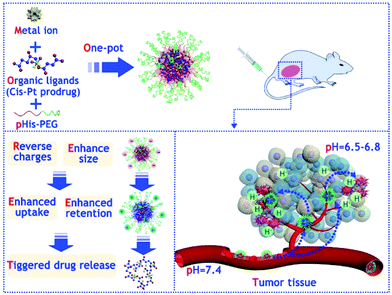 | ||
| Fig. 13 Scheme illustrating the one-pot synthesis of PEGylated nanoscale CPs and their use for tumor microenvironment (TME)-responsive anticancer therapy [reprinted with permission from ref. 156. Copyright 2018, Elsevier]. | ||
Zinc CPs with the drug ibuprofen as a ligand have a high drug content and exhibit high stability, low cytotoxicity, good biodegradability and high biocompatibility.157 For the delivery of small-molecule chemotherapeutic agents, such as 5-fluorouracil (5-FU), one-dimensional (1D) zinc CP based on benzene-1,3,5-tricarboxylic acid (trimesic acid) was used.158 The 5-FU-loaded CP exhibited a time-dependent cytotoxicity effect against breast cancer cell lines MCF-7 and 4T1. The intratumor treatment with 5-FU-loaded CP showed favorable antitumor efficacy by inhibiting tumor growth.
Particular attention is drawn to nanoscale CPs that overcome many of the drawbacks of existing DDSs due to varying compositions, sizes and shapes, high drug loading, ease of surface modification and intrinsic biodegradability.111 CPs, as an alternative to other nanocarriers, have many advantages due to the nature of the coordination polymer, such as high adaptability and diversity. In addition, CPs can combine the advantages of both organic polymer nanoparticles, such as compositional variability, biodegradability and high drug loading, and those of inorganic nanoparticles, such as well-defined particle morphologies and ease of surface functionalization, to create a completely new drug delivery platform.
2.4 Metal–organic frameworks
Over the past few years, MOFs with high surface area, large pore volume, and tunable composition have attracted much attention from researchers as DDSs.159–169 For example, MOFs were functionalized with lipid bilayers for flexible encapsulation of drug molecules in the MOF cavities and used for smart drug delivery.170 Multivariate MOFs, in which multiple functional groups can be introduced into a single MOF crystal without changing the underlying topology, are of interest as DDDs for studying the interaction between MOF pores and drug molecules.171 In particular, a multivariate strategy provides access to a continuum of energy states that correspond to existing discrete energy levels specified by pristine MOFs, thereby adjusting the release profile of guest molecules accurately and over a wide range (Fig. 14).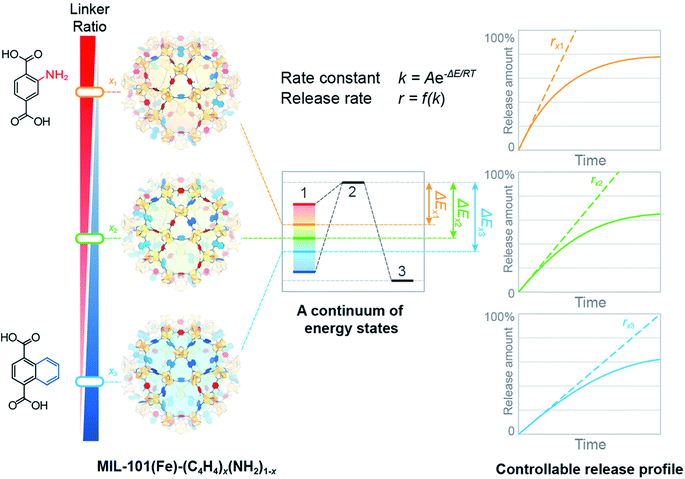 | ||
| Fig. 14 Multivariate MOFs with various linker ratios present a series of continuous energy states from which guest release kinetics can be dialed-in over a wide range [reprinted with permission from ref. 171. Copyright 2017, American Chemical Society]. | ||
MOF nanoparticles can be incorporated into exosomes to efficiently load and seal drugs and facilitate drug release through endogenous exosomal release and degradation of MOFs in the body.172 In addition, the resulting particles can be successfully loaded with one or several drugs, which makes liposome-coated MOF NPs interesting candidates for combination therapy.173 It is of interest to use MOFs as carriers for the delivery of chemotherapeutic drugs for the cancer therapy, such as 5-FU, DOX, CPT, platinum drugs and methotrexate (MTX).165,166,174–178 Thus, 5-FU-loaded MOFs exhibit excellent anticancer activity against human spinal cord tumor cells.179 DOX-loaded nano-sized MOFs (UiO-68) were modified with folic acid (FA) as a targeting agent and were used to effectively treat hepatoma by intravenous injection. Importantly, nanoscale MOFs have much higher anticancer efficacy in in vivo studies.180
The zeolite imidazolate framework (ZIF-8) has been used as biocompatible MOFs for co-delivery of an anticancer drug (DOX) and a P-glycoprotein inhibitor of verapamil hydrochloride (VER) to overcome MDR and deliver an active targeting drug. The resulting design was further modified by PEG-FA to obtain better stability and active targeting ability. In particular, the multifunctional nanoplatform of PEG-FA/DOX-VER/ZIF-8 can efficiently overcome MDR in in vitro studies.181 Hollow MOFs microspheres with MOFs shells with inherent pores as windows and deep submicron-scale cavities were made in combination with FA on the surface of the microsphere as the targeting agent, which is a promising DDS for the cancer treatment.182
In addition, MOFs-based nanocomposites with gold nanoclusters were used to real-time monitor drug release through its external porosity and pH-dependent luminescence. Composites demonstrated good biocompatibility and high luminescence for potential use in the cancer treatment.183
The superior properties of MOFs, such as distinct pore aperture, customizable composition and structure, adjustable size, versatile functionality, high drug loading, and improved biocompatibility, have made them promising candidates as DDSs. However, an important task is the manufacture of universal nontoxic or low-toxic and good biocompatible MOFs to ensure prolonged blood circulation and to ensure that degradation products can be processed through the body's metabolic system. In addition, systematic in vivo studies are needed to optimize MOF performance before clinical use.
2.5 Metallodendrimers
In recent years, a variety of multifunctional metallodendrimer systems has been used as DDSs.183 In particular, the inclusion of metallodrugs in dendritic frameworks can increase the activity and selectivity of these drugs. Indeed, metallodendrimers can combine the anticancer potential of metallodrugs with the features of dendrimers as nanocarriers and have been described as having promising cytotoxicity with respect to various cancer cell lines. Metallodendrimers, apparently, act according to a different mechanism of action compared to native metallodrugs, opening up new opportunities for searching for improved anti-cancer agents.184Among the various dendrimers, polyamidoamine (PAMAM) dendrimers modified with PEG that are non-toxic, biocompatible, and non-immune reactions are most noteworthy.185 PEGylation of the dendrimer may provide more benefits, such as good transfection efficiency, tumor localization, better bioavailability, biodistribution, and pharmacokinetics. In the case of drug delivery efficiency, the amount of dendrimer generation is a major factor and can be well controlled. In addition, PAMAM dendrimers are soluble in aqueous solutions, have a large number of surface groups and a unique structure; thus, they are considered as a promising delivery system. The cytotoxicity of PEGylated G4 PAMAM dendrimer with physically incorporated Carbo on L929 fibroblasts demonstrated that the PEGylation of G4 can eliminate the toxicity caused by the amino groups of G4.186 The results of resazurin test and live/dead assay showed that the killing effect of G4-PEG@Carbo on three representative cells was achieved and increased after increasing the concentration of Carbo.
Ru(II) metallodendrimers containing arene fragments were evaluated as an antitumor nanotherapy against resistant prostate cancer.187,188 Promising cytotoxic, antiproliferative and antimetastatic behavior was observed. In particular, after treatment with these nanocompounds, the tumor volume of the mouse was significantly reduced compared with untreated animals. In addition, carbosilane metallodendrimers based on arene Ru(II) complexes showed relevant in vitro cytotoxic activity against a number of cisplatin-resistant cancer cell lines in the low micromolar range and low hemotoxicity.189 In general, dendritic polymers showed stronger cytotoxicity than their mononuclear analogues, which emphasizes the effect of the dendritic structure on antitumor activity. Interestingly, the G1 metallodendrimers have significant inhibitory properties against cathepsin-B, the protease is overexpressed in invasive and metastatic processes. Low generation dendrimers modified with an organometallic compound of Ru(II) showed high biological activity in vitro against various cancer cell lines, such as Caco-2 (colon adenocarcinoma), CAL-72 (osteocarcoma), and MCF-7 (breast cancer).190 Diaminobutane (DAB) dendrimers containing Ru(II)-p-cymene, Ru(II)-hexamethylbenzene, Rh(III)-cyclopentadienyl, and Ir(III)-cyclopentadienyl fragments exhibited cytotoxicity, but the most pronounced effects were observed for the ferrocenyl-derived Ru(II)-hexamethylbenzene metallodendrimer (Scheme 1).191 It is important to note that all metallodendrimers showed lower cytotoxicity towards non-cancerous cells.
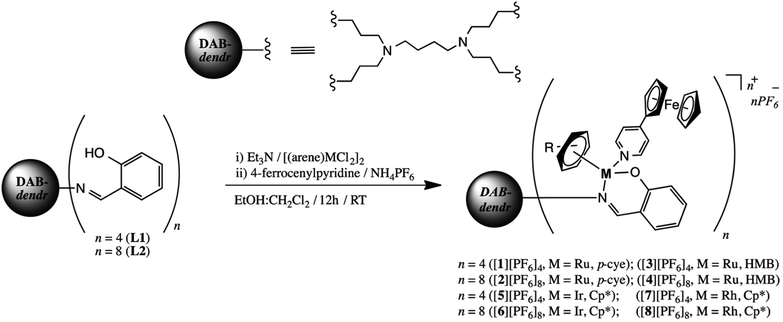 | ||
| Scheme 1 Synthesis of ferrocenyl-derived heterometallic metallodendrimers [1][PF6]4–[8][PF6]8 [reprinted from ref. 191 with permission from The Royal Society of Chemistry]. | ||
Hyperbranched polyglycerol (hPG) was used as a multivalent framework to develop nanocarriers capable of high-affinity encapsulating copper for treatment of various pathological conditions associated with Cu-deficiency.192 Cu-binding domains were attached exclusively in the hPG core by selective differentiation between the primary and secondary hydroxyl groups of the polyol. These Cu-containing nanocomplexes showed a moderate cationic surface charge, which led to controlled protein binding characteristics and a low cellular toxicity profile.
Macromolecular, multivalent, Cu-encapsulating nanocarrier platforms based on hPG have been developed for therapeutic use.193 Copper-containing hPG with EDA fragments can cross the artificial blood–brain barrier (BBB). Cu(II)-renal NPs crossed the BBB model twice as effectively as 14C-sucrose and sodium fluorescein, and up to 60 times better than Evans Blue-labeled albumin. The NP core–shell design allowed the formation of the Cu(II) complex in the outer shell and, therefore, facilitated the pH-dependent release of Cu in contrast to core-multishell NPs, where Cu(II) ions are encapsulated in the core. In addition, Cu can also be released intracellularly from NPs and is available for biological processes.
Thus, the range of MPs used as DDSs is quite wide and is not limited to the examples given here. A huge selection of MPs with a wide arsenal of synthetic methods for their preparation, the simplicity of many formulations, scalability and an acceptable price/quality ratio provide a methodological platform to creating promising DDSs based on them. In particular, one promising area is the use of bioactive motifs (cations, organic ligands, or both) as building blocks for constructing metal biomolecule frameworks (also known as bioMOFs).194 For example, biomolecules, such as amino acids, peptides, and nucleic bases, as well as biocompatible metal cations (Fe, Zn, and Cu), were used as ligands in the construction of this MOF subclass.195,196 A variety of hybrid materials, such as biopolymer@MOFs, are also of considerable interest.197 It should be noted the use of MPs whose polymeric chains include non-traditional heteroatoms, for example, selenium. Thus, selenium-containing polymer (P) micelles were obtained with the drug encapsulated in MOFs (Fig. 15).198 In the presence of external redox agents and pH stimuli, the prepared nanocomposite (P@ZIF-8) drug system showed DOX release ability and good selectivity in DOX release at low pH instead of normal pH.
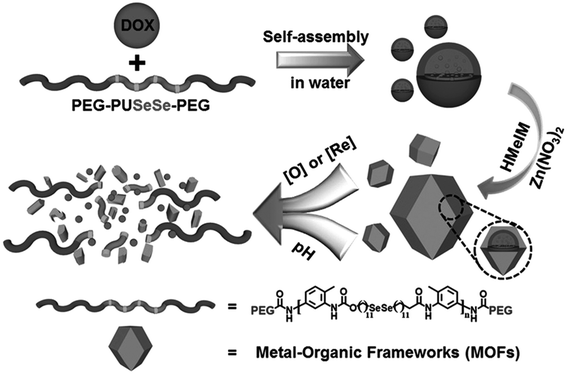 | ||
| Fig. 15 Schematic representation of the synthetic procedures of the P@ZIF-8 nanocomposites as a DDS for the loading and multi-responsive release of DOX. HMeIM: 2-methylimidazolate [reprinted with permission from ref. 198. Copyright 2017, John Wiley and Sons]. | ||
Dendrimer-based DDSs and dendrimers as drugs alone are the two main therapeutic applications of dendrimers.199 A number of significant advantages of dendrimers in drug delivery should be noted:200–204 three-dimensional architecture, high biocompatibility, good solubility in water, biomimicry, controlled sizes, monodispersity, easy modification, reducing the toxicity of the drug itself, extending the route of administration, improvement of the disease outcomes of drugs, improvement of the pharmacokinetic/pharmacodynamic profile of drugs, etc. Dendrimers are excellent nanocarriers for various cargoes, such as drugs and metallodrugs, increasing the selectivity, specificity, stability and biological activity of drugs.205 Interaction of a dendrimer with a drug or drug loading can be achieved in several ways: (1) encapsulation inside the dendrimer; (2) electrostatic encapsulation; (3) covalent conjugation. Currently, interest in metallodrug-dendrimer conjugates and encapsulation of metallodrugs in dendrimers has increased significantly, in particular with the aim of reducing toxicity, increasing bioavailability and overcoming resistance. It should be noted that an undeniable advantage of metallodendrimers as DDSs is the possibility of their functionalization by varied ligands to achieve tumor tissue through various body barriers in the body with minimal loss of activity in the bloodstream. In addition, they have the ability to selectively kill tumor cells without affecting normal cells, and, most importantly, with an actively controlled release mechanism. The disadvantages of metallodendrimers include insufficient drug carrying capacity, biodegradability, toxicity, characteristics of biodistribution and favorable retention with appropriate specificity and bioavailability.
3. Stimuli-responsive metallopolymers-based drug delivery systems
Recently, interest in stimuli-responsive polymers has increased significantly, especially in their applications as DDSs.24,25,206 In the field of DDSs, stimuli-responsive polymers have attracted great attention due to their unique properties, including the enhanced solubility of the drug molecules, the EPR effect, and most importantly, the effective control of chemical and physical characteristics with respect to the field of applications. Over the past two decades, a variety of metallopolymers-based DDSs has been produced that respond to therapeutic stimuli, such as redox, pH, enzyme, light, temperature, etc.98,207 They are divided into three main categories based on the nature of the stimuli, namely: endo-stimuli-responsive DDSs, exo-stimuli-responsive DDSs, and dual-stimuli-responsive DDSs.3.1 Photo-responsive DDSs
Among the many external stimuli, the light trigger strategy attracts more and more attention due to its usefulness for on-demand therapy in well-defined sites that arise due to the non-invasive and spatiotemporal exact mode of action after light irradiation. As an example, we note the integration of CO-releasing complexes and polymers as one of the promising strategies for achieving localized on-demand delivery of CO for therapeutic purposes.208 In particular, a photoactive CO-releasing protein composition has been developed for dose-regulated delivery in living cells.209 Cellular uptake and light-induced (e.g., 456 nm light) CO-releasing properties of this protein cage activate nuclear factor-kB and tumor necrosis factor an in mammalian cells.In another interesting example, a human serum albumin (HSA) protein-based nanocarrier was synthesized, which combined the photoactivatable Pt(IV) antitumor prodrug for controlled release and a fluorescent light-up probe to evaluate the effect and effectiveness of the drug (Fig. 16).210 This platform is locally activated by UV irradiation to release active Pt species, which led to increased cell death in both drug-sensitive A2780 and cisplatin-resistant A2780cis cell lines compared to free prodrug molecules. Cytotoxicity leads to apoptosis by activation of caspase 3, a typical programmed cell death protease, which can be visualized using apoptosis-sensitive probes on the platform.
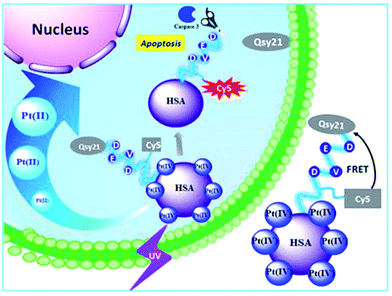 | ||
| Fig. 16 Human transport protein carrier for the controlled photoactivation of an antitumor prodrug and real-time imaging of an intracellular tumor [reprinted with permission from ref. 210. Copyright 2015, American Chemical Society]. | ||
It should be also noted cisplatin DDS with high encapsulation efficiency (EE) and near-infrared (NIR) light stimuli-responsive drug release properties.211 Under the stimuli of a NIR laser, the hydrophobic block of the polymer became more hydrophilic, which led to the rapid release of cisplatin. Photo-sensitive Pt(IV) prodrugs based on cisplatin and oxaliplatin were developed.212 An example is the triblock polymer mPEG-b-poly(ε-caprolactone)-b-poly-L-lysine, mPEG114-b-PCL20-b-PLL10, and the resulting the Pt(IV) drugs can be activated by UV irradiation to release toxic Pt(II) species (Fig. 17).213,214 These photosensitive Pt(IV) nanoformulations exhibit sharply increased cytotoxicity compared to clinically prescribed platinum drugs, reaching 8 times greater cytotoxic activity than cisplatin and 13 times higher than oxaliplatin. In addition, the inclusion of prodrugs in nanoformulation improves blood circulation; the half-life of a micelle in the blood exceeded 30 min, which is more than 10 times higher compared to molecular prodrug.
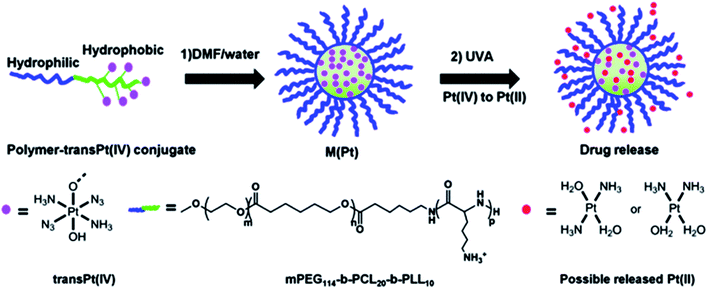 | ||
| Fig. 17 Attachment of trans-Pt(IV) to the mPEG114-b-PCL20-PLL10 copolymer and self-assembly of the polymer-trans Pt(IV) conjugate into micelles (M(Pt)). After UVA irradiation, Pt(IV) prodrugs are rapidly reduced to Pt(II) and released [reprinted from ref. 214 with permission from The Royal Society of Chemistry]. | ||
A simultaneously photo-cleavable and activated prodrug-backboned block copolymer (BCP) micelle was developed using an anticancer prodrug, trans,trans,trans-[Pt(N3)2(OH)2(py)2] (Fig. 18).215 Concurrent chain cleavage of the micelle and the activation of the Pt(IV) prodrug can be temporally and spatially triggered under the influence of UV or even visible light for accurate delivery of anticancer drug. When exposed to light, the micelle is about 1.5–4.0 times more cytotoxic against HeLa cells than in the dark, than and twice as effective as. Even micelles activated by visible light (430 and 500 nm) showed an efficiency comparable to cisplatin.
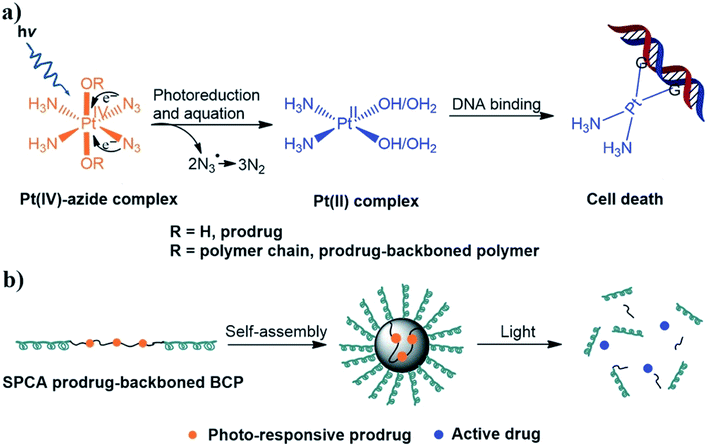 | ||
| Fig. 18 Schematic representation of (a) the anticancer mechanism of photo-responsive Pt(IV)-azide complexes and (b) self-assembly and light-triggered dissociation of simultaneously photo-cleavable and activatable (SPCA) prodrug-backboned BCP micelle [reprinted with permission from ref. 215. Copyright 2016, John Wiley and Sons]. | ||
One very promising strategy for drug encapsulation involves the formation of non-covalent host–guest complexes.216 In particular, due to the high binding constant of ferrocene (Fc) units with β-cyclodextrin (β-CD), a pathway has been developed for the delivery of various drugs and the use of smart materials.217 Fc-based photo-responsive materials have attracted much attention as an integral part of biological applications, such as drug delivery.218 An example is light-responsive hollow nanocapsules (NCs) based on dextran (Dex) with β-CD and Fc fragments, in which the light stimulus significantly increased the encapsulation process of rhodamine B (RhB) compared to the process without a stimulus (Fig. 19).219
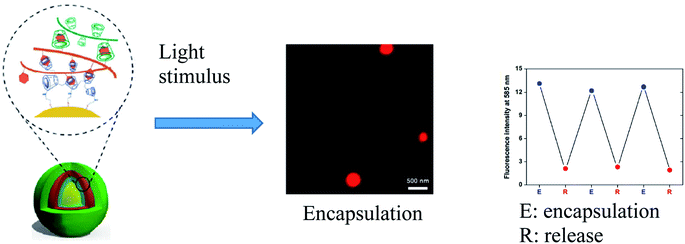 | ||
| Fig. 19 Encapsulation of RhB after the incubation with NCs after the UV-light irradiation and variations in the fluorescence intensity at 585 nm for three sequential encapsulation/release cycles [reprinted with permission from ref. 219. Copyright 2016, Springer Nature]. | ||
3.2 Redox-responsive DDSs
The advantage of a redox stimulus is a change in composition without the addition of chemicals and is attracting increasing attention due to its light trigger mode and the significance of potential changes in biological systems.220,221 For example, the host–guest interaction between β-CD and Fc can respond to redox stimuli and is used in redox-responsive MPs. These supramolecular structures are widely used as DDSs. Thus, the substrate loading ability of the redox-responsive supramolecular Janus device based on β-CD and 2-fold Fc host–guest interactions was confirmed with RhB, and the oxidation of the Fc groups led to the release of loaded cargos.222 The reversible ferrocene/ferricinium (Fc/Fcium) redox couple has attracted considerable attention for the dynamic redox switching of DDSs created using Fc-containing polymers.116 The “on-demand” release of loaded drugs was achieved in response to external redox stimuli.Breathing poly-2-(methacryloyloxy)ethylferrocenecarboxylate (PFcMA)-containing NCs were used for redox-controlled release (Fig. 20a).223 The swollen NCs in acetone were loaded with malachite green as a model payload and transferred to water-containing DDS (Fig. 20b and d). Oxidation-induced release of malachite green was observed when FeCl3 was added as an oxidizing agent for PFcMA shell segments (Fig. 20c).
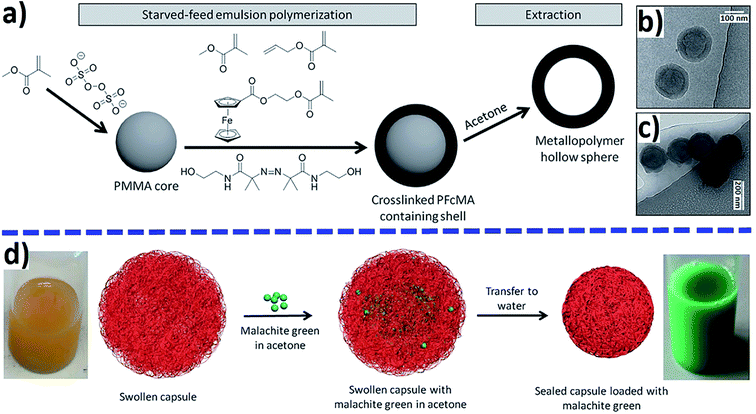 | ||
| Fig. 20 Preparation of PFcMA-containing NCs by sequential starved-feed emulsion polymerization and subsequent extraction (a) and loading malachite green as a model drug (d). Cryo-TEM images of PFcMA capsules (b) and oxidized PFcMA capsules (c) [reprinted with permission from ref. 223. Copyright 2016, John Wiley and Sons]. | ||
A redox-responsive Fc-containing amphiphilic BCP, PEG-b-poly(2-(methacryloyloxy)ethyl ferrocene-carboxylate), was used for controlled drug release.224 Because of the redox-responsive self-assembly and disassembly property, the vesicles were used to load the RhB model drug for controlled release upon external redox stimuli. In particular, rapid release of RhB from vesicles was observed during the oxidation by H2O2 (Fig. 21).
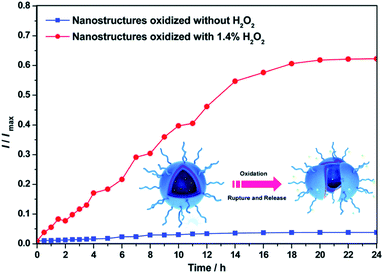 | ||
| Fig. 21 Release kinetics of RhB from Fc-containing amphiphilic BCP. The fluorescence intensity of the oxidized vesicles increases with time, indicating a redox release of RhB [reprinted from ref. 224 with permission from The Royal Society of Chemistry]. | ||
Well-defined redox-responsive Fc-containing amphiphilic BCPs, poly(N-acryloylmorpholine)-b-poly(2-acryloyloxyethyl ferrocenecarboxylate) (PACMO95-b-PAEFc25), and their self-assembled micelles were used as DDSs.225 The copolymer nanomicelles encapsulated model drug paclitaxel (PTX) with EE of 61.4% (Fig. 22a). These redox-responsive PTX-loaded nanomicelles will enter the cancer tissues through blood circulation and endocytosis. The release rate was controlled by the type and concentration of oxidizing agents, as well as by the release media (Fig. 22b and c).
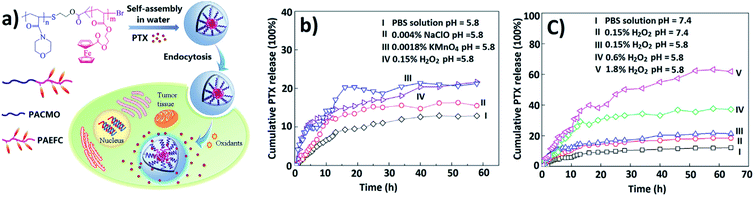 | ||
| Fig. 22 (a) In vitro loading and release chart of a drug from PACMO95-b-PAEFc25 micelles when triggered by oxidative stimuli. Cumulative release of PTX from PACMO95-b-PAEFC25 micelles triggered by various oxidizing agents (b) and different concentrations of H2O2 (c) [reprinted with permission from ref. 225. Copyright 2017, American Chemical Society]. | ||
The water-insoluble model drug Nile Red (NR) was encapsulated in the hydrophobic poly(vinylferrocene) (PVFc) interior of the core–shell nanomicelles based on well-defined PVFc-b-PEG diblock copolymers.226 Through treatment with H2O2, instantaneous and quantitative dye release was observed due to oxidation of the PVFc segments (Fig. 23). After the cross-linking treatment, the residence time of the encapsulated NR was significantly increased, and the newly formed shell around the PVFc core was able to keep the encapsulated model compound in it for several months without any significant diffusion from the hydrophobic interior.
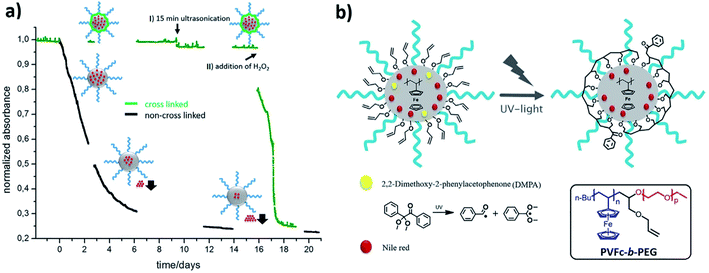 | ||
| Fig. 23 Schematic cross-linking of PVFc-b-PEG micelles and its effect on the stabilization of encapsulated NR. (a) UV-vis measurement of the release of encapsulated NR. Black: uncross-linked micelles, decrease of absorbance due to uncontrolled NR release. Green: cross-linked micelles, no uncontrolled release: (I) no release through ultrasonication; (II) release after oxidation. (b) Scheme of the stage of crosslinking of inserted allyl groups in the corona of PVFc cores. The reaction scheme for the dissociation of a radical photoinitiator into an active benzoyl radical and an inactive acetal radical [reprinted with permission from ref. 226. Copyright 2016, American Chemical Society]. | ||
Redox-responsive porous multicompartment vesicles were constructed from a linear ABC triblock terpolymer, poly[2-(dimethylamino)ethylmethacrylate]-b-poly(benzyl methacrylate)-b-poly(4-vinylbenzylferrocenecarboxylate) (Fig. 24).227 These vesicles can be used as smart DDSs to encapsulate and release payloads when the size of the guest is smaller than the pore diameter.
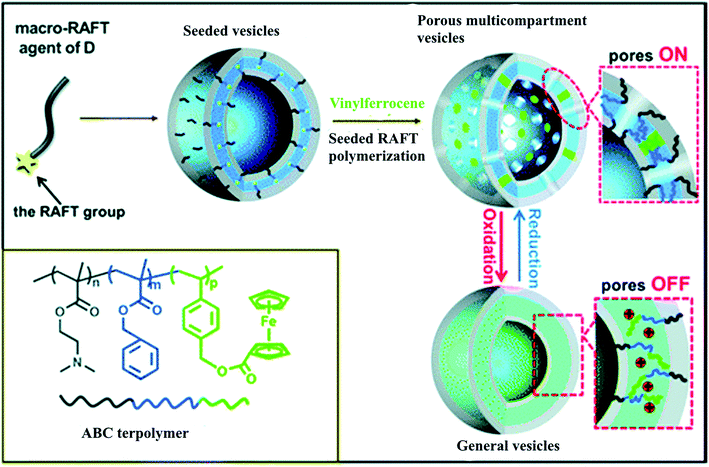 | ||
| Fig. 24 Synthesis of the porous multicompartment vesicles of ABC triblock terpolymer by seeded RAFT polymerization and the schematic on–off switch of the membrane pores of the multicompartment vesicles through oxidation/reduction [reprinted with permission from ref. 227. Copyright 2016, American Chemical Society]. | ||
Fc-modified phospholipid (Fc-DSP)-based vesicles are capable of transferring drugs to target cancer cells and selectively trigger their release (Fig. 25a).228 Both prepared giant and large unilamellar vesicles (GUVs and LUVs) showed effective loading of model drugs, including the anticancer agent DOX. When exposed to an oxidant solution, external Fc moieties of Fc-DSP vesicles were oxidized to positively charged Fcium groups, which led to the release of the encapsulated drugs. The in vitro experiments with DOX-loaded redox GUVs were performed in adenocarcinoma cervical cancer cells (HeLa) and normal lung fibroblast cells (MRC-5) (Fig. 25b). A significant number of DOX groups were only absorbed by HeLa exposed to redox active GUVs (Fig. 25c). Thus, cancer cells trigger the release of a drug from redox vesicles due to a local redox gradient, namely, the selective redox triggering of liposomes uses their cytotoxic payload at the cancer site.
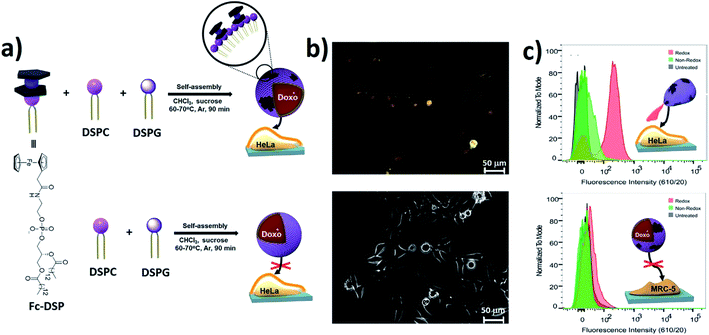 | ||
| Fig. 25 Self-assembly formation of DDSs from Fc-DSP and selective release into cancer cells. (a) Schematic formation of the GUVs. (b) Fluorescence microscopy images of HeLa cells that were exposed to redox-active and non-redox-active GUVs. (c) Flow cytometry of HeLa and MRC-5 cells exposed to control (black), redox-active (red), and non-redox-active (green) active GUVs [reprinted with permission from ref. 228. Copyright 2016, American Chemical Society]. | ||
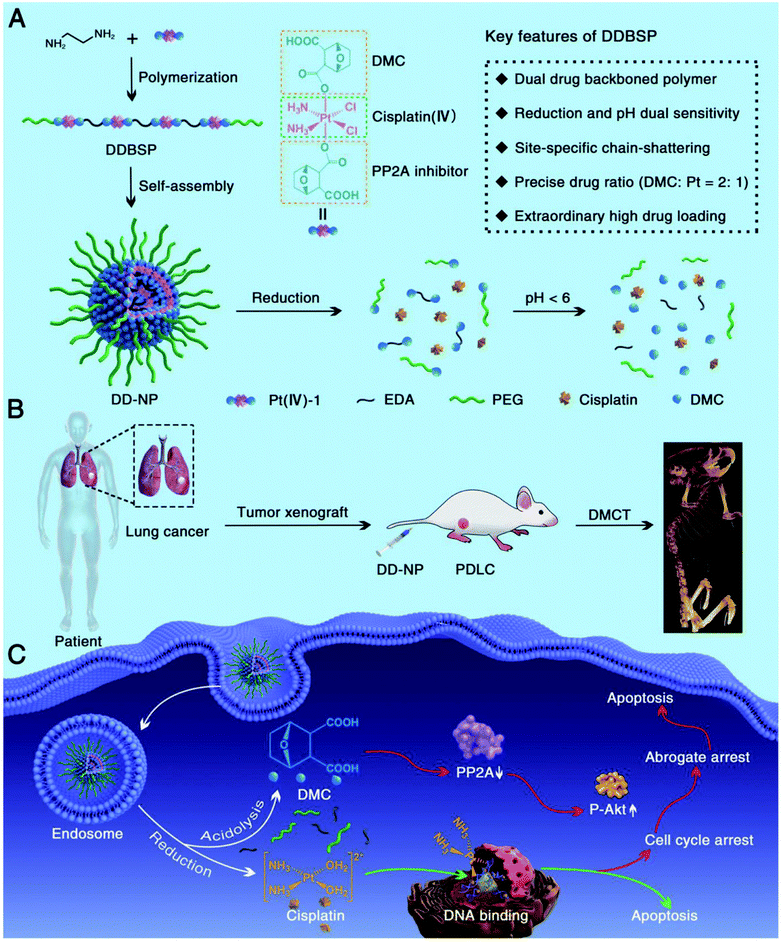
Of interest are redox-responsive Pt(II) prodrug micelles based on FA-PEG-b-poly(α-Pt(II)-SS-caprolactone/caprolactone) copolymers.229 They are relatively stable under physiological conditions, but are sensitive to reduction conditions that can trigger the release of conjugated platinum drugs. A dual drug backbone-shattering polymer (DDBSP) based on demethylcantharidin (DMC) and the Pt(IV) prodrug was developed.230 Under reductive and acidic TME, nano-sized MPs can undergo site-specifically chain-shattered by converting Pt(IV) to Pt(II) and hydrolysis of the DMC precursor, releasing both drugs for a controlled synergistic antitumor effect. The size, composition, and morphology of engineered MOFs can be finely tuned to optimize their properties for drug loading and controlled release, demonstrating the potential to serve as promising DDSs.125
Even though exciting research is occurring in the area of redox stimuli-responsive polymer nanocarriers, it is difficult to achieve specific redox molecular mechanism-based controllability due to the complex biological environment and heterogeneity of tumor cells.
3.3 pH-responsive DDSs
Among a variety of environmental triggers, pH gradients are commonly used to create intelligent responsive DDSs. For example, a class of smart MPs based on platinum prodrug conjugated PAMAM dendrimers with the supersensitive size switching effect in response to pH changes in the TME was developed to increase penetration into the tumor and efficient drug delivery in vivo.231 In another study, a responsive nanocarrier (BLZ-945SCNs/Pt) was obtained, which is able to spatially target both tumor-associated macrophages and tumor cells for chemo-immunotherapy. At pH 6.7–6.8, ionization of the amine groups on the amphiphilic polymer leads to structure collapse of the carrier in the pre-vascular regions of the tumor tissues, which allows the simultaneous release of both platinum prodrug nanoconjugates and small molecule inhibitor BLZ-945 of colony stimulating factor 1 receptor (CSF-1R) of tumor-associated macrophages (TAMs). It turned out that the customized pH-sensitive co-delivery nanocarriers not only induce tumor cell apoptosis, but also modulate the tumor immune environment and eventually augmented the antitumor effect of CD8+ cytotoxic T cells due to TAM depletion.232A method was developed for the manufacture of taste-masked oral DDS based on CP, Fe-4,4′-bipyridine (Fe-bipy) complex, that regulated the release of unpleasant drug taste by changing the pH value in the physiological environment of the alimentary canal (Fig. 26).233 The pH sensitive Fe-bipy was grafted onto MSNPs containing the model bitter drug mequindox (MEQ) in their mesopores. In artificial saliva (pH 6.6), Fe-bipy CPs effectively prevent the leakage of a loaded guest molecule MEQ. On the other hand, in the artificial gastric fluid (pH 1.0), the coordination bonds of the Fe-bipy complex were broken, resulting in the release of MEQ molecules from DDS.
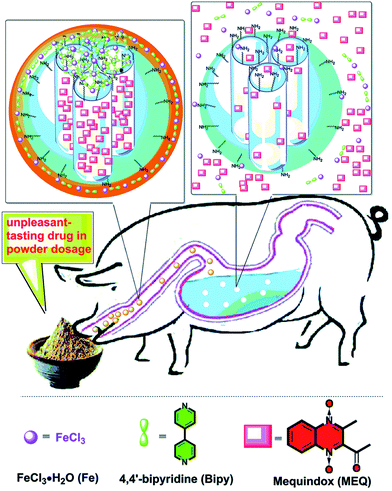 | ||
| Fig. 26 Schematic representation of a working protocol for a taste-masking drug based on physiological pH-responsive release of an unpleasant-tasting drug from MSNPs capped with the CP shell [reprinted from ref. 233 with permission from The Royal Society of Chemistry]. | ||
Epigallocatechin-3-gallatea palmitate was encapsulated in ZIF-8 NPs with functionalization of FA, which is commonly used as pH-responsive DDS.234 By recognizing targets between FA on the surface of NPs and the overexpressed FA receptor (FR) in cancer cells, NPs can efficiently internalize into cells and present targeted effects of inhibition growth on HeLa cells (cancer cells) compared to HEK 293 cells (normal cells), which corresponds to the regulation of the reactive oxygen species (ROS) level and the induction of autophagy. Autophagy flux and autophagosome formation are markedly induced by treating cells by NPs, indicating that pH-responsive NPs with targeted identification for cancer cells can be used as highly effective DDSs for targeting cancer chemotherapy. DOX-loaded ZIF-8 can realize effective pH-responsive release in the cancer treatment, which demonstrates the potential for the production of stimuli-responsive DDSs for widespread use.235
It should be noted the use of smart hydrogel G1c·WP6 obtained by forming inclusion complexes between pillar[6]arene (WP6) and Fc groups of polymer network G1c (Fig. 27).236 Using DOX.HCl as a model drug, drug loading efficiency and pH-responsive release behavior of the smart hydrogel were confirmed. In vitro experiments demonstrated that rapid release of DOX.HCl is achieved in an acidic media (e.g., pH 2.0). Given the acidic microenvironment of tumor cells, the release of DOX.HCl from G1c·WP6 hydrogel can also be triggered in tumor tissues.
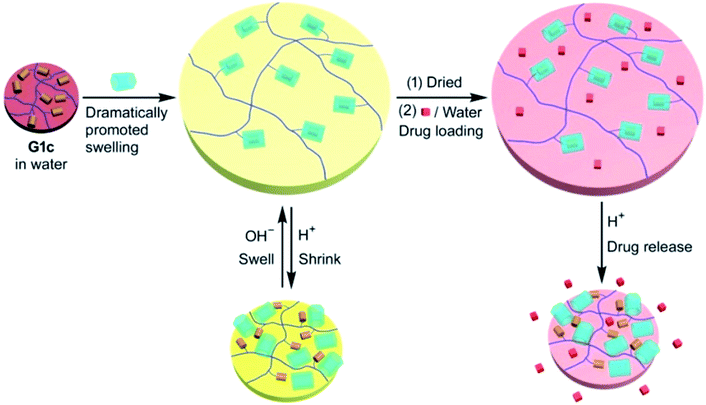 | ||
| Fig. 27 Illustration of a pH-responsive drug (DOX·HCl) release from G1c·WP6 with a loaded drug and corresponding profiles of in vitro cumulative drug release in an aqueous release medium at different pH values at 25 °C [reprinted with permission from ref. 236. Copyright 2016, American Chemical Society]. | ||
Despite the advantages of pH-responsive polymer nanocarriers such as controlled drug release, high specificity to the tumor, intracellular drug delivery, and excellent therapeutic efficacy with reduced side effects, pH-responsive polymer nanocarriers face severe challenges. Although many studies on pH-responsive polymer nanocarriers are still in preliminary stages, in vitro drug release behavior and cytotoxicity data are available. These systems are slow in clinical trials because of defects like polymer-related toxicity and low conjugate bioactivity. In the future, more efforts are needed to develop methods for combinations with other stimuli like redox or temperature for specific targeted release.
3.4 Magnetic stimuli-responsive DDSs
Magnetism is an external non-invasive activation method that has attractive abilities, since magnetic fields rarely interact with the patient's body compared to other traditional stimuli, such as pH and light. Various strategies based on magnetically-responsive MPs have been developed at present, mainly because of their ability to achieve magnetically-guided targeting and hyperthermia-induced drug release in an alternating magnetic field. As a typical example, we note a magnetically active metallopolymer-based DDS loaded with simple magnetic metal complexes for the effective cancer treatment (Fig. 28).237 The magnetothermal properties triggered the release of the encapsulated dual drug (Fe(salen) and DOX) for accurately deliver the drug to achieve improved in vivo tumor treatment, demonstrating its potential as DDS.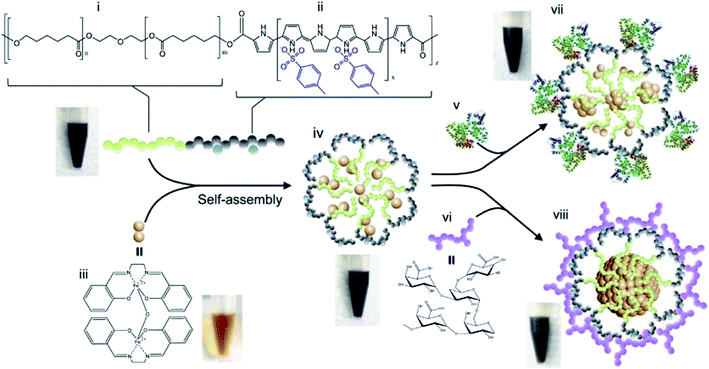 | ||
| Fig. 28 Magnetic stimuli-mediated precision therapeutics. Preparation of Fe(salen)-loaded polypyrrole (PPy)-PCL core–shell nanoassembled composites (iv) and core size modulation induced by biofunctionalization with bovine serum albumin (BSA) (vii) and gum arabic (GA) (viii). (i) PCL; (ii) PPy (benzosulfonate-doped); (iii) Fe(salen); (iv) Fe(salen)-loaded nanoassemblies; (v) BSA; (vi) GA; (vii) BSA-coated Fe(salen)-loaded nanoassemblies; (viii) GA-coated Fe(salen)-loaded nanoassemblies. Photos of the suspension are displayed at each stage of formulation [reprinted with permission from ref. 237. Copyright 2017, Springer Nature]. | ||
3.5 Enzyme-responsive DDSs
Enzyme-responsive DDSs have emerged as a promising option for the accurate delivery of drugs. The use of enzymes as triggers may be useful for the development of therapeutic agents because of their ability to stimulate chemical reactions under physiological conditions. In the past few years, a number of MPs has been used to create enzyme-responsive DDSs. For example, a biocompatible nanostructure of DNA origami was created to deliver the antitumor complex of ruthenium.238 The unique tetrahedral DNA nanocages facilitate intercalation with the Ru(II) complex, increasing the loading efficiency of drugs. Further conjugation with biotin provides specific cellular uptake of the DNA nanocarrier by HepG2 cells by receptor-mediated targeting. In addition, unlike the free Ru(II) complex or only nanocage, this system can translocate to the cell nucleus upon internalization and is degraded in response to DNases, which leads to the controlled release of the drug and subsequent induction of effective cell apoptosis via ROS-mediated signaling pathways (Fig. 29).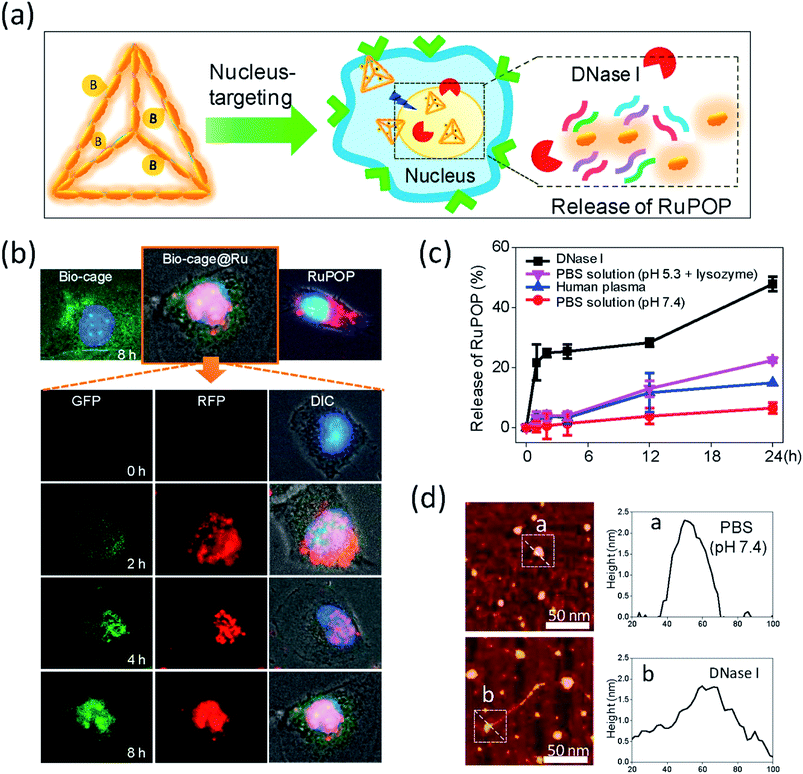 | ||
| Fig. 29 Translocation and drug release behavior of Bio-cage@Ru in nucleus. (a) Schematic illustration of the subcellular localization and release behavior of Bio-cage@Ru in HepG2 cells. (b) The fluorescence images of the trafficking of Bio-cage@Ru in HepG2 cells. The first line of images recorded the localization of free Bio-cage (20 μg mL−1), Bio-cage@Ru (5 μM) and free RuPOP (5 μM) in HepG2 cells at 8 h of incubation. The following images detailed the trafficking of Bio-cage@Ru in HepG2 cells for 8 h of incubation. The GFP images recorded the trafficking of Cy3-labeled Bio-cage from Bio-cage@Ru. The RFP images represented the fluorescence of RuPOP from Bio-cage@Ru. (c) The release rate of RuPOP from Bio-cage@Ru in PBS solution at pH 7.4, human plasma, PBS solution at pH 5.3 supplemented with 1 mg mL−1 lysozymes or DNase I respectively. Values were represented as means ± SD of triplicate. (d) The AFM images and the size changes of Bio-cage@Ru with or without the treatment of DNase I. Scale bar = 50 nm. The size and height of zone a and b were analyzed in histogram a and b respectively [reprinted with permission from ref. 238. Copyright 2016, Elsevier]. | ||
It should be noted DOX-loaded MOFs modified with adenosine triphosphate (ATP)-responsive hydrogel for cancer treatment (Fig. 30).239 This ATP-responsive MOFs/hydrogel showed improved anticancer efficacy and provided an interesting strategy for building stimuli-responsive MOFs-based DDSs.
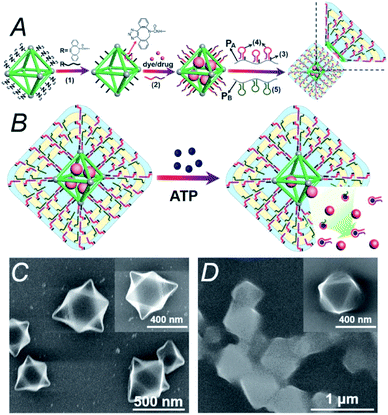 | ||
| Fig. 30 (A) Synthesis of DOX-loaded MOFs modified with ATP-responsive hydrogel. (B) In the presence of ATP, the decomposition of the modified hydrogel accelerates the release of DOX from MOFs. (C) Scanning electron microscopy (SEM) image of the MOFs. (D) SEM image of ATP-responsive hydrogel-modified MOFs [reprinted from ref. 239 with permission from The Royal Society of Chemistry]. | ||
3.6 Multi-stimuli-responsive DDSs
As a typical example of multistimuli-responsive DDS, we note the pH and redox dual-responsive nanovalve with a long stalk on the surface of hollow mesoporous silica NPs (HMSs) designed for size-selective drug delivery.240 The leakage of loaded rhodamine 6G (R6G) is insignificant in a neutral medium, which confirms the excellent sealing effect of the developed nanovalve (Fig. 31). In an acidic solution, the β-CD/Fc supramolecular complexes are removed from the surface of HMSs, which leads to the opening of the nanovalves and the rapid release of R6G molecules from the pores of HMSs. It is important that the resulting long stalk are effective for loading larger-sized cargos, such as DOX and R6G, but are not suitable for encapsulating small sized 5-FU.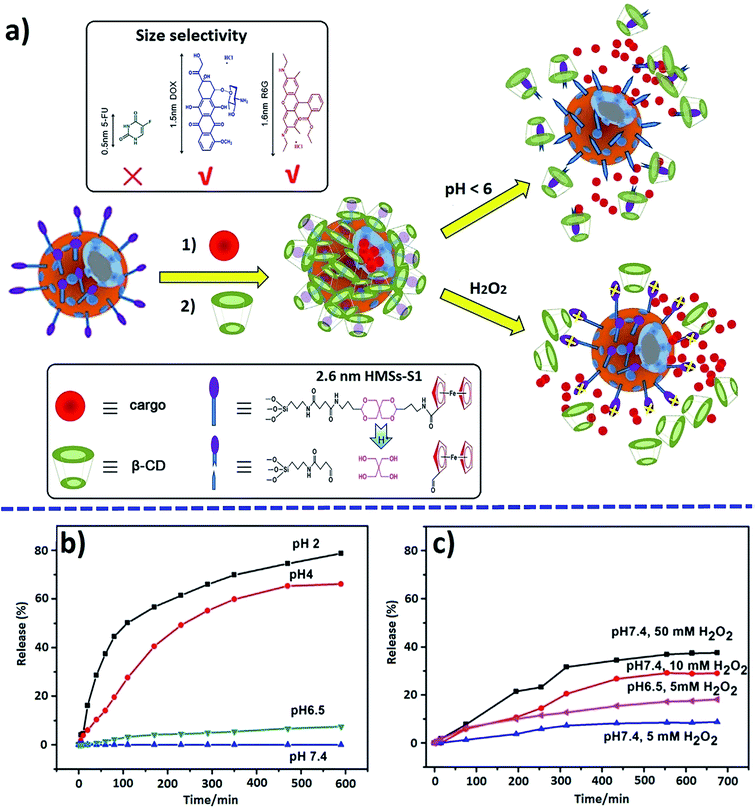 | ||
| Fig. 31 Schematic diagram of drug loading and release for mechanized HMSs (a), and pH- (b) and oxidation-triggered (c) release profiles of encapsulated R6G [reprinted with permission from ref. 240. Copyright 2016, Elsevier]. | ||
ROS and glutathione (GSH) dual redox-responsive supramolecular micelles were developed based on Fc-CPT and mPEG-β-CD (Fig. 32).241 These DDSs exhibit remarkable superfast release of CPT from micelles at high GSH concentration, and controlled release was observed by adjusting ROS (such as H2O2) concentration. In human cancer cells, the amount of ROS is higher than in normal cells, which can oxidize Fc to Fc+ and thus lead to the release of loaded drugs.
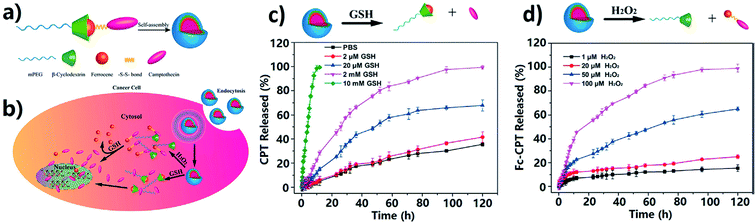 | ||
| Fig. 32 Schematic formation of mPEG-β-CD/Fc-CPT supramolecular complex micelles (a) and their GSH/H2O2 responsive drug release in cancer cells (b). Release profiles of CPT from the mPEG-β-CD/Fc-CPT supramolecular complex micelles at different GSH concentrations (c) and H2O2 concentrations (d) [reprinted with permission from ref. 241. Copyright 2017, American Chemical Society]. | ||
Well-defined dual-redox responsive supramolecular micelles and vesicles were developed, based on a supramolecular amphiphilic BCP containing β-CD/Fc host–guest joints and a traditional hydrophilic homopolymer poly(oligo(ethylene glycol) monomethyl ether methacrylate) with Fc terminus (Fc-POEGMA) (Fig. 33).242 DOX and DOX.HCl as model drugs were successfully encapsulated in the micelles and vesicles, respectively. Both tris(2-carboxyethyl)phosphine (TCEP) as a reducing agent and NaClO as an oxidizing agent triggered structural deformation of the supramolecular micelles and vesicles, contributing to the release of DOX (Fig. 33b and d).
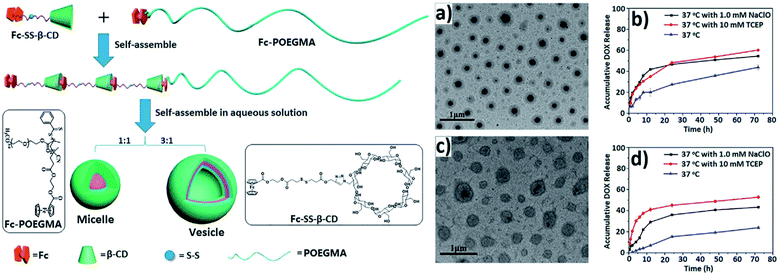 | ||
| Fig. 33 Schematic formation of micelles and vesicles formed by supramolecular self-assembly of Fc-SS-b-CD/Fc-POEGMA. TEM images of micelles (a) and vesicles (c); in vitro release profiles of DOX from micelles (b) and vesicles (d) [reprinted with permission from ref. 242. Copyright 2016, American Chemical Society]. | ||
Multi-stimuli-responsive magnetic nanomicelles were constructed by surface attachment of hydrophilic PEG chains and thermally responsive poly(N-isopropylacrylamide) (PNIPAM) segments (Fig. 34a).243 The PEG/PNIPAM/CD-MNP micelles, where MNP is magnetic nanoparticle, had a high loading capacity for DOX and a high saturation magnetization simultaneously. The tunable release of DOX was achieved in response to temperature, H2O2 or pH independently or of the combined effect of multiple stimuli. The lowering of the temperature triggered a partial release of free DOX (Fig. 34c), and other DOX were retained on the surface of single small PEG/PNIPAM/CD-MNP particles (Fig. 34b). The addition of oxidizing agents such as H2O2 led to the dissociation of the micelles into CD-MNPs, and the encapsulated DOX was consequently released (see Fig. 34b). In addition, a faster release of DOX was observed with increasing H2O2 concentration (Fig. 34d).
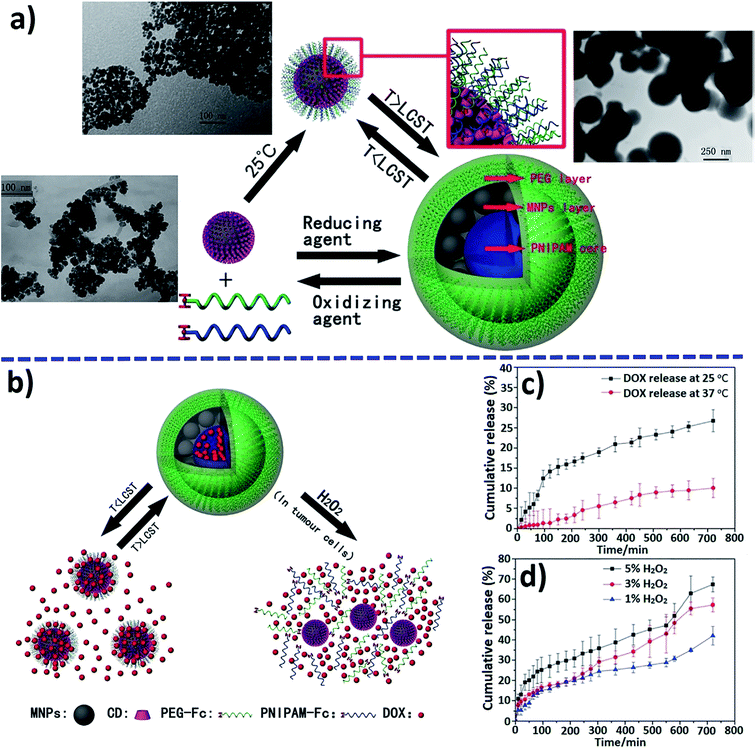 | ||
| Fig. 34 Illustration of the construction (a) of PEG/PNIPAM/CD-MNP micelles and a possible thermo- and redox-triggered mechanism for drug release (b). Cumulative release of DOX from micelles triggered by (c) temperature and (d) H2O2 [reprinted from ref. 243 with permission from The Royal Society of Chemistry]. | ||
Dual-responsive host–guest based colloidal microcapsules (MCs) were made using β-CD-modified polystyrene (PS) NPs (PS-CD NPs) and Fc-grafted polyethylenimine (PEI-Fc) (Fig. 35).244 In the drug-loading experiments, the rhodamine isothiocyanate labeled dextran (Mw 150 kDa) as a model was mainly encapsulated in the pores between NPs in the shell of MCs.
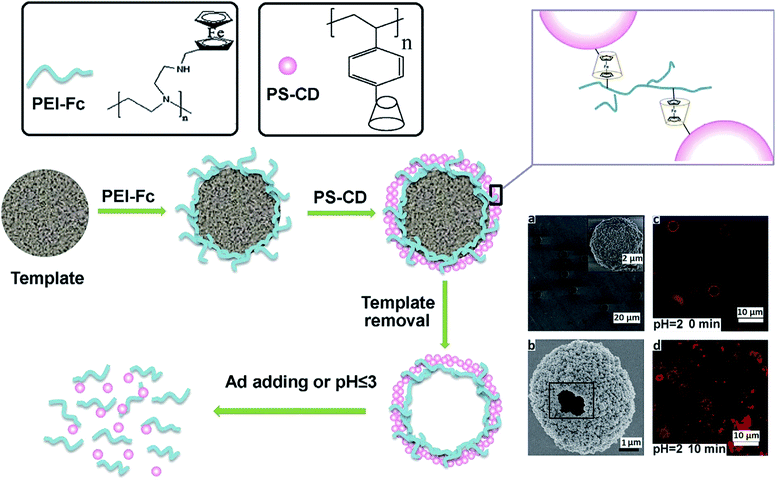 | ||
| Fig. 35 Schematic illustration of the process of manufacturing and disassembling host–guest based colloidal MCs. (a) SEM image of colloidal MCs before template removal; (b) SEM image of one broken colloidal MC dried by CPD method; confocal laser scanning microscopy images of colloidal MCs at pH 2.0 for 0 (c) and 10 (d) min. Ad is amantadine hydrochloride [reprinted with permission from ref. 244. Copyright 2016, Elsevier]. | ||
Both redox- and temperature-responsive supramolecular micelles were developed as tunable releasing nanocarriers for DDSs (Fig. 36a).245 The supramolecular micelles encapsulated hydrophobic drugs, such as DOX, and showed oxidation- and temperature-triggered controllable release behavior. When the ambient temperature was lower than lower critical solution temperature (LCST), for example, 25 °C, a burst of DOX release was observed during the first 15 h, and approximately 91.5% of DOX was released after 48 h (Fig. 36d). In the presence of H2O2, due to the dissociation of β-CD/Fc inclusion complex, the mPEG-Fc/PNIPAM-β-CD micelles dissociated into smaller PNIPAM-β-CD micelles (Fig. 32c), which is accompanied by a partial release of loaded DOX. When the ambient temperature was further reduced to 25 °C after the redox-triggered release, most of the entrapped DOX in the PNIPAM-β-CD micelles was released (Fig. 36e) due to the hydrophobic to hydrophilic transition of PNIPAM and the resulting disassembly of hydrophilic polymers.
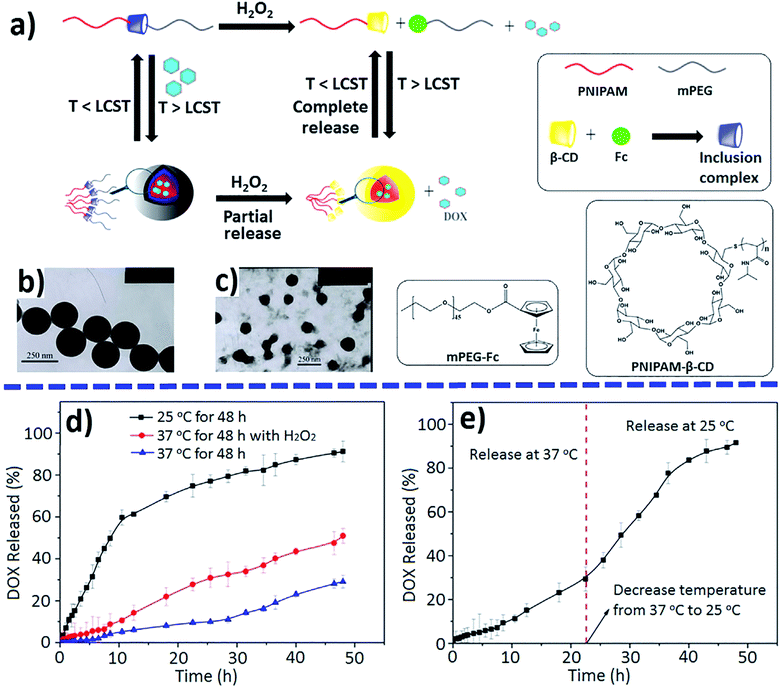 | ||
| Fig. 36 Schematic dual-stimuli-responsive assembly and disassembly of mPEG-Fc/PNIPAM-β-CD micelles (a); TEM images before (b) and after oxidation (c) by H2O2; DOX-released profiles under single (d) or no stimulus and dual stimuli (e) [reprinted with permission from ref. 245. Copyright 2015, American Chemical Society]. | ||
Thermo- and oxidation-responsive supramolecular vesicles for drug delivery application were constructed by self-assembly of pillar[6]arene (P[6])/Fc based amphiphilic supramolecular diblock copolymers (Fig. 37).246 Unique supramolecular vesicles were successfully used to encapsulate an anticancer drug DOX.HCl, which was first mixed with the hydrophilic PNIPAM-P[6]·mPEG-Fc at 25 °C, subjected to thermal shock with pre-heated water, then dialyzed against deionized water at 37 °C to obtain drug-loaded vesicles. The controlled release of DOX.HCl in the vesicles was achieved by cooling the vesicular solution from 37 °C to 25 °C, or by exposure to an oxidizing agent such as AgNO3, NaClO or H2O2.
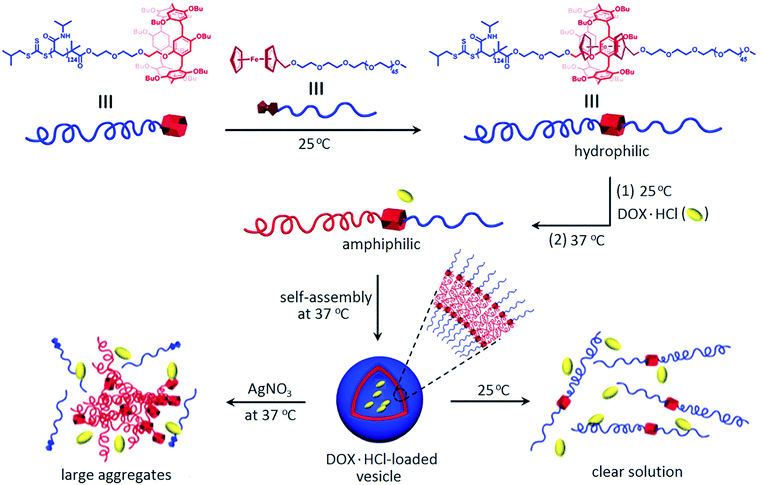 | ||
| Fig. 37 Illustration of drug loading and thermo- or oxidation-responsive drug release from supramolecular polymer vesicles as a result of self-assembly of PNIPAM-P[6]·mPEG-Fc [reprinted from ref. 246 with permission from The Royal Society of Chemistry]. | ||
NCs consisting of the triblock terpolymers PVFc-b-poly(methyl methacrylate)-b-poly(N,N-dimethylaminoethylmethacrylate) show triggered release of the payload under three different stimuli, namely, pH change, oxidizing agent, and temperature.247 Redox/pH dual-responsive supramolecular vesicles based on the host–guest complexation of Fcium carboxylic acid capped pillar[5]arene (FACP5) and a galactose derivative (G) were used for targeted drug delivery (Fig. 38).248 DOX loaded into FACP5G vesicles showed a fast release rate when exposed to GSH in acidic solution (pH 4.0), which makes it possible to control drug release.
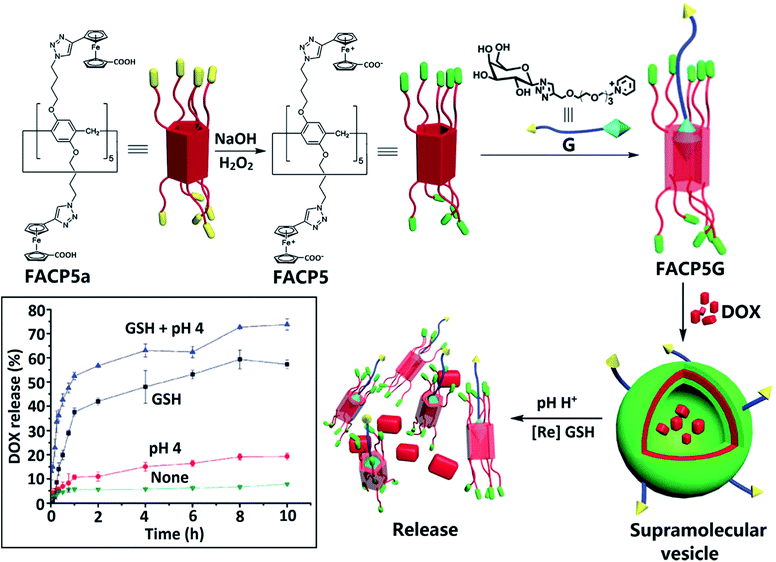 | ||
| Fig. 38 Illustration of the formation of supramolecular vesicles based on the host–guest complexation of FACP5 and the galactose derivative (G) and their redox/pH dual-responsive drug release. The inset shows the DOX release profiles from DOX-loaded FACP5G vesicles in water [reprinted from ref. 248 with permission from The Royal Society of Chemistry]. | ||
Dual stimuli-responsive core–shell microgels based on P(NIPAM-co-vinylferrocene) were used to electrochemically switch the polarity (hydrophilicity/hydrophobicity) of the microgel, which provides the electrochemically triggered uptake and release of drugs.249 Therefore, bactericidal drugs can be released to effectively kill bacteria. In addition, the good biocompatibility of microgels in cell tests indicates the suitability of the new microgel system as DDSs. A redox- and thermos-responsive gated supramolecular star polymers were obtained using the host–guest complexation of a 6-fold β-CD functionalized core molecule and Fc end modified poly(N,N-dimethylacrylamide) and poly(N,N-diethylacrylamide) linear polymers.250
In addition to the similar systems discussed here, it should be noted the development of temperature-,251–253 pressure-,254 humidity-,255 competitive binding agent-,256,257 liposome-responsive258 and other stimuli-responsive metallopolymers used as DDSs. However, using multiple stimuli-responsive polymers is more effective to achieve more precise cancer therapies. Among the most interesting examples, we note pH/GSH-,259,260 NIR/H2O2-,261 and ATP/Mg2+ ion-responsive systems.262
4. Metallopolymers for combination therapy and multimodal systems
In this section, we will consider such promising areas for the use of metallopolymer-based DDSs as combination therapy and multimodal systems.4.1 Metallopolymers for combination therapy
Anticancer drug therapy using a single drug is usually ineffective due to the heterogeneity and complexity of cancer and is therefore rarely used in clinical practice. Potential benefits of combination chemotherapy include reduced drug resistance and reduced dosage when equal or higher levels of effectiveness are achieved, thereby increasing its therapeutic index. These DDSs can take many forms to optimize the co-delivery of a wide range of anticancer drugs with different properties. Among the main methods used for combination drug delivery based on polymer carriers, we note polymer–drug conjugates, polymer–drug NPs and polymer–drug conjugates plus polymer–drug NPs (Fig. 39).263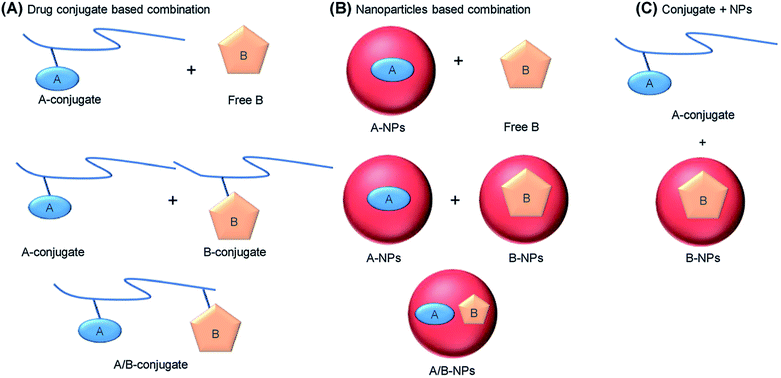 | ||
| Fig. 39 General strategies for combination drug therapy using polymer carriers. (A) The polymer conjugate of drug A can be combined either with the free drug B or with the polymer conjugate of B, on the same or different chains, where the “conjugate” refers to any form of the polymer drug that does not form NPs. (B) Drug A can be loaded into polymer NPs, which are then combined with free B or with B NPs, or both A and B can be co-assembled in the same NPs. (C) Drug A can be administered as a polymer conjugate, while drug B is administered in NPs [reprinted with permission from ref. 123. Copyright 2018, Elsevier]. | ||
A typical example is a polysaccharide-based DDS consisting of Dex decorated with succinic acid (Dex-SA) and loaded with DOX.264 The obtained polymer NPs were then cross-linked using a cisplatin prodrug to form a combination of DOX-cisplatin particles (Fig. 40). These NPs remained in circulation longer and accumulated in the tumor more significantly than non-cross-linked analogues or free DOX. Compared to control groups, the Dex-SA-DOX-cisplatin combination NPs significantly reduced tumor sizes of CT26 colon cancer xenografts. When co-administered with immobilized Arg-Gly-Asp (iRGD), a peptide drug that increases the permeability of tumor tissue,265 Dex-SA-DOX-cisplatin therapy successfully suppressed the growth and metastasis of 4T1 murine mammary carcinoma.
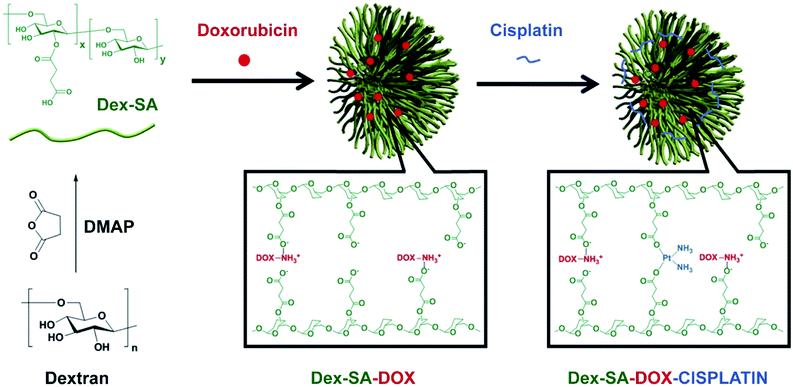 | ||
| Fig. 40 The schematic illustration of the process of preparing Dex-SA, Dex-SA-DOX and Dex-SA-DOX-cisplatin [reprinted with permission from ref. 264. Copyright 2014, Elsevier]. | ||
Recently, a wide variety of biodegradable polymers has been studied for the co-delivery of several anticancer drugs, including those based on Pt(II) drugs.266–268 It should also be noted a graft-type polypeptide micellar system for the combined drug delivery (Fig. 41). This polymer was loaded with cisplatin and docetaxel (DTX), and then was decorated with an αvβ3 integrin targeting peptide c(RGDfK). In vivo results demonstrated surprisingly long circulation time, minimal side effects, and high antitumor and anti-metastasis efficacy.269
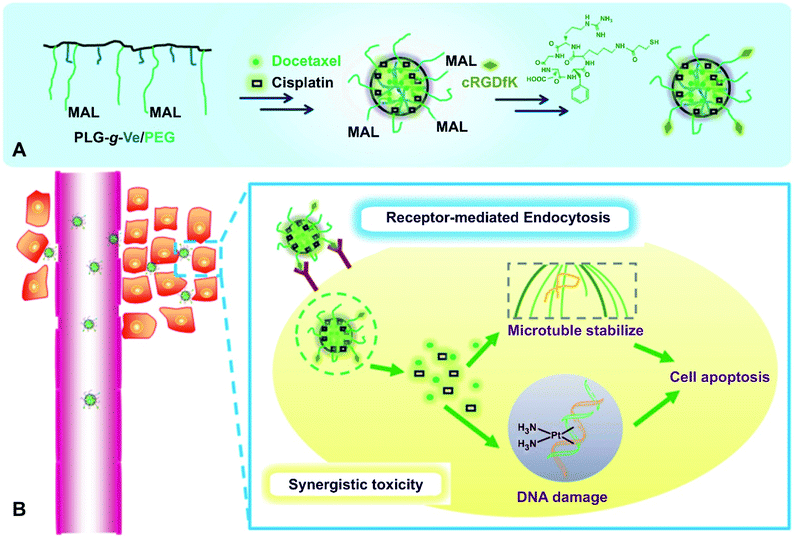 | ||
| Fig. 41 (A) Schematic illustration of preparation of the c(RGDfK)-decorated dual-drug-loaded micelles. (B) The micelles enter tumor cells by receptor-mediated endocytosis, and the loaded two drugs act synergistically intracellularly [reprinted with permission from ref. 269. Copyright 2014, John Wiley and Sons]. | ||
Conjugation of the Pt drug, in which the Pt(IV) pharmacores were coordinated with light-responsive azides as equatorial ligands and with cisplatin-sensitizing norcantharidin as an axial ligand, with biodegradable copolymers led to NPs (Fig. 42).270 The mechanism of action of the drug includes damage to nuclear DNA, oxidative damage to DNA and proteins, as well as preventing the repair of damaged DNA. Compared with other types of combination therapy based on the MP systems, this conjugate has several favorable characteristics, including a combination of multiple therapeutic modalities in one conjugate, a high loading capacity of the carrier for each drug with precise control of their stoichiometric ratio, the synergistic effect of all three drugs and controlled drug release triggered by photo-irradiation.
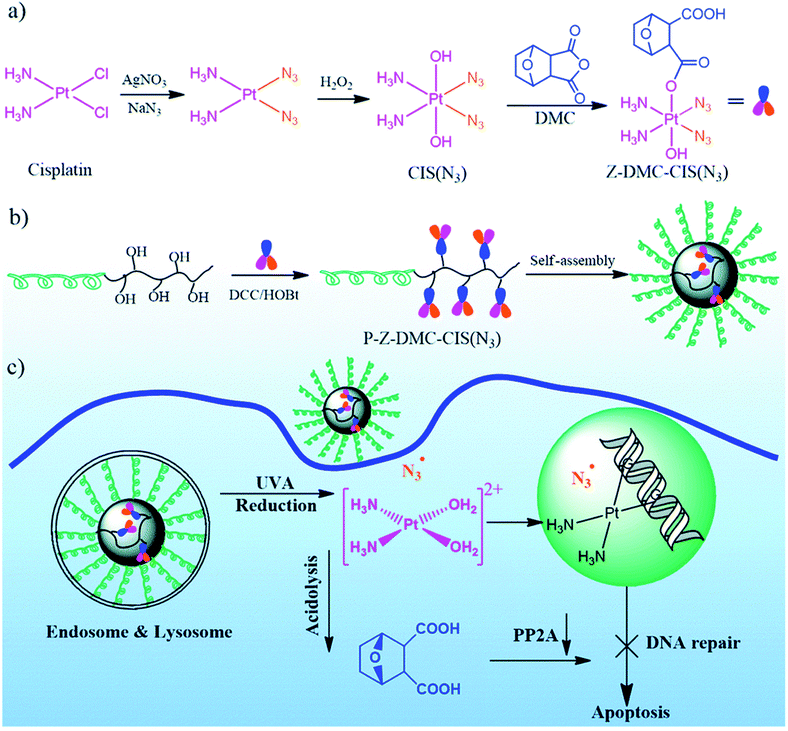 | ||
| Fig. 42 (a) Synthesis of a multifunctional single-drug Z-DMC-CIS(N3), (b) conjugation of a single-drug with a biodegradable amphiphilic BCP to obtain a polymer–(multifunctional single-drug) conjugate micelles, and (c) possible paths after the polymer–drug conjugate micelles enter the cisplatin-resistant cancer cells [reprinted from ref. 270 with permission from The Royal Society of Chemistry]. | ||
The amphiphilic Dex-Pt(IV) conjugate was self-assembled in NPs in an aqueous solution and used as a reduction-responsive carrier for DOX encapsulation.271 Under reducing conditions, the pendant Pt(IV) is reduced to Pt(II), which leads to disintegration of NP and triggered the release of encapsulated DOX. Of interest is the encapsulation of the multifunctional Pt(IV) prodrug, mitaplatin (Fig. 43a), in PEG-b-PLGA micelles. These micelles showed longer blood circulation, lower toxicity, and increased therapeutic efficacy compared with free mitaplatin.272 A multifunctional Pt(IV) prodrug canthaplatin (Fig. 43b) was developed, in which cantharidin (a PP2 A inhibitor that can block DNA repair and inhibit the basic mechanism of platinum drug resistance) was attached to the axial positions of cis-Pt(IV). Canthaplatin was encapsulated in PEG-b-PLGA micelles, forming NPs capable of combination drug delivery. After the micelles were endocytosed, the multifunctional prodrug was chemically reduced and the cisplatin and PP2 A inhibitors were released in a controlled manner. This combination led to inhibition of DNA repair, which significantly contributed to apoptosis, increased antitumor efficacy and reversed cisplatin resistance to human lung cancer both in vitro and in vivo.
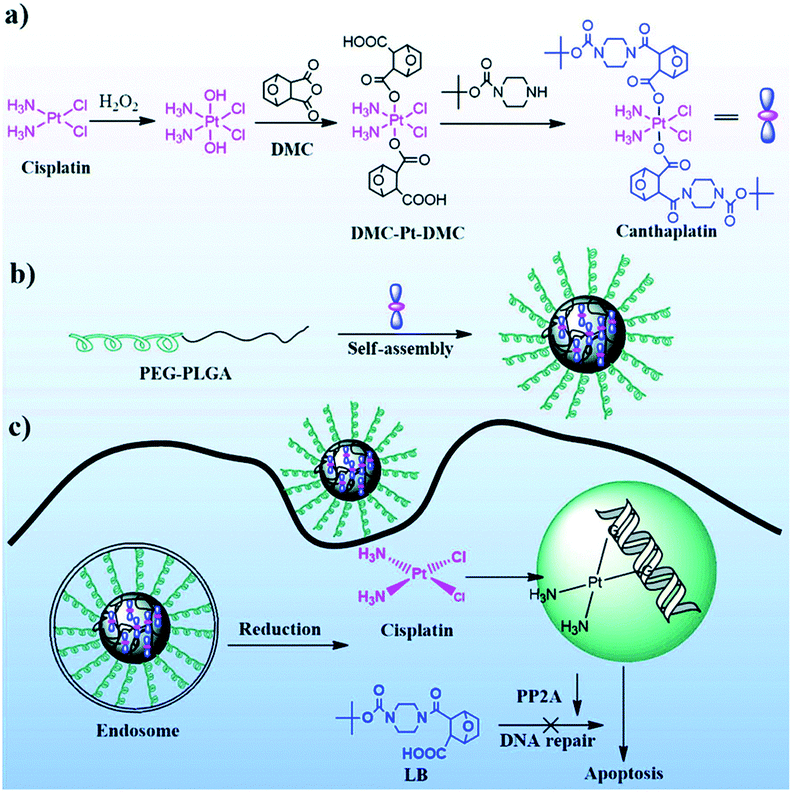 | ||
| Fig. 43 (a) Synthesis of canthaplatin, (b) preparation of polymer/canthaplatin micelles, and (c) schematic representation of the intracellular action after endocytosis of polymer/canthaplatin micelles [reprinted from ref. 272 with permission from The Royal Society of Chemistry]. | ||
It should be noted that such hybrid DDSs significantly improve the dose-specific delivery of each type of drug, increasing their synergistic efficacy. An example is camplatin, a prodrug formed by coining camphoric anhydride and cisplatin, which was delivered in biodegradable NPs. This system for co-delivery of camphoric acid and cisplatin showed increased anticancer activity compared to cisplatin and successfully overcame cisplatin drug resistance.273
A reduction-sensitive DDBSP with dual drugs in its backbone has been developed (Fig. 44A).230 Fixed and accurate drug ratios can be released in response to intracellular reduction or acidic conditions, allowing the drugs to be released in a controlled manner. In addition, DDBSP had a high platinum heavy metal content in the polymer backbone, which made it possible to directly monitor the fate of the drug using drug-mediated computer tomography (DMCT). Finally, DDBSP demonstrated (Fig. 44B and C) significantly improved cancer treatment on a high-fidelity patient-derived lung cancer (PDLC).
 | ||
| Fig. 44 Schematic illustration of DDBSP for synergistic eradication of PDLC. (A) Illustration of the structure of Pt(IV)-1, the polymerization of DDBSP, self-assembly and chain-shattering of DD-NP with the release of active Pt(II) and DMC, as well as key features of DDBSP and DD-NP. (B) Illustration of the creation of a PDLC model and Pt-based DMCT after intravenous injection of DD-NP. (C) Possible dual anticancer mechanisms after endocytosis of DD-NP by cancer cells [reprinted with permission from ref. 230. Copyright 2018, John Wiley and Sons]. | ||
Of interest is a voltage/pH-driven MSNPs-based multidrug (DOX and GEM) delivery system for multimodal controlled drug release (Fig. 45a).274 The unique structure of MSNPs 1 allows two different controlled release methods to be implemented. In particular, when exposed to +1.5 V voltage, an accurate controlled release of small GEM is observed due to the self-dissociation of the Fc-β-CD complex. Raising the ambient pH to an acidic pH, for example, 6.0, 5.0 and 4.0, results in a gradual release of DOX (Fig. 45b), which was associated with the removal of supramolecular nanovalves caused by the splitting of the ketal bond. Being localized in an acidic solution, MSNPs 1 exhibited the concurrent release modality of GEM and DOX (Fig. 45c). Compared to single DOX- or GEM-loaded MSNPs, well-organized MSNPs 1 have improved cytotoxicity to MCF7 cancer cells, indicating potential application in combination therapy to overcome cancer MDR.
 | ||
| Fig. 45 Schematic assembly and multimodal controlled release modalities of MSNPs 1 (a); sequential (b) and concurrent (c) release profiles of encapsulated GEM and DOX [reprinted with permission from ref. 274. Copyright 2015, American Chemical Society]. | ||
The advantages of using metallopolymers-based DDSs in combination therapy include an increase in therapeutic efficacy and a reduction in dosage while achieving equal or higher levels of efficacy and a decrease in drug resistance. In this direction, nanoscale MPs275 are of particular attention for combination therapy.276 Despite the several advantages these DDSs in combination chemotherapy, many combination treatment regimens that have shown promising in vitro results have been affected by clinical translation because they showed little to no improvement in efficacy and often actually caused higher toxicity. Differences in the physicochemical properties of drugs can lead to dissimilarities in the pharmacokinetics and tissue distribution of each drug.
4.2 Metallopolymers-based multimodal systems
Scientists are currently focused on developing multimodal treatment systems to meet the ever-growing demand for effective therapeutic strategies. Over the past few decades, MPs have been synthesized that simultaneously transmit diagnostic information and monitor the in situ therapy process.277 The integration of various cancer treatment strategies, such as chemotherapy, photodynamic therapy (PDT) and photothermal therapy (PTT), into the MP nanoplatform has shown great potential for obtaining a low risk of recurrence and high anticancer efficacy.278As an example, we note the supramolecular dendritic system, including lipoic acid functionalized dendrons, Pt(II)-PEG conjugate, and NIR fluorophore (dye Cy5.5) for in vivo imaging (Fig. 46).279 In vitro studies with lung adenocarcinoma A549 cells showed the internalization of nanostructures due to endocytosis and high cytotoxicity, while in vivo studies demonstrated efficacy comparable to cisplatin with avoiding the nephrotoxicity. NIR imaging revealed the localization of nanostructures in vitro and in vivo, which facilitated the determination of Pt(II) delivery to the target sites.
 | ||
| Fig. 46 (A) Schematic illustrations of molecular and supramolecular engineering of theranostic supramolecular PEGylated dendritic systems. (B) Dynamic Light Scattering (DLS) results (in aqueous solutions) and TEM images for theranostic supramolecular PEGylated dendritic systems (TSPDSs) [reprinted with permission from ref. 279. Copyright 2016, Ivyspring International Publisher]. | ||
The four-armed amphiphilic Pt-PAZMBb-POEGMA copolymer, containing an aggregation-induced emissive fluorescent probe for imaging living cells and the anticancer drug 3,6-bis[trans-Pt(PEt3)2]phenanthrene, was self-assembled into NPs, which were then used for encapsulate DOX to achieve a synergistic anticancer effect.280
Core–shell nanocomposites, based on the controlled growth of the UiO-66 shell on the surface of Fe3O4 core, have been developed for loading therapeutic drugs and simultaneous magnetic resonance imaging (MRI).281 Also noteworthy is a multifunctional nanoplatform based on Fe3O4 NPs, gold NPs and ZIF-8 NPs for integration chemotherapy and multimode imaging, including fluorescence imaging, MRI and X-ray computer tomography. Multifunctional NPs with many advantages, such as easy preparation conditions, a high degree of DOX separation, multimode imaging ability, pH-responsive drug release, and good biocompatibility, demonstrate effective anticancer performance and low systematic toxicity in in vivo studies.282 Of interest are MOFs, which not only include photosensitizers in periodic frameworks, but also have a high specific surface areas and large cavities for drug delivery.283 PDT is based on the excitation of photosensitizers in cancer tissues under light irradiation, which could efficiently produce ROS, causing lipid peroxidation, and increase the permeability of the cell membrane for irreversible killing of cancer cells.
A biocompatible fluorescent imaging-guided therapy system for cancer diagnosis and treatment was identified by integrating the properties of MOFs and chemotherapeutic drugs.284 Thus, biocompatible nanoscale zirconium-porphyrin MOFs-based system showed effective loading of DOX and a pH-responsive drug release for chemotherapy and a high porphyrin content to achieve effective PDT and fluorescent imaging. The imaging-guided dual therapy system is confirmed by the concentration of nanomedicine in the cancer tissues, which leads to low physiological toxicity and effective cancer therapy.
Multifunctional MOFs, which serve as a drug carrier, quenching reagent, and post-synthetic modification site, have been obtained for synergistic chemo-photodynamic therapy and real-time cathepsin B-mediated fluorescence imaging.285
Porphyrin-based MOFs (PCNs) have been used to deliver tirapazamine (TPZ) of the bio-reductive drugs and are coated with cancer cell membrane (Mem) for homologous targeting of cancer (Fig. 47A).286 After exposure to light, PCN-mediated PDT can lead to oxygen consumption in cancer tissues, and the resulting regional hypoxia will additionally activate the bio-reductive drugs of TPZ to improve chemotherapy (Fig. 47B). The synergistic effect of PDT and hypoxia-responsive chemotherapy in TPZ@PCN@Mem can significantly kill cancer cells and also suppress cancer metastasis.
 | ||
| Fig. 47 (A) The process of manufacturing a porphyrin-based MOFs nanoplatform (TPZ@PCN@Mem). (B) Schematic diagram of TPZ@PCN@Mem for synergistic therapy of porphyrin-mediated PDT and hypoxia-induced chemotherapy [reprinted with permission from ref. 286. Copyright 2017, Elsevier]. | ||
Several methods have been developed to improve the anticancer efficacy of PTT and PTT-based synergistic therapies. For example, core–shell PB@MIL-100(Fe) dual metal–organic-frameworks (d-MOFs) NPs, where PB is Prussian blue, can serve as a T1–T2 dual-modal MRI contrast and fluorescence optical imaging agent due to the presence of inner PB MOFs and outer MIL-100(Fe) MOFs.287 High-load artemisinin (a traditional Chinese anticancer drug) is released from d-MOFs during tumor cellular endocytosis due to pH-responsive degradation of outer MOFs in low pH tumor cell lysosomes. In addition, the inner PB MOFs can be used for PTT due to its strong absorbance in the NIR region. In another interesting example, a simple and smart pH/NIR dual-stimulus-responsive degradable mesoporous CoFe2O4@PDA@ZIF-8 sandwich nanocomposite, where PDA is polydopamine, was developed. The CoFe2O4 mesoporous core acts as T2-weighted MRI probe, PTT agent, and a hydrophilic DOX loading platform. The PDA layer is used to prevent premature leakage of DOX prior to arrival at tumor site, increase PTT efficiency, and facilitate ZIF-8 integration. The ZIF-8 shell serves to encapsulate hydrophobic CPT and as a switch for pH and NIR stimulation-responsive release of two drugs.288 Among other synergetic system using core–shell MOFs and organic PTT agents, we note PPy-based MOFs, PPy-MIL-100(Fe). The anticancer drug can be easily loaded into the mesopores of the MIL-100 shell, and the PPy core, as an organic photothermal agent, can photothermally ablate cancer cells and improve the efficacy of chemotherapy under NIR irradiation. The nanocomposites demonstrated outstanding synergistic anticancer ability in vivo.289 The same PPy-MIL-100(Fe) was used for dual-mode MRI/photoacoustic imaging and synergistic chemo-photothermal therapy of cancer cells. The multifunctional nanoplatform also demonstrated the ability to release a pH/NIR-responsive drug.290
In the core–shell porphyrin-based MOFs (AuNR@MOF), where AuNRs are gold nanorods, MOFs are used to load chemotherapeutic drugs such as CPT and diagnostic fluorescence imaging (Fig. 48).291 The AuNR core has an effective photothermal effect when irradiated with NIR light for PTT, which can also contribute to the release of therapeutic drugs for enhanced chemotherapy. In general, AuNR@MOFs have a significant synergistic effect for killing cancer cells and suppressing cancer recurrence and metastasis.
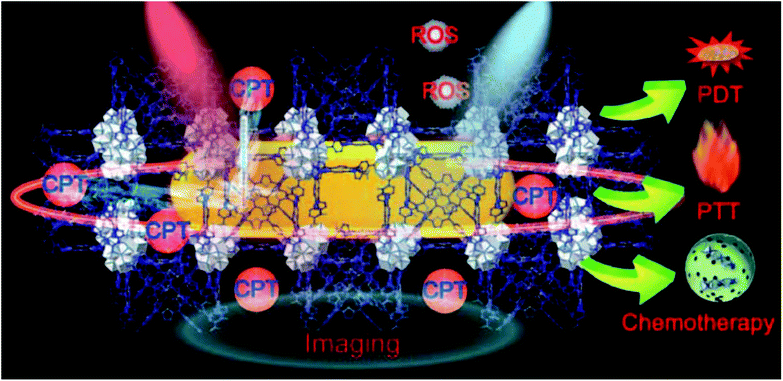 | ||
| Fig. 48 Schematic representation of AuNR@MOFs loaded CPT for synergistic cancer therapy of chemotherapy/PDT/PTT [reprinted with permission from ref. 291. Copyright 2018, John Wiley and Sons]. | ||
A strategy for synergistic cancer therapy based on a combination of chlorin-based MOFs mediated PDT and a small molecule immunotherapeutic agent encapsulated in the MOFs channels should be noted.292 In this case, combination therapy can effectively inhibit indoleamine 2,3-dioxygenase and cause systemic anticancer immunity. In addition, this strategy can be effectively used to treat colorectal cancer.
Benzoporphyrin-based MOFs (TBP-MOF) effectively inhibit cancer metastasis based on the synergistic therapy of PDT and immunotherapy (Fig. 49A).293 To improve the physiological stability, TBP-MOF was modified by PEG, and PEG-modified TBP-MOF can conduct effective PDT in a hypoxic microenvironment. Due to the synergistic effect of TBP-MOF-mediated PDT and the αPD-1 checkpoint blockade immunotherapy, primary cancer can be significantly suppressed, and cancer metastasis can also be efficiently inhibited (Fig. 49B).
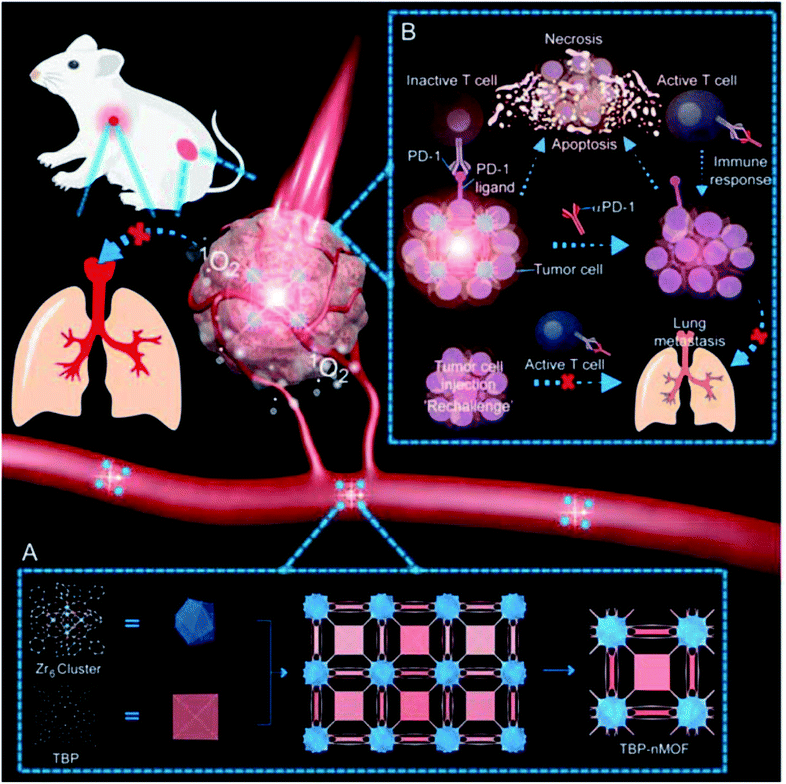 | ||
| Fig. 49 (A) The preparation process of TBP-MOF. (B) Schematic presentation of TBP-MOFs-induced photodynamic cancer immunotherapy for effective inhibition of cancer metastasis [reprinted with permission from ref. 293. Copyright 2018, American Chemical Society]. | ||
Of interest are porphyrin-based MOFs (PCN-224) used as nanocarriers to deliver glucose oxidase (GOx) and catalase, which also served as nanophotosensitizer (Fig. 50A). The enzyme-loaded PCN-224 can be effectively used for synergistic PDT and starvation therapy (Fig. 50B and C). In addition, PCN-224, loaded with GOx and catalase, was further modified with a homotypic cancer cell membrane that could selectively target cancer tissue due to homotypic target talents. As a result, synergistic effects can efficiently kill cancer cells in a controlled manner.294
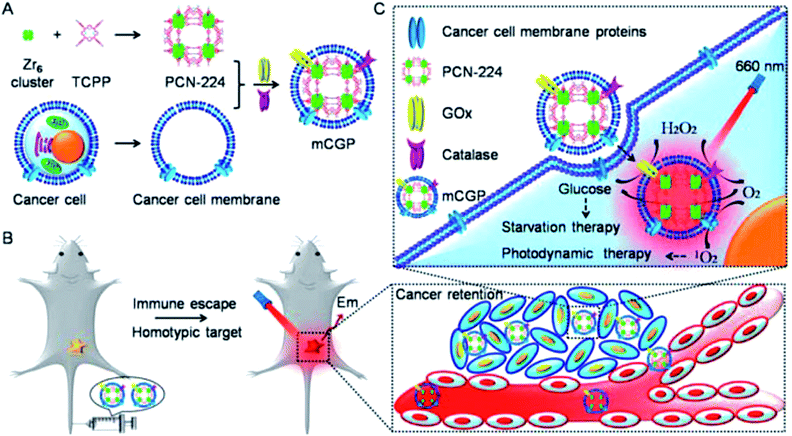 | ||
| Fig. 50 (A) Processes for the preparation of PCN-224 as a nanocarrier for the delivery of GOx and catalase. (B) In vivo immune escape and homotypic cancer targeting of the composites. (C) Multifunctional MOFs-based nanoplatform for synergistic PDT and starvation therapy [reprinted with permission from ref. 294. Copyright 2018, American Chemical Society]. | ||
It should be noted the modification of panitumumab using MPs that are multiple DOTA chelators, providing stable and effective complexation of 111In or 177Lu, which was an EGFR-targeted theranostic for pancreatic cancer.295
Among the advantages of metallopolymers-based DDS with multimodality, such as imaging and targeted drug delivery, it should be noted a decrease in side effects, an improved targeting strategy and biodistribution, enhanced blood circulation and the penetration depth of existing body barriers, as well as a decrease in the required volume needed for distribution.
5. Concluding remarks and outlook
As the contents of this review show, the current stage of the development of metallopolymers-based DDSs has reached its peak in the accumulation of experimental facts and their theoretical interpretation and generalization; all major research groups are involved in this area of science. MPs showed great potential in DDSs due to their unique optical, electrochemical and magnetic properties. In addition, MPs participated in the codelivery of several chemotherapeutics drugs for the treatment of drug-resistant cancer. These functional DDSs have the potential to increase the solubility and efficacy of a drug while reducing side effects.How we see development of this interesting and promising area of using metallopolymers?
First of all, it should be noted that the range of MPs used for these purposes is quite wide and is not limited to the examples given here. A huge selection of MPs with a wide arsenal of synthetic methods for their preparation, the simplicity of many formulations, scalability and an acceptable price/quality ratio provide a methodological platform to creating promising DDSs based on them. Among them, nanoscale MPs attract particular attention, in particular, for combination therapy. One promising area is the use of bioactive motifs (cations, organic ligands, or both) as building blocks for constructing metal biomolecule frameworks (also known as bioMOFs). A variety of hybrid materials, such as biopolymer@MOFs, are also of considerable interest. It is important to develop MPs whose polymeric chains include non-traditional heteroatoms, for example, selenium or tellurium. Stimuli-responsive metallopolymers-based DDSs are one of the most promising class of nanoplatforms that meet the requirements of an effective chemotherapeutic drug formulation.
Despite their enormous potential, very few metallopolymers-based DDSs have been included in clinical trials and no one has yet been approved for the treatment of the disease. Indeed, there are still some problems that must be overcome in order to begin the effective pharmaceutical development of such systems. Unfortunately, for many metallopolymers-based DDSs the exact mechanism of action and behavior in the biological environment are not fully understood. Another major challenge is the assessment of systemic toxicity and in vivo pharmacokinetics. Although a large number of metallopolymer-based DDSs have been studied in vitro with varying cytotoxicity, little is known about their absorption, distribution, metabolism, and excretory properties in humans. Further problems arise due to the requirements for precise control and initiation of the responsive systems. In addition, till now, as a rule, it is impossible to recognize correlations between content, structural features, and properties of metallopolymer-based DDSs, which in many respects restrains scientifically grounded approach to structuring of these systems and prediction of their promising properties.
Conflicts of interest
No potential conflict of interest was reported by the authors.Acknowledgements
This work was performed by Gulzhian I. Dzhardimalieva (Institute of Problems of Chemical Physics RAS) in accordance with the state task, state registration No. 0089-2019-0008.References
- J.-A. Martyn, P. Paliadelis and C. Perry, Nurse Educ. Pract., 2019, 37, 109–114 CrossRef PubMed.
- S. S. Dhruva, J. S. Ross and N. R. Desai, Pharmacy and Therapeutics (P&T), 2018, 43, 464–472 Search PubMed.
- Y.-C. Kim, K. A. Min, D.-J. Jang, T. Y. Ahn, J. H. Min, B. E. Yu and K. H. Cho, J. Pharm. Invest. DOI:10.1007/s40005-019-00452-0.
- A. D. Vadlapudi, D. Kwatra and A. K. Mitra, in Drug Delivery, ed. A. K. Mitra, D. Kwatra and A. D. Vadlapudi, Jones & Bartlett Learning, Burlington, USA, 2015, pp. 1–7 Search PubMed.
- Y. Duarte, V. Márquez-Miranda, M. J. Miossec and F. González-Nilo, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 11, e1554 Search PubMed.
- R. Spitler, S. Zanganeh, T. Jafari, N. Khakpash, M. Erfanzadeh, J. Q. Ho and N. Sakhaie, in Drug Delivery Systems, ed. P. Stroeve and M. Mahmoudi, World Scientific Publ. Co., New Jersey, 2018, pp. 1–52 Search PubMed.
- C. He, D. Liu and W. Lin, Chem. Rev., 2015, 115, 11079–11108 CrossRef CAS PubMed.
- N. Song and Y. W. Yang, Chem. Soc. Rev., 2015, 44, 3474–3504 RSC.
- A. A. Moghanjoughi, D. Khoshnevis and A. Zarrabi, Drug Delivery Transl. Res., 2016, 6, 333–340 CrossRef PubMed.
- M. Daniyal, B. Liu and W. Wang, Curr. Med. Chem. DOI:10.2174/13816128256661902011296290.
- X. Zhao, K. Tian, T. Zhou, X. Jia, J. Li and P. Liu, Colloids Surf., B, 2018, 168, 43–49 CrossRef CAS PubMed.
- J. Wang, J. Fang, P. Fang, X. Li, S. Wu, W. Zhang and S. Li, J. Biomater. Sci., Polym. Ed., 2017, 28, 337–349 CrossRef CAS PubMed.
- M. M. Goswami, Sci. Rep., 2016, 6, 35721 CrossRef PubMed.
- A. Llopis-Lorente, B. Lozano-Torres, A. Bernardos, R. Martínez-Máñez and F. Sancenón, J. Mater. Chem. B, 2017, 5, 3069–3083 RSC.
- H. He, H. Xiao, H. Kuang, Z. Xie, X. Chen, X. Jing and Y. Huang, Colloids Surf., B, 2014, 117, 75–81 CrossRef CAS PubMed.
- M. Yilmaz, A. A. Karanastasis, M. V. Chatziathanasiadou, M. Oguz, A. Kougioumtzi, N. Clemente, T. F. Kellici, N. E. Zafeiropoulos, A. Avgeropoulos, T. Mavromoustakos, U. Dianzani, S. Karakurt and A. G. Tzakos, ACS Appl. Bio Mater., 2019, 2, 2715–2725 CrossRef CAS.
- K. Sztandera, M. Gorzkiewicz and B. Klajnert-Maculewicz, Mol. Pharm., 2019, 16, 1–23 CrossRef CAS PubMed.
- S. Perfahl, M. M. Natile, H. S. Mohamad, C. A. Helm, C. Schulzke, G. Natile and P. J. Bednarski, Mol. Pharmacol., 2016, 13, 2346–2362 CrossRef CAS PubMed.
- X. Wang, C. Liu, Z. Li, C.-Y. Tang, W.-C. Law, X. Gong, Z. Liu, Y. Liao, G. Zhang, S. Long and L. Chen, J. Phys. Chem. C, 2019, 123, 10658–10665 CrossRef CAS.
- H. Pick, A. C. Alves and H. Vogel, Chem. Rev., 2018, 118, 8598–8654 CrossRef CAS PubMed.
- S. Genheden and L. A. Eriksson, J. Chem. Theory Comput., 2016, 12, 4651–4661 CrossRef CAS PubMed.
- M. M. Virk and E. Reimhult, Langmuir, 2018, 34, 395–405 CrossRef PubMed.
- P. Ghasemiyeh and S. Mohammadi-Samani, Results Pharma Sci., 2018, 13, 288–303 CrossRef PubMed.
- M. Rawal, in Stimuli-responsive Drug Delivery Systems, ed. A. Singh and M. M. Amiji, Royal Society of Chemistry, London, 2018, pp. 33–50 Search PubMed.
- S. Indermun, M. Govender, P. Kumar, Y. E. Choonara and V. Pillay, in Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, ed. A. S. H. Makhlouf and N. Y. Abu-Thabit, Elsevier, Amsterdam, 2019, pp. 43–58 Search PubMed.
- N. Kamaly, B. Yameen, J. Wu and O. C. Farokhzad, Chem. Rev., 2016, 116, 2602–2663 CrossRef CAS PubMed.
- P. R. Nair, Polymers, 2019, 11, 630 CrossRef PubMed.
- A. Wicki, D. Witzigmann, V. Balasubramanian and J. Huwyler, J. Controlled Release, 2015, 200, 138–157 CrossRef CAS PubMed.
- T. Stylianopoulos and R. K. Jain, Nanomedicine, 2015, 11, 1893–1907 CrossRef CAS PubMed.
- Z. Li and Y.-W. Yang, J. Mater. Chem. B, 2017, 5, 9278–9290 RSC.
- M.-X. Wu, X. Wang and Y.-W. Yang, Chem. Rec., 2018, 18, 45–54 CrossRef CAS.
- K. Ulbrich, K. Holá, V. Šubr, A. Bakandritsos, J. Tuček and R. Zbořil, Chem. Rev., 2016, 116, 5338–5431 CrossRef CAS PubMed.
- W. Chin, G. Zhong, Q. Pu, C. Yang, W. Lou, P. F. De Sessions, B. Periaswamy, A. Lee, Z. C. Liang, X. Ding, S. Gao, C. W. Chu, S. Bianco, C. Bao, Y. W. Tong, W. Fan, M. Wu, J. L. Hedrick and Y. Y. Yang, Nat. Commun., 2018, 9, 917 CrossRef PubMed.
- G. I. Dzhardimalieva and I. E. Uflyand, Chemistry of Polymeric Metal Chelates, Springer, Cham, 2018 Search PubMed.
- G. I. Dzhardimalieva and I. E Uflyand, J. Inorg. Organomet. Polym. Mater., 2018, 28, 1305–1393 CrossRef CAS.
- R. L. N. Hailes, A. M. Oliver, J. Gwyther, G. R. Whittell and I. Manners, Chem. Soc. Rev., 2016, 45, 5358–5407 RSC.
- Y. Wang, D. Astruc and A. S. Abd-El-Aziz, Chem. Soc. Rev., 2019, 48, 558–636 RSC.
- K. Y. Zhang, S. Liu, Q. Zhao and W. Huang, Coord. Chem. Rev., 2016, 319, 180–195 CrossRef CAS.
- K. C. Bentz and S. M. Cohen, Angew. Chem., Int. Ed., 2018, 57, 14992–15001 CrossRef CAS PubMed.
- H. Li, P. Yang, P. Pageni and C. Tang, Macromol. Rapid Commun., 2017, 38, 1700109 CrossRef PubMed.
- R. A. Musgrave, A. D. Russell, D. W. Hayward, G. R. Whittell, P. G. Lawrence, P. J. Gates, J. C. Green and I. Manners, Nat. Chem., 2017, 9, 743–750 CrossRef CAS.
- M. O. Wolf, Adv. Mater., 2001, 13, 545–553 CrossRef CAS.
- M. O. Wolf, J. Inorg. Organomet. Polym. Mater., 2006, 16, 189–199 CrossRef CAS.
- A. Winter and U. S. Schubert, Chem. Soc. Rev., 2016, 45, 5311–5357 RSC.
- A. Abd-El-Aziz and C. Agatemor, J. Inorg. Organomet. Polym. Mater., 2018, 28, 369–382 CrossRef CAS.
- T. Zhu, Y. Sha, J. Yan, P. Pageni, M. A. Rahman, Y. Yan and C. Tang, Nat. Commun., 2018, 9, 4329 CrossRef PubMed.
- L. Zhao, X. Liu, L. Zhang, G. Qiu, D. Astruc and H. Gu, Coord. Chem. Rev., 2017, 337, 34–79 CrossRef CAS.
- X. Wang, X. Wang and Z. Guo, Acc. Chem. Res., 2015, 48, 2622–2631 CrossRef CAS PubMed.
- J. Xiang, C. L. Ho and W. Y. Wong, Polym. Chem., 2015, 6, 6905–6930 RSC.
- C.-L. Ho, Z.-Q. Yu and W.-Y. Wong, Chem. Soc. Rev., 2016, 45, 5264–5295 RSC.
- A. Alkan and F. R. Wurm, Macromol. Rapid Commun., 2016, 37, 1482–1493 CrossRef CAS.
- T. Zhu, S. Xu, A. Rahman, E. Dogdibegovic, P. Yang, P. Pageni, M. P. Kabir, X.-d. Zhou and C. Tang, Angew. Chem., Int. Ed., 2018, 57, 2388–2392 CrossRef CAS.
- A. S. Abd-El-Aziz, C. Agatemor and N. Etkin, in Functional Metallosupramolecular Materials, ed. J. Hardy and F. Schacher, RSC Publishing, London, 2015, pp. 87–119 Search PubMed.
- J.-F. Lutz, J.-M. Lehn, E. W. Meijer and K. Matyjaszewski, Nat. Rev. Mater., 2016, 1, 16024 CrossRef CAS.
- R. Sakamoto, K.-H. Wu, R. Matsuoka, H. Maeda and H. Nishihara, Chem. Soc. Rev., 2015, 44, 7698–7714 RSC.
- D. A. Pelletier and J. M. Basset, Acc. Chem. Res., 2016, 49, 664–677 CrossRef PubMed.
- O. Pamies, M. Dieguez and J.-E. Backvall, Adv. Synth. Catal., 2015, 357, 1567–1586 CrossRef CAS.
- M. Hoarau, C. Hureau, E. Gras and P. Faller, Coord. Chem. Rev., 2016, 308, 445–459 CrossRef CAS.
- D. F. Sauer, S. Gotzen and J. Okuda, Org. Biomol. Chem., 2016, 14, 9174–9183 RSC.
- Y. W. Lin, Coord. Chem. Rev., 2017, 336, 1–27 CrossRef CAS.
- K. Wieszczycka and K. Staszak, Coord. Chem. Rev., 2017, 351, 160–171 CrossRef CAS.
- F. Schwizer, Y. Okamoto, T. Heinisch, Y. Gu, M. M. Pellizzoni, V. Lebrun, R. Reuter, V. Köhler, J. C. Lewis and T. R. Ward, Chem. Rev., 2018, 118, 142–231 CrossRef CAS PubMed.
- H.-C. Chang, J.-Q. Li, C.-K. Lin, Y.-J. Hsu, T.-H. Tu, Y.-L. Hsieh, H.-H. Hsu, G.-H. Lee, Y.-H. Liu and C.-H. Peng, J. Chin. Chem. Soc., 2019, 66, 1119–1133 CrossRef CAS.
- A. S. Abd-El-Aziz, A. A. Abdelghani, B. D. Wagner and R. Bissessur, Macromol. Rapid Commun., 2018, 40, 1800711 CrossRef.
- J. D. Caraway, M. T. Nguyen, L. A. Mitchell and B. J. Holliday, Macromol. Rapid Commun., 2015, 36, 665–670 CrossRef CAS.
- M. Mauro, S. Bellemin-Laponnaz and C. Cebrian, Chem.–Eur. J., 2017, 23, 17626–17636 CrossRef CAS PubMed.
- K. Albrecht, K. Matsuoka, K. Fujita and K. Yamamoto, Angew. Chem., Int. Ed., 2015, 54, 5677–5682 CrossRef CAS PubMed.
- M. Gallei and C. Rüttiger, Chem.–Eur. J., 2018, 24, 10006–10021 CrossRef CAS PubMed.
- Q. He, C. Wang, J. Xiao, Y. Wang, Y. Zhou, N. Zheng, B. Zhang and W. Bu, J. Mater. Chem. C, 2018, 6, 12187–12191 RSC.
- E. Hobbollahi, M. List and U. Monkowius, Monatsh. Chem., 2019, 150, 877–883 CrossRef CAS.
- M. T. Nguyen, R. A. Jones and B. J. Holliday, Coord. Chem. Rev., 2018, 377, 237–258 CrossRef CAS.
- F. Lu and D. Astruc, Coord. Chem. Rev., 2018, 356, 147–164 CrossRef CAS.
- I. E. Uflyand and G. I. Dzhardimaleva, Nanomaterials Preparation by Thermolysis of Metal Chelates, Springer, Cham, 2018 Search PubMed.
- M. Komiyama, T. Mori and K. Ariga, Bull. Chem. Soc. Jpn., 2018, 91, 1075–1111 CrossRef CAS.
- A. R. Kenaree and J. B. Gilroy, Dalton Trans., 2016, 45, 18229–18240 RSC.
- S.-C. Yiu, C.-L. Ho and W.-Y. Wong, in Polymer-Engineered Nanostructures for Advanced Energy Applications, ed. Z. Lin, Y. Yang and A. Zhang, Springer, Cham, 2017, pp. 51–70 Search PubMed.
- J. Gopinath, R. K. C. Balasubramanyam, V. Santosh, S. K. Swami, D. K. Kumar, S. K. Gupta, V. Dutta, K. R. Reddy, V. Sadhu, A. V. S. Sainath and T. M. Aminabhavi, Chem. Eng. J., 2019, 358, 1166–1175 CrossRef CAS.
- M. J. Dunlop, C. Agatemor, A. S. Abd-El-Aziz and R. Bissessur, J. Inorg. Organomet. Polym. Mater., 2017, 27, 84–89 CrossRef CAS.
- Q. Dong, Z. Meng, C.-L. Ho, H. Guo, W. Yang, I. Manners, L. Xu and W.-Y. Wong, Chem. Soc. Rev., 2018, 47, 4934–4953 RSC.
- Q. Dong, W. Qu, W. Liang, F. Tai, K. Guo, C.-W. Leung, Y. H. Lo and W.-Y. Wong, J. Mater. Chem. C, 2016, 4, 5010–5018 RSC.
- S. Schubert, I. Manners, G. R. Newkome and U. S. Schubert, Macromol. Rapid Commun., 2015, 36, 585 CrossRef CAS PubMed.
- S. G. Song, S. Ha, K.-B. Seo, J. Lee, T.-L. Choi, T. Premkumar and C. Song, RSC Adv., 2016, 6, 107994–107999 RSC.
- A. Alkan, T. Gleede and F. R. Wurm, Organometallics, 2017, 36, 3023–3028 CrossRef CAS.
- C. C. Beto, E. D. Holt, Y. Yang, I. Ghiviriga, K. S. Schanze and A. S. Veige, Chem. Commun., 2017, 53, 9934–9937 RSC.
- C. Rüttiger, H. Hübner, S. Schöttner, T. Winter, G. Cherkashinin, B. Kuttich, B. Stühn and M. Gallei, ACS Appl. Mater. Interfaces, 2018, 10, 4018–4030 CrossRef PubMed.
- L. Babel, K. Baudet, T. N. Y. Hoang, H. Nozary and C. Piguet, Chem.–Eur. J., 2018, 24, 5423–5433 CrossRef CAS PubMed.
- G. R. Newkome, C. N. Moorefield and S. Chakraborty, J. Inorg. Organomet. Polym. Mater., 2018, 28, 360–368 CrossRef CAS.
- I. Dragutan, V. Dragutan, B. C. Simionescu, A. Demonceau and H. Fischer, Beilstein J. Org. Chem., 2015, 11, 2747–2762 CrossRef CAS PubMed.
- P. Yang, P. Pageni, M. P. Kabir, T. Zhu and C. Tang, ACS Macro Lett., 2016, 5, 1293–1300 CrossRef CAS PubMed.
- L. Babel, L. Guénée, C. Besnard, S. V. Eliseeva, S. Petoud and C. Piguet, Chem. Sci., 2018, 9, 325–335 RSC.
- X. Liu, Q. Ling, L. Zhao, G. Qiu, Y. Wang, L. Song, Y. Zhang, J. Ruiz, D. Astruc and H. Gu, Macromol. Rapid Commun., 2017, 38, 1700448 CrossRef PubMed.
- C. Rüttiger, M. Appold, H. Didzoleit, A. Eils, C. Dietz, R. W. Stark, B. Stühn and M. Gallei, Macromolecules, 2016, 49, 3415–3426 CrossRef.
- A. Rahman, Y. Cha, L. Yuan, P. Pageni, T. Zhu, M. S. Jui and C. Tang, J. Polym. Sci., Part A: Polym. Chem. DOI:10.1002/pola.29439.
- T. Imaoka and K. Yamamoto, Bull. Chem. Soc. Jpn., 2019, 92, 941–948 CrossRef CAS.
- P. Kumar, K. Vellingiri, K.-H. Kim, R. J. C. Brown and M. J. Manos, Microporous Mesoporous Mater., 2017, 253, 251–265 CrossRef CAS.
- C. Pettinari, F. Marchetti, N. Mosca, G. Tosi and A. Drozdov, Polym. Int., 2017, 66, 731–744 CrossRef CAS.
- M. J. Rosseinsky, M. W. Smith and C. M. Timperley, Nat. Mater., 2015, 14, 469–470 CrossRef CAS PubMed.
- Q. Yang, Q. Xu and H. L. Jiang, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC.
- W. P. Lustig, S. Mukherjee, N. D. Rudd, A. V. Desai, J. Li and S. K. Ghosh, Chem. Soc. Rev., 2017, 46, 3242–3285 RSC.
- Y. Liu, S. Zhou, S. Liu and M. Xu, ChemElectroChem, 2018, 5, 6–19 CrossRef.
- L. Zhu, X. Q. Liu, H. L. Jiang and L. B. Sun, Chem. Rev., 2017, 117, 8129–8176 CrossRef CAS PubMed.
- W. Wang, X. Xu, W. Zhou and Z. Shao, Adv. Sci., 2017, 4, 1600371 CrossRef PubMed.
- K. K. Gangu, S. Maddila, S. B. Mukkamala and S. B. Jonnalagadda, Inorg. Chim. Acta, 2016, 446, 61–74 CrossRef CAS.
- Y. Cui, B. Li, H. He, W. Zhou, B. Chen and G. Qian, Acc. Chem. Res., 2016, 49, 483–493 CrossRef CAS PubMed.
- C. F. Cogswell, Z. Xie and S. Choi, in Metal-Organic Frameworks: Applications in Separations and Catalysis, ed. H. García and S. Navalón, Wiley, Weinheim, 2018, pp. 297–340 Search PubMed.
- G. Maurin, C. Serre, A. Cooper and G. Férey, Chem. Soc. Rev., 2017, 46, 3104–3107 RSC.
- W. Cai, C. C. Chu, G. Liu and Y. X. Wang, Small, 2015, 11, 4806–4822 CrossRef CAS PubMed.
- Y. Sun and H. C. Zhou, Adv. Mater., 2015, 16, 054202 Search PubMed.
- B. Seoane, S. Castellanos, A. Dikhtiarenko, F. Kapteijn and J. Gascon, Coord. Chem. Rev., 2016, 307, 147–187 CrossRef CAS.
- K. E. Cordova and O. M. Yaghi, Mater. Chem. Front., 2017, 1, 1304–1309 RSC.
- C.-C. Wang, S.-Y. Ke, K.-T. Chen, Y.-F. Hsieh, T.-H. Wang, G.-H. Lee and Y.-C. Chuang, in Structural Design and Properties of Coordination Polymers, ed. G. E. Kostakis, MDPI, Basel, 2018, pp. 1–12 Search PubMed.
- G. I. Dzhardimalieva and I. E. Uflyand, RSC Adv., 2017, 7, 42242–42288 RSC.
- V. V. Butova, M. A. Soldatov, A. A. Guda, K. A. Lomachenko and C. Lamberti, Russ. Chem. Rev., 2016, 85, 280–307 CrossRef CAS.
- Y. Yan, J. Zhang, L. Ren and C. Tang, Chem. Soc. Rev., 2016, 45, 5232–5263 RSC.
- L. Thomi, P. Schaefer, K. Landfester and F. R. Wurm, Macromolecules, 2015, 45, 105–109 Search PubMed.
- A. Zhang, A. Li, W. Zhao and J. Liu, Chem.–Eur. J., 2018, 24, 4215–4227 CrossRef CAS PubMed.
- H. Gu, S. Mu, G. Qiu, X. Liu, L. Zhang, Y. Yuan and D. Astruc, Coord. Chem. Rev., 2018, 364, 51–85 CrossRef CAS.
- X. He, X. He, S. Li, K. Zhuo, W. Qin, S. Dong, J. Chen, L. Ren, G. Liu and H. Xia, Polym. Chem., 2017, 8, 3674–3678 RSC.
- Z. Lu, Y. Cai, Y. Wei, Q. Lin, J. Chen, X. He, S. Li, W. Wu and H. Xia, Polym. Chem., 2018, 9, 2092–2100 RSC.
- Z. Lu, Q. Lin, Y. Cai, S. Chen, J. Chen, W. Wu, X. He and H. Xia, ACS Macro Lett., 2018, 7, 1034–1038 CrossRef CAS.
- E. F. Panarin, Russ. Chem. Bull., 2017, 66, 1812–1820 CrossRef CAS.
- S. Kargozar, S. Ramakrishna and M. Mozafari, Current Opinion in Biomedical Engineering, 2019, 10, 181–190 CrossRef.
- P. Jain, G. Pandey, D. Kumar and S. Chandra, Adv. Sci., Eng. Med., 2019, 11, 144–154 CrossRef CAS.
- H. Xiao, L. Yan, E. M. Dempsey, W. Song, R. Qi, W. Li, Y. Huang, X. Jing, D. Zhou, J. Ding and X. Chen, Prog. Polym. Sci., 2018, 87, 70–106 CrossRef CAS.
- M. Giménez-Marqués, T. Hidalgo, C. Serre and P. Horcajada, Coord. Chem. Rev., 2016, 307, 342–360 CrossRef.
- M. X. Wu and Y. W. Yang, Adv. Mater., 2017, 29, 1606134 CrossRef PubMed.
- G. Parekh, Y. Shi, J. Zheng, X. Zhang and S. Leporatti, Ther. Delivery, 2018, 9, 451–468 CrossRef CAS PubMed.
- L. He, Y. Liu, J. Lau, W. Fan, Q. Li, C. Zhang, P. Huang and X. Chen, Nanomedicine, 2019, 14, 1343–1365 CrossRef CAS PubMed.
- Y. Zhang, Q. Wang, G. Chen and P. Shi, Inorg. Chem., 2019, 58, 6593–6596 CrossRef CAS PubMed.
- A. A. Simagina, M. V. Polynski, A. V. Vinogradov and E. A. Pidko, Russ. Chem. Rev., 2018, 87, 831–858 CrossRef CAS.
- Y. Liu, Y. Zhao and X. Chen, Theranostics, 2019, 9, 3122–3133 CrossRef CAS PubMed.
- Z. Zhang, W. Sang, L. Xie and Y. Dai, Coord. Chem. Rev., 2019, 399, 213022 CrossRef CAS.
- L. Côrte-Real, B. Karas, P. Gírio, A. Moreno, F. Avecilla, F. Marques, B. T. Buckley, K. R. Cooper, C. Doherty, P. Falson, M. H. Garcia and A. Valente, Eur. J. Med. Chem., 2019, 163, 853–863 CrossRef PubMed.
- L. Côrte-Real, M. P. Robalo, F. Marques, G. Nogueira, F. Avecilla, T. J. L. Silva, F. C. Santos, A. I. Tomaz, M. H. Garcia and A. Valente, J. Inorg. Biochem., 2015, 150, 148–159 CrossRef PubMed.
- C. Shi, H. Yu, D. Sun, L. Ma, Z. Tang, Q. Xiao and X. Chen, Acta Biomater., 2015, 18, 68–76 CrossRef CAS PubMed.
- H. Yu, Z. Tang, D. Zhang, W. Song, Y. Zhang, Y. Yang, Z. Ahmad and X. Chen, J. Controlled Release, 2015, 205, 89–97 CrossRef CAS PubMed.
- N. A. N. Hanafy, M. El-Kemary and S. Leporatti, Cancers, 2018, 10, 238 CrossRef PubMed.
- S. Biswas, P. Kumari, P. M. Lakhani and B. Ghosh, Eur. J. Pharm. Sci., 2016, 83, 184–202 CrossRef CAS PubMed.
- J. Ahn, Y. Miura, N. Yamada, T. Chida, X. Liu, A. Kim, R. Sato, R. Tsumura, Y. Koga, M. Yasunaga, N. Nishiyama, Y. Matsumura, H. Cabral and K. Kataoka, Biomaterials, 2015, 39, 23–30 CrossRef CAS PubMed.
- M. Lu, F. Chen, J. M. Noy, H. Lu and M. H. Stenzel, Macromol. Biosci., 2017, 17, 1600513 CrossRef PubMed.
- M. A. Miller, B. Askevold, H. Mikula, R. H. Kohler, D. Pirovich and R. Weissleder, Nat. Commun., 2017, 8, 15906 CrossRef CAS PubMed.
- S. C. Larnaudie, J. C. Brendel, I. Romero-Canelón, C. Sanchez-Cano, S. Catrouillet, J. Sanchis, J. P. C. Coverdale, J.-I. Song, A. Habtemariam, P. J. Sadler, K. A. Jolliffe and S. Perrier, Biomacromolecules, 2018, 19, 239–247 CrossRef CAS PubMed.
- W. Shen, J. Luan, L. Cao, J. Sun, L. Yu and J. Ding, Biomacromolecules, 2015, 16, 105–115 CrossRef CAS PubMed.
- Z. Zhang, Y. Wu, G. Kuang, S. Liu, D. Zhou, X. Chen, X. Jing and Y. Huang, J. Mater. Chem. B, 2017, 5, 2115–2125 RSC.
- Z. Zhang, Y. Li, J. Wan, P. Long, J. Guo, G. Chen and C. Wang, Polym. Chem., 2017, 8, 2410–2422 RSC.
- Y. Zhang, T. Sun and C. Jiang, Acta Pharm. Sin. B, 2018, 8, 34–50 CrossRef PubMed.
- M. T. Wlodarczyk, S. A. Dragulska, O. Camacho-Vanegas, P. R. Dottino, A. A. Jarzęcki, J. A. Martignetti and A. J. Mieszawska, ACS Biomater. Sci. Eng., 2018, 4, 463–467 CrossRef CAS.
- N. Gupta, J. Kancharla, S. Kaushik, A. Ansari, S. Hossain, R. Goyal, M. Pandey, J. Sivaccumar, S. Hussain, A. Sarkar, A. Sengupta, S. K. Mandal, M. Roy and S. Sengupta, Chem. Sci., 2017, 8, 2387–2395 RSC.
- E. J. Cho, B. Sun, K. O. Doh, E. M. Wilson, S. Torregrosa-Allen, B. D. Elzey and Y. Yeo, Biomaterials, 2015, 37, 312–319 CrossRef CAS PubMed.
- S. Liang, Y. Duan, J. Zhang, Z. Xing, X. Chen, Y. Yang and Q. Li, J. Controlled Release, 2015, 213, e95–e96 CrossRef PubMed.
- X. Ling, Y. Shen, R. Sun, M. Zhang, C. Li, J. Mao, J. Xing, C. Sun and J. Tu, Polym. Chem., 2015, 6, 1541–1552 RSC.
- W. Zhang, Z. Zhang and C. H. Tung, Chem. Commun., 2017, 53, 779–782 RSC.
- M. S. Gil, T. Thambi, V. H. G. Phan, S. H. Kim and D. S. Lee, J. Mater. Chem. B, 2017, 5, 7140–7152 RSC.
- C. He, C. Poon, C. Chan, S. D. Yamada and W. Lin, J. Am. Chem. Soc., 2016, 138, 6010–6019 CrossRef CAS PubMed.
- C. Poon, X. Duan, C. Chan, W. Han and W. Lin, Mol. Pharmaceutics, 2016, 13, 3665–3675 CrossRef CAS.
- Y. Yang, L. Xu, W. Zhu, L. Feng, J. Liu, Q. Chen, Z. Dong, J. Zhao, Z. Liu and M. Chen, Biomaterials, 2018, 156, 121–133 CrossRef CAS PubMed.
- A. B. Lago, A. Pino-Cuevas, R. Carballo and E. M. Vázquez-López, Dalton Trans., 2016, 45, 1614–1621 RSC.
- M. Rezaei, A. Abbasi, R. Dinarvand, M. Jeddi-Tehrani and J. Janczak, ACS Appl. Mater. Interfaces, 2018, 10, 17594–17604 CrossRef CAS PubMed.
- J.-Y. Zeng, X.-S. Wang, W.-F. Song, H. Cheng and X.-Z. Zhang, Adv. Ther., 2019, 2, 1800100 CrossRef.
- T. Simon-Yarza, A. Mielcarek, P. Couvreur and C. Serre, Adv. Mater., 2018, 30, 1707365 CrossRef PubMed.
- S. Z. Wang, C. M. McGuirk, A. d'Aquino, J. A. Mason and C. A. Mirkin, Adv. Mater., 2018, 30, 1800202 CrossRef PubMed.
- W. Chen and C. S. Wu, Dalton Trans., 2018, 47, 2114–2133 RSC.
- S. Wuttke, M. Lismont, A. Escudero, B. Rungtaweevoranit and W. J. Parak, Biomaterials, 2017, 123, 172–183 CrossRef CAS PubMed.
- R. Freund, U. Lächelt, T. Gruber, B. Rühle and S. Wuttke, ACS Nano, 2018, 12, 2094–2105 CrossRef CAS PubMed.
- S. K. Alsaiari, S. Patil, M. Alyami, K. O. Alamoudi, F. A. Aleisa, J. S. Merzaban, M. Li and N. M. Khashab, J. Am. Chem. Soc., 2017, 140, 143–149 CrossRef PubMed.
- R. Röder, T. Preiß, P. Hirschle, B. Steinborn, A. Zimpel, M. Höhn, J. O. Rädler, T. Bein, E. Wagner, S. Wuttke and U. Lächelt, J. Am. Chem. Soc., 2017, 139, 2359–2368 CrossRef PubMed.
- T. Simon-Yarza, M. Giménez-Marqués, R. Mrimi, A. Mielcarek, R. Gref, P. Horcajada, C. Serre and P. Couvreur, Angew. Chem., Int. Ed., 2017, 56, 15565–15569 CrossRef CAS PubMed.
- T. Preiß, A. Zimpel, S. Wuttke and J. Rädler, Materials, 2017, 10, 216 CrossRef PubMed.
- L. Wang, M. Zheng and Z. Xie, J. Mater. Chem. B, 2018, 6, 707–717 RSC.
- S. Wuttke, S. Braig, T. Preiß, A. Zimpel, J. Sicklinger, C. Bellomo, J. O. Rädler, A. M. Vollmar and T. Bein, Chem. Commun., 2015, 51, 15752–15755 RSC.
- Z. Y. Dong, Y. Z. S. Sun, J. Chu, X. Z. Zhang and H. X. Deng, J. Am. Chem. Soc., 2017, 139, 14209–14216 CrossRef CAS PubMed.
- B. Illes, P. Hirschle, S. Baenert, V. Cauda, S. Wuttke and H. Engelke, Chem. Mater., 2017, 29, 8042–8046 CrossRef CAS.
- B. Illes, S. Wuttke and H. Engelke, Nanomaterials, 2017, 7, 351 CrossRef PubMed.
- R. Li, S. Liu, Q. Tang, T. Wang, Y. Xie and R. Zhai, Z. Anorg. Allg. Chem., 2018, 644, 317–321 CrossRef CAS.
- H. Zheng, Y. Zhang, L. Liu, W. Wan, P. Guo, A. M. Nyström and X. Zou, J. Am. Chem. Soc., 2016, 138, 962–968 CrossRef CAS PubMed.
- M. Filippousi, S. Turner, K. Leus, P. I. Siafaka, E. D. Tseligka, M. Vandichel, S. G. Nanaki, I. S. Vizirianakis, D. N. Bikiaris, P. V. D. Voort and G. V. Tendeloo, Int. J. Pharm., 2016, 509, 208–218 CrossRef CAS PubMed.
- E. Bellido, T. Hidalgo, M. V. Lozano, M. Guillevic, R. Simón-Vázquez, M. J. Santander-Ortega, Á. González-Fernández, C. Serre, M. J. Alonso and P. Horcajada, Adv. Healthcare Mater., 2015, 4, 1246–1257 CrossRef CAS PubMed.
- A. R. Chowdhuri, D. Bhattacharya and S. K. Sahu, Dalton Trans., 2016, 45, 2963–2973 RSC.
- C. Y. Zou, Z. M. Wang, X. M. Ren, R. R. Li, S. P. Zhang and B. Y. Sheng, Inorg. Chem. Commun., 2018, 91, 91–94 CrossRef CAS.
- Y. A. Li, X. D. Zhao, H. P. Yin, G. J. Chen, S. Yang and Y. B. Dong, Chem. Commun., 2016, 52, 14113–14116 RSC.
- H. Zhang, W. Jiang, R. Liu, J. Zhang, D. Zhang, Z. Li and Y. Luan, ACS Appl. Mater. Interfaces, 2017, 9, 19687–19697 CrossRef CAS PubMed.
- X. Gao, X. Hai, H. Baigude, W. Guan and Z. Liu, Sci. Rep., 2016, 6, 37705 CrossRef CAS PubMed.
- A. S. Abd-El-Aziz, A. A. Abdelghani and A. K. Mishra, J. Inorg. Organomet. Polym. Mater. DOI:10.1007/s10904-019-01293-y.
- A.-M. Caminade, A. Ouali, R. Laurent, C.-O. Turrin and J.-P. Majoral, Coord. Chem. Rev., 2016, 308, 478–497 CrossRef CAS.
- A. Narmani, M. Kamali, B. Amini, A. Salimi and Y. Panahi, Process Biochem., 2018, 69, 178–187 CrossRef CAS.
- D. T. D. Nguyen, L. G. Bach, T. H. Nguyen, M. H. Ho, M. N. Ho, D. H. Nguyen, C. K. Nguyen and T. T. H. Thi, J. Polym. Res., 2019, 26, 116 CrossRef.
- M. Maroto-Diaz, N. Sanz del Olmo, L. Muñoz-Moreno, A. M. Bajo, M. J. Carmena, R. Gómez, S. García-Gallego and F. J. de la Mata, Eur. Polym. J., 2019, 113, 229 CrossRef CAS.
- S. Michlewska, M. Ionov, D. Shcharbin, M. Maroto-Díaz, R. G. Ramirez, F. J. de la Mata and M. Bryszewska, Eur. Polym. J., 2017, 87, 39–47 CrossRef CAS.
- M. Maroto-Diaz, B. T. Elie, P. Gómez-Sal, J. Pérez-Serrano, R. Gómez, M. Contel and F. J. de la Mata, Dalton Trans., 2016, 45, 7049–7066 RSC.
- M. Gouveia, J. Figueira, M. G. Jardim, R. Castro, H. Tomás, K. Rissanen and J. Rodrigues, Molecules, 2018, 23, 1471 CrossRef PubMed.
- P. Govender, T. Riedel, P. J. Dyson and G. S. Smith, Dalton Trans., 2016, 45, 9529–9539 RSC.
- M. Quadir, S. Fehse, G. Multhaup and R. Haag, Molecules, 2018, 23, 1281 CrossRef PubMed.
- R. Albrecht, S. Fehse, K. Pant, S. Nowag, H. Stephan, R. Haag and C. C. Tzschucke, Macromol. Biosci., 2016, 16, 412–419 CrossRef CAS PubMed.
- S. Rojas, T. Devic and P. Horcajada, J. Mater. Chem. B, 2017, 5, 2560–2573 RSC.
- D. Y. Ma, Z. Li, J. X. Xiao, R. Deng, P. F. Lin, R. Q. Chen, Y. Q. Liang, H. F. Guo, B. Liu and J. Q. Liu, Inorg. Chem., 2015, 54, 6719–6726 CrossRef CAS PubMed.
- H. An, M. Li, J. Gao, Z. Zhang, S. Ma and Y. Chen, Coord. Chem. Rev., 2019, 384, 90–106 CrossRef CAS.
- S. El Hankari, M. Bousmina and A. El Kadib, Prog. Mater. Sci., 2019, 106, 100579 CrossRef.
- W. Zhou, L. Wang, F. Li, W. Zhang, W. Huang, F. Huo and H. Xu, Adv. Funct. Mater., 2017, 27, 1605465 CrossRef.
- F. Cao, E. Ju, C. Liu, W. Li, Y. Zhang, K. Dong, Z. Liu, J. Ren and X. Qu, Nanoscale, 2017, 9, 4128–4134 RSC.
- S. Mignani, J. Rodrigues, H. Tomas, A.-M. Caminade, R. Laurent, X. Shi and J.-P. Majoral, Sci. China Mater., 2018, 61, 1367–1386 CrossRef CAS.
- L. Chen, S. Mignani, A.-M. Caminade and J.-P. Majoral, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 11, e1577 Search PubMed.
- G. I. Dzhardimalieva and I. E. Uflyand, Dalton Trans., 2017, 46, 10139–10176 RSC.
- M. Ghaffari, G. Dehghan, F. Abedi-Gaballu, S. Kashanian, B. Baradaran, J. E. N. Dolatabadi and D. Losic, Eur. J. Pharm. Sci., 2018, 122, 311 CrossRef CAS PubMed.
- N. S. Sommerfeld, M. Hejl, M. H. M. Klose, E. Schreiber-Brynzak, A. Bileck, S. M. Meier, C. Gerner, M. A. Jakupec, M. Galanski and B. K. Keppler, Eur. J. Inorg. Chem., 2017, 2017, 1713–1720 CrossRef CAS.
- K. Dave and V. V. K. Venuganti, Ther. Delivery, 2017, 8, 1077–1096 CrossRef CAS PubMed.
- H. L. Che, M. Huo, L. Peng, Q. Q. Ye, J. Guo, K. Wang, Y. Wei and J. Y. Yuan, Polym. Chem., 2015, 6, 2319–2326 RSC.
- W. Cai, J. Wang, C. Chu, W. Chen, C. Wu and G. Liu, Adv. Sci., 2019, 6, 1801526 CrossRef PubMed.
- K. Fujita, Y. Tanaka, S. Abe and T. Ueno, Angew. Chem., Int. Ed., 2016, 55, 1056–1060 CrossRef CAS PubMed.
- D. Nguyen, T. Nguyen, S. A. Rice and C. Boyer, Biomacromolecules, 2015, 16, 2776–2786 CrossRef CAS PubMed.
- X. Li, J. Mu, F. Liu, E. W. P. Tan, B. Khezri, R. D. Webster, E. K. L. Yeow and B. G. Xing, Bioconjugate Chem., 2015, 26, 955–961 CrossRef CAS PubMed.
- F. Li, T. Li, W. Cao, L. Wang and H. Xu, Biomaterials, 2017, 133, 208–218 CrossRef CAS PubMed.
- S. Ding and U. Bierbach, Future Med. Chem., 2015, 7, 911–927 CrossRef CAS PubMed.
- H. Song, X. Kang, J. Sun, X. Jing, Z. Wang, L. Yan, R. Qi and M. Zheng, Chem. Commun., 2016, 52, 2281–2283 RSC.
- H. Song, W. Li, R. Qi, L. Yan, X. Jing, M. Zheng and H. Xiao, Chem. Commun., 2015, 51, 11493–11495 RSC.
- D. Zhou, J. Guo, G. B. Kim, J. Li, X. Chen, J. Yang and Y. Huang, Adv. Healthcare Mater., 2016, 5, 2493–2499 CrossRef CAS PubMed.
- Y. R. Zheng, K. Suntharalingam, T. C. Johnstone and S. J. Lippard, Chem. Sci., 2015, 6, 1189–1193 RSC.
- M. Ma, T. Luan, M. Yang, B. Liu, Y. Wang, W. An, B. Wang, R. Tang and A. Hao, Soft Matter, 2017, 13, 1534–1538 RSC.
- A. Khan, L. Wang, H. Yu, M. Haroon, R. S. Ullah, A. Nazir, T. Elshaarani, M. Usman, S. Fahad and F. Haq, Appl. Organomet. Chem., 2018, 32, e4575 CrossRef.
- E. Wajs, T. T. Nielsen, K. L. Larsen and A. Fragoso, Nano Res., 2016, 9, 2070–2078 CrossRef CAS.
- L. Peng, H. Zhang, A. Feng, M. Huo, Z. Wang, J. Hu, W. Gao and J. Yuan, Polym. Chem., 2015, 6, 3652–3659 RSC.
- S. Szunerits, F. Teodorescu and R. Boukherroub, Eur. Polym. J., 2016, 83, 467–477 CrossRef CAS.
- S. Mu, Q. Ling, X. Liu, J. Ruiz, D. Astruc and H. Gu, J. Inorg. Biochem., 2019, 193, 31–41 CrossRef CAS PubMed.
- D. Scheid, M. von der Lühe and M. Gallei, Macromol. Rapid Commun., 2016, 37, 1573 CrossRef CAS PubMed.
- L. Liu, L. Rui, Y. Gao and W. Zhang, Polym. Chem., 2015, 6, 1817–1829 RSC.
- F. Xu, H. Li, Y.-L. Luo and W. Tang, ACS Appl. Mater. Interfaces, 2017, 9, 5181–5192 CrossRef CAS PubMed.
- J. Morsbach, J. Elbert, C. Rüttiger, S. Winzen, H. Frey and M. Gallei, Macromolecules, 2016, 49, 3406–3414 CrossRef CAS.
- P. Shi, Y. Qu, C. Liu, H. Khan, P. Sun and W. Zhang, ACS Macro Lett., 2016, 5, 88–93 CrossRef CAS.
- T. Noyhouzer, C. L'Homme, I. Beaulieu, S. Mazurkiewicz, S. Kuss, H.-B. Kraatz, S. Canesi and J. Mauzeroll, Langmuir, 2016, 32, 4169–4178 CrossRef CAS PubMed.
- S. J. T. Rezaei, V. Amani, M. R. Nabid, N. Safari and H. Niknejad, Polym. Chem., 2015, 6, 2844–2853 RSC.
- Y. Cong, H. Xiao, H. Xiong, Z. Wang, J. Ding, C. Li, X. Chen, X.-J. Liang, D. Zhou and Y. Huang, Adv. Mater., 2018, 30, 1706220 CrossRef.
- H. J. Li, J. Z. Du, J. Liu, X. J. Du, S. Shen, Y. H. Zhu, X. Y. Wang, X. D. Ye, S. M. Nie and J. Wang, ACS Nano, 2016, 10, 6753–6761 CrossRef CAS PubMed.
- S. Shen, H. J. Li, K. G. Chen, Y. C. Wang, X. Z. Yang, Z. X. Lian, J. Z. Du and J. Wang, Nano Lett., 2017, 17, 3822–3829 CrossRef CAS PubMed.
- G.-M. Bao, L. Wang, H.-Q. Yuan, X.-Y. Wang, T.-X. Mei and M.-R. Qu, RSC Adv., 2016, 6, 109453–109459 RSC.
- X. Chen, Z. Shi, R. Tong, S. Ding, X. Wang, J. Wu, Q. Lei and W. Fang, ACS Biomater. Sci. Eng., 2018, 4, 4183–4192 CrossRef CAS.
- L. Tang, J. F. Shi, X. L. Wang, S. H. Zhang, H. Wu, H. F. Sun and Z. Y. Jiang, Nanotechnology, 2017, 28, 275601 CrossRef PubMed.
- M. Ni, N. Zhang, W. Xia, X. Wu, C. Yao, X. Liu, X.-Y. Hu, C. Lin and L. Wang, J. Am. Chem. Soc., 2016, 138, 6643–6649 CrossRef CAS PubMed.
- J. H. Kim, H. Eguchi, M. Umemura, I. Sato, S. Yamada, Y. Hoshino, T. Masuda, I. Aoki, K. Sakurai, M. Yamamoto and Y. Ishikawa, NPG Asia Mater., 2017, 9, e367 CrossRef CAS.
- Y. Y. Huang, W. Huang, L. Chan, B. W. Zhou and T. F. Chen, Biomaterials, 2016, 103, 183–196 CrossRef CAS PubMed.
- W.-H. Chen, S. Y. Sung, M. Fadeev, A. Cecconello, R. Nechushtai and I. Willner, Nanoscale, 2018, 10, 4650–4657 RSC.
- X. Zhu and C.-Q. Wang, J. Colloid Interface Sci., 2016, 480, 39–48 CrossRef CAS PubMed.
- Y. Kang, X. Ju, L. S. Ding, S. Zhang and B. J. Li, ACS Appl. Mater. Interfaces, 2017, 9, 4475–4484 CrossRef CAS PubMed.
- C. Zuo, X. Y. Dai, S. J. Zhao, X. N. Liu, S. L. Ding, L. W. Ma, M. Z. Liu and H. Wei, ACS Macro Lett., 2016, 5, 873–878 CrossRef CAS.
- X.-M. Zhang, K. Guo, L.-H. Li, S. Zhang and B.-J. Li, J. Mater. Chem. B, 2015, 3, 6026–6031 RSC.
- G. Y. Li, Z. R. Dong, Y. T. Zhu, W. J. Tong and C. Y. Gao, J. Colloid Interface Sci., 2016, 475, 196–202 CrossRef CAS PubMed.
- Y. Kang, Y. Ma, S. Zhang, L. S. Ding and B. J. Li, ACS Macro Lett., 2015, 4, 543–547 CrossRef CAS.
- S. Wang, C. Yao, M. Ni, Z. Xu, M. Cheng, X.-Y. Hu, Y.-Z. Shen, C. Lin, L. Wang and D. Jia, Polym. Chem., 2017, 8, 682–688 RSC.
- S. Behzadi, M. Gallei, J. Elbert, M. Appold, G. Glasser, K. Landfester and D. Crespy, Polym. Chem., 2016, 7, 3434–3443 RSC.
- Y. Chang, C. Hou, J. Ren, X. Xin, Y. Pei, Y. Lu, S. Cao and Z. Pei, Chem. Commun., 2016, 52, 9578–9581 RSC.
- O. Mergel, S. Schneider, R. Tiwari, P. T. Kühn, D. Keskin, M. C. A. Stuart, S. Schöttner, M. de Kanter, M. Noyong, T. Caumanns, J. Mayer, C. Janzen, U. Simon, M. Gallei, D. Wöll, P. van Rijn and F. A. Plamper, Chem. Sci., 2019, 10, 1844–1856 RSC.
- B. V. K. J. Schmidt, D. Kugele, J. von Irmer, J. Steinkoenig, H. Mutlu, C. Rüttiger, C. J. Hawker, M. Gallei and C. Barner-Kowollik, Macromolecules, 2017, 50, 2375–2386 CrossRef CAS.
- S. Nagata, K. Kokado and K. Sada, Chem. Commun., 2015, 51, 8614–8617 RSC.
- M. H. Teplensky, M. Fantham, P. Li, T. C. Wang, J. P. Mehta, L. J. Young, P. Z. Moghadam, J. T. Hupp, O. K. Farha, C. F. Kaminski and D. Fairen-Jimenez, J. Am. Chem. Soc., 2017, 139, 7522–7532 CrossRef CAS PubMed.
- W. X. Lin, Q. Hu, J. C. Yu, K. Jiang, Y. Y. Yang, S. C. Xiang, Y. J. Cui, Y. Yang, Z. Y. Wang and G. D. Qian, ChemPlusChem, 2016, 81, 804–810 CrossRef CAS.
- K. Jiang, L. Zhang, Q. Hu, D. Zhao, T. F. Xia, W. X. Lin, Y. Y. Yang, Y. J. Cui, Y. Yang and G. D. Qian, J. Mater. Chem. B, 2016, 4, 6398–6401 RSC.
- E. Lashkari, H. Wang, L. S. Liu, J. Li and K. Yam, Food Chem., 2017, 221, 926–935 CrossRef CAS PubMed.
- L. L. Tan, H. Li, Y. Zhou, Y. Zhang, X. Feng, B. Wang and Y. W. Yang, Small, 2015, 11, 3807–3813 CrossRef CAS PubMed.
- X. S. Meng, B. Gui, D. Q. Yuan, M. Zeller and C. Wang, Sci. Adv., 2016, 2, e1600480 CrossRef PubMed.
- C. Adhikari and A. Chakraborty, ChemPhysChem, 2016, 7, 1070–1077 CrossRef PubMed.
- X. G. Wang, Z. Y. Dong, H. Cheng, S. S. Wan, W. H. Chen, M. Z. Zou, J. W. Huo, H. X. Deng and X. Z. Zhang, Nanoscale, 2015, 7, 16061–16070 RSC.
- S. Y. Tan, C. Y. Ang, A. Mahmood, Q. Y. Qu, P. Z. Li, R. Q. Zou and Y. L. Zhao, ChemNanoMat, 2016, 2, 504–508 CrossRef CAS.
- H.-J. Cai, T.-T. Shen, J. Zhang, C.-F. Shan, J.-G. Jia, X. Li, W.-S. Liu and Y. Tang, J. Mater. Chem. B, 2017, 5, 2390–2394 RSC.
- W.-H. Chen, W.-C. Liao, Y. S. Sohn, M. Fadeev, A. Cecconello, R. Nechushtai and I. Willner, Adv. Funct. Mater., 2018, 28, 1705137 CrossRef.
- S. Wadhwa and R. J. Mumper, Crit. Rev. Ther. Drug Carrier Syst., 2015, 32, 215–245 CrossRef PubMed.
- M. Li, Z. Tang, S. Lv, W. Song, H. Hong, X. Jing, Y. Zhang and X. Chen, Biomaterials, 2014, 35, 3851–3864 CrossRef CAS PubMed.
- A. Gandioso, E. Shaili, A. Massaguer, G. Artigas, A. González-Cantó, J. A. Woods, P. J. Sadler and V. Marchán, Chem. Commun., 2015, 51, 9169–9172 RSC.
- S. Li, C. Li, S. Jin, J. Liu, X. Xue, A. S. Eltahan, J. Sun, J. Tan, J. Dong and X. J. Liang, Biomaterials, 2017, 144, 119–129 CrossRef CAS PubMed.
- C. Poon, C. He, D. Liu, K. Lu and W. Lin, J. Controlled Release, 2015, 201, 90–99 CrossRef CAS PubMed.
- M. Callari, S. Wong, H. Lu, J. Aldrich-Wright, P. de Souza and M. H. Stenzel, Polym. Chem., 2017, 8, 6289–6299 RSC.
- W. Song, Z. Tang, D. Zhang, Y. Zhang, H. Yu, M. Li, S. Lv, H. Sun, M. Deng and X. Chen, Biomaterials, 2014, 35, 3005–3014 CrossRef CAS.
- D. Zhou, S. He, Y. Cong, Z. Xie, X. Chen, X. Jing and Y. Huang, J. Mater. Chem. B, 2015, 3, 4913–4921 RSC.
- S. He, Y. Cong, D. Zhou, J. Li, Z. Xie, X. Chen, X. Jing and Y. Huang, J. Mater. Chem. B, 2015, 3, 8203–8211 RSC.
- D. Zhou, Y. Cong, Y. Qi, S. He, H. Xiong, Y. Wu, Z. Xie, X. Chen, X. Jing and Y. Huang, Biomater. Sci., 2015, 3, 182–191 RSC.
- R. Qi, H. Xiao, S. Wu, Y. Li, Y. Zhang and X. Jing, J. Mater. Chem. B, 2015, 3, 176–179 RSC.
- T. Wang, G. Sun, M. Wang, B. Zhou and J. Fu, ACS Appl. Mater. Interfaces, 2015, 7, 21295–21304 CrossRef CAS.
- E. Baa, G. M. Watkins, R. W. Krause and D. N. Tantoh, Chin. J. Chem., 2019, 37, 378–404 CrossRef CAS.
- S. Rojas, A. Arenas-Vivo and P. Horcajada, Coord. Chem. Rev., 2019, 388, 202–226 CrossRef CAS.
- Y. Ma, Q. Mou, D. Wang, X. Zhu and D. Yan, Theranostics, 2016, 6, 930–947 CrossRef CAS PubMed.
- S. Chen, Q. Lei, W.-X. Qiu, L.-H. Liu, D.-W. Zheng, J.-X. Fan, L. Rong, Y.-X. Sun and X.-Z. Zhang, Biomaterials, 2017, 117, 92–104 CrossRef CAS PubMed.
- Y. K. Li, Y. C. Li, X. Zhang, X. H. Xu, Z. J. Zhang, C. Hu, Y. Y. He and Z. W. Gu, Theranostics, 2016, 6, 1293–1305 CrossRef CAS PubMed.
- G. Yu, M. Zhang, M. L. Saha, Z. Mao, J. Chen, Y. Yao, Z. Zhou, Y. Liu, C. Gao, F. Huang, X. Chen and P. J. Stang, J. Am. Chem. Soc., 2017, 139, 15940–15949 CrossRef CAS PubMed.
- H.-X. Zhao, Q. Zou, S.-K. Sun, C. Yu, X. Zhang, R.-J. Li and Y.-Y. Fu, Chem. Sci., 2016, 7, 5294–5301 RSC.
- R. Bian, T. Wang, L. Zhang, L. Li and C. Wang, Biomater. Sci., 2015, 3, 1270–1278 RSC.
- C. J. Zhang, Q. L. Hu, G. X. Feng, R. Y. Zhang, Y. Y. Yuan, X. M. Lu and B. Liu, Chem. Sci., 2015, 6, 4580–4586 RSC.
- W. Liu, Y. Wang, Y. Li, S. Cai, X. Yin, X. He and Y. Zhang, Small, 2017, 13, 1603459 CrossRef PubMed.
- J. Liu, L. Zhang, J. Lei, H. Shen and H. Ju, ACS Appl. Mater. Interfaces, 2017, 9, 2150–2158 CrossRef CAS PubMed.
- S. Y. Li, H. Cheng, W. X. Qiu, L. Zhang, S. S. Wan, J. Y. Zeng and X. Z. Zhang, Biomaterials, 2017, 142, 149–161 CrossRef CAS PubMed.
- D. D. Wang, J. J. Zhou, R. H. Chen, R. H. Shi, G. Z. Zhao, G. L. Xia, R. Li, Z. B. Liu, J. Tian, H. J. Wang, Z. Guo, H. B. Wang and Q. W. Chen, Biomaterials, 2016, 100, 27–40 CrossRef CAS PubMed.
- J. C. Yang, Y. Chen, Y. H. Li and X. B. Yin, ACS Appl. Mater. Interfaces, 2017, 9, 22278–22288 CrossRef CAS PubMed.
- Y. D. Zhu, S. P. Chen, H. Zhao, Y. Yang, X. Q. Chen, J. Sun, H. S. Fan and X. D. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 34209–34217 CrossRef CAS PubMed.
- X. Chen, M. Zhang, S. Li, L. Li, L. Zhang, T. Wang, M. Yu, Z. Mou and C. Wang, J. Mater. Chem. B, 2017, 5, 1772–1778 RSC.
- J. Y. Zeng, M. K. Zhang, M. Y. Peng, D. Gong and X. Z. Zhang, Adv. Funct. Mater., 2018, 28, 1705451 CrossRef.
- K. Lu, C. He, N. Guo, C. Chan, K. Ni, R. R. Weichselbaum and W. Lin, J. Am. Chem. Soc., 2016, 138, 12502–12510 CrossRef CAS.
- J. Y. Zeng, M. Z. Zou, M. Zhang, X. S. Wang, X. Zeng, H. Cong and X. Z. Zhang, ACS Nano, 2018, 12, 4630–4640 CrossRef CAS PubMed.
- S.-Y. Li, H. Cheng, B.-R. Xie, W.-X. Qiu, J.-Y. Zeng, C.-X. Li, S.-S. Wan, L. Zhang, W.-L. Liu and X.-Z. Zhang, ACS Nano, 2017, 11, 7006–7018 CrossRef CAS PubMed.
- S. Aghevlian, Y. Lu, M. A. Winnik, D. W. Hedley and R. M. Reilly, Mol. Pharmaceutics, 2018, 15, 1150–1159 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |