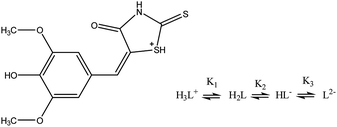
Open Access Article
Open Access ArticleDevelopment of a selective and sensitive colour reagent for gold and silver ions and its application to desktop scanner analysis†
Ashraf A. Mohamed *,
Eslam H. A. Mahmoud and
Mostafa M. H. Khalil
*,
Eslam H. A. Mahmoud and
Mostafa M. H. Khalil
Department of Chemistry, Faculty of Science, Ain Shams University, Abbassia, Cairo-11566, Egypt. E-mail: aamohamd@sci.asu.edu.eg; Fax: +20 224831836; Tel: +20 1001578849
First published on 8th November 2019
Abstract
Desktop scanners can be favorable alternatives to sophisticated spectrophotometers for the assessment of analytes in complex real samples. Distinctively, our method has been thoroughly investigated, optimized, validated and successfully applied to the assessment of silver and gold in complex real samples, applying syringal rhodanine (SR) as a novel specifically tailored chromogenic reagent and using a desktop scanner as a versatile sensor. Maximum colour absorbance was obtained in the presence of cetylpyridinium chloride (CPC) and cetyltrimethylammonium chloride (CTAC) for silver and gold chelates, respectively. For each metal ion, two ternary complexes were formed depending on the SR concentration with stoichiometries of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Ag–SR–CPC) and 1
3 (Ag–SR–CPC) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Au–SR–CTAC), respectively. The methods adhered to Beer's law for 0.15–2.5 and 0.15–2.25 μg mL−1 with detection limits of 0.0089 and 0.0163 μg mL−1 for silver and gold, respectively. The molar absorptivities were 3.63 × 104 and 6.15 × 104 L mol−1 cm−1 at 550 nm and 554 nm, with Sandell's sensitivity indexes of 0.0029 and 0.0032 μg cm−2, respectively. The method was successfully applied to the assessment of silver and gold in a wide range of complex environmental samples.
4 (Au–SR–CTAC), respectively. The methods adhered to Beer's law for 0.15–2.5 and 0.15–2.25 μg mL−1 with detection limits of 0.0089 and 0.0163 μg mL−1 for silver and gold, respectively. The molar absorptivities were 3.63 × 104 and 6.15 × 104 L mol−1 cm−1 at 550 nm and 554 nm, with Sandell's sensitivity indexes of 0.0029 and 0.0032 μg cm−2, respectively. The method was successfully applied to the assessment of silver and gold in a wide range of complex environmental samples.
1. Introduction
Spectrophotometry is a popular and commonly used technique for quantitative chemical analysis for gold and silver. However, UV/vis spectrophotometers are frequently unavailable, especially in locally deprived communities and/or under resource-limited settings.Sophisticated analytical techniques including inductively coupled plasma-mass spectrometry (ICP-MS),1–4 inductively coupled plasma optical emission spectrometry (ICP-OES),5,6 neutron activation analysis (NAA),3,7,8 X-ray fluorescence (XRF),9 and atomic absorption spectrometry (AAS)10–14 enable sensitive and selective determination of trace amounts of Ag(I) and Au(III) in a wide range of matrices. However, conventional spectrophotometry stands out as a relatively low cost, simple and easy-to-use technique. The spectrophotometric determination of silver is usually achieved using its reaction with dithizone,15 4-(2-quinolylazo)phenol (p-QAP),16 4,4′-bis(dimethylamino)thiobenzophenone,17 2-cyano-3-iminodithiobutyrate (CIDT),18 or 2′,3′-dihydroxypyridyl-4′-azobenzene-4-arsonate (DHP-4A);19 however, that of gold is achieved using dithizone,20 4-(2-pyridylazo)resorcinol (PAR),21 4-(2-thiazolylazo)resorcinol (TAR),22 methiomeprazine hydrochloride (MMH),23 4-(2-quinolylazo)phenol (p-QAP),24 or dithiodiantipyrylmethane.25 Nevertheless, the poor selectivity, sensitivity and/or precision restrains the use of most of these reagents. But, rhodanine derivatives surpassed many of these reagents and therefore, have found vast applications in the determination of silver26–29 and gold.26,29–32 However, some difficulties were noted with the use of many rhodanine derivatives, including the relatively poor solubility of reagents and their chelates and/or the poor stability of the developed colours. Thus, surfactants were used to solubilize and stabilize the pseudo-solutions containing the ligands and/or their metal-chelates; thereby enhancing both the selectivity and sensitivity of these methods.33,34
On the other hand, methods of digital image-based analysis (DIBA) can be simple, easy-to-use and low-cost alternatives.35,36 In DIBA, the analyte is allowed to react with a chromogenic reagent to give a coloured product whose digital images are captured and analysed to give the red, green and blue intensity values (IR, IG, and IB). The RGB intensities,37–44 and the RGB absorbances (AR, AG, AB)45,46 or thereof35,36 have been used as analytical signals in DIBA.
Herein, syringal-rhodanine (SR) was synthesized, characterized and applied as a novel and specifically tailored chromogenic reagent for the sensitive and highly selective assessment of silver(I) and gold(III) based on monitoring with a desktop scanner as a versatile low-cost sensor due to its relatively fixed light source intensity and the lack of outdoor light interference. Further, the reaction variables affecting the spectral characteristics of Ag–SR and Au–SR chelates were thoroughly investigated, optimized and incorporated into respective recommended procedures for the assessment of Ag(I) and Au(III) in antiseptic and burn cream, electroplating wastewaters, and rock samples.
2. Experimental
2.1. Apparatus and software
Spectrophotometric measurements were carried out in 10 mm matched glass cells in the range of 200–1100 nm using a Shimadzu (Kyoto, Japan) 1650 UV-vis spectrophotometer that is controlled by a UV-probe 2.5 software. Eppendorf 20–200 μL and 100–1000 μL vary-pipettes (Westbury, NY, USA) and a calibrated EDT pH-mV meter (Dover Kent, UK) were used.A flat bottom 96-micro-well plate was used for colour absorbance measurements using an Hp-Deskjet F4200 all in one printer-scanner-copier. A conventional HP X7H29EA#ABV Notebook running under Windows 10 Home 64 bit was used for data treatment and analysis. Photoshop CS6 was used for digital image processing and ImageJ2x software 2.1.4.7 was used for RGB channel intensities calculations. Then, for each image, the RGB colour absorbance (AR, AG, AB) was calculated.35,36
2.2. Materials and chemicals
All reagents were of ACS grade and were used as received from Merck (Darmstadt, Germany) or Sigma-Aldrich (St. Louis, MO, USA). Unless otherwise stated, deionized water and aqueous solutions were used throughout.Syringal-rhodanine (SR) was synthesized according to Julian and Sturgis47 by adding 3.0 g of freshly prepared, fused and powdered sodium acetate to 30 mL glacial acetic acid containing 0.03 mol rhodanine and 0.03 mol 4-hydroxy-3,5-dimethoxy benzaldehyde (syringaldehyde). The mixture was refluxed for 4 hours with occasional shaking. The solid crude SR was poured into a 1 L beaker containing iced water, triturated vigorously and then filtered. To the filtrate, Na2CO3 was added stepwise until no effervescence was observed. The resulting second crop of crude SR was collected and added to the first crop. The melting point of crude SR was 244–248°. Double recrystallization from glacial acetic acid yielded orange-yellow fine crystals of pure SR with mp 255–256° which is in excellent agreement with the previously reported data.48 The purity of the reagent was confirmed by thin-layer chromatography with benzene–petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) as eluent. A single yellow band was obtained for the pure product.
4 v/v) as eluent. A single yellow band was obtained for the pure product.
An ethanolic stock solution of 5.0 mmol L−1 SR was prepared by dissolving the appropriate amount of SR in absolute ethanol. This solution was stable for more than 2 months. A 1.0 mmol L−1 SR working solution was prepared daily by appropriate dilution of the stock solution with absolute ethanol.
A stock standard 0.01 mol L−1 Ag(I) solution was prepared by dissolving the suitable amount of dried silver nitrate in 0.02 mol L−1 nitric acid. More dilute working silver(I) solutions were prepared daily by appropriate dilution of its stock solution. A stock standard 1000 μg mL−1 Au(III) solution in hydrochloric acid, was purchased from Sigma-Aldrich (St. Louis, MO, USA). More dilute working gold(III) solutions were prepared by appropriate dilution of its stock solution.
A composite surfactant-buffer solution that is 4.0 mmol L−1 cetylpyridinium chloride (CPC) and 50.0 mmol L−1 borate buffer of pH 9.80 was prepared by dissolving the appropriate amounts of CPC and borax in water and adjusting the pH to 9.80 ± 0.05.
Cetyltrimethylammonium chloride (CTAC) was prepared as a 20.0 mmol L−1 solution by dissolving the appropriate amount in deionized water.
A borate buffer series of pH 7.8–10.8 was prepared by adjusting the pH of 50.0 mmol L−1 sodium tetraborate solution with hydrochloric acid or sodium hydroxide solution using a pre-calibrated pH meter.
2.3. Treatment of burn cream for silver determination
A silver-sulfadiazine burn cream was purchased from the local market. About 0.30 g of the cream was exactly weighed and dissolved in 10 mL of 0.10 mol L−1 nitric acid. The solution was filtered to remove any insoluble matter. The filtrate was quantitatively transferred to a 100 mL volumetric flask, the residue was washed with deionized water, the washing was added to the flask and the volume was diluted to the mark with deionized water. This solution was directly analysed following the recommended procedure.2.4. Treatment of wastewater samples for gold determination
Electroplating wastewater was collected from Asfour company, Cairo. The pH was adjusted to 2.0 ± 0.1 using dilute HNO3 and then filtered using a 0.45 μm membrane filer. A 100 mL of this solution was preconcentrated by evaporation to 1/10 its volume, cooled, diluted to the mark in a 10 mL volumetric flask and analyzed following the recommended procedure.2.5. Treatment of rock samples for silver and gold determination
Rock samples were ground in an automatic mortar grinder, sieved through a 2 mm stainless steel sieve to exclude gruff materials. A 1.00 g of the finely powdered sample was dissolved in a Teflon beaker in an HF/HNO3/H2SO4 mixture49 and analysed following the respective recommended procedure.2.6. Recommended procedure for silver and gold assessment
An aliquot of the sample solution or the working silver(I) standard solution containing not more than 25 μg Ag was transferred to a 10 mL volumetric flask. A 5.0 mL of the composite CPC-borate solution and 500 μL of the working SR solution were added. The reacting solution was diluted to the mark with water, mixed well, left at room temperature for 10 min for complete colour development, and the absorbance was measured at 550 nm against a similarly prepared reagent blank. However, for gold determination: an aliquot of the sample solution or the working gold(III) standard solution containing not more than 22.5 μg Au was transferred into a 10 mL volumetric flask. A 1000 μL the working CTAC solution and 500 μL of the working SR solution were added. The reacting solution was diluted to the mark with borate buffer, mixed well, left at room temperature for 10 min for complete colour development, and the absorbance was measured at 554 nm against a similarly prepared reagent blank.From each flask, a 300 μL aliquot was pipetted into the micro-well plate and an image was scanned using the desktop scanner. As previously reported,35,36 various scan resolutions of 300, 600 and 1200 dpi gave essentially the same response; therefore, a scan resolution of 300 dpi was adopted for simplicity. From each image, a representative homogenous circular area (60 pixels in diameter) was cropped using Photoshop CS6, and the RGB colour intensities (IR, IG, IB) were registered by ImageJ2x and then the colour absorbance for each channel (AR, AG, AB) was calculated from [ARGB = log(Io/I)]; where Io and I are the channel intensity of the blank and sample, respectively.35,36 The silver or gold concentration was determined from a similarly prepared calibration graph obtained with a series of standard solutions.
3. Results and discussion
3.1. Dissociation constants of SR
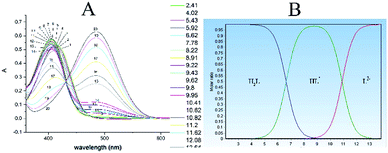
Protonated syringal-rhodanine (SR) has the general formula H3L+.Therefore, four species are involved in the SR acid dissociation process, according to the above equation. The respective dissociation constants were determined spectrophotometrically in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water. Where, K1, K2, K3 were assigned to the deprotonation of SH+, NH, and OH groups, respectively. The deprotonation constant K1, couldn't be determined due to the instability of the reagent in strongly acidic HCl media (pH < 3.0). However, K2 and K3 were determined by spectral measurements of 0.03 mmol L−1 SR solutions adjusted to various pH values between 3.0–13.0, Fig. 1A. The obtained spectral data were treated with Datan software,50 Fig. 1B. The molar ratios distribution of various dissociable species showed intersections corresponding to the dissociation constants K2 and K3 with values of 6.61 and 10.87, respectively. However, the dissociation constants can be also derived graphically or mathematically from the conventional A–pH relation at the respective wavelength maxima.
1 ethanol–water. Where, K1, K2, K3 were assigned to the deprotonation of SH+, NH, and OH groups, respectively. The deprotonation constant K1, couldn't be determined due to the instability of the reagent in strongly acidic HCl media (pH < 3.0). However, K2 and K3 were determined by spectral measurements of 0.03 mmol L−1 SR solutions adjusted to various pH values between 3.0–13.0, Fig. 1A. The obtained spectral data were treated with Datan software,50 Fig. 1B. The molar ratios distribution of various dissociable species showed intersections corresponding to the dissociation constants K2 and K3 with values of 6.61 and 10.87, respectively. However, the dissociation constants can be also derived graphically or mathematically from the conventional A–pH relation at the respective wavelength maxima.
3.2. Effect of pH
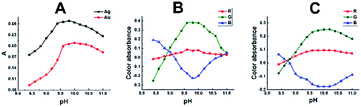
Preliminary experiments showed that the Ag–SR–CPC and Au–SR–CTAC complexes are best formed in alkaline media. Therefore, the effect of pH on the absorbance of the Ag- and Au-chelates were studied over the pH range 8.0–12.0. Maximum spectrophotometric absorbances and colour absorbances of the Ag- and Au-chelates were exhibited in the pH ranges of 9.6–10.2 and 9.6–10.4, for the Ag- and Au-chelates, respectively, Fig. 2A–C. Therefore, borate buffers of pH 9.8 and 10 were adopted in the respective recommended procedures. The green channel colour absorbances showed the same behaviour as that of the spectrophotometric absorbances. The corresponding digital images and their cropped images are given in Fig. S2A and B.† Over pH 10.4, appreciable fraction of OH− ions is present to compete with SR to form hydroxo-complexes with Ag(I) and Au(III) and therefore the absorbances decreased.3.3. Effect of syringal-rhodanine reagent
The effect of syringal-rhodanine reagent concentration was investigated, at the optimum pH, by measuring the spectrophotometric absorbance and digital image colour absorbance of solutions containing 1 or 2 μg mL−1 of silver or gold, following the recommended procedure and using various amounts of SR ranging between 0.01 and 0.10 mmol L−1 SR, respectively. Maximum absorbances were obtained in the presence of 0.05 mmol L−1 SR reagent, as shown in Fig. S3A–C.† The corresponding scanner digital images and their cropped areas are shown in Fig. S4A–D.† At high SR concentrations, association of ligand occurs resulting in exponential increase of the blank absorbance with the simultaneous decrease in the net sample absorbance.3.4. Effect of surfactant's type and concentration
The effects of various surfactants of the cationic, anionic and nonionic types on the spectral characteristics of the binary Ag–SR and Au–SR chelates were studied. Surfactants were used at concentrations above their critical micellization concentration (c.m.c.). Maximum colour absorbance of the chelates was attained with cationic surfactants, where CPC and CTAC were the optimum sensitizers for the colour reactions of silver and gold, respectively, as shown in Fig. 3A–F. The corresponding scanner images and their cropped areas are shown in Fig. S5A–D.†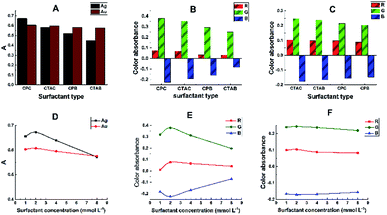 | ||
| Fig. 3 Effect of some cationic surfactants on the spectral characteristics of Ag–SR and Au–SR chelates. CTAB, cetyltrimethyl ammonium bromide; CPB, cetyl pyridinium bromide; except for the abscissa variable, other conditions and symbols are those of Fig. 2. (A) Spectrophotometric data. (B and C) Corresponding DIBA results for the Ag–SR–CPC and Au–SR–CTAC chelates, respectively. (D) Spectrophotometric data for the effects of surfactant's concentration. (E and F) Corresponding DIBA results for Ag–SR and Au–SR chelates, respectively. | ||
The binary Ag–SR and Au–SR chelates were solubilized in presence of ≥0.5 mmol L−1 of CPC or CTAC and using other conditions of the recommended procedure. Moreover, the maximum absorbances of the sensitized complexes were obtained at concentrations of 2.0 and 1.0 mmol L−1 CPC and CTAC, respectively, and these were therefore adopted in the recommended procedure.
Further, the sensitization of the colour reactions was exhibited at surfactant's concentrations laying well below its own critical micellar concentration. (The c.m.c. of CPC and CTAC have been reported as 0.90 and 0.98 mmol L−1, respectively51). Consequently, the surfactant interacts with the binary chelate to form true ternary complexes.
3.5. Effect of solvent's type and concentration
Various miscible solvents were tested to fully solubilize the reagent and its Ag–SR–CPC and Au–SR–CTAC chelates. Preliminary experiments were conducted at a fixed final solvent concentration of 10%. However, maximum enhancement of the absorbances was obtained in the presence of 5% ethyl alcohol; and therefore, was adopted in the recommended procedure. The results are shown in Fig. S6A–F.† The corresponding digital images and their cropped areas are shown in Fig. S7A–D.†3.6. Effect of the order of addition of reagents
The effect of possible orders of addition of all reactants was studied to figure out the optimum order that results in maximum colour intensity of the chelates. Fig. S8A–D† summarizes the obtained results obtained using various orders of additions. Data revealed that the optimum order was Ag(I) followed by a composite CPC–borate solution and then the SR reagent; while for gold the order should be Au(III), followed by CTAC and borate buffer and then the SR reagent.3.7. Stoichiometry of the complexes
The molar ratio and Job's continuous variation methods were utilized to determine the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio in the complexes at the adopted optimum conditions of the recommended procedure. Experiments were performed using 1 × 10−5 mol L−1 Ag(I) or Au(III). The results showed that Ag(I) ion forms 1
L ratio in the complexes at the adopted optimum conditions of the recommended procedure. Experiments were performed using 1 × 10−5 mol L−1 Ag(I) or Au(III). The results showed that Ag(I) ion forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with absorption maxima at 560 and 550 nm, respectively; while Au(III) forms 1
2 complexes with absorption maxima at 560 and 550 nm, respectively; while Au(III) forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 complexes with absorption maxima at 580 and 554 nm, respectively, depending on the ligand (SR) concentration Fig. 4A–H, the scanned and cropped images of various M
3 complexes with absorption maxima at 580 and 554 nm, respectively, depending on the ligand (SR) concentration Fig. 4A–H, the scanned and cropped images of various M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios are shown in Fig. S9.† From these figures, it's clear that the green channel perfectly represents the orange-red 1
L ratios are shown in Fig. S9.† From these figures, it's clear that the green channel perfectly represents the orange-red 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Ag
2 (Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SR) and 1
SR) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Au
3 (Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SR) complexes; while the red channel perfectly represents the purplish-blue 1
SR) complexes; while the red channel perfectly represents the purplish-blue 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Ag
1 (Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SR) and 1
SR) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Au
2 (Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SR) complexes, respectively.
SR) complexes, respectively.
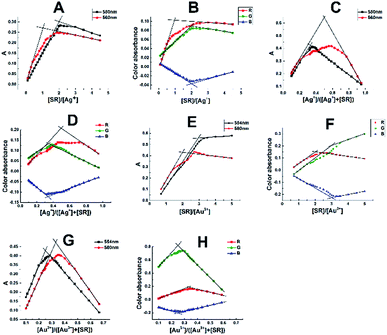 | ||
| Fig. 4 Molar ratio and continuous variation plots of the Ag–SR (A–D) and Au–SR (E–H) ternary complexes. | ||
Similarly, the surfactant ratios in the ternary complexes were determined in a similar way by molar ratio method, by increasing the CPC or CTAC concentration while maintaining a constant M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios of 1
L ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ag–SR, 1
1 Ag–SR, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ag–SR, 1
2 Ag–SR, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Au–SR, or 1
2 Au–SR, or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 Au–SR complexes. The study confirmed the formation of 1
3 Au–SR complexes. The study confirmed the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Ag–SR–CPC) and 1
3 (Ag–SR–CPC) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Au–SR–CTAC) complexes, respectively.
4 (Au–SR–CTAC) complexes, respectively.
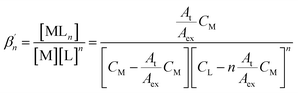
Further, the conditional stability constants  of the M–L complexes were determined from the molar ratio and the continuous variation plots using the equation52
of the M–L complexes were determined from the molar ratio and the continuous variation plots using the equation52
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Ag–SR) and 1
2 (Ag–SR) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Au–SR) complexes derived from the spectrophotometric and digital image-based measurements were given in Table S1,† showing the excellent agreement between the spectrophotometric and scanner-based measurements. Further, the data show that, the higher 1
3 (Au–SR) complexes derived from the spectrophotometric and digital image-based measurements were given in Table S1,† showing the excellent agreement between the spectrophotometric and scanner-based measurements. Further, the data show that, the higher 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Ag–SR) and 1
2 (Ag–SR) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Au–SR) complexes exhibited a four-orders-of-magnitude stability compared to the lower 1
3 (Au–SR) complexes exhibited a four-orders-of-magnitude stability compared to the lower 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Ag–SR) and 1
1 (Ag–SR) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Au–SR) complexes, respectively.
2 (Au–SR) complexes, respectively.
3.8. Calibration and precision
The suggested method adhered to Beer's law for 0.15–2.50 and 0.15–2.25 μg mL−1 of silver and gold, at 550 and 554 nm, respectively. The corresponding molar absorptivities, as determined by a least-squares fit for 17 results, were 3.6 × 104 and 6.1 × 104 L mol−1 cm−1, for the Ag- and Au-based chelates, respectively, Fig. 5A. The Sandell's sensitivities were 0.0029 and 0.0032 μg cm−2, respectively. A plot of the green colour absorbance versus silver or gold concentration exhibited excellent linearity, typical to spectrophotometric measurements, Fig. 5B, C and Table S2.† The detection limits were 0.0089 and 0.0163 μg mL−1 for silver and gold, respectively. The quantification limits were 0.0296 and 0.0544 μg mL−1 for silver and gold, respectively. The spectrophotometric and digital image-based data were in excellent agreement as shown in Fig. 5A–E and Table S2,† with the advantage of the low-cost, availability, and affordability of scanners compared to sophisticated spectrophotometers, showing the high efficacy of scanner image-based measurements.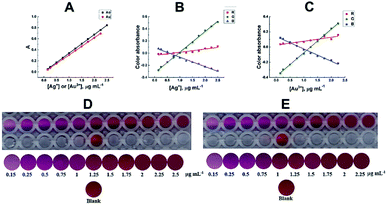 | ||
| Fig. 5 Calibration plots for the determination of Ag(I) and Au(III); (A) spectrophotometrically; (B) and (C) DIBA-based data; (D) and (E) are scanned and cropped images of Ag–SR–CPC and Au–SR–CTAC chelates, respectively. Except for the abscissa variable, other conditions are those of Fig. 2. Captured images were arbitrarily compressed to fit into the page margins; however, for image processing, the original uncompressed images were used. | ||
3.9. Effects of diverse ions
The effects of foreign ions on the determination of 2 μg mL−1 Ag(I) or Au(III) were thoroughly studied, following the recommended procedure. The tolerance limit was taken as the ratio of the foreign ion to the analyte that produced a ±5% error in the absorbance value.Despite the extraordinary selectivity of SR towards silver and gold, few precious metal ions interfered with the determination.
Therefore, some masking agents were adopted to impart extra selectivity to the developed methods. The results obtained are illustrated in Table 1. However, in the implemented method, an EDTA–citrate masking solution was adopted to enhance the tolerance limits of the proposed highly selective methods towards some metal ions, e.g., Cu(II) and Fe(II, III). Dimethylglyoxime (DMG) was effective in masking Pd(II) and Pt(II). Ascorbic acid was used to mask Hg(II) and Au(III) in the determination of silver.
| Ternary silver complex method | Ternary gold complex method | ||
|---|---|---|---|
| Foreign ionb | Tolerance limit [ion]/[Ag] | Foreign ionb | Tolerance limit [ion]/[Au] |
| a Conditions were those of the recommended procedure using 2 μg mL−1 Ag(I) or Au(III).b Masking agents: (a) 1 mL 0.01 mol L−1 EDTA/citrate solution; (b) 0.1 mL of 0.25 mol L−1 ethanolic solution of DMG; (c) 0.1 mL of 0.1 mol L−1 ascorbic acid; (d) 0.1 mL of 0.1 mol L−1 iodide/Cl–NH3OH. | |||
| Glycine, tartrate, citrate, succinate, acetate, benzoate | >5000 | Glycine, tartrate, citrate, succinate, acetate, benzoate, Cl− | >5000 |
| Ascorbic acid, Mo(VI), W(VI) | 3000 | Mo(VI), W(VI), Br−, I− | 4000 |
| Ca(II)a, Cu(II)a, NO2− | 2500 | Ca(II)a, Cu(II)a, NH2OH·HCl | 2500 |
| Mg(II)a, Mn(II)a, Co(II)a, Ni(II)a, Zn(II)a, Cd(II)a, PO42−, | 1500 | PO42−, SO42− | 2000 |
| Fe(II)a Al(III)a, NO3−, Cl− | 500 | Mg(II)a, Mn(II)a, Co(II)a, Ni(II)a, Zn(II)a, Cd(II)a, NO2− | 1500 |
| Pb(II)a, SO42−, CO32− | 250 | Al(III)a, Fe(II)a, NO3, CO32− | 500 |
| ClO3−, SCN−, NH4+ | 150 | Pb(II)a, Ag(I)d, Hg(II)d | 200 |
| Pt(II)b, Pd(II)b, Hg(II)c, Au(III)c, BrO3− | 100 | Pt(II)b, Pd(II)b, ClO3−, NH4+ | 150 |
| BrO3−, SCN− | 100 | ||
A 0.1 mol L−1 solution of sodium iodide was used to mask Hg(I, II) and Ag(I) in the determination of gold. The presence of hydroxylamine hydrochloride didn't affect the spectral characteristics of the Au–SR ternary complex indicating that Au(III) has been already reduced to Au(I). However; the presence of ascorbic acid severely suppressed the absorbance of the complex denoting the reduction of Au(III) to the metallic gold. This finding replenishes the findings of Cotton and Woolf,30 and Borissova.53 Traces of an oxidizing agent such as hydrogen peroxide altered the spectral characteristics of the complex consolidating the previous findings.
Thus, in the implemented methods most interfering species were tolerated at three orders of magnitude compared to silver and gold. Moreover, a 100-fold excess of Hg(II), Pt(II), and Au(III) did not interfere in the determination of Ag(I). In addition, a 200-fold excess of Ag(I), and Hg(II) and 150-fold excess of Pt(II), and Pd(II) did not interfere in the determination of Au(III); showing the extraordinary selectivity of the implemented methods.
3.10. Applications
The proposed method was conveniently applied to the assessment of silver in a pharmaceutical sample, gold in electroplating wastewater, and both silver and gold in rock samples. The pharmaceutical silver sulfadiazine burn cream under trade name Dermazin™; manufactured by Medical Union Pharmaceuticals (Abu-Sultan, Ismailia, Egypt) under license from Sandoz GmbH (Austria) was used. Electroplating wastewater was collected from Asfour Crystal (Shoubra El-khema, Cairo, Egypt). The geological samples were collected from some goldmines in Umm Qurayyat and Haimour (Wadi Allaqi, Aswan Governate), mongul, fatiri and Abu Marawat (Safaga-Qena Road, Red Sea Governate), and Um Atoud (Marsa Alam, Red Sea Governate). The analytical data obtained by the proposed method was validated by comparison with the corresponding spectrophotometric and the standard ICP-AES data.The result obtained for silver in the pharmaceutical sample, as determined by the proposed procedure for seven replicates, was 0.987 ± 0.019 whereas the corresponding values obtained using ICP-AE spectrometry was 1.010 ± 0.004. For the determination of gold in two samples of electroplating wastewater, the results obtained were 1.285 ± 0.011 and 0.546 ± 0.003 and the corresponding values obtained with the ICP-AES were 1.271 ± 0.007 and 0.529 ± 0.001 for seven replicates each. The results of silver and gold determination in the goldmines samples obtained using the proposed DIBA and spectrophotometric methods along with those of the ICP-AES method are summarized in Table 2.
| Source | ICP | Spectrophotometer | DIBA (G) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (μg g−1) | Mean ± SD (μg g−1) | F0.05 Ftable = 6.388 | t0.05 ttable = 2.306 | P0.05 | Mean ± SD (μg g−1) | F0.05 Ftable = 6.388 | t0.05 ttable = 2.306 | P0.05 | |
| Gold in presence of other precious metals | |||||||||
| Umm urayyat | 31.66 ± 0.36 | 31.56 ± 0.36 | 1.00 | 0.43 | 0.67 | 31.57 ± 0.36 | 1.00 | 0.41 | 0.69 |
| Haimour | 12.58 ± 0.01 | 12.49 ± 0.02 | 4.00 | 1.31 | 0.06 | 12.61 ± 0.01 | 1.00 | 1.18 | 0.08 |
| Mongul | 16.18 ± 0.09 | 16.24 ± 0.12 | 1.78 | 0.96 | 0.09 | 16.42 ± 0.14 | 2.42 | 0.98 | 0.10 |
| Fatiri | 3.57 ± 0.01 | 3.62 ± 0.01 | 1.00 | 1.59 | 0.09 | 3.63 ± 0.02 | 4.00 | 1.63 | 0.08 |
| Abu Marawat | 12.74 ± 0.02 | 13.03 ± 0.03 | 2.25 | 0.63 | 0.61 | 13.13 ± 0.04 | 4.00 | 0.87 | 0.57 |
| Um Atoud | 20.12 ± 0.14 | 20.36 ± 0.14 | 1.00 | 1.63 | 0.11 | 20.52 ± 0.15 | 1.15 | 1.80 | 0.11 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Silver in presence of other precious metals | |||||||||
| Um qurayyat | 0.52 ± 0.01 | 0.51 ± 0.01 | 1.619 | 0.63 | 0.53 | 0.50 ± 0.01 | 1.691 | 0.66 | 0.51 |
| Haimour | 3.37 ± 0.08 | 3.32 ± 0.07 | 1.433 | 0.97 | 0.21 | 3.29 ± 0.08 | 1.024 | 0.99 | 0.19 |
| Mongul | 5.69 ± 0.11 | 5.66 ± 0.09 | 1.471 | 1.82 | 0.06 | 5.57 ± 0.09 | 1.535 | 1.98 | 0.07 |
| Fatiri | 12.48 ± 0.30 | 12.10 ± 0.24 | 1.623 | 1.21 | 0.09 | 12.23 ± 0.15 | 3.896 | 1.52 | 0.10 |
| Abu Marawat | 59.75 ± 0.66 | 60.34 ± 0.86 | 1.699 | 2.00 | 0.11 | 60.97 ± 0.77 | 1.367 | 2.13 | 0.13 |
| Um Atoud | 0.74 ± 0.02 | 0.73 ± 0.01 | 2.367 | 1.96 | 0.06 | 0.71 ± 0.02 | 1.562 | 1.99 | 0.08 |
The statistical t-test and F-test at 95% confidence level clearly show that there is no statistical difference between the means and variances of the proposed methods and the standard ICP-AES methods.
4. Conclusion
This work proves the ability of scanner image analysis as an efficient competitor to conventional spectrophotometers for the optimization, validation, and application to the assessment of trace amounts of silver and gold in complex real samples using a novel and specifically tailored rhodanine derivative.A comparison between the analytical characteristics of the proposed method, some of the well-established reagents for silver and gold, and some of the well-known rhodanine derivatives is clarified in Table 3. This work introduces competitive selectivity and precision and even greater simplicity, affordability and sensitivity compared to most of the previously reported spectrophotometric methods.
| Reagent | pH | λmax, nm | ε × 10−4 L mol−1 cm−1 | Sensitivity μg cm−2 | Comments | Ref. |
|---|---|---|---|---|---|---|
| Selected methods of silver determination | ||||||
| Dithizone | ≤4 mol L−1 H2SO4 | 462 | 3.1 | 0.003 | Extraction with CCl4 | 15 |
| 4-(2-Quinolylazo)phenol | 9.2 | 530 | 8.3 | 0.001 | Cu, co, Fe, Ni, and Pd interfere | 16 |
| 4,4′-Bis(dimethylamino)thiobenzophenone | 3 | 520 | 9.3 | 0.001 | Noble metals interfere | 17 |
| 2-Cyano-3-iminodithiobutyrate | 4–6 | 565 | 1.3 | 0.008 | Hg interferes | 18 |
| 2′,3′-Dihydroxypyridyl-4 ′-azobenzene-4-arsenate | 535 | 3.0 | 0.004 | Complex formation in strongly alkaline medium | 19 | |
| p-Dimethylaminobenzylidenerhodanine | 0.05 mol L−1 HNO3 | 580 | 2.0 | 0.005 | With long pathlength cuvettes | 26 |
| 5-(4-Hydroxybenzylidene)rhodanine | Citrate buffer | 490 | 1.5 | 0.007 | Pt metal interferes | 27 |
| 5-[4-(2-Methyl-3-hydroxy-5-hydroxymethyl)pyridylene]rhodanine | 8.2 | 530 | 1.5 | 0.007 | Pt metal interferes | 28 |
| 5-(2,4-Dihydroxybenzylidene)rhodanine | 10 | 547 | 7.1 | 0.002 | 29 | |
| Syringal rhodanine–CPC | 9.8 | 550 | 3.63 | 0.003 | Spectrophotometer is not needed | This work |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Selected methods of gold determination | ||||||
| Dithizone | 420 | 2.8 | 0.007 | Extraction with chloroform | 20 | |
| 4-(2-Pyridylazo)resorcinol | 2.5 | 540 | 8.3 | 0.002 | Extraction; strong interferences | 21 |
| 4-(2-Thiazolylazo)resorcinol | 1.5 | 520 | 1.5 | 0.013 | Extraction; strong interferences | 22 |
| 5-(4-Dimethylaminobenzylidene)rhodanine | 0.12 mol L−1 HCl | 500 | — | — | 26 | |
| 5-(2.4-Dihydroxybenzylidene)rhodanine | 10 | 558 | 8.5 | 0.002 | 29 | |
| 5-(4-Dimethylaminobenzylidene)rhodanine | 0.12 mol L−1 HCl | 515 | 3.9 | 0.005 | Extraction with isoamyl acetate | 30 |
| 5-(4-Dimethylaminobenzylidene)rhodanine | 3 | 515 | 3.8 | 0.005 | Pt metal interferes | 31 |
| 5-(o-Hydroxyphenyl)methylenerhodanine | 446 | 0.9 | 0.022 | Pt metal interferes | 32 | |
| 5-(6-Methylpyridyl)methylenerhodanine | 420 | 1.1 | 0.018 | Pt metal interferes | 32 | |
| Syringal rhodanine–CTAC | 10 | 554 | 6.15 | 0.003 | Spectrophotometer is not needed | This work |
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Aznar, F. Barahona, O. Geiss, J. Ponti, T. Jose Luis and J. Barrero-Moreno, Talanta, 2017, 175, 200–208 CrossRef CAS.
- D. Tao, W. Guo, W. Xie, L. Jin, Q. Guo and S. Hu, Microchem. J., 2017, 135, 221–225 CrossRef CAS.
- Y. H. Liu, B. Wan and D. S. Xue, Molecules, 2019, 24, 3840 CrossRef.
- S. Kilic, Environ. Monit. Assess., 2019, 191, 452 CrossRef.
- P. Anekthirakun and A. Imyim, Microchem. J., 2019, 145, 470–475 CrossRef CAS.
- M. M. Rahman, S. B. Khan, H. M. Marwani, A. M. Asiri, K. A. Alamry and A. O. Al-Youbi, Talanta, 2013, 104, 75–82 CrossRef CAS.
- J. Mizera, Z. Řanda and J. Kučera, J. Radioanal. Nucl. Chem., 2008, 278, 599–602 CrossRef CAS.
- A. El-Taher, K. L. Kratz, A. Nossair and A. H. Azzam, Radiat. Phys. Chem., 2003, 68, 751–755 CrossRef CAS.
- M. Ghosh, K. K. Swain, T. A. Chavan, D. N. Wagh and R. Verma, X-Ray Spectrom., 2015, 44, 13–15 CrossRef CAS.
- M. Amjadi, A. Samadi, J. L. Manzoori and N. Arsalani, Anal. Methods, 2015, 7, 5847–5853 RSC.
- S. L. Ramesh, P. V. Sunder Raju, K. V. Anjaiah, R. Mathur, T. Gnaneswara Rao, B. Dasaram, S. N. Charan, D. V. Subba Rao, D. S. Sarma, R. Mekala and V. Balaram, At. Spectrosc., 2001, 22, 263 CAS.
- X. Tang, H. Li, H. Liu, B. Li, Y. Zhao, J. Lu, J. Zhou and Q. Liu, J. Anal. Methods Chem., 2019, 2019, 1792792 CrossRef PubMed.
- F. Salimi and M. Ramezani, Sep. Sci. Technol., 2019, 54, 2274–2282 CrossRef CAS.
- T. G. Choleva, G. Z. Tsogas and D. L. Giokas, Talanta, 2019, 196, 255–261 CrossRef CAS PubMed.
- G. K. Schweitzer and F. F. Dyer, Anal. Chim. Acta, 1960, 22, 172–180 CrossRef CAS.
- S. Barua, B. S. Garg, R. P. Singh and I. Singh, Analyst, 1980, 105 Search PubMed.
- K. L. Cheng, Mikrochim. Acta, 1967, 55, 820–827 CrossRef.
- M. Muraoka, T. Yamamoto and T. Takeshima, Analyst, 1979, 104, 87–90 RSC.
- Y. S. Varma, I. Singh, B. S. Garg and R. P. Singh, Microchem. J., 1984, 29, 326–332 CrossRef CAS.
- A. W. Titley, Analyst, 1962, 87, 349–355 RSC.
- S. G. Nagarkar and M. C. Eshwar, Anal. Chim. Acta, 1974, 71, 461–463 CrossRef CAS.
- B. Subrahmanyam and M. C. Eshwar, Anal. Chim. Acta, 1976, 82, 435–437 CrossRef CAS.
- H. S. Gowda, K. A. Padmaji and K. N. Thimmaiah, Analyst, 1981, 106, 198–205 RSC.
- I. Singh, P. S. Kadyan, B. S. Garg and S. Barua, Bull. Chem. Soc. Jpn., 1989, 62, 1316–1320 CrossRef CAS.
- A. V. Dolgorev and Y. G. Lysak, Dithiopyrylmethane and its analogs as analytical reagents. Study of complex formation between dithiodiantipyrylmethane and gold, bismuth, and molybdenum, 1974 Search PubMed.
- R. Borissova, Talanta, 1975, 22, 797–802 CrossRef CAS.
- Y. Tanaka, Y. Saito, J. Odo and H. Omori, Bunseki Kagaku, 1980, 29, 381–384 CrossRef CAS.
- R. E. Godoy and A. G. Pérez, Analyst, 1986, 111, 1297–1299 RSC.
- F. M. El-Zawawy, M. F. El-Shahat, A. A. Mohamed and M. T. M. Zaki, Analyst, 1995, 120, 549–554 RSC.
- T. M. Cotton and A. A. Woolf, Anal. Chim. Acta, 1960, 22, 192–194 CrossRef CAS.
- I. E. Lichtenstein, Anal. Chem., 2002, 47, 465–468 CrossRef.
- G. G. Alfonso and J. L. G. Ariza, Microchem. J., 1981, 26, 574–585 CrossRef.
- K. Ohshita, H. Wada and G. Nakasawa, Anal. Chim. Acta, 1986, 182, 157–162 CrossRef CAS.
- M. C. Alvarez-Coque, R. M. Camanas, M. C. Vaya, G. R. Ramos and C. M. Fernandez, Talanta, 1986, 33, 697–699 CrossRef CAS.
- A. A. Mohamed, A. A. Shalaby and A. M. Salem, RSC Adv., 2018, 8, 10673–10679 RSC.
- A. A. Mohamed and A. A. Shalaby, Food Chem., 2019, 274, 360–367 CrossRef CAS.
- H. Kudo, K. Yamada, D. Watanabe, K. Suzuki and D. Citterio, Micromachines, 2017, 8, 127 CrossRef.
- P. Masawat, A. Harfield, N. Srihirun and A. Namwong, Anal. Lett., 2016, 50, 173–185 CrossRef.
- S. K. Vashist, T. van Oordt, E. M. Schneider, R. Zengerle, F. von Stetten and J. H. Luong, Biosens. Bioelectron., 2015, 67, 248–255 CrossRef CAS.
- C. L. M. Morais, K. M. G. Lima and F. L. Martin, Anal. Lett., 2019, 52, 2239–2250 CrossRef CAS.
- X. X. Zhang, Y. Z. Song, F. Fang and Z. Y. Wu, Anal. Bioanal. Chem., 2018, 410, 2665–2669 CrossRef CAS.
- M. Jafari, J. Tashkhourian and G. Absalan, Talanta, 2018, 178, 870–878 CrossRef CAS.
- C. d. L. Medeiros de Morais and K. M. G. de Lima, Talanta, 2014, 126, 145–150 CrossRef CAS.
- C. d. L. M. de Morais, S. R. B. Silva, D. S. Vieira and K. M. G. Lima, J. Chem. Educ., 2016, 93, 1760–1765 CrossRef CAS.
- J. A. V. A. Barros, F. M. d. Oliveira, G. d. O. Santos, C. Wisniewski and P. O. Luccas, Anal. Lett., 2016, 50, 414–430 CrossRef.
- D. C. Christodouleas, A. Nemiroski, A. A. Kumar and G. M. Whitesides, Anal. Chem., 2015, 87, 9170–9178 CrossRef CAS.
- P. L. Julian and B. M. Sturgis, J. Am. Chem. Soc., 1935, 57, 1126–1128 CrossRef CAS.
- E. W. Gorecki and J. M. Pepper, Can. J. Chem., 1959, 37, 2089–2092 CrossRef CAS.
- P. G. Jeffery and D. Hutchison, Chemical methods of rock analysis, Pergamon Press, New York, 3rd edn, 1981 Search PubMed.
- https://multid.se/datan/https://multid.se/datan/, accessed 23-7-2019, 2019.
- M. J. Rosen and J. T. Kunjappu, Surfactants and interfacial phenomena, Wiley, 4th edn, 2012 Search PubMed.
- J. Inczédy, Analytical Applications of Complex Equilibria, Wiley, New York, 1976 Search PubMed.
- R. Borissova, Talanta, 1975, 22, 6 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures and tables. See DOI: 10.1039/c9ra06840f |
| This journal is © The Royal Society of Chemistry 2019 |