Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Combined inorganic base promoted N-addition/[2,3]-sigmatropic rearrangement to construct homoallyl sulfur-containing pyrazolones†
Shou-Jie Shen *a,
Xiao-Li Dua,
Xiao-Li Xub,
Yue-Hua Wub,
Ming-gang Zhaoa and
Jin-Yan Liang*b
*a,
Xiao-Li Dua,
Xiao-Li Xub,
Yue-Hua Wub,
Ming-gang Zhaoa and
Jin-Yan Liang*b
aKey Laboratory of Magnetic Molecules, Magnetic Information Materials Ministry of Education, The School of Chemical and Material Science, Shanxi Normal University, Linfen, 041004, China. E-mail: shoujie_shen@outlook.com; jinyan_liang@outlook.com
bCollege of Life Science, Shanxi Normal University, Linfen, 041004, China
First published on 29th October 2019
Abstract
The first sequentially combined inorganic base promoted N-addition/[2,3]-sigmatropic rearrangement reaction of α-alkylidene pyrazolinones and propargyl sulfonium salts has been reported to construct homoallyl sulfur-containing pyrazolones with moderate to excellent yields. α-Alkylidene pyrazolinones function as N-nucleophilic agents distinguished from the reported C-addition reactions. Propargyl sulfonium salts were first involved in the [2,3]-sigmatropic rearrangement protocol differentiated from the well-established annulation reactions. The excellent regioselectivity, the broad scope of substrates, gram-scale synthesis and convenient transformation embody the synthetic superiority of this cascade process.
Introduction
Organosulfur compounds that exist broadly in biologically active natural products as well as pharmaceuticals and are engaged in numerous chemical transformations, have been attracting vivid interest from academia and industry.1 Sulfur ylides are among the most versatile class of structural motifs, having widespread applications ranging from classical cyclopropanation, epoxidation and aziridination to more complicated [n + 1]-cycloadditions, domino reactions and rearrangement reactions based on transition metal catalysis, organocatalysis and photocatalysis.2 As an efficient C–C bond-forming strategy, [2,3]-sigmatropic rearrangements of sulfur ylides have been widely explored and applied since their discovery in the late 1960s.3,4 The transition metal carbenoid-mediated rearrangement reactions between allyl sulfides and diazo species named Doyle–Kirmse reactions are representative examples and have made impressive progress especially in to the content of catalytic and asymmetric variants in the past decade (Scheme 1a).5 Metal-free generation of sulfur ylides in situ between allyl thioethers and arynes followed by [2,3]-sigmatropic rearrangements are also alternative methods (Scheme 1b).6 Despite the aforementioned excellent work, the attempt to utilize propargyl sulfonium salts to form key species of sulfur ylides accompanied by subsequent [2,3]-sigmatropic rearrangements process has not been achieved.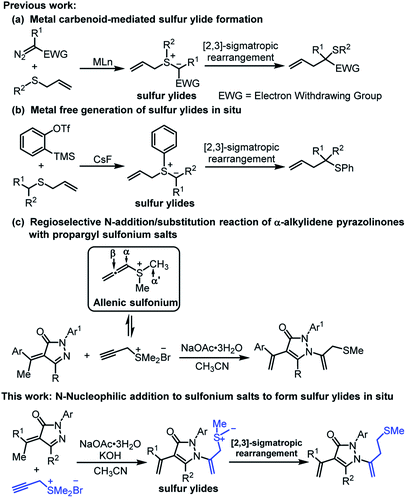 | ||
| Scheme 1 [2,3]-Sigmatropic rearrangements of sulfur ylides (a and b) and N-2 initiating nucleophilic reaction of α,β-unsaturated pyrazolone (c). | ||
Propargyl sulfonium salts, because of their easy acquirements and multiple reaction sites, are versatile and promising building blocks. Generally, propargyl sulfonium salts can isomerize to allenic sulfonium salts in the presence of the base, which are active forms and possess three reactive sites of α-carbon, β-carbon and α′-carbon (Scheme 1c). Kanematsu's,7 Huang's8 and our group9 have reported the [n + 2] or [n + 1] cascade annulation reactions based on α-carbon and β-carbon sites. Meanwhile, pyrazolones represent a class of privileged heterocycles that exhibit extensive physiological and pharmacological activities and are valuable drug candidates.10 Accordingly, fruitful protocols have been explored to access versatile pyrazolones architectures based on the multiple reactive sites of pyrazolin-5-ones and α,β-unsaturated pyrazolones.11,12 As to α,β-unsaturated pyrazolones having γ-H, the preferential γ-C nucleophilic property facilities their functioning as C3 synthons to construct spiro-pyrazolones by [3 + n] annulation.13 In contrast, the N-2 initiating nucleophilic reaction of α,β-unsaturated pyrazolones was less investigated. Recently our group reported the first regioselective NaOAc·3H2O-promoted N-addition/substitution reaction between α-alkylidene pyrazolinones and propargyl sulfonium salts (Scheme 1c).14 Accidentally, we found that strong inorganic bases can efficiently promoted the rearrangement of sulfur salts. Based on our processive interests on constructing functionalized pyrazolones and exploring the diverse reactive pathway of propargyl sulfonium salts,9,15 we herein report the realization of regioselective NaOAc·3H2O/KOH-promoted N-addition/[2,3]-sigmatropic rearrangement reaction of α-alkylidene pyrazolinones and propargyl sulfonium salts, delivering bioactive homoallyl sulfur-containing pyrazolones in moderate to excellent yields (Scheme 1d).
Results and discussion
We began our investigation by selecting α-alkylidene pyrazolinone 1a and propargyl sulfonium salt 2a as model substrates (Table 1). When 1a (0.2 mmol, 1.0 equiv.), 2a (0.24 mmol, 1.2 equiv.) and NaOAc·3H2O (0.1 mmol, 0.5 equiv.) in CH3CN (2 mL) were mixed and stirred for 10 minutes at 20 °C, the α-alkylidene pyrazolinone 1a was consumed completely. After the reaction temperature was decreased to 0 °C, KOH (0.4 mmol, 2.0 equiv.) was added and the reaction was stirred for 6 h at 0 °C to afford N-addition/[2,3]-sigmatropic rearrangement product 3a with 82% yield (Table 1, entry 4). When the mixture of NaOAc·3H2O and KOH was added in one portion, α-alkylidene pyrazolinone could not be consumed completely even after 12 h and the operation caused decreased yield. Extensive exploration of a range of bases indicated that NaOAc·3H2O or anhydrous NaOAc was most efficient to promote the process (entries 4–5 vs. 1–3 and 6–10 in Table 1). The combined usage of bases NaOAc·3H2O and KOH was crucial for the high efficiency while NaOAc·3H2O or KOH was utilized separately to give the desired 3a in 0% and 46% yields, respectively (Table 1, entries 11–12). Replacement of KOH with NaOH or LiOH gave related 74% and 68% yields (Table 1, entries 13–14). Screening of solvents including CH2Cl2, CHCl3, MeOH, THF and toluene did not give a better yield (entries 4 vs. 15–19). We also probed the influence of the amount of sulfonium salts 2a, NaOAc·3H2O and KOH. Increasing the content of propargyl sulfonium salt 2a to 2.0 equiv. has little influence on yield (entry 20). Decreasing the amount of NaOAc·3H2O to 0.25 equiv. or increasing to 1.0 equiv. had a slight effect on yield (entries 21–22). Increasing the quantities of KOH to 4.0 equiv. did not improve the yield (entry 23). The yield was declined from 82% to 78% when the reaction was stirred at 20 °C (entry 24).| Entry | Base1/base2 | Solvent | 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) base1 base1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) base2 base2 |
Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise noted, the reactions were performed under air and α-alkylidene pyrazolinones 1 (0.2 mmol, 1.0 equiv.), sulfonium salt 2a (0.24 mmol, 1.2 equiv.) and NaOAc·3H2O (0.1 mmol, 0.5 equiv.) in CH3CN (2.0 mL) were mixed and stirred for 10–40 minutes at 20 °C until starting material 1a disappeared (monitored by TLC), then the reaction temperature was decreased to 0 °C and KOH (2.0 equiv.) was added to keep stirring at 0 °C for 6–10 h.b Isolated yield. | ||||
| 1 | Na2CO3/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
69 |
| 2 | K2CO3/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
58 |
| 3 | Cs2CO3/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
75 |
| 4 | NaOAc·3H2O/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
82 |
| 5 | NaOAc/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
82 |
| 6 | KOAc/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
68 |
| 7 | NEt3/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
39 |
| 8 | DABCOc/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
68 |
| 9 | DBUd/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
60 |
| 10 | DMAPe/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
75 |
| 11 | NaOAc·3H2O | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0 |
| 12 | KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
46 |
| 13 | NaOAc·3H2O/NaOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
74 |
| 14 | NaOAc·3H2O/LiOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
68 |
| 15 | NaOAc·3H2O/KOH | CH2CI2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
74 |
| 16 | NaOAc·3H2O/KOH | CHCI3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
53 |
| 17 | NaOAc·3H2O/KOH | MeOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
64 |
| 18 | NaOAc·3H2O/KOH | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
48 |
| 19 | NaOAc·3H2O/KOH | Toluene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
36 |
| 20 | NaOAc·3H2O/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
83 |
| 21 | NaOAc·3H2O/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 |
| 22 | NaOAc·3H2O/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
82 |
| 23 | NaOAc·3H2O/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
81 |
| 24 | NaOAc·3H2O/KOH | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
78 |
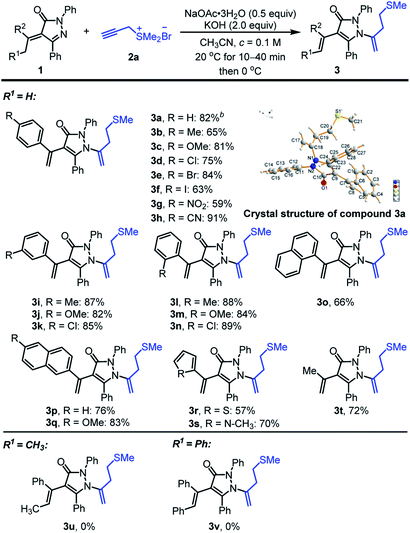
Having established the optimized conditions (Table 1, entry 4), we commenced to explore the substrate scope of the reaction (Scheme 2). Generally, the existence of methyl group (R1 = H) at α-position of alkylidene pyrazolinones was pivotal for the success of the reaction and various aryl and alkyl-substituted alkylidene pyrazolinones 1 was adaptable to the transformation. Acetophenones derived alkylidene pyrazolinones 1 with methyl, methoxy, chloro-, bromo-, iodo-, nitro- and cyano-groups on ortho-, meta- or para-positions, could react effectively with propargyl sulfonium salt 2a to furnish the related homoallyl sulfur-containing pyrazolones in 59–91% yields (3a–3n in Scheme 2). As to the same substituent on phenyl group, such as methyl, methoxy and chloro-, ortho- and meta-positions exhibited higher yields than para-position (3b vs. 3i, 3l; 3c vs. 3j, 3m; 3d vs. 3k, 3n). Naphthyl-substituted α-alkylidene pyrazolinones were well-tolerated to provide 3o, 3p and 3q in 66, 76 and 83% yields, respectively. Hetero-aromatic unsaturated pyrazolinones containing thiophene and N-methyl protected pyrrole could participate in the reaction to afford corresponding product 3r and 3s in 57 and 70% yields, respectively. Double alkyl-substituted alkylidene pyrazolinone could also be engaged in the reaction to produce the predicted 3t with 72% yield. In contrast, when the methyl group was replaced by ethyl (R1 = CH3) or benzyl groups (R1 = Ph), the desired reaction was sluggish, abundant alkylidene pyrazolinones were recovered and no target products 3u or 3v could be separated. In addition, the structure of homoallyl sulfur-containing pyrazolone derivative 3a was assigned unambiguously by using single crystal X-ray analysis.16
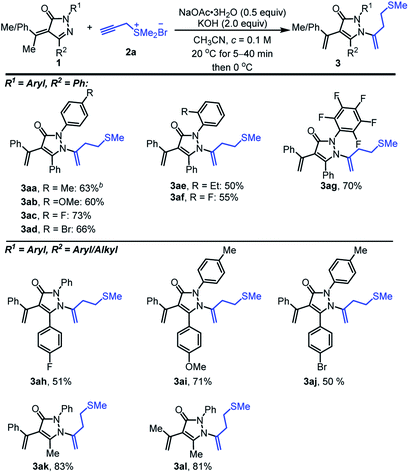
Subsequently, we went on to evaluate the effect of different substituents on pyrazolinone ring (Scheme 3). α-Alkylidene pyrazolinones 1 with the para-substituted phenyl-ring of R1 worked well to deliver the corresponding products 3aa, 3ab, 3ac and 3ad with moderate yields of 63, 60, 73 and 66%, respectively. Electron-withdrawing groups on the phenyl group gave better yields than electron-donating groups (3ac, 3ad vs. 3aa, 3ab). ortho-Ethyl, fluoro-substituted phenyl ring of R1 gave relatively lower yields of 50 and 55% partially because of the instability of 1. It is noteworthy that the substrate having electron-withdrawing group C6F5- supplied the desired product 3ag with a moderate yield of 70%. α-Alkylidene pyrazolinones 1 with 4-fluoro-, 4-methoxy and 4-bromo-substituted phenyl ring of R2 could also be applied to the reaction and provide the related homoallyl sulfur-containing pyrazolones 3ah, 3ai and 3aj with 51, 71 and 50% yields. α-Alkylidene pyrazolinones 1 including alkyl group of R2 showed excellent compatibility and afforded 3ak with 83% yield. Moreover, trimethyl involved alkylidene pyrazolinone displayed proof of tolerance and 81% yield was obtained (3al).
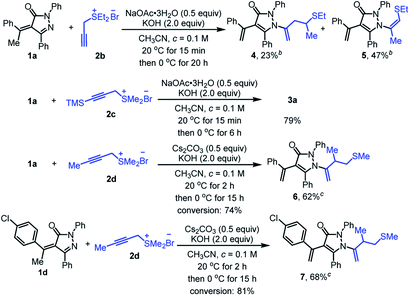
To further broaden the scope of the reaction, other representative propargyl sulfonium salts were also investigated (Scheme 4). Diethyl thioether derived propargyl sulfonium salt 2b was adaptable to give the predicted homoallyl sulfur-containing pyrazolone 4 in 23% yield, together with additional isomerization product 5 in 47% yield. Trimethylsilyl-containing propargyl sulfonium salt 2c can also be applied to the reaction but the desilylation product 3a was obtained with a yield of 79%. Methyl substituted propargyl sulfonium salt 2d did not engage in the reaction under standard condition, mainly because NaOAc·3H2O was not suitable to transform propargyl sulfonium salt 2d into active allenic form and alkylidene pyrazolinone 1a was nearly fully recovered after stirred at 20 °C for 20 h. When NaOAc·3H2O was replaced by Cs2CO3, the reaction could proceed to afford the desired product 6 with 62% yield. Substrate 1d could also react with 2d smoothly to provide 7 with 68% yield under the same conditions.
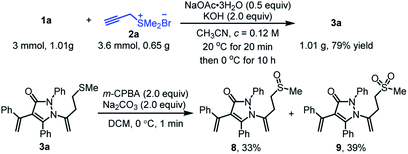
To demonstrate the further synthetic utility of this protocol, we performed the large-scale operation using α-alkylidene pyrazolinone 1a (1.01 g, 3 mmol) and propargyl sulfonium salt 2a (1.2 equiv.) as the representative substrates under the optimized conditions, providing the related product 3a (1.00 g) with 79% yield (Scheme 5). The typical transformation was also conducted by oxidation of 3a with m-chloro peroxybenzoic acid (2.0 equiv.), sulfinyl product 8 and sulfonyl product 9 were obtained in Scheme 5 with 33% and 39% yields, respectively.
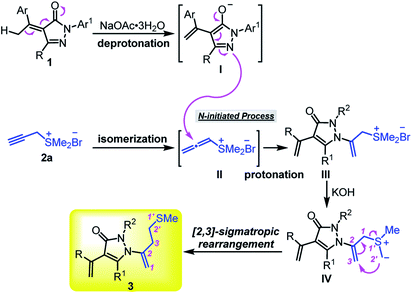
According to the experimental observations and previous reports,8,9,14 a possible mechanism is proposed to account for the formation of homoallyl sulfur-containing pyrazolone derivatives 3 (Scheme 6). Under the activation of inorganic base NaOAc·3H2O, α-alkylidene pyrazolone 1 can form intermediate I and propargyl sulfonium salt 2a can isomerize to allenic sulfonium salts II. The N-nucleophilic attack of I to allenic sulfonium salts II initiates the reaction and gives intermediate III after protonation. Subsequently, the deprotonation of methyl-carbon by KOH provides the key sulfur ylide IV. Finally, the [2,3]-sigmatropic rearrangement of key species IV affords the desired product 3.
Conclusions
In summary, we have developed a sequentially combined inorganic bases promoted N-addition/[2,3]-sigmatropic rearrangement reaction between α-alkylidene pyrazolinones and propargyl sulfonium salts for the first time, delivering bioactive homoallyl sulfur-containing pyrazolones in moderate to excellent yields. In this reaction, α-alkylidene pyrazolinones function as N-nucleophilic agents distinguished from reported C-addition reactions. Meanwhile, propargyl sulfonium salts were first involved in [2,3]-sigmatropic rearrangement protocols differentiated from the well-established annulation reactions. Gram-scale synthesis and convenient transformations are furnished. The proposed mechanism is also discussed. Excellent regioselectivity, the broad scope of substrates, gram-scale synthesis and convenient transformation embody the synthetic superiority of this reaction process.Experimental
General information
All reactions were performed in oven-dried or flame-dried round-bottom flasks and vials. Stainless steel syringes and cannula were used to transfer air- and moisture-sensitive liquids. Flash chromatography was performed using silica gel 60 (230–400 mesh) from Aladdin. Commercial reagents were purchased from Aladdin, J&K, Macklin and Meryer and used as received. All solvents were used after being freshly distilled unless otherwise noted. Proton nuclear magnetic resonance (1H NMR) spectra and carbon nuclear magnetic resonance (13C NMR) spectra were recorded on Bruker UltraShield-600 (600 MHz). The mass spectroscopic data were obtained using a Micromass Platform II single quadrupole instrument. Infrared (IR) spectra were obtained using a PerkinElmer Spectrum 100 FT-IR spectrometer.General procedure for the synthesis of α-alkylidene pyrazolinones (1)
α-Alkylidene pyrazolinones 1 were prepared through a known procedure:12b,13b,14,17 aryl formyl acetate (5.5 mmol, 1.1 equiv.) was slowly added to the mixture of the corresponding hydrazine (5 mmol, 1.0 equiv.) in glacial acetic acid (2 mL). The mixture was stirred at room temperature for 24 h. NEt3 (5 mmol, 1.0 equiv.) was added to neutralize the hydrochloride while phenylhydrazine hydrochloride was used. After the reaction was completed, ethyl (50 mL) was added. The precipitate was filtered and washed with 5 mL of ether (three times). The corresponding pyrozolone products were obtained as solid and used in the following step.Under nitrogen atmosphere, a mixture of pyrazolone (5 mmol, 1.0 equiv.), acetophenone (6 mmol, 1.2 equiv.) and pyridine (0.8 mL, 10 mmol) in THF (10 mL) was stirred for 10 min followed by slow addition (30 min) of Titanium isopropoxide (4.3 mL, 15 mmol). The mixture was stirred at room temperature for 24 h. The resulting reaction mixture was diluted with EtOAc (100 mL) and washed with 1 N aqueous HCl, saturated aqueous solution of NaHCO3 and brine. The organic layer was dried over Na2SO4, concentrated, and purified by column chromatography to provide α-alkylidene pyrazolinone derivatives 1. If the products are mixed with excess liquid acetophenes, they can be further purified by washing with petroleum ether.
Propargyl Sulfonium Salts (2a, 2b, 2c, 2d and 2e) were prepared through a known procedure.18
General procedure for the reaction of unsaturated pyrazolones with propargyl sulfide ylide
To a flame-dried sealable 3-dram vial equipped with a stir bar was added unsaturated pyrazolinones 1 (0.2 mmol, 1.0 equiv.), NaOAc·3H2O (0.1 mmol, 0.5 equiv.) and 2a (44 mg, 0.24 mmol, 1.2 equiv.) under air. Subsequently treated CH3CN (2 mL, c = 0.1 M) was added to vial via syringe. The reaction mixture was stirred for 10–40 min at 20 °C until unsaturated pyrazolones 1 was fully consumed (monitored by TLC). Then the reaction was stirred at 0 °C for 6–10 h. The organic solvent was removed under reduced pressure and purified through column chromatography (eluent: petroleum ether and EtOAc) to afford the desired product 3.1-(4-(Methylthio)but-1-en-2-yl)-2,5-diphenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3a)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3a was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a white solid (75 mg, 85% yield). mp 174.7–175.6 °C. IR νmax (neat)/cm−1: 3062, 2985, 1677, 1568, 1452, 1391, 1183, 1026, 955; 1H NMR (600 MHz, CDCl3): δ 7.62 (dd, J = 1.2, 7.8 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.34–7.29 (m, 5H), 7.26 (d, J = 7.2 Hz, 1H), 7.23 (t, J = 7.2 Hz, 2H), 7.17 (t, J = 7.8 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 5.69 (d, J = 1.2 Hz, 1H), 5.65 (d, J = 1.2 Hz, 1H), 5.45 (s, 1H), 5.06 (s, 1H), 2.24 (t, J = 7.8 Hz, 2H), 2.05 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 155.6, 145.1, 140.2, 138.3, 135.9, 130.0, 129.6, 129.2, 128.7, 127.9, 127.9, 127.2, 126.9, 126.4, 124.2, 118.9, 117.9, 114.3, 31.3, 31.1, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H26N2NaOS+, [M + Na]+, 461.1658, found 461.1662.
1) and obtained as a white solid (75 mg, 85% yield). mp 174.7–175.6 °C. IR νmax (neat)/cm−1: 3062, 2985, 1677, 1568, 1452, 1391, 1183, 1026, 955; 1H NMR (600 MHz, CDCl3): δ 7.62 (dd, J = 1.2, 7.8 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.34–7.29 (m, 5H), 7.26 (d, J = 7.2 Hz, 1H), 7.23 (t, J = 7.2 Hz, 2H), 7.17 (t, J = 7.8 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 5.69 (d, J = 1.2 Hz, 1H), 5.65 (d, J = 1.2 Hz, 1H), 5.45 (s, 1H), 5.06 (s, 1H), 2.24 (t, J = 7.8 Hz, 2H), 2.05 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 155.6, 145.1, 140.2, 138.3, 135.9, 130.0, 129.6, 129.2, 128.7, 127.9, 127.9, 127.2, 126.9, 126.4, 124.2, 118.9, 117.9, 114.3, 31.3, 31.1, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H26N2NaOS+, [M + Na]+, 461.1658, found 461.1662.
1-(4-(Methylthio)but-1-en-2-yl)-2,5-diphenyl-4-(1-(p-tolyl)vinyl)-1,2-dihydro-3H-pyrazol-3-one (3b)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3b was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (59 mg, 65% yield). IR νmax (neat)/cm−1: 3102, 2899, 1698, 1571, 1433, 1367, 1256, 1142, 1030, 979; 1H NMR (600 MHz, CDCl3): δ 7.62 (dd, J = 0.6, 8.4 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.36 (d, J = 8.4 Hz, 2H), 7.31–7.30 (m, 2H), 7.28–7.23 (m, 4H), 7.00 (d, J = 7.8 Hz, 2H), 5.64 (d, J = 1.2 Hz, 1H), 5.49 (d, J = 1.2 Hz, 1H), 5.44 (s, 1H), 5.06 (s, 1H), 2.28 (s, 3H), 2.26 (t, J = 7.8 Hz, 2H), 2.06 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.7, 155.6, 145.3, 138.2, 137.4, 137.0, 136.1, 130.1, 129.6, 129.4, 128.7, 127.9, 126.8, 126.4, 124.1, 118.0, 117.8, 114.8, 31.4, 31.1, 21.0, 15.4. HRMS (ESI-TOF, m/z): calcd for C29H28N2ONaS+, [M + Na]+, 475.1815, found 475.1812.
1) and obtained as a foam solid (59 mg, 65% yield). IR νmax (neat)/cm−1: 3102, 2899, 1698, 1571, 1433, 1367, 1256, 1142, 1030, 979; 1H NMR (600 MHz, CDCl3): δ 7.62 (dd, J = 0.6, 8.4 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.36 (d, J = 8.4 Hz, 2H), 7.31–7.30 (m, 2H), 7.28–7.23 (m, 4H), 7.00 (d, J = 7.8 Hz, 2H), 5.64 (d, J = 1.2 Hz, 1H), 5.49 (d, J = 1.2 Hz, 1H), 5.44 (s, 1H), 5.06 (s, 1H), 2.28 (s, 3H), 2.26 (t, J = 7.8 Hz, 2H), 2.06 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.7, 155.6, 145.3, 138.2, 137.4, 137.0, 136.1, 130.1, 129.6, 129.4, 128.7, 127.9, 126.8, 126.4, 124.1, 118.0, 117.8, 114.8, 31.4, 31.1, 21.0, 15.4. HRMS (ESI-TOF, m/z): calcd for C29H28N2ONaS+, [M + Na]+, 475.1815, found 475.1812.
4-(1-(4-Methoxyphenyl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3c)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3c was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (76 mg, 81% yield). IR νmax (neat)/cm−1: 3087, 2977, 1664, 1582, 1456, 1361, 1129, 1057, 933, 854; 1H NMR (600 MHz, CDCl3): δ 7.62 (d, J = 7.2 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.36 (d, J = 7.2 Hz, 2H), 7.30–7.24 (m, 6H), 6.72 (d, J = 9.0 Hz, 2H), 5.60 (d, J = 1.2 Hz, 1H), 5.48 (d, J = 1.2 Hz, 1H), 5.45 (s, 1H), 5.06 (s, 1H), 3.75 (s, 3H), 2.24 (t, J = 7.8 Hz, 2H), 2.05 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 159.1, 155.5, 145.1, 137.6, 135.9, 132.8, 129.9, 129.5, 129.2, 128.6, 127.9, 127.8, 126.3, 124.0, 117.8, 117.1, 114.6, 113.5, 55.2, 31.3, 31.0, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H29N2O2S+, [M + H]+, 469.1944, found 469.1943.
1) and obtained as a foam solid (76 mg, 81% yield). IR νmax (neat)/cm−1: 3087, 2977, 1664, 1582, 1456, 1361, 1129, 1057, 933, 854; 1H NMR (600 MHz, CDCl3): δ 7.62 (d, J = 7.2 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.36 (d, J = 7.2 Hz, 2H), 7.30–7.24 (m, 6H), 6.72 (d, J = 9.0 Hz, 2H), 5.60 (d, J = 1.2 Hz, 1H), 5.48 (d, J = 1.2 Hz, 1H), 5.45 (s, 1H), 5.06 (s, 1H), 3.75 (s, 3H), 2.24 (t, J = 7.8 Hz, 2H), 2.05 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 159.1, 155.5, 145.1, 137.6, 135.9, 132.8, 129.9, 129.5, 129.2, 128.6, 127.9, 127.8, 126.3, 124.0, 117.8, 117.1, 114.6, 113.5, 55.2, 31.3, 31.0, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H29N2O2S+, [M + H]+, 469.1944, found 469.1943.
4-(1-(4-Chlorophenyl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3d)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3d was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (71 mg, 75% yield). IR νmax (neat)/cm−1: 3073, 2956, 1680, 1479, 1364, 1182, 1036, 937; 1H NMR (600 MHz, CDCl3): δ 7.60 (dd, J = 1.2, 8.4 Hz, 2H), 7.47 (t, J = 8.4 Hz, 2H), 7.30 (d, J = 8.4 Hz, 4H), 7.25 (d, J = 7.8 Hz, 2H), 7.23–7.21 (m, 2H), 7.11 (d, J = 8.4 Hz, 2H), 5.69 (d, J = 1.2 Hz, 1H), 5.65 (d, J = 1.2 Hz, 1H), 5.44 (s, 1H), 5.06 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.5, 144.9, 138.8, 137.3, 135.7, 133.1, 130.0, 129.7, 129.1, 128.8, 128.2, 128.0, 128.0, 126.5, 124.2, 119.3, 118.0, 113.6, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H26ClN2OS+, [M + H]+, 473.1449, found 473.1451.
1) and obtained as a foam solid (71 mg, 75% yield). IR νmax (neat)/cm−1: 3073, 2956, 1680, 1479, 1364, 1182, 1036, 937; 1H NMR (600 MHz, CDCl3): δ 7.60 (dd, J = 1.2, 8.4 Hz, 2H), 7.47 (t, J = 8.4 Hz, 2H), 7.30 (d, J = 8.4 Hz, 4H), 7.25 (d, J = 7.8 Hz, 2H), 7.23–7.21 (m, 2H), 7.11 (d, J = 8.4 Hz, 2H), 5.69 (d, J = 1.2 Hz, 1H), 5.65 (d, J = 1.2 Hz, 1H), 5.44 (s, 1H), 5.06 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.5, 144.9, 138.8, 137.3, 135.7, 133.1, 130.0, 129.7, 129.1, 128.8, 128.2, 128.0, 128.0, 126.5, 124.2, 119.3, 118.0, 113.6, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H26ClN2OS+, [M + H]+, 473.1449, found 473.1451.
4-(1-(4-Bromophenyl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3e)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3e was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (87 mg, 84% yield). IR νmax (neat)/cm−1: 3102, 2941, 1668, 1603, 1490, 1377, 1268, 1146, 1021, 919, 832; 1H NMR (600 MHz, CDCl3): δ 7.60 (d, J = 7.8 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.31–7.26 (m, 5H), 7.25–7.22 (m, 3H), 7.15 (d, J = 8.4 Hz, 2H), 5.69 (d, J = 0.6 Hz, 1H), 5.64 (d, J = 0.6 Hz, 1H), 5.42 (s, 1H), 5.05 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.4, 144.8, 139.3, 137.3, 135.7, 131.0, 129.9, 129.7, 129.0, 128.7, 128.5, 128.0, 126.5, 124.1, 121.2, 119.2, 118.0, 113.4, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H26BrN2OS+, [M + H]+, 517.0944, found 517.0945.
1) and obtained as a foam solid (87 mg, 84% yield). IR νmax (neat)/cm−1: 3102, 2941, 1668, 1603, 1490, 1377, 1268, 1146, 1021, 919, 832; 1H NMR (600 MHz, CDCl3): δ 7.60 (d, J = 7.8 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.31–7.26 (m, 5H), 7.25–7.22 (m, 3H), 7.15 (d, J = 8.4 Hz, 2H), 5.69 (d, J = 0.6 Hz, 1H), 5.64 (d, J = 0.6 Hz, 1H), 5.42 (s, 1H), 5.05 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.4, 144.8, 139.3, 137.3, 135.7, 131.0, 129.9, 129.7, 129.0, 128.7, 128.5, 128.0, 126.5, 124.1, 121.2, 119.2, 118.0, 113.4, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H26BrN2OS+, [M + H]+, 517.0944, found 517.0945.
4-(1-(4-Iodophenyl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3f)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3f was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (71 mg, 63% yield). IR νmax (neat)/cm−1: 3082, 2986, 1677, 1598, 1465, 1343, 1188, 1067, 962; 1H NMR (600 MHz, CDCl3): δ 7.60 (dd, J = 1.2, 7.8 Hz, 2H), 7.48–7.45 (m, 4H), 7.32–7.28 (m, 4H), 7.25 (d, J = 7.2 Hz, 2H), 7.03 (d, J = 8.4 Hz, 2H), 5.67 (d, J = 1.2 Hz, 1H), 5.64 (d, J = 1.2 Hz, 1H), 5.42 (s, 1H), 5.06 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.5, 144.9, 139.9, 137.5, 137.0, 135.8, 130.0, 129.7, 129.1, 128.8, 128.7, 128.0, 126.5, 124.2, 119.3, 117.9, 113.4, 92.7, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H25IN2ONaS+, [M + Na]+, 587.0624, found 587.0620.
1) and obtained as a foam solid (71 mg, 63% yield). IR νmax (neat)/cm−1: 3082, 2986, 1677, 1598, 1465, 1343, 1188, 1067, 962; 1H NMR (600 MHz, CDCl3): δ 7.60 (dd, J = 1.2, 7.8 Hz, 2H), 7.48–7.45 (m, 4H), 7.32–7.28 (m, 4H), 7.25 (d, J = 7.2 Hz, 2H), 7.03 (d, J = 8.4 Hz, 2H), 5.67 (d, J = 1.2 Hz, 1H), 5.64 (d, J = 1.2 Hz, 1H), 5.42 (s, 1H), 5.06 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.5, 144.9, 139.9, 137.5, 137.0, 135.8, 130.0, 129.7, 129.1, 128.8, 128.7, 128.0, 126.5, 124.2, 119.3, 117.9, 113.4, 92.7, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H25IN2ONaS+, [M + Na]+, 587.0624, found 587.0620.
1-(4-(Methylthio)but-1-en-2-yl)-4-(1-(4-nitrophenyl)vinyl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3g)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3g was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (57 mg, 59% yield). IR νmax (neat)/cm−1: 3076, 2977, 1668, 1599, 1472, 1338, 1169, 1027, 912, 835; 1H NMR (600 MHz, CDCl3): δ 7.98 (d, J = 9.0 Hz, 2H), 7.60 (dd, J = 1.2, 8.4 Hz, 2H), 7.49 (t, J = 7.8 Hz, 2H), 7.39 (d, J = 8.4 Hz, 2H), 7.33 (t, J = 7.8 Hz, 1H), 7.27–7.25 (m, 3H), 7.22 (t, J = 7.2 Hz, 2H), 5.96 (d, J = 0.6 Hz, 1H), 5.77 (d, J = 0.6 Hz, 1H), 5.45 (s, 1H), 5.08 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.1, 155.4, 147.1, 144.7, 137.0, 135.7, 130.1, 130.0, 128.9, 128.2, 128.0, 127.9, 126.8, 124.4, 123.2, 121.9, 118.2, 112.5, 31.5, 31.3, 15.4. HRMS (ESI-TOF, m/z): calcd for C28H25N3O3NaS+, [M + Na]+, 506.1509, found 506.1509.
1) and obtained as a foam solid (57 mg, 59% yield). IR νmax (neat)/cm−1: 3076, 2977, 1668, 1599, 1472, 1338, 1169, 1027, 912, 835; 1H NMR (600 MHz, CDCl3): δ 7.98 (d, J = 9.0 Hz, 2H), 7.60 (dd, J = 1.2, 8.4 Hz, 2H), 7.49 (t, J = 7.8 Hz, 2H), 7.39 (d, J = 8.4 Hz, 2H), 7.33 (t, J = 7.8 Hz, 1H), 7.27–7.25 (m, 3H), 7.22 (t, J = 7.2 Hz, 2H), 5.96 (d, J = 0.6 Hz, 1H), 5.77 (d, J = 0.6 Hz, 1H), 5.45 (s, 1H), 5.08 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.1, 155.4, 147.1, 144.7, 137.0, 135.7, 130.1, 130.0, 128.9, 128.2, 128.0, 127.9, 126.8, 124.4, 123.2, 121.9, 118.2, 112.5, 31.5, 31.3, 15.4. HRMS (ESI-TOF, m/z): calcd for C28H25N3O3NaS+, [M + Na]+, 506.1509, found 506.1509.
4-(1-(1-(4-(Methylthio)but-1-en-2-yl)-3-oxo-2,5-diphenyl-2,3-dihydro-1H-pyrazol-4-yl)vinyl) benzonitrile (3h)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3h was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (84 mg, 91% yield). IR νmax (neat)/cm−1: 3065, 2963, 2023, 1672, 1564, 1491, 1336, 1287, 1142, 1053, 965; 1H NMR (600 MHz, CDCl3): δ 7.58 (d, J = 7.2 Hz, 2H), 7.48 (t, J = 7.8, 2H), 7.39 (d, J = 7.2, 2H), 7.30–7.34 (m, 3H), 7.28 (d, J = 3.6 Hz, 1H), 7.27–7.25 (m, 2H), 7.22 (d, J = 7.2 Hz, 2H), 5.90 (s, 1H), 5.72 (s, 1H), 5.44 (s, 1H), 5.07 (s, 1H), 2.22 (t, J = 7.8 Hz, 2H), 2.03 (t, J = 7.8 Hz, 2H), 1.92 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.1, 155.4, 145.1, 144.7, 137.2, 135.7, 131.7, 130.0, 128.9, 128.1, 127.7, 126.8, 124.3, 121.4, 118.7, 118.2, 112.5, 110.9, 31.4, 31.2, 15.4. HRMS (ESI-TOF, m/z): calcd for C29H25IN3ONaS+, [M + Na]+, 486.1611, found 486.1610.
1) and obtained as a foam solid (84 mg, 91% yield). IR νmax (neat)/cm−1: 3065, 2963, 2023, 1672, 1564, 1491, 1336, 1287, 1142, 1053, 965; 1H NMR (600 MHz, CDCl3): δ 7.58 (d, J = 7.2 Hz, 2H), 7.48 (t, J = 7.8, 2H), 7.39 (d, J = 7.2, 2H), 7.30–7.34 (m, 3H), 7.28 (d, J = 3.6 Hz, 1H), 7.27–7.25 (m, 2H), 7.22 (d, J = 7.2 Hz, 2H), 5.90 (s, 1H), 5.72 (s, 1H), 5.44 (s, 1H), 5.07 (s, 1H), 2.22 (t, J = 7.8 Hz, 2H), 2.03 (t, J = 7.8 Hz, 2H), 1.92 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.1, 155.4, 145.1, 144.7, 137.2, 135.7, 131.7, 130.0, 128.9, 128.1, 127.7, 126.8, 124.3, 121.4, 118.7, 118.2, 112.5, 110.9, 31.4, 31.2, 15.4. HRMS (ESI-TOF, m/z): calcd for C29H25IN3ONaS+, [M + Na]+, 486.1611, found 486.1610.
1-(4-(Methylthio)but-1-en-2-yl)-2,5-diphenyl-4-(1-(m-tolyl)vinyl)-1,2-dihydro-3H-pyrazol-3-one (3i)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3i was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (79 mg, 87% yield). IR νmax (neat)/cm−1: 3106, 2932, 1688, 1492, 1356, 1149, 1028, 972, 855; 1H NMR (600 MHz, CDCl3): δ 7.64 (dd, J = 1.2, 8.4 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.34 (d, J = 7.2 Hz, 2H), 7.29 (t, J = 7.8 Hz, 1H), 7.26 (d, J = 7.2 Hz, 1H), 7.23 (t, J = 7.2 Hz, 2H), 7.13 (d, J = 7.8 Hz, 1H), 7.09 (d, J = 6.0 Hz, 1H), 7.06 (d, J = 7.2 Hz, 1H), 6.94 (d, J = 7.2 Hz, 1H), 5.67 (d, J = 1.2 Hz, 1H), 5.64 (d, J = 1.2 Hz, 1H), 5.44 (s, 1H), 5.05 (s, 1H), 2.26 (t, J = 7.8 Hz, 2H), 2.25 (s, 3H), 2.06 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 155.5, 145.1, 140.1, 138.4, 137.2, 136.0, 130.0, 129.5, 129.3, 128.7, 128.0, 127.8, 127.8, 127.7, 126.3, 124.1, 118.5, 117.8, 114.3, 31.3, 31.1, 21.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H29N2OS+, [M + H]+, 453.1995, found 453.1993.
1) and obtained as a foam solid (79 mg, 87% yield). IR νmax (neat)/cm−1: 3106, 2932, 1688, 1492, 1356, 1149, 1028, 972, 855; 1H NMR (600 MHz, CDCl3): δ 7.64 (dd, J = 1.2, 8.4 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.34 (d, J = 7.2 Hz, 2H), 7.29 (t, J = 7.8 Hz, 1H), 7.26 (d, J = 7.2 Hz, 1H), 7.23 (t, J = 7.2 Hz, 2H), 7.13 (d, J = 7.8 Hz, 1H), 7.09 (d, J = 6.0 Hz, 1H), 7.06 (d, J = 7.2 Hz, 1H), 6.94 (d, J = 7.2 Hz, 1H), 5.67 (d, J = 1.2 Hz, 1H), 5.64 (d, J = 1.2 Hz, 1H), 5.44 (s, 1H), 5.05 (s, 1H), 2.26 (t, J = 7.8 Hz, 2H), 2.25 (s, 3H), 2.06 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 155.5, 145.1, 140.1, 138.4, 137.2, 136.0, 130.0, 129.5, 129.3, 128.7, 128.0, 127.8, 127.8, 127.7, 126.3, 124.1, 118.5, 117.8, 114.3, 31.3, 31.1, 21.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H29N2OS+, [M + H]+, 453.1995, found 453.1993.
4-(1-(3-Methoxyphenyl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3j)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3j was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (77 mg, 82% yield). IR νmax (neat)/cm−1: 3088, 2898, 1667, 1584, 1492, 1336, 1177, 1062, 946; 1H NMR (600 MHz, CDCl3): δ 7.62 (d, J = 7.2 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.34 (d, J = 7.2 Hz, 2H), 7.30–7.26 (m, 2H), 7.23 (t, J = 7.2 Hz, 2H), 7.08 (t, J = 7.8 Hz, 1H), 6.92 (d, J = 7.8 Hz, 1H), 6.83 (s, 1H), 6.68 (dd, J = 1.8, 7.8 Hz, 1H), 5.68 (d, J = 1.2 Hz, 1H), 5.67 (s, 1H), 5.43 (s, 1H), 5.05 (s, 1H), 3.74 (s, 3H), 2.24 (t, J = 7.8 Hz, 2H), 2.05 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 159.4, 155.5, 145.1, 141.7, 138.2, 135.9, 130.0, 129.6, 129.2, 128.9, 128.7, 127.8, 126.4, 124.1, 119.7, 118.9, 117.9, 114.2, 113.0, 112.9, 55.1, 31.3, 31.1, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaO2S+, [M + Na]+, 491.1764, found 491.1764.
1) and obtained as a foam solid (77 mg, 82% yield). IR νmax (neat)/cm−1: 3088, 2898, 1667, 1584, 1492, 1336, 1177, 1062, 946; 1H NMR (600 MHz, CDCl3): δ 7.62 (d, J = 7.2 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.34 (d, J = 7.2 Hz, 2H), 7.30–7.26 (m, 2H), 7.23 (t, J = 7.2 Hz, 2H), 7.08 (t, J = 7.8 Hz, 1H), 6.92 (d, J = 7.8 Hz, 1H), 6.83 (s, 1H), 6.68 (dd, J = 1.8, 7.8 Hz, 1H), 5.68 (d, J = 1.2 Hz, 1H), 5.67 (s, 1H), 5.43 (s, 1H), 5.05 (s, 1H), 3.74 (s, 3H), 2.24 (t, J = 7.8 Hz, 2H), 2.05 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 159.4, 155.5, 145.1, 141.7, 138.2, 135.9, 130.0, 129.6, 129.2, 128.9, 128.7, 127.8, 126.4, 124.1, 119.7, 118.9, 117.9, 114.2, 113.0, 112.9, 55.1, 31.3, 31.1, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaO2S+, [M + Na]+, 491.1764, found 491.1764.
4-(1-(3-Chlorophenyl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3k)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3k was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (80 mg, 85% yield). IR νmax (neat)/cm−1: 3074, 2973, 1679, 1563, 1455, 1383, 1257, 1061, 977; 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 7.2 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.31–7.30 (m, 3H), 7.29–7.21 (m, 4H), 7.15 (dt, J = 1.8, 4.8 Hz, 1H), 7.05 (d, J = 4.8 Hz, 2H), 5.81 (d, J = 1.2 Hz, 1H), 5.66 (d, J = 1.2 Hz, 1H), 5.42 (s, 1H), 5.05 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.4, 144.8, 142.2, 137.2, 135.8, 133.8, 130.0, 129.7, 129.1, 128.8, 127.9, 127.3, 127.1, 126.5, 125.2, 124.2, 119.8, 118.0, 113.1, 31.3, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H25ClN2NaOS+, [M + Na]+, 495.1268, found 495.1265.
1) and obtained as a foam solid (80 mg, 85% yield). IR νmax (neat)/cm−1: 3074, 2973, 1679, 1563, 1455, 1383, 1257, 1061, 977; 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 7.2 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.31–7.30 (m, 3H), 7.29–7.21 (m, 4H), 7.15 (dt, J = 1.8, 4.8 Hz, 1H), 7.05 (d, J = 4.8 Hz, 2H), 5.81 (d, J = 1.2 Hz, 1H), 5.66 (d, J = 1.2 Hz, 1H), 5.42 (s, 1H), 5.05 (s, 1H), 2.23 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.4, 144.8, 142.2, 137.2, 135.8, 133.8, 130.0, 129.7, 129.1, 128.8, 127.9, 127.3, 127.1, 126.5, 125.2, 124.2, 119.8, 118.0, 113.1, 31.3, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C28H25ClN2NaOS+, [M + Na]+, 495.1268, found 495.1265.
1-(4-(Methylthio)but-1-en-2-yl)-2,5-diphenyl-4-(1-(o-tolyl)vinyl)-1,2-dihydro-3H-pyrazol-3-one (3l)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3l was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (80 mg, 88% yield). IR νmax (neat)/cm−1: 3106, 3023, 2984, 1688, 1492, 1356, 1149, 1028, 972, 855; 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 7.2 Hz, 2H), 7.47 (t, J = 7.2 Hz, 2H), 7.30 (t, J = 7.2 Hz, 1H), 7.19–7.16 (m, 1H), 7.12–7.09 (m, 4H), 6.95 (dd, J = 1.2, 7.2 Hz, 1H), 6.90 (dt, J = 1.2, 7.2 Hz, 1H), 6.86 (t, J = 7.2 Hz, 1H), 6.81 (d, J = 7.8 Hz, 1H), 6.25 (d, J = 1.8 Hz, 1H), 5.37 (d, J = 1.8 Hz, 1H), 5.22 (s, 1H), 4.93 (s, 1H), 2.20 (t, J = 7.8 Hz, 2H), 2.10 (s, 3H), 2.00 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.2, 154.4, 144.5, 141.1, 138.3, 135.9, 135.7, 134.8, 129.7, 129.6, 129.5, 129.1, 128.7, 127.3, 126.7, 126.4, 124.9, 124.0, 120.1, 117.0, 112.9, 31.5, 31.0, 19.9, 15.2. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaOS+, [M + Na]+, 475.1815, found 475.1813.
1) and obtained as a foam solid (80 mg, 88% yield). IR νmax (neat)/cm−1: 3106, 3023, 2984, 1688, 1492, 1356, 1149, 1028, 972, 855; 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 7.2 Hz, 2H), 7.47 (t, J = 7.2 Hz, 2H), 7.30 (t, J = 7.2 Hz, 1H), 7.19–7.16 (m, 1H), 7.12–7.09 (m, 4H), 6.95 (dd, J = 1.2, 7.2 Hz, 1H), 6.90 (dt, J = 1.2, 7.2 Hz, 1H), 6.86 (t, J = 7.2 Hz, 1H), 6.81 (d, J = 7.8 Hz, 1H), 6.25 (d, J = 1.8 Hz, 1H), 5.37 (d, J = 1.8 Hz, 1H), 5.22 (s, 1H), 4.93 (s, 1H), 2.20 (t, J = 7.8 Hz, 2H), 2.10 (s, 3H), 2.00 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.2, 154.4, 144.5, 141.1, 138.3, 135.9, 135.7, 134.8, 129.7, 129.6, 129.5, 129.1, 128.7, 127.3, 126.7, 126.4, 124.9, 124.0, 120.1, 117.0, 112.9, 31.5, 31.0, 19.9, 15.2. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaOS+, [M + Na]+, 475.1815, found 475.1813.
4-(1-(2-Methoxyphenyl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3m)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3m was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (79 mg, 84% yield). IR νmax (neat)/cm−1: 3078, 2983, 1660, 1576, 1469, 1337, 1147, 1053, 983; 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 7.2 Hz, 2H), 7.45 (t, J = 7.8 Hz, 2H), 7.27–7.25 (m, 3H), 7.21 (t, J = 7.2 Hz, 1H), 7.15 (t, J = 7.2 Hz, 2H), 7.13 (d, J = 7.2 Hz, 1H), 7.04 (dt, J = 1.8, 8.4 Hz, 1H), 6.75 (dt, J = 0.6, 7.2 Hz, 1H), 6.53 (d, J = 8.4 Hz, 1H), 6.03 (d, J = 1.8 Hz, 1H), 5.44 (d, J = 1.8 Hz, 1H), 5.30 (s, 1H), 4.96 (s, 1H), 3.68 (s, 3H), 2.22 (t, J = 7.8 Hz, 2H), 2.02 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 156.4, 154.4, 145.1, 136.2, 130.6, 130.1, 129.4, 129.1, 128.6, 128.4, 127.3, 126.1, 123.9, 120.2, 117.1, 113.8, 110.5, 55.3, 31.2, 15.2. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaO2S+, [M + Na]+, 491.1764, found 491.1766.
1) and obtained as a foam solid (79 mg, 84% yield). IR νmax (neat)/cm−1: 3078, 2983, 1660, 1576, 1469, 1337, 1147, 1053, 983; 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 7.2 Hz, 2H), 7.45 (t, J = 7.8 Hz, 2H), 7.27–7.25 (m, 3H), 7.21 (t, J = 7.2 Hz, 1H), 7.15 (t, J = 7.2 Hz, 2H), 7.13 (d, J = 7.2 Hz, 1H), 7.04 (dt, J = 1.8, 8.4 Hz, 1H), 6.75 (dt, J = 0.6, 7.2 Hz, 1H), 6.53 (d, J = 8.4 Hz, 1H), 6.03 (d, J = 1.8 Hz, 1H), 5.44 (d, J = 1.8 Hz, 1H), 5.30 (s, 1H), 4.96 (s, 1H), 3.68 (s, 3H), 2.22 (t, J = 7.8 Hz, 2H), 2.02 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 156.4, 154.4, 145.1, 136.2, 130.6, 130.1, 129.4, 129.1, 128.6, 128.4, 127.3, 126.1, 123.9, 120.2, 117.1, 113.8, 110.5, 55.3, 31.2, 15.2. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaO2S+, [M + Na]+, 491.1764, found 491.1766.
4-(1-(2-Chlorophenyl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3n)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3n was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (84 mg, 89% yield). IR νmax (neat)/cm−1: 3056, 2933, 1679, 1586, 1453, 1332, 1175, 1026, 944; 1H NMR (600 MHz, CDCl3): δ 7.58 (d, J = 7.2 Hz, 2H), 7.45 (t, J = 7.2 Hz, 2H), 7.29 (t, J = 7.2 Hz, 1H), 7.23–7.18 (m, 3H), 7.17 (d, J = 6.6 Hz, 2H), 7.07 (dd, J = 1.8, 6.6 Hz, 1H), 7.03 (dd, J = 1.8, 6.6 Hz, 1H), 6.96–6.92 (m, 2H), 6.23 (d, J = 1.2 Hz, 1H), 5.43 (d, J = 1.2 Hz, 1H), 5.24 (s, 1H), 4.94 (s, 1H), 2.22 (t, J = 7.8 Hz, 2H), 2.02 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.2, 154.2, 144.5, 139.9, 136.5, 135.9, 132.3, 131.3, 129.9, 129.2, 129.1, 128.7, 128.0, 127.5, 126.4, 126.0, 124.1, 121.1, 117.1, 112.0, 31.7, 31.0, 15.2. HRMS (ESI-TOF, m/z): calcd for C28H25ClN2NaOS+, [M + Na]+, 495.1268, found 495.1270.
1) and obtained as a foam solid (84 mg, 89% yield). IR νmax (neat)/cm−1: 3056, 2933, 1679, 1586, 1453, 1332, 1175, 1026, 944; 1H NMR (600 MHz, CDCl3): δ 7.58 (d, J = 7.2 Hz, 2H), 7.45 (t, J = 7.2 Hz, 2H), 7.29 (t, J = 7.2 Hz, 1H), 7.23–7.18 (m, 3H), 7.17 (d, J = 6.6 Hz, 2H), 7.07 (dd, J = 1.8, 6.6 Hz, 1H), 7.03 (dd, J = 1.8, 6.6 Hz, 1H), 6.96–6.92 (m, 2H), 6.23 (d, J = 1.2 Hz, 1H), 5.43 (d, J = 1.2 Hz, 1H), 5.24 (s, 1H), 4.94 (s, 1H), 2.22 (t, J = 7.8 Hz, 2H), 2.02 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.2, 154.2, 144.5, 139.9, 136.5, 135.9, 132.3, 131.3, 129.9, 129.2, 129.1, 128.7, 128.0, 127.5, 126.4, 126.0, 124.1, 121.1, 117.1, 112.0, 31.7, 31.0, 15.2. HRMS (ESI-TOF, m/z): calcd for C28H25ClN2NaOS+, [M + Na]+, 495.1268, found 495.1270.
1-(4-(Methylthio)but-1-en-2-yl)-4-(1-(naphthalen-2-yl)vinyl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3o)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3o was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (65 mg, 66% yield). IR νmax (neat)/cm−1: 3113, 2965, 1680, 1566, 1473, 1357, 1121, 1073, 961; 1H NMR (600 MHz, CDCl3): δ 7.87 (dd, J = 2.4, 7.2 Hz, 1H), 7.62 (d, J = 4.8 Hz, 1H), 7.61 (t, J = 7.2 Hz, 2H), 7.49 (t, J = 7.2 Hz, 2H), 7.44 (d, J = 7.8 Hz, 1H), 7.38–7.36 (m, 2H), 7.32 (t, J = 7.2 Hz, 1H), 7.13–7.08 (m, 2H), 6.95 (t, J = 7.2 Hz, 1H), 6.79 (t, J = 7.2 Hz, 2H), 6.68 (d, J = 7.8 Hz, 2H), 6.61 (d, J = 2.4 Hz, 1H), 5.58 (d, J = 2.4 Hz, 1H), 5.06 (s, 1H), 4.83 (s, 1H), 2.16 (t, J = 7.8 Hz, 2H), 1.98 (t, J = 7.8 Hz, 2H), 1.91 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 154.8, 144.3, 139.5, 137.4, 136.0, 133.4, 131.4, 129.5, 128.8, 128.7, 127.9, 127.3, 127.0, 126.8, 126.5, 126.0, 125.5, 125.2, 124.8, 124.1, 120.8, 116.7, 112.9, 32.0, 31.1, 15.3. HRMS (ESI-TOF, m/z): calcd for C32H28ClN2NaOS+, [M + Na]+, 511.1815, found 511.1818.
1) and obtained as a foam solid (65 mg, 66% yield). IR νmax (neat)/cm−1: 3113, 2965, 1680, 1566, 1473, 1357, 1121, 1073, 961; 1H NMR (600 MHz, CDCl3): δ 7.87 (dd, J = 2.4, 7.2 Hz, 1H), 7.62 (d, J = 4.8 Hz, 1H), 7.61 (t, J = 7.2 Hz, 2H), 7.49 (t, J = 7.2 Hz, 2H), 7.44 (d, J = 7.8 Hz, 1H), 7.38–7.36 (m, 2H), 7.32 (t, J = 7.2 Hz, 1H), 7.13–7.08 (m, 2H), 6.95 (t, J = 7.2 Hz, 1H), 6.79 (t, J = 7.2 Hz, 2H), 6.68 (d, J = 7.8 Hz, 2H), 6.61 (d, J = 2.4 Hz, 1H), 5.58 (d, J = 2.4 Hz, 1H), 5.06 (s, 1H), 4.83 (s, 1H), 2.16 (t, J = 7.8 Hz, 2H), 1.98 (t, J = 7.8 Hz, 2H), 1.91 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 154.8, 144.3, 139.5, 137.4, 136.0, 133.4, 131.4, 129.5, 128.8, 128.7, 127.9, 127.3, 127.0, 126.8, 126.5, 126.0, 125.5, 125.2, 124.8, 124.1, 120.8, 116.7, 112.9, 32.0, 31.1, 15.3. HRMS (ESI-TOF, m/z): calcd for C32H28ClN2NaOS+, [M + Na]+, 511.1815, found 511.1818.
1-(4-(Methylthio)but-1-en-2-yl)-4-(1-(naphthalen-2-yl)vinyl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3p)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3p was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (74 mg, 76% yield). IR νmax (neat)/cm−1: 3039, 2976, 1677, 1562, 1473, 1390, 1124, 973, 837; 1H NMR (600 MHz, CDCl3): δ 7.76 (d, J = 4.8 Hz, 2H), 7.74 (d, J = 7.2 Hz, 1H), 7.68–7.65 (m, 3H), 7.51–7.48 (m, 3H), 7.44–7.40 (m, 2H), 7.38–7.37 (m, 2H), 7.31 (t, J = 7.2 Hz, 1H), 7.16–7.14 (m, 3H), 5.84 (s, 1H), 5.74 (s, 1H), 5.47 (s, 1H), 5.07 (s, 1H), 2.29 (t, J = 7.8 Hz, 2H), 2.11 (t, J = 7.8 Hz, 2H), 1.97 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 155.7, 145.1, 138.3, 137.7, 135.9, 133.2, 132.8, 129.9, 129.5, 129.1, 128.7, 128.1, 127.8, 127.5, 127.3, 126.4, 126.0, 125.7, 125.6, 125.1, 124.1, 119.2, 117.9, 114.3, 31.3, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C32H28ClN2NaOS+, [M + Na]+, 511.1815, found 511.1816.
1) and obtained as a foam solid (74 mg, 76% yield). IR νmax (neat)/cm−1: 3039, 2976, 1677, 1562, 1473, 1390, 1124, 973, 837; 1H NMR (600 MHz, CDCl3): δ 7.76 (d, J = 4.8 Hz, 2H), 7.74 (d, J = 7.2 Hz, 1H), 7.68–7.65 (m, 3H), 7.51–7.48 (m, 3H), 7.44–7.40 (m, 2H), 7.38–7.37 (m, 2H), 7.31 (t, J = 7.2 Hz, 1H), 7.16–7.14 (m, 3H), 5.84 (s, 1H), 5.74 (s, 1H), 5.47 (s, 1H), 5.07 (s, 1H), 2.29 (t, J = 7.8 Hz, 2H), 2.11 (t, J = 7.8 Hz, 2H), 1.97 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 155.7, 145.1, 138.3, 137.7, 135.9, 133.2, 132.8, 129.9, 129.5, 129.1, 128.7, 128.1, 127.8, 127.5, 127.3, 126.4, 126.0, 125.7, 125.6, 125.1, 124.1, 119.2, 117.9, 114.3, 31.3, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C32H28ClN2NaOS+, [M + Na]+, 511.1815, found 511.1816.
4-(1-(6-Methoxynaphthalen-2-yl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3q)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3q was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (86 mg, 83% yield). IR νmax (neat)/cm−1: 3027, 2917, 1673, 1510, 1445, 1307, 1011, 904, 816; 1H NMR (600 MHz, CDCl3): δ 7.69–7.65 (m, 4H), 7.56 (d, J = 9.0 Hz, 1H), 7.50–7.47 (m, 3H), 7.39–7.38 (m, 2H), 7.30 (t, J = 7.2 Hz, 1H), 7.17–7.16 (m, 4H), 7.11 (dd, J = 2.4, 9.0 Hz, 1H), 7.05 (d, J = 2.4 Hz, 1H), 5.81 (d, J = 0.6 Hz, 1H), 5.67 (s, 1H), 5.47 (s, 1H), 5.07 (s, 1H), 3.89 (s, 3H), 2.28 (t, J = 7.8 Hz, 2H), 2.10 (t, J = 7.8 Hz, 2H), 1.96 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 157.6, 155.6, 145.0, 138.2, 135.9, 135.4, 133.9, 129.8, 129.5, 129.1, 128.7, 127.7, 126.4, 126.3, 125.7, 125.4, 124.0, 118.4, 118.4, 117.9, 114.5, 105.8, 55.1, 31.2, 31.1, 15.3. HRMS (ESI-TOF, m/z): calcd for C33H30N2NaO2S+, [M + Na]+, 541.1920, found 541.1922.
1) and obtained as a foam solid (86 mg, 83% yield). IR νmax (neat)/cm−1: 3027, 2917, 1673, 1510, 1445, 1307, 1011, 904, 816; 1H NMR (600 MHz, CDCl3): δ 7.69–7.65 (m, 4H), 7.56 (d, J = 9.0 Hz, 1H), 7.50–7.47 (m, 3H), 7.39–7.38 (m, 2H), 7.30 (t, J = 7.2 Hz, 1H), 7.17–7.16 (m, 4H), 7.11 (dd, J = 2.4, 9.0 Hz, 1H), 7.05 (d, J = 2.4 Hz, 1H), 5.81 (d, J = 0.6 Hz, 1H), 5.67 (s, 1H), 5.47 (s, 1H), 5.07 (s, 1H), 3.89 (s, 3H), 2.28 (t, J = 7.8 Hz, 2H), 2.10 (t, J = 7.8 Hz, 2H), 1.96 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 157.6, 155.6, 145.0, 138.2, 135.9, 135.4, 133.9, 129.8, 129.5, 129.1, 128.7, 127.7, 126.4, 126.3, 125.7, 125.4, 124.0, 118.4, 118.4, 117.9, 114.5, 105.8, 55.1, 31.2, 31.1, 15.3. HRMS (ESI-TOF, m/z): calcd for C33H30N2NaO2S+, [M + Na]+, 541.1920, found 541.1922.
1-(4-(Methylthio)but-1-en-2-yl)-2,5-diphenyl-4-(thiophen-2-yl)-1,2-dihydro-3H-pyrazol-3-one (3r)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3r was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (51 mg, 57% yield). IR νmax (neat)/cm−1: 3032, 2987, 1681, 1527, 1397, 1262, 1098, 1002, 935, 882; 1H NMR (600 MHz, CDCl3): δ 7.63 (d, J = 7.8 Hz, 2H), 7.48–7.45 (m, 4H), 7.37–7.29 (m, 4H), 7.09 (d, J = 5.4 Hz, 1H), 6.95 (d, J = 3.6 Hz, 1H), 6.84 (dd, J = 3.6, 5.4 Hz, 1H), 5.73 (s, 1H), 5.48 (s, 1H), 5.34 (s, 1H), 5.08 (s, 1H), 2.26 (t, J = 7.8 Hz, 2H), 2.06 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.1, 155.7, 145.0, 143.9, 135.8, 131.8, 129.8, 129.6, 129.0, 128.7, 128.0, 127.0, 126.4, 125.1, 124.9, 124.4, 124.3, 124.2, 118.1, 117.5, 114.2, 31.2, 31.0, 15.3. HRMS (ESI-TOF, m/z): calcd for C26H25N2OS+, [M + H]+, 445.1403, found 445.1405.
1) and obtained as a foam solid (51 mg, 57% yield). IR νmax (neat)/cm−1: 3032, 2987, 1681, 1527, 1397, 1262, 1098, 1002, 935, 882; 1H NMR (600 MHz, CDCl3): δ 7.63 (d, J = 7.8 Hz, 2H), 7.48–7.45 (m, 4H), 7.37–7.29 (m, 4H), 7.09 (d, J = 5.4 Hz, 1H), 6.95 (d, J = 3.6 Hz, 1H), 6.84 (dd, J = 3.6, 5.4 Hz, 1H), 5.73 (s, 1H), 5.48 (s, 1H), 5.34 (s, 1H), 5.08 (s, 1H), 2.26 (t, J = 7.8 Hz, 2H), 2.06 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.1, 155.7, 145.0, 143.9, 135.8, 131.8, 129.8, 129.6, 129.0, 128.7, 128.0, 127.0, 126.4, 125.1, 124.9, 124.4, 124.3, 124.2, 118.1, 117.5, 114.2, 31.2, 31.0, 15.3. HRMS (ESI-TOF, m/z): calcd for C26H25N2OS+, [M + H]+, 445.1403, found 445.1405.
4-(1-(1-Methyl-1H-pyrrol-2-yl)vinyl)-1-(4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (3s)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3s was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (62 mg, 70% yield). IR νmax (neat)/cm−1: 3072, 2976, 1672, 1476, 1331, 1229, 1152, 1034, 913, 826; 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 7.8 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.31 (t, J = 7.8 Hz, 4H), 7.28–7.25 (m, 2H), 6.29 (t, J = 2.4 Hz, 1H), 5.92 (dd, J = 1.8, 3.0 Hz, 1H), 5.85 (d, J = 1.8 Hz, 1H), 5.84 (s, 1H), 5.43 (d, J = 1.8 Hz, 1H), 5.38 (s, 1H), 5.02 (s, 1H), 3.42 (s, 3H), 2.22 (t, J = 7.8 Hz, 2H), 2.01 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.0, 144.9, 135.8, 133.2, 129.7, 129.5, 128.7, 127.6, 126.4, 124.1, 122.8, 119.4, 117.7, 114.0, 109.8, 107.2, 34.5, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C27H27N3NaOS+, [M + Na]+, 464.1767, found 464.1772.
1) and obtained as a foam solid (62 mg, 70% yield). IR νmax (neat)/cm−1: 3072, 2976, 1672, 1476, 1331, 1229, 1152, 1034, 913, 826; 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 7.8 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.31 (t, J = 7.8 Hz, 4H), 7.28–7.25 (m, 2H), 6.29 (t, J = 2.4 Hz, 1H), 5.92 (dd, J = 1.8, 3.0 Hz, 1H), 5.85 (d, J = 1.8 Hz, 1H), 5.84 (s, 1H), 5.43 (d, J = 1.8 Hz, 1H), 5.38 (s, 1H), 5.02 (s, 1H), 3.42 (s, 3H), 2.22 (t, J = 7.8 Hz, 2H), 2.01 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 155.0, 144.9, 135.8, 133.2, 129.7, 129.5, 128.7, 127.6, 126.4, 124.1, 122.8, 119.4, 117.7, 114.0, 109.8, 107.2, 34.5, 31.2, 15.3. HRMS (ESI-TOF, m/z): calcd for C27H27N3NaOS+, [M + Na]+, 464.1767, found 464.1772.
1-(4-(Methylthio)but-1-en-2-yl)-2,5-diphenyl-4-(prop-1-en-2-yl)-1,2-dihydro-3H-pyrazol-3-one (3t)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3t was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (54 mg, 72% yield). IR νmax (neat)/cm−1: 3051, 2982, 1672, 1568, 2448, 1379, 1253, 1062, 937, 784; 1H NMR (600 MHz, CDCl3): δ 7.55 (d, J = 7.8 Hz, 2H), 7.47–7.43 (m, 7H), 7.28 (t, J = 7.2 Hz, 1H), 5.46 (s, 1H), 5.31 (s, 1H), 5.15 (s, 1H), 4.98 (s, 1H), 2.21 (t, J = 7.8 Hz, 2H), 1.99 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H), 1.77 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 153.7, 144.9, 135.9, 133.8, 130.2, 129.8, 128.7, 128.2, 126.4, 124.1, 117.4, 117.2, 114.7, 31.3, 31.2, 22.6, 15.3. HRMS (ESI-TOF, m/z): calcd for C23H25N2OS+, [M + H]+, 377.5255, found 377.5256.
1) and obtained as a foam solid (54 mg, 72% yield). IR νmax (neat)/cm−1: 3051, 2982, 1672, 1568, 2448, 1379, 1253, 1062, 937, 784; 1H NMR (600 MHz, CDCl3): δ 7.55 (d, J = 7.8 Hz, 2H), 7.47–7.43 (m, 7H), 7.28 (t, J = 7.2 Hz, 1H), 5.46 (s, 1H), 5.31 (s, 1H), 5.15 (s, 1H), 4.98 (s, 1H), 2.21 (t, J = 7.8 Hz, 2H), 1.99 (t, J = 7.8 Hz, 2H), 1.93 (s, 3H), 1.77 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.3, 153.7, 144.9, 135.9, 133.8, 130.2, 129.8, 128.7, 128.2, 126.4, 124.1, 117.4, 117.2, 114.7, 31.3, 31.2, 22.6, 15.3. HRMS (ESI-TOF, m/z): calcd for C23H25N2OS+, [M + H]+, 377.5255, found 377.5256.
1-(4-(Methylthio)but-1-en-2-yl)-2,5-diphenyl-4-(thiophen-2-yl)-1,2-dihydro-3H-pyrazol-3-one (3aa)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3aa was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (57 mg, 63% yield). IR νmax (neat)/cm−1: 3022, 2918, 1672, 1580, 1397, 1301, 1189, 1011, 934, 781; 1H NMR (600 MHz, CDCl3): δ 7.49 (d, J = 8.4 Hz, 2H), 7.34–7.30 (m, 4H), 7.28–7.24 (m, 3H), 7.21 (t, J = 7.2 Hz, 2H), 7.16 (t, J = 7.2 Hz, 2H), 7.11 (t, J = 7.2 Hz, 1H), 5.68 (d, J = 1.2 Hz, 1H), 5.67 (d, J = 1.2 Hz, 1H), 5.41 (s, 1H), 5.04 (s, 1H), 2.40 (s, 3H), 2.25 (t, J = 7.8 Hz, 2H), 2.05 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 155.1, 145.1, 140.3, 138.4, 136.3, 133.4, 130.0, 129.5, 129.3, 127.8, 127.8, 127.1, 126.9, 124.3, 118.7, 117.8, 114.1, 31.2, 20.9, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaOS+, [M + Na]+, 475.1815, found 475.1817.
1) and obtained as a foam solid (57 mg, 63% yield). IR νmax (neat)/cm−1: 3022, 2918, 1672, 1580, 1397, 1301, 1189, 1011, 934, 781; 1H NMR (600 MHz, CDCl3): δ 7.49 (d, J = 8.4 Hz, 2H), 7.34–7.30 (m, 4H), 7.28–7.24 (m, 3H), 7.21 (t, J = 7.2 Hz, 2H), 7.16 (t, J = 7.2 Hz, 2H), 7.11 (t, J = 7.2 Hz, 1H), 5.68 (d, J = 1.2 Hz, 1H), 5.67 (d, J = 1.2 Hz, 1H), 5.41 (s, 1H), 5.04 (s, 1H), 2.40 (s, 3H), 2.25 (t, J = 7.8 Hz, 2H), 2.05 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 155.1, 145.1, 140.3, 138.4, 136.3, 133.4, 130.0, 129.5, 129.3, 127.8, 127.8, 127.1, 126.9, 124.3, 118.7, 117.8, 114.1, 31.2, 20.9, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaOS+, [M + Na]+, 475.1815, found 475.1817.
2-(4-Methoxyphenyl)-1-(4-(methylthio)but-1-en-2-yl)-5-phenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3ab)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3ab was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (56 mg, 60% yield). IR νmax (neat)/cm−1: 3030, 2944, 1677, 1589, 1491, 1270, 1086, 933, 812; 1H NMR (600 MHz, CDCl3): δ 7.50 (d, J = 9.0 Hz, 2H), 7.32–7.30 (m, 4H), 7.25 (t, J = 7.2 Hz, 1H), 7.20 (t, J = 7.2 Hz, 2H), 7.15 (t, J = 7.2 Hz, 2H), 7.11 (t, J = 7.2 Hz, 1H), 6.99 (d, J = 9.0 Hz, 2H), 5.67 (s, 1H), 5.66 (s, 1H), 5.39 (s, 1H), 5.05 (s, 1H), 3.84 (s, 3H), 2.25 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.7, 158.5, 154.7, 145.1, 140.4, 138.5, 130.0, 129.5, 129.4, 128.9, 127.9, 127.8, 127.2, 127.0, 126.3, 118.7, 118.0, 114.3, 114.0, 55.5, 31.3, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H29N2OS+, [M + H]+, 469.1944, found 469.1946.
1) and obtained as a foam solid (56 mg, 60% yield). IR νmax (neat)/cm−1: 3030, 2944, 1677, 1589, 1491, 1270, 1086, 933, 812; 1H NMR (600 MHz, CDCl3): δ 7.50 (d, J = 9.0 Hz, 2H), 7.32–7.30 (m, 4H), 7.25 (t, J = 7.2 Hz, 1H), 7.20 (t, J = 7.2 Hz, 2H), 7.15 (t, J = 7.2 Hz, 2H), 7.11 (t, J = 7.2 Hz, 1H), 6.99 (d, J = 9.0 Hz, 2H), 5.67 (s, 1H), 5.66 (s, 1H), 5.39 (s, 1H), 5.05 (s, 1H), 3.84 (s, 3H), 2.25 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.7, 158.5, 154.7, 145.1, 140.4, 138.5, 130.0, 129.5, 129.4, 128.9, 127.9, 127.8, 127.2, 127.0, 126.3, 118.7, 118.0, 114.3, 114.0, 55.5, 31.3, 15.3. HRMS (ESI-TOF, m/z): calcd for C29H29N2OS+, [M + H]+, 469.1944, found 469.1946.
2-(4-Fluorophenyl)-1-(4-(methylthio)but-1-en-2-yl)-5-phenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3ac)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3ac was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (67 mg, 73% yield). IR νmax (neat)/cm−1: 3037, 2975, 1658, 1589, 1452, 1358, 1284, 1031, 982; 1H NMR (600 MHz, CDCl3): δ 7.60–7.57 (m, 2H), 7.32 (dd, J = 7.2, 8.4 Hz, 2H), 7.29 (t, J = 6.6 Hz, 2H), 7.26 (d, J = 7.2 Hz, 1H), 7.22 (t, J = 7.2 Hz, 2H), 7.18–7.15 (m, 4H), 7.13 (t, J = 7.2 Hz, 1H), 5.68 (d, J = 1.2 Hz, 1H), 5.64 (d, J = 1.2 Hz, 1H), 5.42 (s, 1H), 5.07 (s, 1H), 2.26 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.8, 161.9, 160.3, 155.7, 145.2, 140.2, 138.4, 132.1, 130.0, 129.7, 129.2, 128.0, 127.3, 126.9, 126.1, 126.1, 119.0, 118.0, 115.8, 115.6, 114.3, 31.4, 31.1, 15.4. 19F NMR (600 MHz, CDCl3): δ −114.98. HRMS (ESI-TOF, m/z): calcd for C28H26FN2OS+, [M + H]+, 457.1744, found 457.1746.
1) and obtained as a foam solid (67 mg, 73% yield). IR νmax (neat)/cm−1: 3037, 2975, 1658, 1589, 1452, 1358, 1284, 1031, 982; 1H NMR (600 MHz, CDCl3): δ 7.60–7.57 (m, 2H), 7.32 (dd, J = 7.2, 8.4 Hz, 2H), 7.29 (t, J = 6.6 Hz, 2H), 7.26 (d, J = 7.2 Hz, 1H), 7.22 (t, J = 7.2 Hz, 2H), 7.18–7.15 (m, 4H), 7.13 (t, J = 7.2 Hz, 1H), 5.68 (d, J = 1.2 Hz, 1H), 5.64 (d, J = 1.2 Hz, 1H), 5.42 (s, 1H), 5.07 (s, 1H), 2.26 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.8, 161.9, 160.3, 155.7, 145.2, 140.2, 138.4, 132.1, 130.0, 129.7, 129.2, 128.0, 127.3, 126.9, 126.1, 126.1, 119.0, 118.0, 115.8, 115.6, 114.3, 31.4, 31.1, 15.4. 19F NMR (600 MHz, CDCl3): δ −114.98. HRMS (ESI-TOF, m/z): calcd for C28H26FN2OS+, [M + H]+, 457.1744, found 457.1746.
2-(4-Bromophenyl)-5-(4-methoxyphenyl)-1-(4-(methylthio)but-1-en-2-yl)-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3ad)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3ad was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (68 mg, 66% yield). IR νmax (neat)/cm−1: 3033, 2989, 1668, 1565, 1487, 1312, 1220, 1101, 979, 856; 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 7.8 Hz, 2H), 7.52 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 7.2 Hz, 2H), 7.30 (d, J = 7.2 Hz, 2H), 7.26 (d, J = 7.2 Hz, 1H), 7.23 (t, J = 7.2 Hz, 2H), 7.17 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 5.68 (d, J = 0.6 Hz, 1H), 5.62 (d, J = 0.6 Hz, 1H), 5.44 (s, 1H), 5.08 (s, 1H), 2.26 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 156.1, 145.2, 140.1, 138.2, 135.1, 131.9, 130.0, 129.8, 129.7, 129.0, 127.9, 127.3, 126.9, 125.3, 119.8, 119.0, 118.0, 114.5, 31.4, 30.9, 15.4. HRMS (ESI-TOF, m/z): calcd for C28H25BrN2NaOS+, [M + Na]+, 539.0763, found 539.0763.
1) and obtained as a foam solid (68 mg, 66% yield). IR νmax (neat)/cm−1: 3033, 2989, 1668, 1565, 1487, 1312, 1220, 1101, 979, 856; 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 7.8 Hz, 2H), 7.52 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 7.2 Hz, 2H), 7.30 (d, J = 7.2 Hz, 2H), 7.26 (d, J = 7.2 Hz, 1H), 7.23 (t, J = 7.2 Hz, 2H), 7.17 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 5.68 (d, J = 0.6 Hz, 1H), 5.62 (d, J = 0.6 Hz, 1H), 5.44 (s, 1H), 5.08 (s, 1H), 2.26 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 156.1, 145.2, 140.1, 138.2, 135.1, 131.9, 130.0, 129.8, 129.7, 129.0, 127.9, 127.3, 126.9, 125.3, 119.8, 119.0, 118.0, 114.5, 31.4, 30.9, 15.4. HRMS (ESI-TOF, m/z): calcd for C28H25BrN2NaOS+, [M + Na]+, 539.0763, found 539.0763.
2-(2-Ethylphenyl)-1-(4-(methylthio)but-1-en-2-yl)-5-phenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3ae)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3ae was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (47 mg, 50% yield). IR νmax (neat)/cm−1: 3038, 2933, 1681, 157, 1392, 1267, 1083, 962, 828; 1H NMR (600 MHz, CDCl3): δ 7.42 (d, J = 6.6 Hz, 1H), 7.39 (dt, J = 1.2, 7.2 Hz, 1H), 7.35 (d, J = 7.2 Hz, 1H), 7.32–7.29 (m, 5H), 7.23 (d, J = 7.2 Hz, 1H), 7.19 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 2H), 7.09 (t, J = 7.2 Hz, 1H), 5.74 (d, J = 1.2 Hz, 1H), 5.72 (d, J = 1.2 Hz, 1H), 5.20 (s, 1H), 4.99 (s, 1H), 2.91–2.84 (m, 1H), 2.73–2.67 (m, 1H), 2.27–2.24 (m, 2H), 2.11–2.07 (m, 2H), 1.94 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ 165.6, 154.1, 144.8, 142.8, 140.5, 138.5, 134.4, 129.8, 129.5, 129.4, 129.0, 127.9, 127.8, 127.1, 127.0, 126.2, 118.8, 118.0, 113.0, 32.1, 31.1, 23.9, 15.4, 14.0. HRMS (ESI-TOF, m/z): calcd for C30H30N2NaOS+, [M + Na]+, 489.1971, found 489.1972.
1) and obtained as a foam solid (47 mg, 50% yield). IR νmax (neat)/cm−1: 3038, 2933, 1681, 157, 1392, 1267, 1083, 962, 828; 1H NMR (600 MHz, CDCl3): δ 7.42 (d, J = 6.6 Hz, 1H), 7.39 (dt, J = 1.2, 7.2 Hz, 1H), 7.35 (d, J = 7.2 Hz, 1H), 7.32–7.29 (m, 5H), 7.23 (d, J = 7.2 Hz, 1H), 7.19 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 2H), 7.09 (t, J = 7.2 Hz, 1H), 5.74 (d, J = 1.2 Hz, 1H), 5.72 (d, J = 1.2 Hz, 1H), 5.20 (s, 1H), 4.99 (s, 1H), 2.91–2.84 (m, 1H), 2.73–2.67 (m, 1H), 2.27–2.24 (m, 2H), 2.11–2.07 (m, 2H), 1.94 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ 165.6, 154.1, 144.8, 142.8, 140.5, 138.5, 134.4, 129.8, 129.5, 129.4, 129.0, 127.9, 127.8, 127.1, 127.0, 126.2, 118.8, 118.0, 113.0, 32.1, 31.1, 23.9, 15.4, 14.0. HRMS (ESI-TOF, m/z): calcd for C30H30N2NaOS+, [M + Na]+, 489.1971, found 489.1972.
2-(2-Fluorophenyl)-1-(4-(methylthio)but-1-en-2-yl)-5-phenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3af)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3af was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (50 mg, 55% yield). IR νmax (neat)/cm−1: 3062, 2983, 1668, 1549, 1433, 1327, 1252, 1098, 972, 819; 1H NMR (600 MHz, CDCl3): δ 7.51 (t, J = 6.0 Hz, 1H), 7.41–7.37 (m, 1H), 7.33–7.25 (m, 4H), 7.23 (d, J = 7.8 Hz, 3H), 7.20 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 2H), 7.09 (t, J = 7.2 Hz, 1H), 5.74 (d, J = 1.2 Hz, 1H), 5.70 (d, J = 1.2 Hz, 1H), 5.39 (s, 1H), 5.05 (s, 1H), 2.27 (t, J = 7.8 Hz, 2H), 2.08 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 165.2, 158.8, 157.2, 155.2, 144.8, 140.3, 138.3, 130.1, 130.0, 129.8, 129.6, 129.1, 127.9, 127.8, 127.1, 127.0, 124.3, 118.8, 118.4, 116.8, 116.7, 113.1, 31.5, 31.2, 15.3. 19F NMR (600 MHz, CDCl3): δ −118.90. HRMS (ESI-TOF, m/z): calcd for C28H25FN2NaOS+, [M + Na]+, 479.1564, found 479.1566.
1) and obtained as a foam solid (50 mg, 55% yield). IR νmax (neat)/cm−1: 3062, 2983, 1668, 1549, 1433, 1327, 1252, 1098, 972, 819; 1H NMR (600 MHz, CDCl3): δ 7.51 (t, J = 6.0 Hz, 1H), 7.41–7.37 (m, 1H), 7.33–7.25 (m, 4H), 7.23 (d, J = 7.8 Hz, 3H), 7.20 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 2H), 7.09 (t, J = 7.2 Hz, 1H), 5.74 (d, J = 1.2 Hz, 1H), 5.70 (d, J = 1.2 Hz, 1H), 5.39 (s, 1H), 5.05 (s, 1H), 2.27 (t, J = 7.8 Hz, 2H), 2.08 (t, J = 7.8 Hz, 2H), 1.94 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 165.2, 158.8, 157.2, 155.2, 144.8, 140.3, 138.3, 130.1, 130.0, 129.8, 129.6, 129.1, 127.9, 127.8, 127.1, 127.0, 124.3, 118.8, 118.4, 116.8, 116.7, 113.1, 31.5, 31.2, 15.3. 19F NMR (600 MHz, CDCl3): δ −118.90. HRMS (ESI-TOF, m/z): calcd for C28H25FN2NaOS+, [M + Na]+, 479.1564, found 479.1566.
1-(4-(Methylthio)but-1-en-2-yl)-2-(perfluorophenyl)-5-phenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3ag)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3ag was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (74 mg, 70% yield). IR νmax (neat)/cm−1: 3066, 2952, 1561, 1445, 1270, 1184, 1077, 977, 835; 1H NMR (600 MHz, CDCl3): δ 7.32 (d, J = 6.6 Hz, 2H), 7.28–7.26 (m, 3H), 7.20 (t, J = 7.2 Hz, 2H), 7.14 (dd, J = 6.0, 7.8 Hz, 2H), 7.10 (t, J = 7.2 Hz, 1H), 5.75 (d, J = 1.2 Hz, 1H), 5.71 (d, J = 1.2 Hz, 1H), 5.40 (s, 1H), 5.14 (s, 1H), 2.33 (t, J = 7.8 Hz, 2H), 2.11 (t, J = 7.8 Hz, 2H), 1.96 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 165.9, 157.2, 145.9, 145.3, 144.2, 141.3, 139.9, 138.9, 137.8, 137.2, 130.2, 130.0, 129.7, 129.6, 128.6, 128.1, 128.0, 127.4, 126.9, 119.3, 118.5, 113.1, 111.8, 31.1, 31.1, 15.3. 19F NMR (600 MHz, CDCl3): δ −142.99, −143.02, −151.13, −151.17, −151.21, −161.11, −161.14, −161.14, −161.18. HRMS (ESI-TOF, m/z): calcd for C28H21F5N2NaOS+, [M + Na]+, 551.1187, found 551.1188.
1) and obtained as a foam solid (74 mg, 70% yield). IR νmax (neat)/cm−1: 3066, 2952, 1561, 1445, 1270, 1184, 1077, 977, 835; 1H NMR (600 MHz, CDCl3): δ 7.32 (d, J = 6.6 Hz, 2H), 7.28–7.26 (m, 3H), 7.20 (t, J = 7.2 Hz, 2H), 7.14 (dd, J = 6.0, 7.8 Hz, 2H), 7.10 (t, J = 7.2 Hz, 1H), 5.75 (d, J = 1.2 Hz, 1H), 5.71 (d, J = 1.2 Hz, 1H), 5.40 (s, 1H), 5.14 (s, 1H), 2.33 (t, J = 7.8 Hz, 2H), 2.11 (t, J = 7.8 Hz, 2H), 1.96 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 165.9, 157.2, 145.9, 145.3, 144.2, 141.3, 139.9, 138.9, 137.8, 137.2, 130.2, 130.0, 129.7, 129.6, 128.6, 128.1, 128.0, 127.4, 126.9, 119.3, 118.5, 113.1, 111.8, 31.1, 31.1, 15.3. 19F NMR (600 MHz, CDCl3): δ −142.99, −143.02, −151.13, −151.17, −151.21, −161.11, −161.14, −161.14, −161.18. HRMS (ESI-TOF, m/z): calcd for C28H21F5N2NaOS+, [M + Na]+, 551.1187, found 551.1188.
5-(4-Fluorophenyl)-1-(4-(methylthio)but-1-en-2-yl)-2-phenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3ah)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3ah was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (47 mg, 51% yield). IR νmax (neat)/cm−1: 3036, 2942, 1670, 1589, 1433, 1362, 1098, 937, 828; 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 7.8 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.32–7.30 (m, 3H), 7.27 (dd, J = 1.2, 7.8 Hz, 2H), 7.17–7.13 (m, 3H), 6.90 (t, J = 9.0 Hz, 2H), 5.72 (d, J = 1.2 Hz, 1H), 5.70 (d, J = 1.2 Hz, 1H), 5.44 (s, 1H), 5.08 (s, 1H), 2.24 (t, J = 7.8 Hz, 2H), 2.03 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 164.2, 162.5, 154.3, 145.2, 140.2, 138.2, 135.9, 132.0, 132.0, 128.8, 128.0, 127.4, 127.0, 126.5, 125.3, 124.2, 119.1, 118.2, 115.2, 115.1, 114.5, 31.3, 31.0, 15.4. 19F NMR (600 MHz, CDCl3): δ −110.07. HRMS (ESI-TOF, m/z): calcd for C28H26FN2OS+, [M + H]+, 457.1744, found 457.1744.
1) and obtained as a foam solid (47 mg, 51% yield). IR νmax (neat)/cm−1: 3036, 2942, 1670, 1589, 1433, 1362, 1098, 937, 828; 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 7.8 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.32–7.30 (m, 3H), 7.27 (dd, J = 1.2, 7.8 Hz, 2H), 7.17–7.13 (m, 3H), 6.90 (t, J = 9.0 Hz, 2H), 5.72 (d, J = 1.2 Hz, 1H), 5.70 (d, J = 1.2 Hz, 1H), 5.44 (s, 1H), 5.08 (s, 1H), 2.24 (t, J = 7.8 Hz, 2H), 2.03 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 164.2, 162.5, 154.3, 145.2, 140.2, 138.2, 135.9, 132.0, 132.0, 128.8, 128.0, 127.4, 127.0, 126.5, 125.3, 124.2, 119.1, 118.2, 115.2, 115.1, 114.5, 31.3, 31.0, 15.4. 19F NMR (600 MHz, CDCl3): δ −110.07. HRMS (ESI-TOF, m/z): calcd for C28H26FN2OS+, [M + H]+, 457.1744, found 457.1744.
5-(4-Methoxyphenyl)-1-(4-(methylthio)but-1-en-2-yl)-4-(1-phenylvinyl)-2-(p-tolyl)-1,2-dihydro-3H-pyrazol-3-one (3ai)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3ai was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (69 mg, 71% yield). IR νmax (neat)/cm−1: 3052, 2989, 1671, 1489, 1345, 1287, 1176, 1044, 929, 817; 1H NMR (600 MHz, CDCl3): δ 7.48 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 7.2 Hz, 2H), 7.27 (t, J = 8.4 Hz, 4H), 7.17 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 6.73 (d, J = 8.4 Hz, 2H), 5.70 (d, J = 1.2 Hz, 1H), 5.64 (s, 1H), 5.43 (s, 1H), 5.06 (s, 1H), 3.75 (s, 3H), 2.39 (s, 3H), 2.25 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.8, 160.6, 155.1, 145.4, 140.2, 138.5, 136.2, 133.5, 131.4, 129.3, 127.8, 127.1, 126.8, 124.3, 121.4, 118.5, 117.9, 113.4, 55.1, 31.3, 31.0, 20.9, 15.3. HRMS (ESI-TOF, m/z): calcd for C30H30N2NaO2S+, [M + Na]+, 505.1920, found 505.1920.
1) and obtained as a foam solid (69 mg, 71% yield). IR νmax (neat)/cm−1: 3052, 2989, 1671, 1489, 1345, 1287, 1176, 1044, 929, 817; 1H NMR (600 MHz, CDCl3): δ 7.48 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 7.2 Hz, 2H), 7.27 (t, J = 8.4 Hz, 4H), 7.17 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 6.73 (d, J = 8.4 Hz, 2H), 5.70 (d, J = 1.2 Hz, 1H), 5.64 (s, 1H), 5.43 (s, 1H), 5.06 (s, 1H), 3.75 (s, 3H), 2.39 (s, 3H), 2.25 (t, J = 7.8 Hz, 2H), 2.04 (t, J = 7.8 Hz, 2H), 1.95 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.8, 160.6, 155.1, 145.4, 140.2, 138.5, 136.2, 133.5, 131.4, 129.3, 127.8, 127.1, 126.8, 124.3, 121.4, 118.5, 117.9, 113.4, 55.1, 31.3, 31.0, 20.9, 15.3. HRMS (ESI-TOF, m/z): calcd for C30H30N2NaO2S+, [M + Na]+, 505.1920, found 505.1920.
5-(4-Bromophenyl)-1-(4-(methylthio)but-1-en-2-yl)-4-(1-phenylvinyl)-2-(p-tolyl)-1,2-dihydro-3H-pyrazol-3-one (3aj)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3aj was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (53 mg, 50% yield). IR νmax (neat)/cm−1: 3039, 2962, 1670, 1527, 1363, 1299, 1108, 1036, 898; 1H NMR (600 MHz, CDCl3): δ 7.46 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 8.4 Hz, 2H), 7.28–7.26 (m, 4H), 7.19–7.16 (m, 5H), 5.69 (s, 1H), 5.68 (s, 1H), 5.40 (s, 1H), 5.07 (s, 1H), 2.40 (s, 3H), 2.25 (t, J = 7.8 Hz, 2H), 2.02 (t, J = 7.8 Hz, 2H), 1.96 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ 164.4, 153.7, 145.2, 140.3, 138.3, 136.6, 133.3, 132.0, 131.5, 131.2, 131.1, 129.5, 128.3, 128.1, 127.4, 127.0, 124.4, 124.2, 124.1, 119.1, 118.8, 118.1, 114.8, 31.4, 31.2, 21.0, 15.4. HRMS (ESI-TOF, m/z): calcd for C29H28BrN2OS+, [M + H]+, 531.1100, found 531.1103.
1) and obtained as a foam solid (53 mg, 50% yield). IR νmax (neat)/cm−1: 3039, 2962, 1670, 1527, 1363, 1299, 1108, 1036, 898; 1H NMR (600 MHz, CDCl3): δ 7.46 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 8.4 Hz, 2H), 7.28–7.26 (m, 4H), 7.19–7.16 (m, 5H), 5.69 (s, 1H), 5.68 (s, 1H), 5.40 (s, 1H), 5.07 (s, 1H), 2.40 (s, 3H), 2.25 (t, J = 7.8 Hz, 2H), 2.02 (t, J = 7.8 Hz, 2H), 1.96 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ 164.4, 153.7, 145.2, 140.3, 138.3, 136.6, 133.3, 132.0, 131.5, 131.2, 131.1, 129.5, 128.3, 128.1, 127.4, 127.0, 124.4, 124.2, 124.1, 119.1, 118.8, 118.1, 114.8, 31.4, 31.2, 21.0, 15.4. HRMS (ESI-TOF, m/z): calcd for C29H28BrN2OS+, [M + H]+, 531.1100, found 531.1103.
5-Methyl-1-(4-(methylthio)but-1-en-2-yl)-2-phenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (3ak)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3ak was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (62 mg, 83% yield). IR νmax (neat)/cm−1: 3072, 2916, 1667, 1539, 1420, 1331, 1298, 1032, 919, 827; 1H NMR (600 MHz, CDCl3): δ 7.49 (d, J = 7.2 Hz, 2H), 7.49–7.40 (m, 2H), 7.34 (t, J = 7.2 Hz, 2H), 7.29 (t, J = 7.2 Hz, 1H), 7.25 (t, J = 7.2 Hz, 1H), 5.73 (d, J = 1.2 Hz, 1H), 5.66 (d, J = 1.2 Hz, 1H), 5.38 (s, 1H), 5.27 (s, 1H), 2.36 (t, J = 7.2 Hz, 2H), 2.23 (t, J = 7.2 Hz, 2H), 2.03 (s, 3H), 1.98 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.8, 152.1, 143.7, 141.0, 138.6, 136.4, 128.7, 128.3, 127.5, 126.9, 126.0, 123.2, 117.9, 115.4, 110.8, 32.6, 30.9, 15.4, 13.0. HRMS (ESI-TOF, m/z): calcd for C23H25N2OS+, [M + H]+, 377.1682, found 377.1686.
1) and obtained as a foam solid (62 mg, 83% yield). IR νmax (neat)/cm−1: 3072, 2916, 1667, 1539, 1420, 1331, 1298, 1032, 919, 827; 1H NMR (600 MHz, CDCl3): δ 7.49 (d, J = 7.2 Hz, 2H), 7.49–7.40 (m, 2H), 7.34 (t, J = 7.2 Hz, 2H), 7.29 (t, J = 7.2 Hz, 1H), 7.25 (t, J = 7.2 Hz, 1H), 5.73 (d, J = 1.2 Hz, 1H), 5.66 (d, J = 1.2 Hz, 1H), 5.38 (s, 1H), 5.27 (s, 1H), 2.36 (t, J = 7.2 Hz, 2H), 2.23 (t, J = 7.2 Hz, 2H), 2.03 (s, 3H), 1.98 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.8, 152.1, 143.7, 141.0, 138.6, 136.4, 128.7, 128.3, 127.5, 126.9, 126.0, 123.2, 117.9, 115.4, 110.8, 32.6, 30.9, 15.4, 13.0. HRMS (ESI-TOF, m/z): calcd for C23H25N2OS+, [M + H]+, 377.1682, found 377.1686.
5-Methyl-1-(4-(methylthio)but-1-en-2-yl)-2-phenyl-4-(prop-1-en-2-yl)-1,2-dihydro-3H-pyrazol-3-one (3al)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 3al was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (51 mg, 81% yield). IR νmax (neat)/cm−1: 3046, 2982, 1670, 1533, 1478, 1324, 1278, 1109, 1082, 952; 1H NMR (600 MHz, CDCl3): δ 7.43–7.39 (m, 4H), 7.25–7.22 (m, 1H), 5.38 (s, 1H), 5.27 (s, 1H), 5.20 (d, J = 1.2 Hz, 1H), 5.09 (d, J = 1.2 Hz, 1H), 2.32 (s, 3H), 2.31 (t, J = 7.8 Hz, 2H), 2.18 (t, J = 7.8 Hz, 2H), 2.14 (s, 3H), 2.00 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.7, 150.4, 144.0, 136.4, 135.9, 128.7, 126.0, 123.3, 115.8, 115.3, 112.5, 32.5, 31.0, 22.3, 15.4, 12.8. HRMS (ESI-TOF, m/z): calcd for C18H22N2NaOS+, [M + Na]+, 337.1345, found 337.1345.
1) and obtained as a foam solid (51 mg, 81% yield). IR νmax (neat)/cm−1: 3046, 2982, 1670, 1533, 1478, 1324, 1278, 1109, 1082, 952; 1H NMR (600 MHz, CDCl3): δ 7.43–7.39 (m, 4H), 7.25–7.22 (m, 1H), 5.38 (s, 1H), 5.27 (s, 1H), 5.20 (d, J = 1.2 Hz, 1H), 5.09 (d, J = 1.2 Hz, 1H), 2.32 (s, 3H), 2.31 (t, J = 7.8 Hz, 2H), 2.18 (t, J = 7.8 Hz, 2H), 2.14 (s, 3H), 2.00 (s, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.7, 150.4, 144.0, 136.4, 135.9, 128.7, 126.0, 123.3, 115.8, 115.3, 112.5, 32.5, 31.0, 22.3, 15.4, 12.8. HRMS (ESI-TOF, m/z): calcd for C18H22N2NaOS+, [M + Na]+, 337.1345, found 337.1345.
Reaction of unsaturated pyrazolones 1a with propargyl sulfide ylide 2b
To a flame-dried sealable 3-dram vial equipped with a stir bar was added unsaturated pyrazolinones 1a (68 mg, 0.2 mmol, 1.0 equiv.), NaOAc·3H2O (14 mg, 0.1 mmol, 0.5 equiv.) and 2b (50 mg, 0.24 mmol, 1.2 equiv.) under air. Subsequently treated CH3CN (2 mL, c = 0.1 M) was added to vial via syringe. The reaction mixture was stirred for 15 min at 20 °C until unsaturated pyrazolones 1a was fully consumed (monitored by TLC). Then the reaction was stirred at 0 °C for 20 h. The organic solvent was removed under reduced pressure and purified through column chromatography (eluent: petroleum ether and EtOAc) to afford the desired product 4 (23% yield, 22 mg) and 5 (47% yield, 41 mg).1-(4-(Ethylthio)pent-1-en-2-yl)-2,5-diphenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (4)
IR νmax (neat)/cm−1: 3082, 2977, 2864, 1705, 1594, 1466, 1272, 1042, 967, 832; 1H NMR (600 MHz, CDCl3): δ 7.63 (dd, J = 1.2, 8.4 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.36 (d, J = 8.4 Hz, 2H), 7.32–7.25 (m, 4H), 7.22 (t, J = 7.2 Hz, 2H), 7.17–7.11 (m, 3H), 5.69 (d, J = 1.2 Hz, 1H), 5.63 (d, J = 1.2 Hz, 1H), 5.52 (s, 1H), 5.09 (s, 1H), 2.64–2.59 (m, 1H), 2.41 (q, J = 7.8 Hz, 2H), 2.19 (dd, J = 4.8, 15.6 Hz, 1H), 1.77 (dd, J = 9.6, 15.6 Hz, 1H), 1.18 (t, J = 7.2 Hz, 3H), 0.78 (d, J = 7.2 Hz, 1H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.7, 155.6, 144.1, 140.3, 138.4, 136.2, 130.3, 129.7, 129.3, 128.7, 128.0, 127.9, 127.3, 127.0, 126.4, 124.2, 119.2, 118.9, 114.3, 39.2, 36.4, 24.6, 20.5, 14.7. HRMS (ESI-TOF, m/z): calcd for C30H30N2NaOS+, [M + Na]+, 489.1971, found 489.1972.(E)-1-(1-(ethylthio)prop-1-en-2-yl)-2,5-diphenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (5)
IR νmax (neat)/cm−1: 3057, 2973, 2824, 1698, 1572, 1376, 1269, 1187, 1028, 932; 1H NMR (600 MHz, CDCl3): δ 7.58 (d, J = 7.2 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.33–7.30 (m, 5H), 7.26 (t, J = 7.2 Hz, 1H), 7.21 (t, J = 7.2 Hz, 2H), 7.16 (t, J = 7.2 Hz, 2H), 7.12 (t, J = 7.2 Hz, 1H), 6.34 (s, 1H), 5.69 (d, J = 1.2 Hz, 1H), 5.67 (d, J = 1.2 Hz, 1H), 2.53 (q, J = 7.2 Hz, 2H), 1.38 (s, 3H), 1.03 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.4, 155.5, 140.4, 138.6, 135.9, 133.3, 130.5, 129.8, 129.6, 129.5, 128.7, 127.9, 127.2, 127.1, 126.5, 124.7, 118.8, 114.8, 28.1, 15.4, 13.5. HRMS (ESI-TOF, m/z): calcd for C28H27N2OS+, [M + H]+, 439.1839, found 439.1838.Reaction of unsaturated pyrazolones 1a with trimethylsilyl propargyl sulfide ylide 2c
To a flame-dried sealable 3-dram vial equipped with a stir bar was added unsaturated pyrazolinones 1a (68 mg, 0.2 mmol, 1.0 equiv.), NaOAc·3H2O (14 mg, 0.1 mmol, 0.5 equiv.) and 2c (61 mg, 0.24 mmol, 1.2 equiv.) under air. Subsequently treated CH3CN (2 mL, c = 0.1 M) was added to vial via syringe. The reaction mixture was stirred for 15 min at 20 °C until unsaturated pyrazolones 1a was fully consumed (monitored by TLC). Then the reaction was stirred at 0 °C for 6 h. The organic solvent was removed under reduced pressure and purified through column chromatography (eluent: petroleum ether and EtOAc) to afford the desired product 3a (79% yield, 70 mg).Reaction of unsaturated pyrazolones 1a with methyl propargyl sulfide ylide 2d
To a flame-dried sealable 3-dram vial equipped with a stir bar was added unsaturated pyrazolinones 1a (68 mg, 0.2 mmol, 1.0 equiv.), Cs2CO3 (33 mg, 0.1 mmol, 0.5 equiv.) and 2d (47 mg, 0.24 mmol, 1.2 equiv.) under air. Subsequently treated CH3CN (2 mL, c = 0.1 M) was added to vial via syringe. The reaction mixture was stirred for 2 h at 20 °C until unsaturated pyrazolones 1a was fully consumed (monitored by TLC). Then the reaction was stirred at 0 °C for 15 h. The organic solvent was removed under reduced pressure and purified through column chromatography (eluent: petroleum ether and EtOAc) to afford the desired product 6 (36 mg, 62% yield, conversion: 74%).1-(3-Methyl-4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (6)
The reaction was carried out on a 0.2 mmol scale following the General procedure. The product 6 was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (56 mg, 62% yield). IR νmax (neat)/cm−1: 3062, 2933, 1668, 1574, 1379, 1259, 1149, 1028, 932, 834; 1H NMR (600 MHz, CDCl3): δ 7.63 (d, J = 7.2 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.36 (t, J = 7.2 Hz, 2H), 7.32 (t, J = 7.2 Hz, 2H), 7.29–7.22 (m, 4H), 7.17 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 5.68 (d, J = 1.2 Hz, 1H), 5.60 (d, J = 1.2 Hz, 1H), 5.51 (s, 1H), 5.10 (s, 1H), 2.19 (dd, J = 2.4, 13.2 Hz, 1H), 2.13–2.10 (m, 1H), 1.97 (dd, J = 10.2, 13.2 Hz, 1H), 1.89 (s, 3H), 0.81 (d, J = 6.6 Hz, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.8, 155.6, 150.6, 140.3, 138.4, 136.2, 130.4, 129.6, 129.3, 128.7, 128.0, 127.9, 127.3, 126.9, 126.3, 124.1, 123.2, 118.8, 117.0, 114.0, 40.4, 35.1, 18.8, 15.9. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaOS+, [M + Na]+, 475.1815, found 475.1818.
1) and obtained as a foam solid (56 mg, 62% yield). IR νmax (neat)/cm−1: 3062, 2933, 1668, 1574, 1379, 1259, 1149, 1028, 932, 834; 1H NMR (600 MHz, CDCl3): δ 7.63 (d, J = 7.2 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.36 (t, J = 7.2 Hz, 2H), 7.32 (t, J = 7.2 Hz, 2H), 7.29–7.22 (m, 4H), 7.17 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 5.68 (d, J = 1.2 Hz, 1H), 5.60 (d, J = 1.2 Hz, 1H), 5.51 (s, 1H), 5.10 (s, 1H), 2.19 (dd, J = 2.4, 13.2 Hz, 1H), 2.13–2.10 (m, 1H), 1.97 (dd, J = 10.2, 13.2 Hz, 1H), 1.89 (s, 3H), 0.81 (d, J = 6.6 Hz, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.8, 155.6, 150.6, 140.3, 138.4, 136.2, 130.4, 129.6, 129.3, 128.7, 128.0, 127.9, 127.3, 126.9, 126.3, 124.1, 123.2, 118.8, 117.0, 114.0, 40.4, 35.1, 18.8, 15.9. HRMS (ESI-TOF, m/z): calcd for C29H28N2NaOS+, [M + Na]+, 475.1815, found 475.1818.
Reaction of unsaturated pyrazolones 1d with methyl propargyl sulfide ylide 2d
To a flame-dried sealable 3-dram vial equipped with a stir bar was added unsaturated pyrazolinones 1d (72 mg, 0.2 mmol, 1.0 equiv.), Cs2CO3 (33 mg, 0.1 mmol, 0.5 equiv.) under air. Subsequently treated CH3CN (2 mL, c = 0.1 M) was added to vial via syringe. The reaction mixture was stirred for 2 h at 20 °C until unsaturated pyrazolones 1d was fully consumed (monitored by TLC). Then the reaction was stirred at 0 °C for 15 h. The organic solvent was removed under reduced pressure and purified through column chromatography (eluent: petroleum ether and EtOAc) to afford the desired product 7 (65 mg, 68% yield, conversion: 81%).4-(1-(4-Chlorophenyl)vinyl)-1-(3-methyl-4-(methylthio)but-1-en-2-yl)-2,5-diphenyl-1,2-dihydro-3H-pyrazol-3-one (7)
The reaction was carried out on a 0.2 mmol scale. The product 7 was purified through column chromatography (PE/EtOAc: from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a foam solid (64 mg, 66% yield). IR νmax (neat)/cm−1: 3086, 2982, 1688, 1559, 1437, 1322, 1251, 1067, 972, 853; 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 7.8 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.33 (d, J = 7.2 Hz, 2H), 7.32–7.30 (m, 2H), 7.25 (d, J = 7.2 Hz, 2H), 7.22 (t, J = 7.2 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H), 5.65 (s, 1H), 5.64 (s, 1H), 5.50 (s, 1H), 5.10 (s, 1H), 2.18 (dd, J = 1.8, 7.2 Hz, 1H), 2.12–2.08 (m, 1H), 1.97 (dd, J = 10.8, 13.2 Hz, 1H), 1.88 (s, 3H), 0.81 (t, J = 6.6 Hz, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 155.5, 150.4, 138.9, 137.4, 136.1, 133.2, 130.3, 129.8, 129.1, 128.8, 128.3, 128.1, 128.0, 126.5, 124.1, 119.3, 117.1, 113.3, 40.4, 35.3, 18.7, 15.9. HRMS (ESI-TOF, m/z): calcd for C29H27ClN2NaOS+, [M + Na]+, 509.1425, found 509.1425.
1) and obtained as a foam solid (64 mg, 66% yield). IR νmax (neat)/cm−1: 3086, 2982, 1688, 1559, 1437, 1322, 1251, 1067, 972, 853; 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 7.8 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.33 (d, J = 7.2 Hz, 2H), 7.32–7.30 (m, 2H), 7.25 (d, J = 7.2 Hz, 2H), 7.22 (t, J = 7.2 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H), 5.65 (s, 1H), 5.64 (s, 1H), 5.50 (s, 1H), 5.10 (s, 1H), 2.18 (dd, J = 1.8, 7.2 Hz, 1H), 2.12–2.08 (m, 1H), 1.97 (dd, J = 10.8, 13.2 Hz, 1H), 1.88 (s, 3H), 0.81 (t, J = 6.6 Hz, 3H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.5, 155.5, 150.4, 138.9, 137.4, 136.1, 133.2, 130.3, 129.8, 129.1, 128.8, 128.3, 128.1, 128.0, 126.5, 124.1, 119.3, 117.1, 113.3, 40.4, 35.3, 18.7, 15.9. HRMS (ESI-TOF, m/z): calcd for C29H27ClN2NaOS+, [M + Na]+, 509.1425, found 509.1425.
Gram-scale synthesis of 3a
To a flame-dried sealable 3-dram vial equipped with a stir bar was added unsaturated pyrazolinones 1a (1.01 g, 3.0 mmol, 1.0 equiv.), NaOAc·3H2O (204 mg, 1.5 mmol, 0.5 equiv.) and 2a (0.65 g, 3.6 mmol, 1.2 equiv.) under air. Subsequently treated CH3CN (25 mL, c = 0.12 M) was added to vial via syringe. The reaction mixture was stirred for 20 min at 20 °C until unsaturated pyrazolones 1a was fully consumed (monitored by TLC). Then the reaction was stirred at 0 °C for 10 h. The organic solvent was removed under reduced pressure and purified through column chromatography (eluent: petroleum ether and EtOAc) to afford the desired product 3a with a yield of 79% (1.01 g).Oxidation of 3a with m-CPBA
To a flame-dried sealable 2-dram vial equipped with a stir bar was added pyrazolones 3a (302 mg, 0.68 mmol, 1.0 equiv.), Na2CO3 (145 mg, 1.36 mmol, 3 equiv.) and CH2Cl2 (5 mL, c = 0.14 M). After stirred at 0 °C for 10 min, m-CPBA (313 mg, 1.36 mmol, 75%, 2.0 equiv.) was added to the mixture slowly. The reaction mixture was kept stirring for 1 min at 0 °C until unsaturated pyrazolones 3a was fully consumed (monitored by TLC). The reaction was quenched with aqueous Na2CO3 and extracted with CH2Cl2 (5 mL × 3). The combined organic solvent was dried with anhydrous Na2SO4, removed under reduced pressure and purified through column chromatography (eluent: petroleum ether and EtOAc) to afford the desired product 8 (101 mg, 33%) and 9 (125 mg, 39%).1-(4-(Methylsulfinyl)but-1-en-2-yl)-2,5-diphenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (8)
IR νmax (neat)/cm−1: 3162, 2958, 1672, 1587, 1421, 1356, 1151, 1077, 946; 1H NMR (600 MHz, CDCl3): δ 7.60 (d, J = 7.8 Hz, 2H), 7.46 (t, J = 7.2 Hz, 2H), 7.31 (d, J = 7.2 Hz, 3H), 7.29–7.24 (m, 3H), 7.21 (t, J = 7.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 2H), 7.10 (t, J = 7.2 Hz, 1H), 5.68 (s, 2H), 5.52 (s, 1H), 5.11 (s, 1H), 2.41 (t, J = 6.6 Hz, 2H), 2.36 (s, 3H), 2.22 (t, J = 6.6 Hz, 2H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.6, 155.5, 143.9, 140.1, 138.2, 135.8, 129.9, 129.8, 128.8, 128.1, 128.0, 127.9, 127.2, 126.9, 126.5, 124.1, 119.1, 114.5, 51.5, 38.4, 23.9. HRMS (ESI-TOF, m/z): calcd for C28H26N2NaO2S+, [M + Na]+, 477.1607, found 477.1609.1-(4-(Methylsulfonyl)but-1-en-2-yl)-2,5-diphenyl-4-(1-phenylvinyl)-1,2-dihydro-3H-pyrazol-3-one (9)
IR νmax (neat)/cm−1: 3055, 2985, 1663, 1578, 1412, 1365, 1155, 1062, 964; 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 1.2 Hz, 2H), 7.60 (d, J = 1.2 Hz, 2H), 7.48 (t, J = 7.8 Hz, 3H), 7.34–7.27 (m, 3H), 7.23 (t, J = 7.2 Hz, 2H), 7.15 (t, J = 7.2 Hz, 2H), 7.12 (t, J = 7.2 Hz, 1H), 5.70 (s, 2H), 5.54 (s, 1H), 5.09 (d, J = 0.6 Hz, 1H), 2.73 (t, J = 8.4 Hz, 2H), 2.68 (s, 3H), 2.29 (t, J = 8.4 Hz, 2H); 13C{1H} NMR (150 MHz, CDCl3): δ 164.7, 155.5, 143.2, 140.1, 138.1, 135.8, 130.0, 129.0, 128.2, 128.0, 127.4, 127.0, 126.8, 124.1, 119.3, 115.1, 52.4, 40.5, 24.0. HRMS (ESI-TOF, m/z): calcd for C28H26N2NaO3S+, [M + Na]+, 493.1556, found 493.1558.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the research program from Natural Science Foundation of Shanxi Province (201801D221067 and 201801D121048), the Natural Science Foundation of Shanxi Normal University (0505/02080160 and 0109/01053018), and the 1331 Engineering of Shanxi Province. The instruments were performed using the Analytical Test Center of Shanxi Normal University, Linfen, China.References
- For related reviews and books, see: (a) T. Kondo and T.-A. Mitsudo, Chem. Rev., 2000, 100, 3205–3220 CrossRef CAS; (b) H. Liu and X. Jiang, Chem.–Asian J., 2013, 8, 2546–2563 CrossRef CAS; (c) M. Feng, B. Tang, S. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef CAS; (d) N. Kharasch, The Chemistry of Organic Sulfur Compounds, Pergamon, London, 1996 Search PubMed; (e) L. A. Damani, Sulfur-Containing Drugs and Related Organic Compounds: Chemistry, Biochemistry, And Toxicology, Ellis Horwood, Ltd., Chichester, U.K., 1989, vol. 1, part B Search PubMed.
- For related reviews, see: (a) A.-H. Li, L.-X. Dai and V. K. Aggarwal, Chem. Rev., 1997, 97, 2341–2372 CrossRef CAS; (b) L.-X. Dai, X.-L. Hou and Y.-G. Zhou, Pure Appl. Chem., 1999, 71, 369–376 CAS; (c) V. K. Aggarwal and C. L. Winn, Acc. Chem. Res., 2004, 37, 611–620 CrossRef CAS; (d) E. M. McGarrigle, E. L. Myers, O. Illa, M. A. Shaw, S. L. Riches and V. K. Aggarwal, Chem. Rev., 2007, 107, 5841–5883 CrossRef CAS; (e) X.-L. Sun and Y. Tang, Acc. Chem. Res., 2008, 41, 937–948 CrossRef CAS PubMed; (f) L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Acc. Chem. Res., 2012, 45, 1278–1293 CrossRef CAS; (g) A. C. B. Burtoloso, R. M. P. Dias and I. A. Leonarczyk, Eur. J. Org. Chem., 2013, 23, 5005–5016 CrossRef; (h) L.-Q. Lu, T.-R. Li, Q. Wang and W.-J. Xiao, Chem. Soc. Rev., 2017, 46, 4135–4149 RSC; (i) J. D. Neuhaus, R. Oost, J. Merad and N. Maulide, Top. Curr. Chem., 2018, 376, 15 CrossRef; (j) D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2019, 119, 8701–8780 CrossRef CAS; (k) X.-P. Wu, S. Sun, J.-T. Yu and J. Cheng, Synlett, 2019, 30, 21–29 CrossRef CAS; (l) J. Vaitla and A. Bayer, Synthesis, 2019, 51, 612–628 CrossRef CAS. For selected synthetic applications of sulfur-containing compounds, see: (m) S. Peng, Y.-X. Song, J.-Y. He, S.-S. Tang, J.-X. Tan, Z. Cao, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.08.002; (n) Z. Cao, Q. Zhu, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.09.041; (o) L.-Y. Xie, S. Peng, F. Liu, G.-R. Chen, W. Xia, X. Yu, W.-F. Li, Z. Cao and W.-M. He, Org. Chem. Front., 2018, 5, 2604–2609 RSC; (p) W.-H. Bao, Z. Wang, X. Tang, Y.-F. Zhang, J.-X. Tan, Q. Zhu, Z. Cao, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.06.052; (q) C. Wu, L.-H. Lu, A.-Z. Peng, G.-K. Jia, C. Peng, Z. Cao, Z. Tang, W.-M. He and X. Xu, Green Chem., 2018, 20, 3683–3688 RSC.
- (a) J. E. Baldwin, R. E. Hackler and D. P. Kelley, J. Am. Chem. Soc., 1968, 90, 4758–4759 CrossRef CAS; (b) G. M. Blackburn, W. D. Ollis, J. D. Plackett, C. Smith and I. O. Sutherland, J. Chem. Soc., Chem. Commun., 1968, 186–188 RSC; (c) J. E. Baldwin, R. E. Hackler and D. P. Kelley, J. Chem. Soc., Chem. Commun., 1968, 537–538 (J. Chem. Soc., Chem. Commun., 1968, 538–539) RSC.
- For related reviews, see: (a) M. P. Doyle and D. C. Forbes, Chem. Rev., 1998, 98, 911–936 CrossRef CAS; (b) D. M. Hodgson, F. Y. T. M. Pierard and P. A. Stupple, Chem. Soc. Rev., 2001, 30, 50–61 RSC; (c) S. Braverman and M. Cherkinsky, Top. Curr. Chem., 2006, 275, 67–101 CrossRef; (d) M. Reggelin, Top. Curr. Chem., 2007, 275, 1–65 CrossRef CAS; (e) Y. Zhang and J. Wang, Coord. Chem. Rev., 2010, 254, 941–953 CrossRef CAS; (f) S.-F. Zhu and Q.-L. Zhou, Natl. Sci. Rev., 2014, 1, 580–603 CrossRef CAS; (g) T. H. West, S. S. M. Spoehrle, K. Kasten, J. E. Taylor and A. D. Smith, ACS Catal., 2015, 5, 7446–7479 CrossRef CAS; (h) Z. Sheng, Z. Zhang, C. Chu, Y. Zhang and J. Wang, Tetrahedron, 2017, 73, 4011–4022 CrossRef CAS.
- For selected examples, see: (a) M. P. Doyle, J. H. Griffin, M. S. Chinn and D. Van Leusen, J. Org. Chem., 1984, 49, 1917–1925 CrossRef CAS; (b) T. Fukuda and T. Katsuki, Tetrahedron Lett., 1997, 38, 3435–3438 CrossRef CAS; (c) D. S. Carter and D. L. Van Vranken, Org. Lett., 2000, 2, 1303–1305 CrossRef CAS; (d) K. L. Greenman, D. S. Carter and D. L. Van Vranken, Tetrahedron, 2001, 57, 5219–5225 CrossRef CAS; (e) C.-Y. Zhou, W.-Y. Yu, P. W. H. Chan and C.-M. Che, J. Org. Chem., 2004, 69, 7072–7082 CrossRef CAS; (f) M. Ma, L. Peng, C. Li, X. Zhang and J. Wang, J. Am. Chem. Soc., 2005, 127, 15016–15017 CrossRef CAS; (g) L. Peng, X. Zhang, M. Ma and J. Wang, Angew. Chem., Int. Ed., 2007, 46, 1905–1908 CrossRef CAS PubMed; (h) I. Aviv and Z. Gross, Chem.–Eur. J., 2008, 14, 3995–4005 CrossRef CAS; (i) M. Liao, L. Peng and J. Wang, Org. Lett., 2008, 10, 693–696 CrossRef CAS; (j) P. W. Davies, S. J.-C. Albrecht and G. Assanelli, Org. Biomol. Chem., 2009, 7, 1276–1279 RSC; (k) Y. Li, Y. Shi, Z. Huang, X. Wu, P. Xu, J. Wang and Y. Zhang, Org. Lett., 2011, 13, 1210–1213 CrossRef CAS; (l) M. S. Holzwarth, I. Alt and B. Plietker, Angew. Chem., Int. Ed., 2012, 51, 5351–5354 CrossRef CAS; (m) V. Tyagi, G. Sreenilayam, P. Bajaj, A. Tinoco and R. Fasan, Angew. Chem., Int. Ed., 2016, 55, 13562–13566 CrossRef CAS; (n) K. J. Hock and R. M. Koenigs, Angew. Chem., Int. Ed., 2017, 56, 13566–13568 CrossRef CAS; (o) Z. Zhang, Z. Sheng, W. Yu, G. Wu, R. Zhang, W.-D. M. Chu, Y. Zhang and J. Wang, Nat. Chem., 2017, 9, 970–976 CrossRef CAS; (p) K. J. Hock, L. Mertens, R. Hommelsheim, R. Spitzner and R. M. Koenigs, Chem. Commun., 2017, 53, 6577–6580 RSC; (q) X. Lin, Y. Tang, W. Yang, F. Tan, L. Lin, X. Liu and X. Feng, J. Am. Chem. Soc., 2018, 140, 3299–3305 CrossRef CAS; (r) H. Zhang, B. Wang, H. Yi, Y. Zhang and J. Wang, Org. Lett., 2015, 17, 3322–3325 CrossRef CAS; (s) X. Zhang, Z. Qu, Z. Ma, W. Shi, X. Jin and J. Wang, J. Org. Chem., 2002, 67, 5621–5625 CrossRef CAS.
- (a) M. Thangaraj, R. N. Gaykar, T. Roy and A. T. Biju, J. Org. Chem., 2017, 82, 4470–4476 CrossRef CAS; (b) J. Tan, T. Zheng, K. Xu and C. Liu, Org. Biomol. Chem., 2017, 15, 4946–4950 RSC.
- (a) M. Aso, M. Sakamoto, N. Urakawa and K. Kanemastsu, Heterocycles, 1990, 31, 1003–1006 CrossRef CAS; (b) M. Aso, A. Ojida, G. Yang, O.-J. Cha, E. Osawa and K. Kanematsu, J. Org. Chem., 1993, 58, 3960–3968 CrossRef CAS; (c) A. Ojida, F. Tanoue and K. Kanematsu, J. Org. Chem., 1994, 59, 5970–5976 CrossRef CAS.
- (a) P. Jia, Q. Zhang, H. Jin and Y. Huang, Org. Lett., 2017, 19, 412–415 CrossRef CAS; (b) P. Jia, Q. Zhang, Q. Ou and Y. Huang, Org. Lett., 2017, 19, 4664–4667 CrossRef CAS; (c) P. Jia, Q. Zhang, Y. Zhuge, X. Liwei and Y. Huang, Adv. Synth. Catal., 2018, 360, 438–443 CrossRef CAS; (d) P. Jia and Y. Huang, Adv. Synth. Catal., 2018, 360, 3044–3048 CrossRef CAS.
- S. Shen, Y. Yang, J. Duan, Z. Jia and J. Liang, Org. Biomol. Chem., 2018, 16, 1068–1072 RSC.
- For an excellent book on pyrazolone chemistry, see: (a) G. Varvounis, Pyrazol-3-ones. Part IV: Synthesis and Applications, in Advances in Heterocyclic Chemistry, ed. A. R. Katritzky, Academic Press, New York, 2009, vol. 98, pp. 143–224 Search PubMed. For related reviews, see: (b) B. Ke, M. Tian, J. Li, B. Liu and G. He, Med. Res. Rev., 2016, 36, 983–1035 CrossRef; (c) V. Kumar, K. Kaur, G. K. Gupta and A. K. Sharma, Eur. J. Med. Chem., 2013, 69, 735–753 CrossRef CAS; (d) S. Fustero, M. Sánchez-Roselló, P. Barrio and A. Simón-Fuentes, Chem. Rev., 2011, 111, 6984–7034 CrossRef CAS; (e) A. Schmidt and A. Dreger, Curr. Org. Chem., 2011, 15, 1423–1463 CrossRef CAS; (f) S. Sondhi, M. Dinodia, J. Singh and R. Rani, Curr. Bioact. Compd., 2007, 3, 91–108 CrossRef CAS; (g) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893–930 CrossRef CAS, and the references therein. For selected synthetic applications of N-heterocycles, see: (h) Z. Yang, Z. Song, L. Jie, L. Wang and X. Cui, Chem. Commun., 2019, 55, 6094–6097 RSC; (i) L.-Y. Xie, S. Peng, T.-G. Fan, Y.-F. Liu, M. Sun, L.-L. Jiang, X.-X. Wang, Z. Cao and W.-M. He, Science China Chemistry, 2019, 62, 460–464 CrossRef CAS; (j) L.-Y. Xie, L.-L. Jiang, J.-X. Tan, Y. Wang, X.-Q. Xu, B. Zhang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 14153–14160 CrossRef CAS; (k) K. Sun, S. Wang, R. Feng, Y. Zhang, X. Wang, Z. Zhang and B. Zhang, Org. Lett., 2019, 21, 2052–2055 CrossRef CAS; (l) K. Sun, Z. Shi, Z. Liu, B. Luan, J. Zhu and Y. Xue, Org. Lett., 2018, 20, 6687–6690 CrossRef CAS; (m) K. Sun, B. Luan, Z. Liu, J. Zhu, J. Du, E. Bai, Y. Fang and B. Zhang, Org. Biomol. Chem., 2019, 17, 4208–4211 RSC; (n) X. Wang, C. Li, Y. Zhang, B. Zhang and K. Sun, Org. Biomol. Chem., 2019, 17, 8364–8368 RSC.
- For recent reviews, see: (a) P. Chauhan, S. Mahajan and D. Enders, Chem. Commun., 2015, 51, 12890–12907 RSC; (b) S. Liu, X. Bao and B. Wang, Chem. Commun., 2018, 54, 11515–11529 RSC; (c) X. Xie, L. Xiang, C. Peng and B. Han, Chem. Rec., 2019, 19, 1–28 CrossRef; (d) X. Xie, W. Huang, C. Peng and B. Han, Adv. Synth. Catal., 2018, 360, 194–228 CrossRef CAS; (e) R. Rios, Chem. Soc. Rev., 2012, 41, 1060–1074 RSC.
- For recent selected asymmetric examples: see: (a) J.-X. Zhang, N.-K. Li, Z.-M. Liu, X.-F. Huang, Z.-C. Geng and X.-W. Wang, Adv. Synth. Catal., 2013, 355, 797–808 CrossRef CAS; (b) X. Han, W. Yao, T. Wang, Y. R. Tan, Z. Yan, J. Kwiatkowski and Y. Lu, Angew. Chem., Int. Ed., 2014, 53, 5643–5647 CrossRef CAS; (c) P. Chauhan, S. Mahajan, C. C. J. Loh, G. Raabe and D. Enders, Org. Lett., 2014, 16, 2954–2957 CrossRef CAS; (d) S. Wang, C. Rodriguez-Escrich and M. A. Pericas, Org. Lett., 2016, 18, 556–559 CrossRef CAS; (e) C. Ni and X. Tong, J. Am. Chem. Soc., 2016, 138, 7872–7875 CrossRef CAS; (f) D. Hack, A. B. Dürr, K. Deckers, P. Chauhan, N. Seling, L. Rübenach, L. Mertens, G. Raabe, F. Schoenebeck and D. Enders, Angew. Chem., Int. Ed., 2016, 55, 1797–1800 CrossRef CAS; (g) F. Vetica, S. Bailey, P. Chauhan, M. Turberg, A. Ghaur, G. Raabe and D. Enders, Adv. Synth. Catal., 2017, 359, 3729–3734 CrossRef CAS; (h) U. Kaya, P. Chauhan, S. Mahajan, K. Deckers, A. Valkonen, K. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2017, 56, 15358–15362 CrossRef CAS PubMed; (i) J. Zheng, S.-B. Wang, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 4540–4544 CrossRef CAS; (j) S.-W. Li, Q. Wan and Q. Kang, Org. Lett., 2018, 20, 1312–1315 CrossRef CAS; (k) X. Bao, S. Wei, J. Qu and B. Wang, Chem. Commun., 2018, 54, 2028–2031 RSC; (l) B.-L. Zhao and D.-M. Du, Org. Lett., 2018, 20, 3797–3800 CrossRef CAS; (m) X. Bao, S. Wei, X. Qian, J. Qu, B. Wang, L. Zou and G. Ge, Org. Lett., 2018, 20, 3394–3398 CrossRef CAS PubMed; (n) B. Mondal, R. Maity and S. C. Pan, J. Org. Chem., 2018, 83, 8645–8654 CrossRef CAS; (o) S. Meninno, A. Mazzanti and A. Lattanzi, Adv. Synth. Catal., 2019, 361, 79–84 CrossRef CAS.
- (a) G. Rassu, V. Zambrano, L. Pinna, C. Curti, L. Battistini, A. Sartori, G. Pelosi, G. Casiraghi and F. Zanardi, Adv. Synth. Catal., 2014, 356, 2330–2336 CrossRef CAS; (b) S. R. Yetra, S. Mondal, S. Mukherjee, R. G. Gonnade and A. T. Biju, Angew. Chem., Int. Ed., 2016, 55, 268–272 CrossRef CAS; (c) S. Mondal, S. Mukherjee, S. R. Yetra, R. G. Gonnade and A. T. Biju, Org. Lett., 2017, 19, 4367–4370 CrossRef CAS PubMed; (d) J.-Y. Liu, J. Zhao, J.-L. Zhang and P.-F. Xu, Org. Lett., 2017, 19, 1846–1849 CrossRef CAS PubMed; (e) W. Yang, W. Sun, C. Zhang, Q. Wang, Z. Guo, B. Mao, J. Liao and H. Guo, ACS Catal., 2017, 7, 3142–3146 CrossRef CAS; (f) H.-J. Leng, Q.-Z. Li, R. Zeng, Q.-S. Dai, H.-P. Zhu, Y. Liu, W. Huang, B. Han and J.-L. Li, Adv. Synth. Catal., 2018, 360, 229–234 CrossRef CAS; (g) J. Zheng, P. Li, M. Gu, A. Lin and H. Yao, Org. Lett., 2017, 19, 2829–2832 CrossRef CAS; (h) H. Li, R. Gontla, J. Flegel, C. Merten, S. Ziegler, A. P. Antonchick and H. Waldmann, Angew. Chem., Int. Ed., 2019, 58, 307–311 CrossRef CAS; (i) J. Xu, L. Hu, H. Hu, S. Ge, X. Liu and X. Feng, Org. Lett., 2019, 21, 1632–1636 CrossRef CAS.
- S.-J. Shen, X.-L. Du, X.-L. Xu, Y.-H. Wu, M.-G. Zhao and J.-Y. Liang, J. Org. Chem., 2019, 84, 12520–12531 CrossRef CAS PubMed.
- J.-Y. Liang, S.-J. Shen, X.-Q. Chai and T. Lv, J. Org. Chem., 2018, 83, 12744–12752 CrossRef CAS PubMed.
- Crystallographic data for 3a has been deposited with the Cambridge Crystallographic Data Centre as deposition number CCDC 1943758.†.
- Y. Liu, Y. Yang, Y. Huang, X.-H. Xu and F.-L. Qing, Synlett, 2015, 26, 67–72 CAS.
- A. Terada and Y. Kishida, Chem. Pharm. Bull., 1969, 17, 966–973 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H, 13C and 19F NMR and ESI-HRMS spectra for compounds. CCDC 1943758. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra07610g |
| This journal is © The Royal Society of Chemistry 2019 |