Open Access Article
Open Access ArticleTriarylethylene-indolin-2,3-dione molecular conjugates: design, synthesis, docking studies and anti-proliferation evaluation†
Sumit Kumara,
Gabriella Palmab,
Shanen Perumal b,
Mandeep Kaurb,
Ashona Singh-Pillayc,
Raghu Rajd,
Parvesh Singhc and
Vipan Kumar
b,
Mandeep Kaurb,
Ashona Singh-Pillayc,
Raghu Rajd,
Parvesh Singhc and
Vipan Kumar *a
*a
aDepartment of Chemistry, Guru Nanak Dev University, Amritsar-143005, India. Tel: +91 183 2258802-09 ext. 3320; Fax: +91 183 2258819-20; E-mail: vipan_org@yahoo.com
bSchool of Molecular and Cell Biology, University of the Witswatersrand, Private Bag 3, Wits-2050, Johannesburg, South Africa
cSchool of Chemistry and Physics, University of KwaZulu Natal, P/Bag X54001, Westvile, Durban 4000, South Africa
dDepartment of Chemistry, DAV College, Amritsar-143001, India
First published on 20th December 2019
Abstract
A series of 1H-1,2,3-triazole-linked ospemifene-isatin and O-methylated ospemifene–isatin conjugates were synthesized and assayed for their anti-proliferative activities against estrogen-responsive as well as estrogen-non-responsive cells. The non-cytotoxic conjugate 14e, with an optimal combination of bromo substituents at the C-5/C-7 positions of isatin, proved to be a promising hit with an IC50 value of 31.62 μM against MCF-7 and 19.23 μM against MDA-MB-231. The observed anti-proliferative activities of active conjugates were further corroborated via docking studies carried out on estrogen receptor subtypes α and β.
Introduction
Breast cancer (BC) is the second-leading cancer worldwide, and shows a high incidence of morbidity and mortality. The major risk factor for BC is being of the female sex since a high level of estrogen in females not only fuels the division of breast cells but also escalates the damage to DNA. BC is more prevalent in the age groups 14–30 (younger cells more prone to carcinogens) and above 40 (lifestyle and menopause).1,2 The low rate of breast feeding in nations with high resources is also considered to be a contributing factor for the disease, rendering BC a classical cancer of these nations. The Global Cancer Observatory (GLOBOCAN) estimated that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088
088![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 849 new cases of BC with 626
849 new cases of BC with 626![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 679 deaths occurred worldwide in 2018, with India accounting for 17% of the world's population that suffer from this disease.3
679 deaths occurred worldwide in 2018, with India accounting for 17% of the world's population that suffer from this disease.3
Triarylethylenes constitute a well-known pharmacophore with non-steroidal and non-hormonal properties. The core is present in non-steroidal architecturally varied collections of compounds called selective estrogen receptor modulators (SERMs) that function by binding estrogen receptors. These compounds are different from pure anti-estrogens in their action, as SERMs act variedly in different tissues, selectively inhibiting or stimulating estrogen-like actions.4,5 Tamoxifene (TAM), toremifene (TMF) and clomifene are clinically approved SERMs for the treatment of BC and display estrogenic effects in bones and anti-estrogenic effects in breast and endometrium tissues. Further side effects related to TAM have been shown to include hot flashes, insomnia, depression and dizziness.6–8 Ospemifene, a hydroxylated TAM metabolite Y, is involved in a metabolic pathway similar to that for TAM and is recommended as a first-line drug for moderate to severe dyspareunia associated with vulvar and vaginal atrophy (VVA).9,10
Use of isatin (1H-indole 2,3-dione) as a pharmacophore is of great interest and has been associated with a diverse range of biological activities such as anti-cancer, anti-depressant, anti-convulsant, anti-fungal, anti-HIV and anti-angiogenic activities.11–16 Inhibitions of tyrosine kinase and cyclin-dependent kinases are some of the mechanistic pathways through which isatin exerts its anticancer potential. Bazedoxifene (BZA), an indole-based SERM, has been approved for the prevention and treatment of post-menopausal osteoporosis and displays slightly higher binding affinity to ER-α than to ER-β. Recent studies have shown that BZA, when given in combination with conjugated estrogens, relieves hot flashes and also improves vulvovaginal atrophy and its symptoms.17,18 The structures of various United States Food and Drug Administration (US-FDA)-approved drugs displaying relatively substantial affinity for hormonal receptors are provided in Fig. 1.
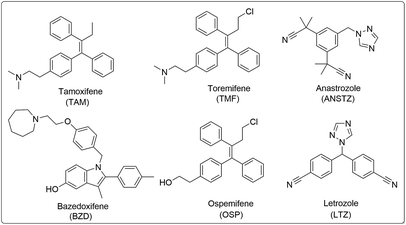 | ||
| Fig. 1 US-FDA-approved active SERMs (TAM, TMF, BZD and OSP) and estrogen synthesis inhibitors (ANSTZ and LTZ). | ||
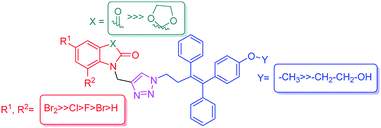
Recent reports from our laboratory have disclosed the synthesis and anti-proliferative activities of ospemifene–isatin conjugates with promising results against MCF-7 cells, in contrast to the results against MDA-MB-231 cells, with the most active conjugate of the series exhibiting an IC50 value of 1.56 μM.22 The currently described work was a continuation and logical extension of our interests19–21 and involved the use of a 1H-1,2,3-triazole core to link C-5-functionalized isatin to the alkyl side chain of ospemifene and methylated ospemifene. The use of triazole as a linker was further supported by its presence in anastrazole, an aromatase inhibitor used in BC chemotherapy.23 The present series of compounds was designed based on these literature rationales, and this design is depicted in Fig. 2.
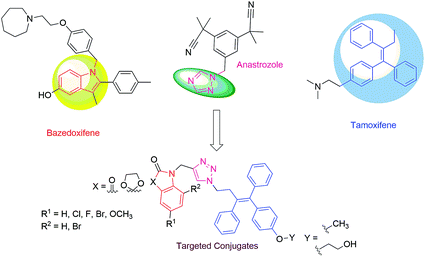 | ||
| Fig. 2 Design of the targeted 1H-1,2,3-triazole-linked ospemifene–isatin and O-methylated ospemifene–isatin conjugates. | ||
Results and discussion
Synthetic chemistry
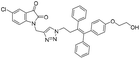
The methodology for the synthesis of the click chemistry precursors (Z)-2-(4-(4-azido-1,2-diphenylbut-1-en-1-yl)phenoxy)ethan-1-ol 7 and its derivative (Z)-(4-azido-1-(4-methoxyphenyl)but-1-ene-1,2-diyl)dibenzene 6 involved an initial McMurry reaction between 3-chloro-1-phenylpropan-1-one 1 with (4-methoxyphenyl)(phenyl)methanone 2 or (4-(2-hydroxyethoxy)phenyl)(phenyl)methanone 3 under refluxing conditions in a nitrogen atmosphere using Zn/TiCl4 in anhydrous tetrahydrofuran (THF) to afford 4 and 5, in a Z/E ratio of >98%.24 Sodium azide promoted nucleophilic substitution of 4 and 5 in anhydrous dimethylformamide (DMF) and gave the desired precursors 6 and 7, respectively (Scheme 1).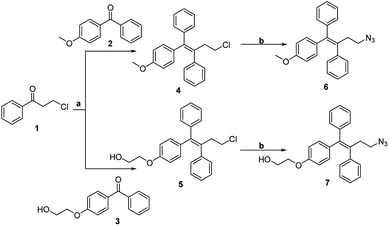 | ||
| Scheme 1 Synthesis of ospemifene and the O-methoxy derivative of ospemifene. Reagents and conditions: (a) Zn/TiCl4, anhydrous THF, rt to reflux, 6 h. (b) NaN3, DMF, 60 °C, 4 h. | ||
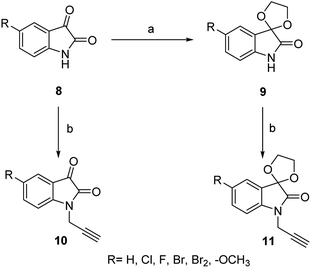
The second precursors, viz. N-propargylated isatin 10 and N-propargylated isatin-spiroketal 11, were obtained by deploying a previously reported methodology25,26 as shown in Scheme 2.
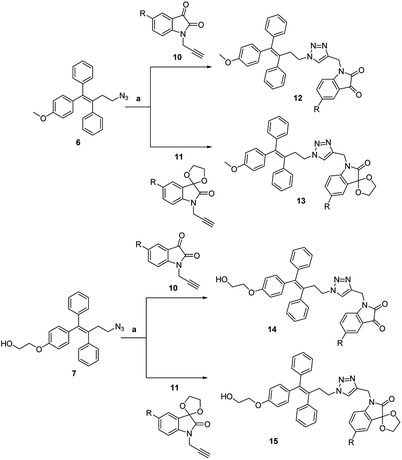
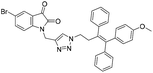
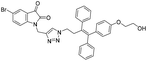
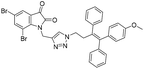
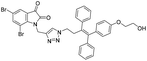
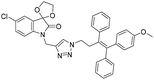
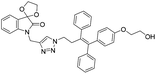
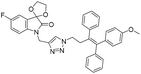
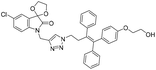
A Cu(I)-promoted azide–alkyne 1,3-dipolar cycloaddition reaction of appropriate precursors, namely 6 with 10 and 11, afforded the targeted 1H-1,2,3-triazole-linked O-methylated ospemifene–isatin/spiroisatins 12 and 13, while similar cycloaddition reactions of 7 with 10 and 11 gave ospemifen–isatin/spiroisatin conjugates 14 and 15 (Scheme 3).
The synthesized conjugates were purified via column chromatography using an ethylacetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (70
hexane (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) mixture as the eluent. The structure to the synthesized compounds was assigned on the basis of spectral data and analytical evidence. Compound 14b, for example, exhibited a molecular ion peak at m/z 604.1881 (M+) in its high-resolution mass spectrum (HRMS). Its 1H NMR spectrum included the characteristic signals at δ 2.97 (t, J = 7.2 Hz), 3.86 (t, J = 4.0 Hz), 3.94 (t, J = 4.7 Hz), 4.26 (t, J = 7.4 Hz) and 4.94 (s), corresponding to its different methylenes. Characteristic absorptions at δ 157.3 and 182.4 in its 13C NMR spectrum, corresponding to isatin ring carbonyls, further corroborated the assigned structure.
30) mixture as the eluent. The structure to the synthesized compounds was assigned on the basis of spectral data and analytical evidence. Compound 14b, for example, exhibited a molecular ion peak at m/z 604.1881 (M+) in its high-resolution mass spectrum (HRMS). Its 1H NMR spectrum included the characteristic signals at δ 2.97 (t, J = 7.2 Hz), 3.86 (t, J = 4.0 Hz), 3.94 (t, J = 4.7 Hz), 4.26 (t, J = 7.4 Hz) and 4.94 (s), corresponding to its different methylenes. Characteristic absorptions at δ 157.3 and 182.4 in its 13C NMR spectrum, corresponding to isatin ring carbonyls, further corroborated the assigned structure.
Anti-proliferative activities of synthesized conjugates against MCF-7 (ER+) and MDA-MB-231 (ER−) cells
The synthesized ospemifene and O-methylated ospemifene–isatin conjugates were evaluated for their anti-proliferative activities against estrogen-dependent ER(+) and non-estrogen-dependent ER(−) cells using the MTT assay with plumbagin as a positive control. Their percentage growth inhibitions for various concentrations viz. 1, 5, 10, 20, 40, 50 and 100 μM were determined and the results are provided in Fig. S1–S4 (see ESI†), while the IC50 data are listed in Table 1.Analysis of these data revealed an interesting structure activity relationship (SAR), one depending on the nature of the substituents at the C-5/C-7 positions as well as the nature of the functional group at the C-3 position of the isatin ring. Of the O-methylated ospemifene–isatin conjugates, 12a and 12b were selective against MDA-MB-231 cells while 12c, having bromo substituents at the C-5 and C-7 positions of the isatin ring, displayed IC50 values of 13.06 and 14.79 μM against estrogen-responsive and estrogen-non-responsive cells, respectively. The replacement of the keto-carbonyl with a spiroketal at the C-3 position of the isatin core in conjugates 13a–c proved to be detrimental, except in the case of 13a, which exhibited IC50 values of 21 and 20.39 μM against MCF-7 and MDA-MB-231 cells, respectively. Among the ospemifene–isatin conjugates 14a–e, the presence of a halogen substituent at the C-5 position of the isatin ring improved the anti-proliferative activities, with the order of preference being –Cl > –Br > –F. The replacement of keto-carbonyl with spiroketal at the C-3 position of the isatin ring again resulted in a deterioration of the anti-proliferative activities, as evident by compounds 15a–d, which were inactive against cells from both cell lines. Of the most active conjugates, 14b and 14e were selective for the estrogen-independent MDA-MB-231 cells.
Based on the observed activity profiles, a generalized SAR for the series of synthesized ospemiphene–isatin/isatinspiroketal and O-methylated ospemiphene–isatin/isatinspiroketal compounds was derived, and this SAR is illustrated in Fig. 3. The results indicated that the inclusion of bromo substituents at C-5/C-7, a keto-carbonyl at the C-3 position of the isatin ring, and O-methylation at the C-4 position of ospemifene improved the anti-proliferative activity.
Molecular docking studies
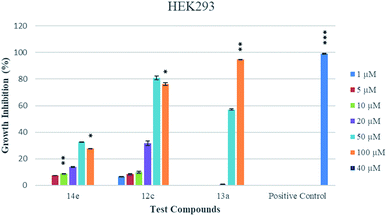
Molecular docking was performed on a set of test compounds viz. 12c, 13a, 14b and 14e against the two estrogen receptor subtypes α and β in order to identify the critical ligand–protein interactions. Compound 12c was the most potent inhibitor of the cells from both cell lines. In the docking, the binding of 12c to the β-subtype was supported by two hydrophobic interactions from residue Glu108 and the ligand at bond distances of 3.64 and 3.78 Å. These interactions were accompanied by three hydrogen bonds involving residues Glu28 and Gly109 as H-bond donors and residue Cys106 and the oxygen moiety of the ligand as H-bond acceptors (with bond distances of 2.33, 2.57 and 1.59 Å, respectively, and bond angles of 115.58°, 127.29° and 175.71°, respectively). According to the dockings, compound 13a shared a similar binding profile with 12c and also showed two hydrophobic interactions with residue Glu108, here with bonding distances of 3.68 and 3.82 Å, and a single hydrogen bond with donor residue Gly109, with a bond distance of 3.11 Å and bond angle of 133.67°. Compound 14b displayed two hydrophobic interactions with Gly108 and the ligand (with bond distances of 3.57 and 3.92 Å). These interactions were accompanied by three hydrogen bonds between donor residues Glu28, Gly109 and Glu112 and the oxygen of the ligand (with bond distances of 2.95, 2.96 and 2.91 Å and bond angles of 146.92°, 148.12° and 160.53°, respectively). Compound 14e displayed, according to the dockings, a more extensive binding interaction network: including two hydrophobic interactions with residue Glu108 at bonding distances of 3.47 and 3.94 Å; five hydrogen bonds observed between residues Glu28, Ala29, Gly109 and Glu112 and the oxygen groups of the ligand (bond distances of 3.04, 3.96, 2.87/2.99 and 2.94 Å, and bond angles of 136.45°, 155.75°, 129.58°/154.86° and 165.31°, respectively); and a single electrostatic interaction between the bromine of the ligand and Lys214 (bond distance of 3.43 Å and bond angle of 151.48°) (Fig. S5, see ESI†).The volume of the ligand-binding domain of ER-α has been found to be much greater than that of ER-β, and this greater volume allowed for an improved interaction with the ligand in our dockings. Here, the interaction profile of compound 12c comprised three distinct sets of features: six hydrophobic interactions, five hydrogen bonds and a π-stacking. The hydrophobic interactions were observed between residues Leu49, Trp78, Lys224, Val228, Pro230 and Leu234 and the hydrophobic pharmacophores of the ligand (bond distances of 3.90, 3.92, 3.73, 3.34, 3.92 and 3.72 Å, respectively). The five hydrogen bonds involved residues Gly75, Lys224 and Tyr232 as donors and residue Glu75 as the acceptor (bond distances of 2.44, 3.77, 3.18 and 2.44/2.50 Å, and bond angles of 102.63°, 119.34°, 158.60° and 171.15/161.49°, respectively). A P-type π-stacking was established between residue Trp78 and the ligand at a distance of 4.05 Å and angle of 24.79°. Compound 14e displayed a similar binding landscape with extensive hydrophobic interactions between residues Trp78, Leu220, Tyr221 and Lys224 at bond distances of 3.56, 3.96/3.85, 3.76 and 3.99/3.51 Å. Hydrogen bond interactions were identified between donor residue Leu231 and acceptor residue Glu218 (bond distances of 4.02 and 3.00 Å, and bond angles of 155.26° and 158.19°, respectively) (Fig. S6, see ESI†). A T and P-type π-stacking was measured between Tyr221 and the ligand (distances of 5.24 and 4.17 Å, and angles of 89.17° and 24.76°, respectively). Compounds 13a and 14b shared similar binding profiles, differing only in the presence of a hydrogen bond network in compound 13a between residues Ala45 and Leu231 (bond distances of 3.23 and 3.11 Å, and bond angles of 111.57° and 164.55°, respectively) and an electrostatic interaction in compound 14b incorporating the chloride group and Arg89 (distance of 3.81 Å, angle of 142.51°). The complexes of 12c, 13a, 14b and 14e showed high binding affinities during the dockings in the active sites of estrogen receptors (PDB ID: 3OLS/PDB ID: 3ERT) and these results are shown in Table S1 (see ESI†). Three of the most active conjugates, namely 12c, 13a and 14e, were selected for determining their cytotoxicity levels against the non-tumorigenic HEK-293 cells. The percentage growth inhibitions of these compounds are presented in Fig. 4 while their IC50 values are listed in Table 2. Conjugate 14e proved to be non-cytotoxic against the HEK-293 cells while the conjugates 12c and 13a were moderately cytotoxic.
| Compound | IC50 value |
|---|---|
| 12c | 21.61 |
| 13a | 43.87 |
| 14e | >100 |
Conclusion
In conclusion, a series of ospemifene–isatin and O-methylated ospemifene–isatin conjugates were synthesized using the Mc-Murray reaction as the key synthetic step. The synthesized conjugates were assayed for their anti-proliferative activities against ER+ (MCF-7) and ER− (MDA-MB-231) cells. The replacement of the carbonyl core with a spiroketal at the C-3 position of the isatin ring resulted in the loss of anti-proliferative activity, except in the case of 13a, which exhibited IC50 values of 21 and 20.39 μM against MCF-7 and MDA-MB-231 cells. The most potent compound of the series, 12c, with an optimum combination of bromo substituents at C-5/C-7 and a keto-carbonyl at C-3 of the isatin ring linked to O-methylated ospemiphene, displayed IC50 values of 13.06 and 14.79 μM against MCF-7 and MDA-MB-231 cells, respectively. However 12c was moderately cytotoxic to HEK 293 cells. On the other hand, the non-cytotoxic conjugate 14e, with similar structural characteristics of an isatin ring linked to ospemifene, was more selective towards the ER-cells, with which it displayed an IC50 of 19.23 μM, and can hence be regarded as a promising hit.Abbreviations
| BC | Breast cancer |
| ER | Estrogen receptor |
| GLOBOCAN | Global cancer observatory |
| SERMs | Selective estrogen receptor modulators |
| TAM | Tamoxifene |
| TMF | Toremifene |
| VVA | Vulvar and vaginal atrophy |
| BZA | Bazedoxifene |
| THF | Tetrahydrofuran |
| DMF | Dimethylformamide |
| SAR | Structure activity relationship |
| DMEM | Dulbecco's modified Eagle's medium |
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Financial assistance from the Science and Engineering Research Board (SERB) under Start-Up Research Grant Scheme No. YSS/2015/000879/CS (RR) is gratefully acknowledged. The authors would like to thank the National Research Foundation of South Africa for their support in facilitating our research (UID: 99563). We would also like to thank the Centre for High Performance Computing based in Cape Town for access to computational resources.Notes and references
- B. Su, S. Landini, D. D. Davis and W. R. Brueggemeier, J. Med. Chem., 2017, 50, 1635 CrossRef PubMed.
- American Cancer Society, Cancer facts and figures 2017, https://www.cancer.org/research/cancer-facts-statistics/allcancer-facts-figures/cancer-factsfigures-2017.html Search PubMed.
- F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre and A. Jemal, Ca-Cancer J. Clin., 2018, 68, 394 CrossRef PubMed.
- G. Kaur, M. P. Mahajan, M. K. Pandey, P. Singh, S. R. Ramisetti and A. K. Sharma, Eur. J. Med. Chem., 2014, 86, 211 CrossRef CAS PubMed.
- G. Kaur, M. P. Mahajan, M. K. Pandey, P. Singh, S. R. Ramisetti and A. K. Sharma, Bioorg. Med. Chem. Lett., 2016, 26, 1963 CrossRef CAS PubMed.
- J. Ellmen, P. Hakulinen, A. Partanen and D. F. Hayes, Breast Cancer Res. Treat., 2003, 82, 103 CrossRef CAS PubMed.
- T. L. Taras, G. T. Wurz and M. W. DeGregorio, J. Steroid Biochem. Mol. Biol., 2001, 77, 271–279 CrossRef CAS PubMed.
- A. DeMichele, A. Bn. Troxel, J. A. Berlin, A. L. Weber, G. R. Bunin, E. Turzo, R. Schinnar, D. Burgh, M. Berlin, S. C. Rubin, T. R. Rebbeck and B. L. Strom, J. Clin. Oncol., 2008, 26, 4151–4159 CrossRef PubMed.
- C. Descoteaux, V. Leblanc, G. Belanger, S. Parent, E. Asselin and G. Berube, Steroids, 2008, 73, 1077 CrossRef CAS PubMed.
- S. Adsule, S. Banerjee, F. Ahmed, S. Padhye and F. H. Sarkar, Bioorg. Med. Chem. Lett., 2010, 20, 1247 CrossRef CAS PubMed.
- H. S. Ibrahim, S. M. Abou-Seri, M. Tanc, M. M. Elaasser, H. A. Abdel-Aziz and C. T. Supuran, Eur. J. Med. Chem., 2015, 103, 583 CrossRef CAS PubMed.
- R. Rohini, P. M. Reddy, K. Shanker, K. Kanthaiah, V. Ravinder and A. Hu, Arch. Pharmacal Res., 2011, 34, 1077 CrossRef CAS PubMed.
- M. Verma, S. N. Pandeya, K. N. Singh and J. P. Stables, Acta Pharm., 2004, 54, 49 CAS.
- S. N. Pandeya, P. Yogeeshwari, D. Sriram and G. Nath, Indian J. Pharm. Sci., 2002, 64, 209 CAS.
- P. Selvam, N. Murugesh, M. Chandramohan, Z. Debyser and M. Witvrouw, Indian J. Pharm. Sci., 2008, 70, 779 CrossRef CAS PubMed.
- S. K. Sridhar and A. Ramesh, Biol. Pharm. Bull., 2001, 24, 1149 CrossRef CAS PubMed.
- D. M. Biskobing, Clin. Interventions Aging, 2007, 2, 299 CAS.
- R. Kagan, R. S. Williams, K. Pan, S. Mirkin and J. H. Pickar, Menopause, 2010, 2, 281 CrossRef PubMed.
- S. Kumar, L. Gu, G. Palma, M. Kaur, A. S. Pillay, P. Singh and V. Kumar, New J. Chem., 2018, 42, 3703 RSC.
- A. Singh, S. T. Saha, S. Perumal, M. Kaur and V. Kumar, ACS Omega, 2018, 3, 1263 CrossRef CAS PubMed.
- B. Sharma, A. Singh, L. Gu, S. T. Saha, A. S. Pillay, N. Cele, P. Singh, M. Kaur and V. Kumar, RSC Adv., 2019, 9, 9809 RSC.
- S. Kumar, S. T. Saha, L. Gu, G. Palma, S. Perumal, A. S. Pillay, P. Singh, A. Anand, M. Kaur and V. Kumar, ACS Omega, 2018, 3, 12106 CrossRef CAS PubMed.
- A. Mojaddami, A. Sakhteman, M. Fereidoonnezhad, Z. Faghih, A. Najdian, S. Khabnadideh, H. Sadeghpour and Z. Rezaei, Res. Pharma. Sci., 2017, 12, 21 CrossRef PubMed.
- L. Eklund and J. Nilsson, New Processes for Producing Benzophenone Derivatives, PCT WO 2011/089385 A1, 2011 Search PubMed.
- K. Kumar, S. C. Kremer, L. Kremer, Y. Guérardel, C. Biot and V. Kumar, Organometallics, 2013, 32, 5713 CrossRef CAS.
- K. Kumar, C. Biot, S. C. Kremer, L. Kremer, Y. Guérardel, P. Roussel and V. Kumar, Organometallics, 2013, 32, 7386 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR data of all the synthesized conjugates along with scanned (1H, 13C, DEPT, D2O) NMR spectra for representative compounds viz. 13a, 13b, 14a, 14b, 14c, 14e, 15c, 15d. See DOI: 10.1039/c9ra08776a |
| This journal is © The Royal Society of Chemistry 2019 |