Open Access Article
Open Access ArticleMoS2/carbon composites prepared by ball-milling and pyrolysis for the high-rate and stable anode of lithium ion capacitors†
Chong Wanga,
Changzhen Zhana,
Xiaolong Rena,
Ruitao Lv a,
Wanci Shena,
Feiyu Kangbc and
Zheng-Hong Huang
a,
Wanci Shena,
Feiyu Kangbc and
Zheng-Hong Huang *ab
*ab
aState Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, P. R. China. E-mail: zhhuang@tsinghua.edu.cn
bKey Laboratory of Advanced Materials (MOE), School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China
cEngineering Laboratory for Functionalized Carbon Materials, Graduate School at Shenzhen, Tsinghua University, Shenzhen 518055, P. R. China
First published on 20th December 2019
Abstract
Lithium ion capacitors (LICs), bridging the advantages of batteries and electrochemical capacitors, are regarded as one of the most promising energy storage devices. Nevertheless, it is always limited by the anodes that accompany with low capacity and poor rate performance. Here, we develop a versatile and scalable method including ball-milling and pyrolysis to synthesize exfoliated MoS2 supported by N-doped carbon matrix derived from chitosan, which is encapsulated by pitch-derived carbon shells (MoS2/CP). Because the carbon matrix with high nitrogen content can improve the electron conductivity, the robust carbon shells can suppress the volume expansion during cycles, and the sufficient exfoliation of lamellar MoS2 can reduce the ions transfer paths, the MoS2/CP electrode delivers high specific capacity (530 mA h g−1 at 100 mA g−1), remarkable rate capability (230 mA h g−1 at 10 A g−1) and superior cycle performance (73% retention after 250 cycles). Thereby, the LICs, composed of MoS2/CP as the anode and commercial activated carbon (21 KS) as the cathode, exhibit high power density of 35.81 kW kg−1 at 19.86 W h kg−1 and high energy density of 87.74 W h kg−1 at 0.253 kW kg−1.
Introduction
With the increasingly serious environmental concerns and the shortage of clean and sustainable resources, there are an increasing number of scientific research works concentrating on constructing superior energy storage devices.1–4 Over the years, lithium ion batteries and supercapacitors have been two dominant energy storage devices, which attract unprecedented attentions. Because LIBs and ECs store energy by faradaic reactions and electrostatic charge adsorption respectively, LIBs deliver high energy density (>180 W h kg−1) but unoptimistic power density (<1000 W kg−1) with short cycle period (<500 cycles), and ECs exhibit excellent power density (>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1) and remarkable cycle stability (>10
000 W kg−1) and remarkable cycle stability (>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) but inferior energy density (<10 W h kg−1).5–8 Therefore, lithium ion capacitors (LICs), which can bridge the merits of LIBs and ECs, is promising to be new generation of advanced energy storage devices in the future.9–11
000 cycles) but inferior energy density (<10 W h kg−1).5–8 Therefore, lithium ion capacitors (LICs), which can bridge the merits of LIBs and ECs, is promising to be new generation of advanced energy storage devices in the future.9–11
There are two basic types of lithium ion capacitors (LICs). One is the configuration of battery-type cathode and capacitor-type anode, such as (−) AC//LiMn2O4 (+).12 The kind of LICs has narrow voltage windows because the stable potential range of AC is 1.5–4.5 V. Therefore, it has low energy density. As for power density, the battery-type cathode cannot satisfy the rate demand so that the introduction of conductive materials is necessary. However, the measure would decrease the capacity of cathode and the energy of LICs. The other is the construction of capacitive cathode and battery-type anode.9,13–16 In 2001, Amatucci et al. had developed the system which consists of nano-Li4Ti5O12 as the battery-type anode and N-doped activated carbon as the capacitor-type cathode.17 However, on account of the different mechanisms of energy storage, the power and energy densities depends on the faradaic-type anode with sluggish reaction kinetics and the adsorption-type cathode with low capacity, respectively.18,19 It is an urgent and everlasting goal to construct an anode with high rate performance and long cycle life so that the whole device can deliver better performance by remedying the mismatch of kinetics and capacity between the cathode and anode.
MoS2 (molybdenum disulfide), as one of the family of bidimensional layered metal sulfides, has attracted many researchers' attention.20–22 It possesses large interlayers distance (0.615 nm) and well-defined 2D plane structure. MoS2 demonstrates low reaction potential and high theoretical capacity of 669 mA h g−1 as promising anode materials.23 Nonetheless, the electrochemical performance of MoS2 is subject to its terrible intrinsic conductivity and severe volume changes during cycles, which will bring about the pulverization of electrode and the failure of active materials.24–26 Recently, there are some strategies, such as preparing nano-sized MoS2,27,28 mixed with conductive carbon matrix,29–31 and increasing the interlayer distance,22,32 to compensate the above shortage. However, many above synthetic methods are complicated and difficult for scale production so that researchers are looking forward to finding simple and scalable ways to synthesizing MoS2-based anode materials with superior rate property and long cycle life. Sun et al.33 obtain MoS2/graphene nanosheets from bulk MoS2 and commercial graphite through two-step ball-milling method and the materials deliver outstanding electrochemical properties. According to our survey findings, there are few reports about exfoliating and then coating MoS2 by other carbon sources in the up-down methods so far.
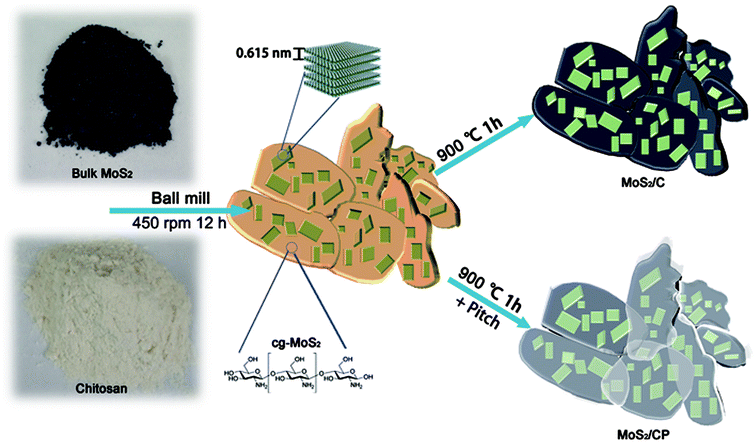
Here, we used a ball-milling approach to prepare chitosan-grafted MoS2 with few layers (cg-MoS2) from chitosan and commercial bulk MoS2. Then we successfully synthesized the exfoliated MoS2 supported by N-doped carbon matrix derived from chitosan, which is encapsulated by pitch-derived carbon shells (MoS2/CP) through one-step heat treatment of the cg-MoS2 mixed with the pitch (Scheme 1). Because the few-layered MoS2 is uniformly distributed in the conductive carbon matrix with high nitrogen content, it possesses superior electron conductivity and short ion/electron transfer paths. What's more, the pitch melts and flows onto the surface of the cg-MoS2 particles at about 270 °C during pyrolysis process so that a close contact is formed between the MoS2 nanosheets and the robust carbon shells, which can restrain the volume expansion and pulverization. For LIBs, MoS2/CP exhibits high capacity (530 mA h g−1 at 100 mA g−1), ultrahigh rate capability (230 mA h g−1 at 10 A g−1) and stable cycle (73% capacity retention after 250 cycles). When matched with commercial AC (21 KS) as cathode, the fabricated LICs deliver high energy density of 87.74 W h kg−1 at 0.253 kW kg−1, superb power density of 35.81 kW kg−1 even at 19.86 W h kg−1 and superior cycling durability (79.61% capacity retention after 1000 cycles). Consequently, we demonstrate the composites synthesized by the versatile methods show superb electrochemical property and have certain application prospects in LIBs and LICs.
Experimental
Preparation of N-doped carbon matrix, MoS2/C and MoS2/CP
In a typical ball-milling process, 5 g commercial bulk MoS2 (Aladdin, 99.5%, <2 μm), 10 g chitosan (Biochemical Reagent) and four sorts of ZrO2 balls with diameters of 2 mm (100 g), 4 mm (60 g), 6 mm (20 g) and 10 mm (20 g) were mixed into a ZrO2 tank (volume = 140 mL). The weight ratio of ball to sample was between 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and each of them accounted for 1/3 of the tank volume. Then the tank operated at a rotational rate of 450 rpm for 12 h in a planetary ball-milling machine (MITR-YXQM-0.4L) to produce cg-MoS2. Then 1.5 g petroleum pitch powder was mixed with 15 g cg-MoS2 in the tank and they were rotated at a speed of 300 rpm for 1 hour to get homogeneous mixture. After the mixture was heated at 900 °C for 1 hour at a ramp rate of 2 °C min−1, we got the products-MoS2/CP. The contrast sample-MoS2/C was synthesized in the same way without pitch. The N-doped carbon matrix was synthesized with the chitosan through the same heat treatment.
1 and each of them accounted for 1/3 of the tank volume. Then the tank operated at a rotational rate of 450 rpm for 12 h in a planetary ball-milling machine (MITR-YXQM-0.4L) to produce cg-MoS2. Then 1.5 g petroleum pitch powder was mixed with 15 g cg-MoS2 in the tank and they were rotated at a speed of 300 rpm for 1 hour to get homogeneous mixture. After the mixture was heated at 900 °C for 1 hour at a ramp rate of 2 °C min−1, we got the products-MoS2/CP. The contrast sample-MoS2/C was synthesized in the same way without pitch. The N-doped carbon matrix was synthesized with the chitosan through the same heat treatment.
Characterization
The scanning electron microscopy (LEO 1530), transmission electron microscopy (Tecnai G20) and X-ray diffraction (Bruker D8) were employed to characterize the morphology and structure of the samples. The valence states and element types on the sample surface were analyzed by X-ray photoelectron spectroscopy (PHI Quantera Imaging). Raman scattering (Renishaw, In Via-Reflex, λ = 532 nm) was mainly used to reveal the structural features of MoS2 and carbon. The content of each constituent was measured by Thermo-gravimetric Analysis (Mettler Toledo) in air at a ramp rate of 5 °C min−1 from 25 °C to 700 °C. The Brunauer–Emmett–Teller method and density functional theory (DFT) were utilized to calculate the specific surface areas and pore size distribution through the N2 sorption/desorption isotherms. The DFT describes the distribution characteristics of adsorbed molecules in the pore, which is based on the statistic mechanics. We can obtain reliable pore size analysis in the whole pore range (including micropore and mesoporous) in the DFT-based method.Fabrication of the cells
Active materials (80%), super P (10%) and poly (vinylidene fluoride) (10%) were mixed in excess NMP solvent to form a homogenous slurry. Then it was spread on the Cu foil for the anode and on the Al foil for the cathode. The foils were dried for 24 h in vacuum oven at 120 °C and then punched into discs for cell fabrication. The mass loading density of cathode was controlled from 1.7 mg cm−2 to 2.8 mg cm−2 and that of anode was fixed at about 1.1 mg cm−2. Half-cells were assembled in coin cell CR-2032 while the counter electrode was the Li metal sheet and the electrolyte was 1 M LiPF6 in EC/DEC (vol% = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For LICs, the anode was discharged to the cut-off potential 0.7 V after 3 cycles at 0.1 A g−1 and then assembled with cathode of different mass for the capacity balance.
1). For LICs, the anode was discharged to the cut-off potential 0.7 V after 3 cycles at 0.1 A g−1 and then assembled with cathode of different mass for the capacity balance.
Electrochemical measurements
The galvanostatic charge/discharge (GCD) test of half cells was operated by a LAND battery system (Jinnuo Electronics Co.) and the voltage window was set in 0.01–3.0 V vs. Li/Li+. The Arbin-BT 2000 test equipment and VSP-300 electrochemical analyzer were used to accomplish the GCD and cyclic voltammetry (CV) tests of LICs in the voltage windows of 1.0–4.0 V. Electrochemical impedance spectroscopy (EIS) test was performed in the frequency area of 1 M Hz to 10 m Hz with an AC amplitude of 10 mV.Results and discussion
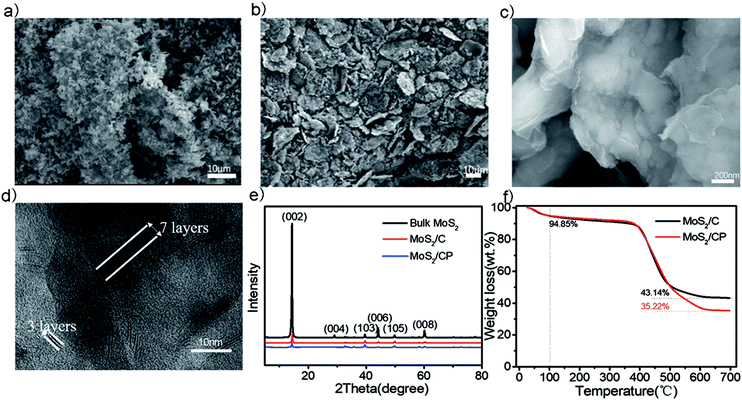
The chitosan is chosen as the carbon precursor due to its high carbon and nitrogen content. Moreover, many –OHs also facilitate the peeling of bulk MoS2 through the bonding between oxygen atom and Mo atom and preventing exfoliated MoS2 to stack with each other.34 As shown in Fig. 1a, the bulk MoS2 is clastic with a length of 1–2 μm. The SEM image (Fig. S1†) of chitosan presents a dense nubby structure with a diameter of 10–30 μm. The cg-MoS2 (Fig. S2†) has smaller particle sizes and the MoS2 sheets are attached uniformly on the chitosan after ball-milling process. The pitch can melt at about 270 °C and flow onto the surface of cg-MoS2 to form compact shells after carbonization.35,36 Therefore the MoS2/CP particles present a lenticular shape on the whole and a layer of hazy membrane on its surface in Fig. 1b and c. MoS2/C is carbonized from cg-MoS2 without pitch and presents bare MoS2 nanosheets on the carbon matrix (Fig. S3†).The X-ray diffraction (XRD) results (Fig. 1e) shows that the crystal lattice of as-prepared MoS2/CP and MoS2/C are in accord with that of 2H-MoS2 (JCPDS PDF#37-1492) and the peak intensity of (002) crystal plane presents prominent recede while that of other-directional crystal planes becomes more obvious.27,37,38 As shown in Fig. 1d, the transmission electron microscopy images explain that the exfoliated MoS2 is mostly 3–7 layers and the interlayer spacing of 0.62 nm corresponds to the (002) crystal face of 2H-MoS2.39 It also demonstrates the exfoliation of bulk MoS2 is fairly sufficient, which is consistent with the XRD results. The Raman spectra (Fig. S4†) has obvious characteristics of MoS2, namely peaks at 403 cm−1 and 375 cm−1 reflect the A1g and E12g vibration modes of Mo–S bond, respectively.40 The G band at 1581 cm−1 shows relatively intense in comparison to the D band at 1345 cm−1, which suggests that the dominant form of carbon in the samples is disordered carbon.41 TGA curves are showed in the Fig. 1f. It must also be mentioned that the final residue is MoO3 converted from MoS2 and other components are oxidized to gases. According to this reaction process, we calculate the mass ratio of MoS2 in MoS2/C and MoS2/CP is 50.56% and 41.44%, respectively.26,32
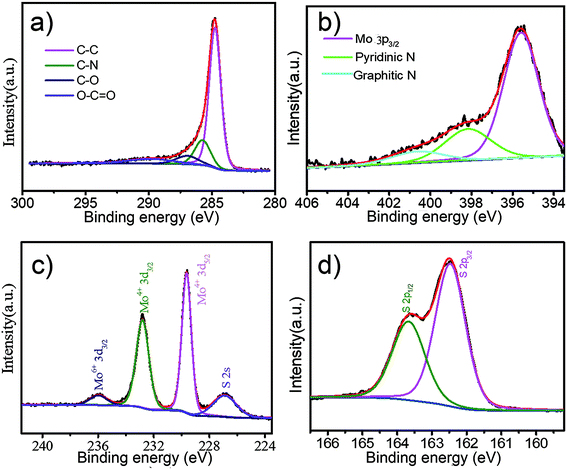
According to the X-ray photoelectron spectroscopy shown in Fig. 2a–d, the surface chemical states of MoS2/CP are examined. The peaks at about 284.8 eV, 285.7 eV, 286.9 eV and 289.7 eV can be separated from the C 1s region, which are assigned to the C–C, C–N, C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species.42,43 The peak at 398.3 eV in the N 1s spectrum corresponds to the pyridinic N, which is the main form of nitrogen in the sample while the peaks at 400.7 eV is deemed to the graphitic N.27,42 Due to the kinetics-controlled lithium storage process, the introduction of heteroatoms can cause some vacancies and/or redundant electrons so that the conductivity may be improved.44 As for nitrogen element, the pyridinic N can also provide more capacity.45 The intense peak at 395.4 eV is attributed to the Mo 3p3/2.46 We can see from Fig. 3c that Mo element have two oxidation states. Mo4+ at the edge of MoS2 sheets is easier to be oxidized to Mo6+ during ball-milling and pyrolysis process.25,27 The XPS curves of N-doped carbon matrix and MoS2/C are shown in Fig. S5 and S6.† The atom ratio of three samples is shown in Table S1.† It is calculated from the integral peak areas. The Mo
O species.42,43 The peak at 398.3 eV in the N 1s spectrum corresponds to the pyridinic N, which is the main form of nitrogen in the sample while the peaks at 400.7 eV is deemed to the graphitic N.27,42 Due to the kinetics-controlled lithium storage process, the introduction of heteroatoms can cause some vacancies and/or redundant electrons so that the conductivity may be improved.44 As for nitrogen element, the pyridinic N can also provide more capacity.45 The intense peak at 395.4 eV is attributed to the Mo 3p3/2.46 We can see from Fig. 3c that Mo element have two oxidation states. Mo4+ at the edge of MoS2 sheets is easier to be oxidized to Mo6+ during ball-milling and pyrolysis process.25,27 The XPS curves of N-doped carbon matrix and MoS2/C are shown in Fig. S5 and S6.† The atom ratio of three samples is shown in Table S1.† It is calculated from the integral peak areas. The Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atom ratio is about 2.02, which is consistent with the raw material-MoS2. Notably, the carbon to oxygen ratio of carbon matrix is clearly higher than that of MoS2/C and MoS2/CP. Thus the oxygen atoms in the chitosan are really combined with the Mo atoms at the edge zone so that the confined oxygen atoms would remain in the sample during the heat treatment according to the previous literature.33 The nitrogen atom ratio of MoS2/C and MoS2/CP is about 6.1%, which is beneficial to the electrochemical property.
S atom ratio is about 2.02, which is consistent with the raw material-MoS2. Notably, the carbon to oxygen ratio of carbon matrix is clearly higher than that of MoS2/C and MoS2/CP. Thus the oxygen atoms in the chitosan are really combined with the Mo atoms at the edge zone so that the confined oxygen atoms would remain in the sample during the heat treatment according to the previous literature.33 The nitrogen atom ratio of MoS2/C and MoS2/CP is about 6.1%, which is beneficial to the electrochemical property.
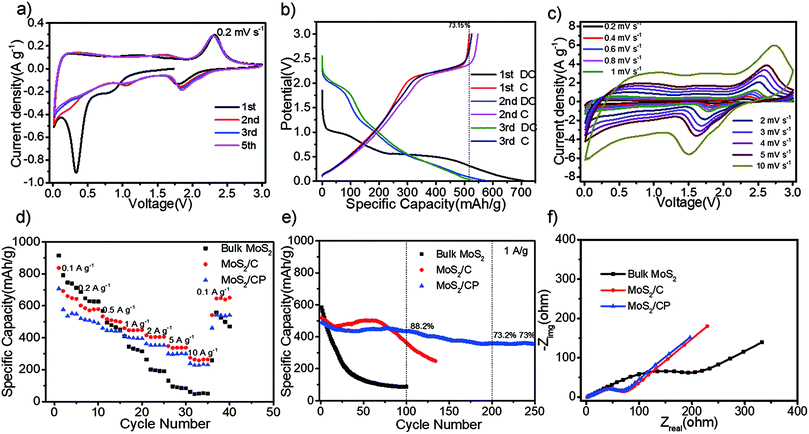
According to the CV curves tested at 0.2 mV s−1 in Fig. 3a, the electrode reactions are investigated. In the 1st cathodic sweep, the broad peak at about 0.85 V corresponds to the formation of LixMoS2 with octahedral structure due to the insertion of Li+ into MoS2 interlayers, and the peak at about 0.3 V is attributed to the conversion reaction from LixMoS2 to Mo atom and the formation of solid electrolyte interphase resulted from the decomposition reaction of electrolyte.47 In the 2nd and 3rd cathodic sweeps, two new peaks at 1.8 V and 1.0 V may arise from the multistep Li+ insertion mechanism as follows:47,48
| 2Li+ + 2 e− + S → Li2S | (1) |
| MoS2 + xLi+ + xe− → LixMoS2 | (2) |
The kinetics analysis of three samples is carried out by the CV curves at diverse scan rates, aiming to comprehend the electrochemistry behaviours. A valid method has been reported previously to analyse the Li+ storage kinetics.50 There is a relationship between peak current density (i) and scan rates (ν) as follows:
| i = avb | (3) |
The CV curves of MoS2/CP at various scan rates (Fig. 3c) display similar shapes even at 10 mV s−1. It predicts superior rate performances. As for MoS2/C, it presents roughly the same characteristics shown in Fig. S7b.† In contrast, the CV curves of bulk MoS2 (Fig. S8b†) present obvious shape change and is not able to find its whole anodic peak while the scan rates increase to 10 mV s−1. The phenomenon is attributed to the introduction of the conductive carbon matrix and the sufficient exfoliation of bulk MoS2, which promote the rapid transfer of electrons and ions even in the face of biggish voltage/current changes.
The cathodic peak at 1.8 V and the anodic peak at 2.3 V are chosen to evaluate the b value. The results are shown in Fig. S9.† The b values of bulk MoS2 are 0.49 and 0.50, which belongs to clear battery behaviour. The b values of MoS2/C and MoS2/CP are between 0.8 and 0.9, suggesting that they possess quicker response to the current changes compared with bulk MoS2. The difference depends on the structural changes. After the ball-milling of MoS2 and pyrolysis of carbon source, the ultrathin MoS2 nanosheets with 3–7 layers are closely anchored on the carbon matrix through the Mo–O bond. The Li ion diffusion path is drastically reduced and more active sites in the plane and edge are exposed to Li+ in the electrolyte. Thus the surface-controlled capacitive behavior is more obvious. While the bulk MoS2 powder presents mostly battery behavior due to the integrated crystal structure. The conductive carbon matrix can accelerate the electron transport and prevent the delaminated MoS2 nanosheets from aggregating together so that the b value of MoS2/C and MoS2/CP in the kinetics analysis is closer to 1.0. The performance improvement is achieved by the synergy between the MoS2 and carbon.
When the three samples are tested by the galvanostatic charge/discharge measurement (GCD) with gradually increased current densities, MoS2/C and MoS2/CP both present superior rate performance than bulk MoS2 and their capacity retention is about 43% even at the 100-times of initial current density as can be seen from Fig. 3d. These results imply that few-layered MoS2 coated on the carbon matrix with high nitrogen content has short ion diffusion path, high electron conductivity and good interfacial wettability.52 The consistent result can be obtained from the EIS data (Fig. 3f) where MoS2/C and MoS2/CP show smaller semicircles than bulk MoS2 in the high frequency zone resulted from low charge transfer resistance (Rct).39,53 It should be pointed out that MoS2/C shows higher capacity from above data because it has higher content of nitrogen (Table S1†) and MoS2 mass ratio (Fig. 1f) than those of MoS2/CP.4,54 The role of carbon derived from pitch is to restrain the volume expansion for better cycling property.35 We can see from Fig. 4e that the capacity of two samples increased gradually due to the activation,55 and MoS2/CP delivered superior cycle stability than MoS2/C with the addition of pitch. The capacity retention of MoS2/CP is 88.2% and 73% after 100 and 250 cycles at 1 A g−1, respectively. The above results exhibit MoS2/CP have potential advantages in LICs due to its high capacity, superior rate and cycle performance and rapid reaction kinetics.
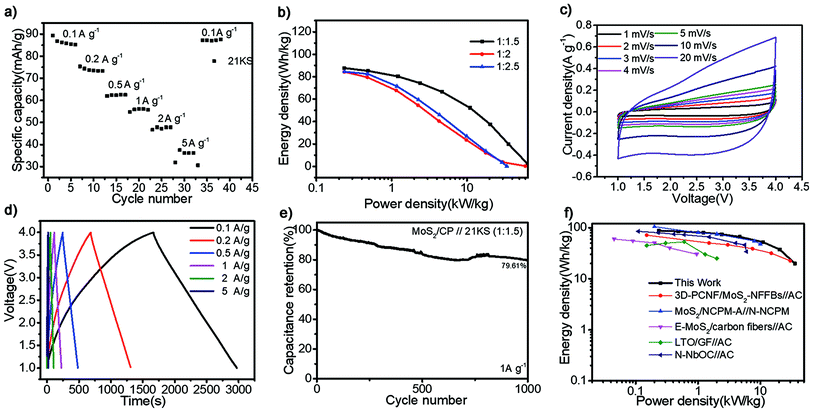
As shown in Fig. 4a, the commercial AC (21 KS), which delivers superior rate property in the voltage windows of 1.5–4.5 V due to its great SSA (Fig. S10a†) and energy storage mode of physical adsorption, has been considered as a terrific capacitive electrode for LICs. The GCD curves of 21 KS (Fig. S11a†) present ideal linear and the capacity retention (Fig. S11b†) can maintain at 80% after 500 cycles. Therefore, the LICs are assembled by 21 KS and as-prepared MoS2/CP in the organic electrolyte and the configuration of device is shown in Fig. S12a.† According to the potential distribution curves of the electrodes shown in Fig. S12b,† the voltage window of devices is optimized by using the pre-lithiated anode and finally set as 1.0–4.0 V conservatively to prevent Li metal from precipitating on the anode. The mass ratio of 21 KS to MoS2/CP is adjusted in detail between 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2.5
1 and 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the charge balance of cathode to anode (Qcathode = Qanode) and the optimum of energy and power property (Fig. 4b). Therefore, the optimum ratio 1.5
1 for the charge balance of cathode to anode (Qcathode = Qanode) and the optimum of energy and power property (Fig. 4b). Therefore, the optimum ratio 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 among them is determined in this device and the following tests are operated under the condition.
1 among them is determined in this device and the following tests are operated under the condition.
The CV curves at increased scan rates (from 1 mV s−1 to 20 mV s−1) are shown in Fig. 4c. At all scan rates, it exhibits regular rectangle without obvious redox peaks, indicative the typical capacitive behaviour and well-matching kinetics of the two electrodes.6,56 The GCD profiles (Fig. 4d) present a standard triangle at various current densities with negligible IR drops, which is ascribed to the convenient ion diffusion and rapid charge transfer profited from N-doped carbon matrix and the adequate exfoliation of MoS2.45,57,58 The cycle stability (79.61% of initial capacity after 1000 cycles at 1 A g−1) is shown in Fig. 4e. The power/energy property of device is shown in the Ragone plots (Fig. 4f). The LICs deliver the maximum energy density of 87.74 W h kg−1 at 253 W kg−1 and high power density of 35.81 kW kg−1 even at 19.86 W h kg−1, which precedes other similar LICs systems reported recently, especially in the zone of high power density.59–63
Conclusions
In summary, we successfully synthesized the exfoliated MoS2 supported by N-doped carbon matrix derived from chitosan, which is encapsulated by pitch-derived carbon shells by a simple ball-milling and pyrolysis method. It delivers high rate capability (530 mA h g−1 at 0.1 A g−1 and 230 mA h g−1 at 10 A g−1) and cycle performance(73% capacity retention after 250 cycles) as the anode for LIBs, mainly ascribed to the sufficient exfoliation of MoS2, conductive carbon matrix with high nitrogen content and robust carbon shells. Notably, the close contact between MoS2 and carbon matrix through Mo–O bonds also have significant effect on the electrons and ions transfer. The LICs assembled by commercial AC (21 KS) as the cathode and MoS2/CP as the anode present high energy density (87.74 W h kg−1) and high power density (35.81 kW kg−1) mainly profited from the fast Li+ storage kinetics of the anode. The work provides an idea for constructing the devices with high energy and power densities by versatile and scalable methods.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No. 51672151).References
- D. G. Nocera, Chem. Soc. Rev., 2009, 38, 13–15 RSC.
- Z. Zhang, S. C. Mu, B. W. Zhang, L. Tao, S. F. Huang, Y. Z. Huang, F. M. Gao and Y. F. Zhao, J. Mater. Chem. A, 2016, 4, 2137–2146 RSC.
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917–918 CrossRef CAS PubMed.
- X. W. Dou, I. Hasa, D. Saurel, C. Vaalma, L. M. Wu, D. Buchholz, D. Bresser, S. Komaba and S. Passerini, Mater. Today, 2019, 23, 87–104 CrossRef CAS.
- L. Ji, M. Gu, Y. Shao, X. Li, M. H. Engelhard, B. W. Arey, W. Wang, Z. Nie, J. Xiao, C. Wang, J. G. Zhang and J. Liu, Adv. Mater., 2014, 26, 2901–2908 CrossRef CAS PubMed.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- X. Li and L. Zhi, Chem. Soc. Rev., 2018, 47, 3189–3216 RSC.
- S. Lee, G. Kwon, K. Ku, K. Yoon, S. K. Jung, H. D. Lim and K. Kang, Adv. Mater., 2018, 30, e1704682 CrossRef PubMed.
- J. Ding, W. Hu, E. Paek and D. Mitlin, Chem. Rev., 2018, 118, 6457–6498 CrossRef CAS PubMed.
- J. Zhao, G. Wang, R. Hu, K. Zhu, K. Cheng, K. Ye, D. Cao and Z. Fan, J. Mater. Chem. A, 2019, 7, 4047–4054 RSC.
- X. Shen, J. He, K. Wang, X. Li, X. Wang, Z. Yang, N. Wang, Y. Zhang and C. Huang, ChemSusChem, 2019, 12, 1342–1348 CrossRef CAS PubMed.
- P. Han, G. Xu, X. Han, J. Zhao, X. Zhou and G. Cui, Adv. Energy Mater., 2018, 8, 1801243 CrossRef.
- S. Zheng, Z.-S. Wu, S. Wang, H. Xiao, F. Zhou, C. Sun, X. Bao and H.-M. Cheng, Energy Storage Materials, 2017, 6, 70–97 CrossRef.
- F. Yu, T. Huang, P. Zhang, Y. Tao, F.-Z. Cui, Q. Xie, S. Yao and F. Wang, Energy Storage Materials, 2019, 22, 235–255 CrossRef.
- A. Bhowmik, I. E. Castelli, J. M. Garcia-Lastra, P. B. Jørgensen, O. Winther and T. Vegge, Energy Storage Materials, 2019, 21, 446–456 CrossRef.
- C. Choi, D. S. Ashby, D. M. Butts, R. H. DeBlock, Q. Wei, J. Lau and B. Dunn, Nat. Rev. Mater., 2019 DOI:10.1038/s41578-019-0142-z.
- G. G. Amatucci, F. Badway, A. Du Pasquier and T. Zheng, J. Electrochem. Soc., 2001, 148, A930–A939 CrossRef CAS.
- H. Wang, C. Zhu, D. Chao, Q. Yan and H. J. Fan, Adv. Mater., 2017, 29, 1702093 CrossRef PubMed.
- D. P. Dubal, K. Jayaramulu, J. Sunil, Š. Kment, P. Gomez-Romero, C. Narayana, R. Zbořil and R. A. Fischer, Adv. Funct. Mater., 2019, 29, 1900532 CrossRef.
- S. Li, H. Tang, P. Ge, F. Jiang, J. Zhou, C. Zhang, H. Hou, W. Sun and X. Ji, ACS Appl. Mater. Interfaces, 2018, 10, 6378–6389 CrossRef CAS PubMed.
- P. Wang, J. Tian, J. Hu, X. Zhou and C. Li, ACS Nano, 2017, 11, 7390–7400 CrossRef CAS PubMed.
- C. Z. Zhan, W. Liu, M. X. Hu, Q. H. Liang, X. L. Yu, Y. Shen, R. T. Lv, F. Y. Kang and Z. H. Huang, NPG Asia Mater., 2018, 10, 775–787 CrossRef CAS.
- T. Stephenson, Z. Li, B. Olsen and D. Mitlin, Energy Environ. Sci., 2014, 7, 209–231 RSC.
- X. Zhou, L. J. Wan and Y. G. Guo, Chem. Commun., 2013, 49, 1838–1840 RSC.
- L. Ma, B. Zhao, X. Wang, J. Yang, X. Zhang, Y. Zhou and J. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 22067–22073 CrossRef CAS PubMed.
- Y. Teng, H. Zhao, Z. Zhang, Z. Li, Q. Xia, Y. Zhang, L. Zhao, X. Du, Z. Du, P. Lv and K. Swierczek, ACS Nano, 2016, 10, 8526–8535 CrossRef CAS PubMed.
- S. S. Xia, Y. R. Wang, Y. Liu, C. H. Wu, M. H. Wu and H. J. Zhang, Chem. Eng. J., 2018, 332, 431–439 CrossRef CAS.
- S. M. Cao, L. Y. Shi, M. Miao, J. H. Fang, H. B. Zhao and X. Feng, Electrochim. Acta, 2019, 298, 22–30 CrossRef CAS.
- Y. G. Huang, Y. Wang, X. H. Zhang, F. Y. Lai, Y. N. Sun, Q. Y. Li and H. Q. Wang, Mater. Lett., 2019, 243, 84–87 CrossRef CAS.
- H. L. Xue, S. Yue, J. Wang, Y. Zhao, Q. Li, M. M. Yin, S. S. Wang, C. H. Feng, Q. Wu, H. S. Li, D. X. Shi and Q. Z. Jiao, J. Electroanal. Chem., 2019, 840, 230–236 CrossRef CAS.
- X. Wang, S. M. Fei, S. S. Huang, C. H. Wu, J. R. Zhao, Z. W. Chen, K. Uvdal and Z. J. Hu, Carbon, 2019, 150, 363–370 CrossRef CAS.
- N. Feng, R. Meng, L. Zu, Y. Feng, C. Peng, J. Huang, G. Liu, B. Chen and J. Yang, Nat. Commun., 2019, 10, 1372 CrossRef PubMed.
- D. Sun, D. L. Ye, P. Liu, Y. G. Tang, J. Guo, L. Z. Wang and H. Y. Wang, Adv. Energy Mater., 2018, 8, 11 Search PubMed.
- S. Chen, R. Xu, J. Liu, X. Zou, L. Qiu, F. Kang, B. Liu and H. M. Cheng, Adv. Mater., 2019, 31, e1804810 CrossRef PubMed.
- G. Li, J. Y. Li, F. S. Yue, Q. Xu, T. T. Zuo, Y. X. Yin and Y. G. Guo, Nano Energy, 2019, 60, 485–492 CrossRef CAS.
- F. Xie, Z. Xu, A. C. S. Jensen, H. Au, Y. Lu, V. Araullo-Peters, A. J. Drew, Y. S. Hu and M. M. Titirici, Adv. Funct. Mater., 2019, 29, 1901072 CrossRef.
- Y. C. Jiao, A. Mukhopadhyay, Y. Ma, L. Yang, A. M. Hafez and H. L. Zhu, Adv. Energy Mater., 2018, 8, 1702779 CrossRef.
- S. Wang, R. Wang, Q. Zhao, L. Ren, J. Wen, J. Chang, X. Fang, N. Hu and C. Xu, J. Colloid Interface Sci., 2019, 544, 37–45 CrossRef CAS PubMed.
- L. Q. Xu, Z. Jiao, P. F. Hu, Y. Wang, Y. J. Wang and H. J. Zhang, Chemelectrochem, 2016, 3, 1503–1512 CrossRef CAS.
- M. R. Gao, M. K. Chan and Y. Sun, Nat. Commun., 2015, 6, 7493 CrossRef PubMed.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS PubMed.
- J. Liu, Y. Zhang, L. Zhang, F. Xie, A. Vasileff and S. Z. Qiao, Adv. Mater., 2019, 31, e1901261 CrossRef PubMed.
- W. Zhang, Y. Fu, W. Liu, L. Lim, X. Wang and A. Yu, Nano Energy, 2019, 57, 48–56 CrossRef CAS.
- D. Xie, X. Xia, Y. Wang, D. Wang, Y. Zhong, W. Tang, X. Wang and J. Tu, Chemistry, 2016, 22, 11617–11623 CrossRef CAS PubMed.
- J. X. Wu, Z. Y. Pan, Y. Zhang, B. J. Wang and H. S. Peng, J. Mater. Chem. A, 2018, 6, 12932–12944 RSC.
- R. Tian, W. Wang, Y. Huang, H. Duan, Y. Guo, H. Kang, H. Li and H. Liu, J. Mater. Chem. A, 2016, 4, 13148–13154 RSC.
- Z. Lei, L. Xu, Y. Jiao, A. Du, Y. Zhang and H. Zhang, Small, 2018, 14, e1704410 CrossRef.
- G. Huang, T. Chen, W. Chen, Z. Wang, K. Chang, L. Ma, F. Huang, D. Chen and J. Y. Lee, Small, 2013, 9, 3693–3703 CrossRef CAS PubMed.
- X. P. Fang, X. Q. Yu, S. F. Liao, Y. F. Shi, Y. S. Hu, Z. X. Wang, G. D. Stucky and L. Q. Chen, Microporous Mesoporous Mater., 2012, 151, 418–423 CrossRef CAS.
- V. Augustyn, J. Come, M. A. Lowe, J. W. Kim, P. L. Taberna, S. H. Tolbert, H. D. Abruna, P. Simon and B. Dunn, Nat. Mater., 2013, 12, 518–522 CrossRef CAS PubMed.
- H.-S. Kim, J. B. Cook, H. Lin, J. S. Ko, S. H. Tolbert, V. Ozolins and B. Dunn, Nat. Mater., 2016, 16, 454–460 CrossRef PubMed.
- M. Wang, Z. Yang, W. Li, L. Gu and Y. Yu, Small, 2016, 12, 2559–2566 CrossRef CAS PubMed.
- G. A. Elia, U. Ulissi, S. Jeong, S. Passerini and J. Hassoun, Energy Environ. Sci., 2016, 9, 3210–3220 RSC.
- J. Y. Jin, Z. W. Wang, R. Wang, J. L. Wang, Z. D. Huang, Y. W. Ma, H. Li, S. H. Wei, X. Huang, J. X. Yan, S. Z. Li and W. Huang, Adv. Funct. Mater., 2019, 29, 1807441 CrossRef.
- Y. Y. Liu, L. Zhang, Y. C. Zhao, T. D. Shen, X. L. Yan, C. Yu, H. Q. Wang and H. Zeng, J. Alloys Compd., 2019, 787, 996–1003 CrossRef CAS.
- Y. Gogotsi and R. M. Penner, ACS Nano, 2018, 12, 2081–2083 CrossRef CAS PubMed.
- D. Ni, W. Sun, Z. Wang, Y. Bai, H. Lei, X. Lai and K. Sun, Adv. Energy Mater., 2019, 9, 1900036 CrossRef.
- D. F. Xu, C. J. Chen, J. Xie, B. Zhang, L. Miao, J. Cai, Y. H. Huang and L. N. Zhang, Adv. Energy Mater., 2016, 6, 1501929 CrossRef.
- S. Hemmati, G. Li, X. L. Wang, Y. L. Ding, Y. Pei, A. P. Yu and Z. W. Chen, Nano Energy, 2019, 56, 118–126 CrossRef CAS.
- J. Jiang, Y. Zhang, Y. An, L. Wu, Q. Zhu, H. Dou and X. Zhang, Small Methods, 2019, 1900081 CrossRef.
- C. T. Zhao, C. Yu, M. D. Zhang, Q. Sun, S. F. Li, M. N. Banis, X. T. Han, Q. Dong, J. Yang, G. Wang, X. L. Sun and J. S. Qiu, Nano Energy, 2017, 41, 66–74 CrossRef CAS.
- Y. Qian, X. Y. Cai, C. Y. Zhang, H. F. Jiang, L. J. Zhou, B. S. Li and L. F. Lai, Electrochim. Acta, 2017, 258, 1311–1319 CrossRef CAS.
- J. G. Ju, L. T. Zhang, H. S. Shi, Z. J. Li, W. M. Kang and B. W. Cheng, Appl. Surf. Sci., 2019, 484, 392–402 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM spectra of chitosan, cg-MoS2 and MoS2/C. XPS data of N-doped carbon matrix and MoS2/C. Raman images, GCD curves and CV curves of bulk MoS2 and MoS2/C. N2 adsorption/desorption isotherms, pore size distribution (PSD), CV and GCD curves of 21 KS. Element atom ratio of three samples. See DOI: 10.1039/c9ra09411c |
| This journal is © The Royal Society of Chemistry 2019 |