Open Access Article
Open Access ArticleBa4Bi2(Si8−xB4+xO29) (x = 0.09): a new acentric metal borosilicate as a promising nonlinear optical material†‡
Ru-Ling
Tang
abc,
Chun-Li
Hu
a,
Fei-Fei
Mao
a,
Jiang-He
Feng
a and
Jiang-Gao
Mao
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China. E-mail: mjg@fjirsm.ac.cn
bSchool of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China
cUniversity of the Chinese Academy of Sciences, Beijing, 100049, China
First published on 31st October 2018
Abstract
A new acentric metal borosilicate, namely Ba4Bi2(Si8−xB4+xO29) (x = 0.09), has been synthesized by a standard solid-state reaction. The title compound crystallizes in noncentrosymmetric (NCS) space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m with lattice parameters a = 11.0254(4) Å and c = 10.3961(9) Å. Structure refinements indicate that mixing of B atoms and Si atoms exists for a few atomic sites. In the “ideal” Ba4Bi2(Si8B4O29), BO4 or SiO4 tetrahedra are inter-connected by corner-sharing to cyclic B4O12 or Si4O12 units. These B4O12 and Si4O12 units are further interconnected via corner-sharing to an “ideal” [Si8B4O29]14− 3D network. The Ba2+ and Bi3+ act as the counter cations and are located at the cavities of the structure. Ba4Bi2(Si8−xB4+xO29) (x = 0.09) melts incongruently at a high temperature of 929 °C. Powder second-harmonic generation (SHG) measurements reveal that Ba4Bi2(Si8−xB4+xO29) (x = 0.09) is a type I phase-matching compound with a good SHG response of about 5.1 times that of KDP (KH2PO4), which is the highest among the borosilicates reported so far. The SHG source has been studied by DFT theoretical calculations. Our preliminary results indicate that Ba4Bi2(Si8−xB4+xO29) (x = 0.09) is a new second-order nonlinear-optical crystalline material candidate.
2m with lattice parameters a = 11.0254(4) Å and c = 10.3961(9) Å. Structure refinements indicate that mixing of B atoms and Si atoms exists for a few atomic sites. In the “ideal” Ba4Bi2(Si8B4O29), BO4 or SiO4 tetrahedra are inter-connected by corner-sharing to cyclic B4O12 or Si4O12 units. These B4O12 and Si4O12 units are further interconnected via corner-sharing to an “ideal” [Si8B4O29]14− 3D network. The Ba2+ and Bi3+ act as the counter cations and are located at the cavities of the structure. Ba4Bi2(Si8−xB4+xO29) (x = 0.09) melts incongruently at a high temperature of 929 °C. Powder second-harmonic generation (SHG) measurements reveal that Ba4Bi2(Si8−xB4+xO29) (x = 0.09) is a type I phase-matching compound with a good SHG response of about 5.1 times that of KDP (KH2PO4), which is the highest among the borosilicates reported so far. The SHG source has been studied by DFT theoretical calculations. Our preliminary results indicate that Ba4Bi2(Si8−xB4+xO29) (x = 0.09) is a new second-order nonlinear-optical crystalline material candidate.
Introduction
Noncentrosymmetric solid materials have always been an active research field due to their unique physical performances, such as second-order nonlinear optics (NLO), piezoelectricity, pyroelectricity, ferroelectricity, etc.1–9 In the second-harmonic generation domain particularly, a variety of excellent acentric materials have been synthesized including the famous NLO materials, such as β-BaB2O4 (β-BBO) and LiB3O5 (LBO).10,11 Recently, fluorooxoborates have aroused great interest due to their short ultraviolet cutoff edge. Some new acentric fluorooxoborates have been discovered, such as AB4O6F (A = NH4, Na, K, Rb, or Cs).12–16As for the NLO materials used in the deep-ultraviolet (DUV) region, the KBe2BO3F2 (KBBF) crystal is the only material that can produce coherent light wavelengths below 200 nm by direct SHG.17,18 After continuous efforts, a number of new beryllium borate crystals of the KBBF family have been discovered including β-KBe2B3O7, γ-KBe2B3O7, RbBe2B3O7, Na2Be4B4O11 and LiNa5Be12B12O33.19–21 Unfortunately, due to the weak interlayer bonding force and the toxicity of Be2+ in these materials, their crystal growth and wide industrial applications are still restricted. Hence, finding an appropriate composition to substitute beryllium has aroused widespread research interest.
Considering the coordination environment of beryllium atoms, a very effective strategy is to replace BeO4 tetrahedra with PO4, SiO4 and GeO4, which led to the formation of a variety of metal borophosphates, borogermanates and borosilicates, including Ba3(ZnB5O10)PO4, Na4MB2P3O13 (M = Rb, Cs), A2EB4O9 (A = Cs, Rb) (E = Si, Ge), AEB3O7 (A = Cs, Rb) (E = Si, Ge), and Ba4(BO3)3(SiO4)·Ba3X (X = Cl, Br).22–29 Recently, the partial disorder between Ge atoms and B atoms in tetrahedral positions has led to borogermanates with interesting structures, as shown by CsBxGe6−xO12 (x = 1) and Sr3−x/2B2−xGe4+xO14 (x = 0.32).30,31
A2EB4O9 (A = Cs, Rb) (E = Si, Ge) and AEB3O7 (A = Cs, Rb) (E = Si, Ge) possess relatively large SHG responses in inorganic metal borogermanate and borosilicate systems; for example, Cs2GeB4O9 has a SHG response of 2.8 × KDP and Cs2SiB4O9 exhibits a SHG response of 4.6 × KDP.24,27 A2EB4O9 (A = Cs, Rb) (E = Si, Ge) features a three-dimensional anionic open framework formed by corner-sharing EO4 tetrahedra and B4O9 clusters, with A+ cations filling in the anionic channels formed by nine-/ten-membered rings. It is noticed that alkaline-earth metal borogermanates and borosilicates display a relatively weaker SHG response, for example, Ba4(BO3)3(SiO4)·Ba3X (X = Cl, Br) (1 × KDP) and Ba3[Ge2B7O16(OH)2](OH)(H2O) (0.3 × KDP).32,33
Compared with metal borates and fluorooxoborates, it is obvious that the SHG responses of most acentric borosilicates and borogermanates are relatively weak. To increase their SHG responses, one useful method is to introduce SHG active cations with stereo-active lone pairs, such as Pb2+, Sn2+, and Bi3+.34–36
We have been trying to introduce cations with lone pairs into metal borogermanates and borosilicates to obtain materials with enhanced SHG performance. In this work, we have successfully synthesized Ba4Bi2(Si8−xB4+xO29) (x = 0.09) (BBSBO) by solid state reactions. It exhibits an SHG response of 5.1 × KDP. In this compound, mixing of B atoms and Si atoms for some atomic sites occurred, which is similar to what was observed for a few borogermanates previously reported.30,31 In this work, the synthesis, crystal structure, second-order nonlinear-optical effects, and thermal stability of this compound are reported.
Results and discussion
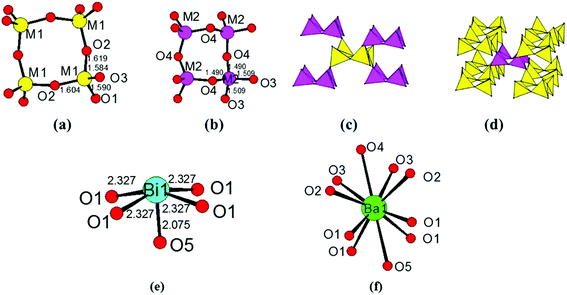
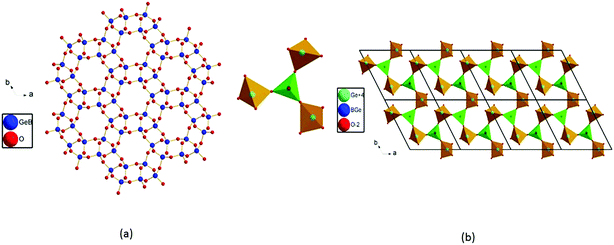
The title compound crystallizes in noncentrosymmetric space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m (no. 121) with lattice parameters of a = 11.034(3) Å and c = 10.410(6) Å (Table S1‡). The asymmetric unit of this compound contains two B3+/Si4+ mixed sites, one Bi atom, one Ba atom, and five O atoms. The first one at a 16j site is mainly Si4+ (92.6%) mixed with a small amount of B3+ (7.4%), whereas the one at the 8g site is mainly B3+ (87.5%) mixed with 12.5% of Si4+. All of the Si4+ and B3+ atoms are tetrahedrally connected with four oxygen atoms. The M1 position based on mainly Si atoms showed M−O distances from 1.584 Å to 1.619 Å. Four M(1)O4 tetrahedra form a cyclic M(1)4O12 unit (Fig. 2a). The M(2)O4 tetrahedral centers based on mainly B atoms also form similar M(2)4O12 units (Fig. 2b), and the M−O distances are within the range from 1.490 Å to 1.509 Å. Each M(1)4O12 unit is corner-shared with four M(2)4O12 units (Fig. 2c), while each M(2)4O12 unit is corner-shared with eight M(1)4O12 units (Fig. 2d). Such connectivity led to a 3D anionic framework (Fig. 1b). The Ba2+ atom is ten-coordinated with Ba–O distances ranging from 2.734(5) to 3.2092(4) Å (Fig. 2f). Coordinated with five oxygen atoms, the Bi3+ atoms are in a BiO5 square pyramidal geometry with four Bi–O1 (2.327(3) Å) and one Bi–O5 (2.0748(3) Å) (Fig. 2e). The unpaired electrons of the Bi3+ ion are oriented towards the open side of the BiO4 square (opposite to O5). All the Ba2+ and Bi3+ act as the counter cations and are filled in the tunnels of the 8-member rings of the anionic framework (Fig. 1a).
2m (no. 121) with lattice parameters of a = 11.034(3) Å and c = 10.410(6) Å (Table S1‡). The asymmetric unit of this compound contains two B3+/Si4+ mixed sites, one Bi atom, one Ba atom, and five O atoms. The first one at a 16j site is mainly Si4+ (92.6%) mixed with a small amount of B3+ (7.4%), whereas the one at the 8g site is mainly B3+ (87.5%) mixed with 12.5% of Si4+. All of the Si4+ and B3+ atoms are tetrahedrally connected with four oxygen atoms. The M1 position based on mainly Si atoms showed M−O distances from 1.584 Å to 1.619 Å. Four M(1)O4 tetrahedra form a cyclic M(1)4O12 unit (Fig. 2a). The M(2)O4 tetrahedral centers based on mainly B atoms also form similar M(2)4O12 units (Fig. 2b), and the M−O distances are within the range from 1.490 Å to 1.509 Å. Each M(1)4O12 unit is corner-shared with four M(2)4O12 units (Fig. 2c), while each M(2)4O12 unit is corner-shared with eight M(1)4O12 units (Fig. 2d). Such connectivity led to a 3D anionic framework (Fig. 1b). The Ba2+ atom is ten-coordinated with Ba–O distances ranging from 2.734(5) to 3.2092(4) Å (Fig. 2f). Coordinated with five oxygen atoms, the Bi3+ atoms are in a BiO5 square pyramidal geometry with four Bi–O1 (2.327(3) Å) and one Bi–O5 (2.0748(3) Å) (Fig. 2e). The unpaired electrons of the Bi3+ ion are oriented towards the open side of the BiO4 square (opposite to O5). All the Ba2+ and Bi3+ act as the counter cations and are filled in the tunnels of the 8-member rings of the anionic framework (Fig. 1a).
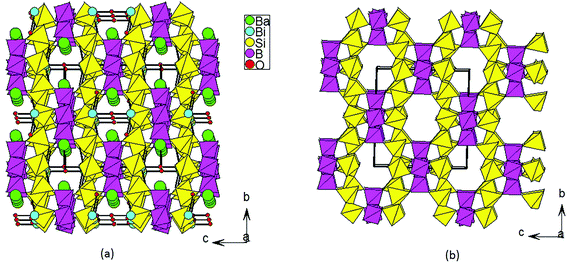 | ||
| Fig. 1 View of the 3D crystal structure of BBSBO down the a axis (a); view of the 3D anionic framework of BBSBO with tunnels of 8-MRs along the a axis (b). | ||
The anionic borosilicate framework in LnBSiO5 (Ln = La, Ce, Nd) is also solely composed of BO4 and SiO4 tetrahedral groups.37 However, the BO4 and SiO4 arrange in a different way. In LnBSiO5, the BO4 tetrahedra form infinite helical chains with the SiO4 tetrahedra grafting on the 1D chain by bridging with two neighboring BO4 tetrahedra, hence forming 1D borosilicate chains based on B2Si three-membered rings.
About the site-mixing of B and Si atoms, it is not very common in the borosilicates reported. However two borogermanates, namely, CsBxGe6−xO12 (x = 1) (CBGO) and Sr3−x/2B2−xGe4+xO14 (x = 0.32) (SBGO), have been reported to exhibit the mixing of the B and Ge atoms at the same site.30,31 There is only one mixed occupied site of B3+/Ge4+ in the structure of CBGO, and it has the percentage of B3+ (16.6%) and Ge4+ (83.3%). B/GeO4 tetrahedra are interconnected leading to an octahedral cage, and each connects with the eight nearest ones (Fig. 3a). Cs+ acts as the counter cation located in each cage, which produces a zeolite SOD-type net.30 As for SBGO, it exhibits a Ca3Ga2Ge4O14-like structure (Fig. 3b). SBGO exhibits both GeO4 tetrahedra and GeO6 octahedra. The mixed Ge4+/B3+ site is tetrahedrally coordinated with Ge4+ (16%) and B3+ (84%). The B/GeO4 tetrahedron connects with three other GeO4 tetrahedra by sharing three corners in a layered structure.31 We deem that the mixing of B and Si atoms at the same site should be more likely compared with the B/Ge mixing due to being closer in size; hence we believe that more similar examples will be found in the future.
 | ||
| Fig. 3 The Ge/BO4 tetrahedral cage in CsBxGe6−xO12 (x = 1) (a); the B/GeO4–GeO4 layer in Sr3−x/2B2−xGe4+xO14 (x = 0.32) (b). | ||
The TG and DSC curves are given in Fig. S4.‡ The thermal behavior of BBSBO is measured from 30 to 1000 °C. The TG results show that there is no apparent weight loss before 1000 °C, which indicates that this compound has very high thermal stability. As shown in the graph, there is one clear endothermic peak at 929 °C but no exothermic peak in the cooling curves, which indicates that this compound melts incongruently. The peak at 929 °C is later proved to be the melting point. The powder XRD pattern for the sample heated at 940 °C does not match the calculated PXRD (Fig. S1‡); hence the material goes through incongruent melting. Through phase analysis, the residuals are mainly Ba0.5Bi1.5O2.16, SiO2 and B2O3.
The UV-Vis absorption spectrum of BBSBO is displayed in Fig. S3.‡ It is shown that the UV cutoff edge of BBSBO is 318 nm. The UV spectrum indicates that the band gap of BBSBO is 3.89 eV (Fig. S3‡). Strong absorption bands are observed with vibration frequencies within 1100 cm−1 in the IR spectrum of BBSBO (Fig. S2‡). According to ref. 38, the high-frequency peaks located at 1100 to 740 cm−1 are well matched with the BO45− and SiO44− unit stretching vibrations. The BO4 and SiO4 group bending vibration bands should be below 660 cm−1 in the figure. Due to some overlap of vibration bands for BO4 and SiO4 groups, these absorption bands cannot be assigned undoubtedly, which is common in the reported ref. 34 and 39.
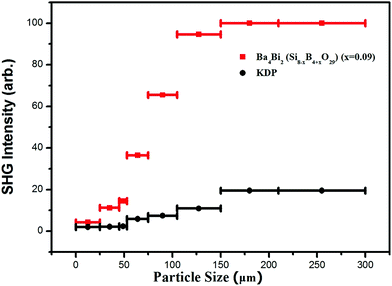
Powder SHG signals of BBSBO crystals at a wavelength of 1064 nm are exhibited in Fig. 4. Comparing the SHG intensity produced by BBSBO and KDP shows that BBSBO has a good SHG effect of 5.1 × KDP, which is the largest among metal borosilicates reported to date, and BBSBO is phase-matching based on the rule established by Kurtz and Perry.40 The SHG source of the title compound may have originated from the synergistic effect of Bi3+, BO4 and SiO4 groups.
To learn about the SHG response origin of BBSBO more deeply, theoretical computations on an ideal “Ba4Bi2(Si8B4O29)” structure were made based on DFT methods. The band structure calculations reveal that BBSBO is an indirect band-gap material (from Z to G) (Fig. S5 and Table S5‡). The theoretical band gap is 4.35 eV, being larger than the value of 3.89 eV obtained by experimental measurement. The result is not unreasonable, because it is common that GGA cannot precisely depict the eigenvalues of the conduction bands.41–44
From the upper five panels in the partial density of states (PDOS) graph of BBSBO (Fig. 5), the bands and the bonding interactions of the structure can be easily assigned and understood. We study the vicinity of the Fermi level from −10 to 8 eV to account for the bonding trait and optical properties of BBSBO. Obviously, the electronic states of Si and B atoms are well overlapped with coordinated O atoms, signifying the firm bonding interactions. Similarly, Bi atoms are greatly overlapped with the O atoms in some regions too. The upper region of the VB from −5.0 to 0 eV is mainly the 2p nonbonding states of oxygen atoms. Particularly, a few of the flat bands at the top of the VB are ascribed to the nonbonding states of oxygen atoms that are bonded to Bi and some of the Bi-6s states; in addition, the bottommost part of the CB originates from the unoccupied Bi-6p and some O-2p states bonded to Bi. Hence the band gap of BBSBO rests with BiO5 anionic groups.
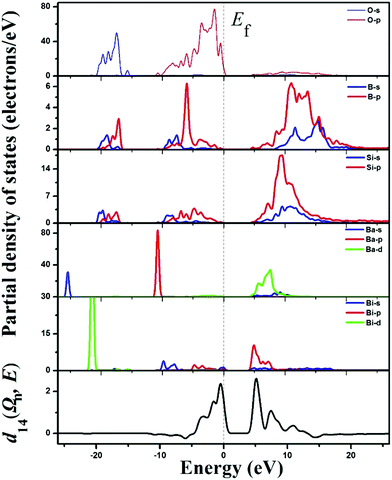 | ||
| Fig. 5 The partial density of states (the upper five panels) and the spectral decomposition of d14 (the bottommost panel) for BBSBO. | ||
We further calculated the second-order nonlinear optical properties of BBSBO. BBSBO crystallized in I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m space group, which belongs to the point group
2m space group, which belongs to the point group ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m and has only one non-zero SHG tensor (d14) in consideration of Kleinman symmetry. The calculated SHG tensor d14 = 5.20 × 10−9 esu coincides with the experimental value of 5.1 times that of KDP.
2m and has only one non-zero SHG tensor (d14) in consideration of Kleinman symmetry. The calculated SHG tensor d14 = 5.20 × 10−9 esu coincides with the experimental value of 5.1 times that of KDP.
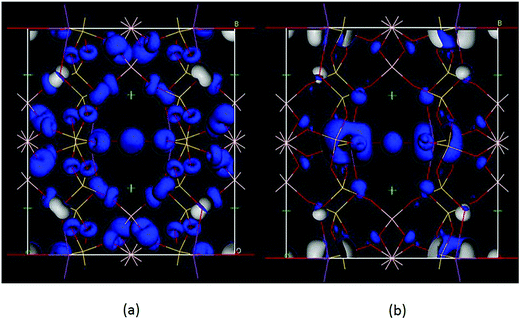
Moreover, we analyzed the SHG source of BBSBO. We performed the spectral decomposition and the SHG density analyses of tensor d14. It is obvious that the upper part of the VB (−5.0–0 eV) and the lower part of the CB (<10 eV) are the most SHG-active energy regions of d14 (Fig. 5), corresponding to O-2p electronic states in the VB and unoccupied Bi-6p, Ba-5d, Si-3p, B-2p, and O-2p in the CB. It is worth noting that the SHG density of d14 (Fig. 6) indicates that the 2p nonbonding states of all O atoms in the VB make a prominent difference to the SHG effect, while the unoccupied Bi-6p and some O-2p orbitals contribute the most to the SHG process in the CB. By calculating the SHG density in amount, the SHG contribution percentages of BBSBO are 27.70%, 34.47%, 19.95% and 17.88% for BiO5, SiO4, BO4 and Ba2+, respectively. Notably, all anionic groups work well in the SHG process. It's also worth noting that as counter-ions, the Ba2+ cations contribute a lot to the SHG effect, which is very similar to the effect of Cs+ in the NLO compound of LiCs2PO4.45 Therefore, the synergistic effect of all the groups/ions makes BBSBO a remarkable SHG crystal.
Conclusions
In summary, a new acentric borosilicate, Ba4Bi2(Si8−xB4+xO29) (x = 0.09), has been synthesized and characterized. For the first time, Bi3+ has been introduced into the borosilicate system. BBSBO exhibits a strong SHG response which is 5.1 times that of KDP (KH2PO4) and possesses high thermal stability. According to first-principles calculations, the synergistic effect of Bi3+ and Ba2+ and SiO4 and BO4 groups led to an outstanding SHG response of the title compound. Our achievement in the case of BBSBO provides a feasible strategy for designing novel borosilicates and borogermanates with excellent SHG performance. Our future research efforts will be committed to the exploration of other borosilicates or borogermanates containing lone pair cations or fluoride anions.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
Our work has been supported by the National Natural Science Foundation of China (21875248, 91622112, and 21701173), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), and the 100 Talents Project of Fujian Province.Notes and references
- H. Yu, J. Young, H. Wu, W. Zhang, J. M. Rondinelli and P. S. Halasyamani, Chem. Mater., 2017, 29, 1845–1855 CrossRef.
- P. Kumar and A. Gaur, Ceram. Int., 2017, 43, 16403–16407 CrossRef.
- H. Yu, W. Zhang, J. Young, J. M. Rondinelli and P. S. Halasyamani, J. Am. Chem. Soc., 2016, 138, 88–91 CrossRef PubMed.
- J. Dalal and B. Kumar, Opt. Mater., 2016, 51, 139–147 CrossRef.
- K. Lin, Z. Zhou, L. Liu, H. Ma, J. Chen, J. Deng, J. Sun, L. You, H. Kasai, K. Kato, M. Takata and X. Xing, J. Am. Chem. Soc., 2015, 137, 13468–13471 CrossRef PubMed.
- C. M. N. Kumar, Y. Xiao, P. Lunkenheimer, A. Loidl and M. Ohl, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 235149 CrossRef.
- G. Zhang, Y. J. Li, K. Jiang, H. Y. Zeng, T. Liu, X. G. Chen, J. G. Qin, Z. S. Lin, P. Z. Fu, Y. C. Wu and C. T. Chen, J. Am. Chem. Soc., 2012, 134, 14818–14822 CrossRef PubMed.
- K. M. Ok, E. O. Chi and P. S. Halasyamani, Chem. Soc. Rev., 2006, 35, 710–717 RSC.
- P. Becker, Adv. Mater., 1998, 10, 979–992 CrossRef.
- C. T. Chen, B. C. Wu, A. D. Jiang and G. M. You, Sci. Sin., Ser. B, 1985, 589, 235–243 Search PubMed.
- C. T. Chen, Y. C. Wu, A. D. Jiang, G. M. You, R. K. Li and S. J. Lin, J. Opt. Soc. Am. B, 1989, 6, 616–621 CrossRef.
- Z. Zhang, Y. Wang, B. Zhang, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2018, 57, 1–6 CrossRef.
- G. Shi, Y. Wang, F. Zhang, B. Zhang, Z. Yang, X. Hou, S. Pan and K. R. Poeppelmeier, J. Am. Chem. Soc., 2017, 139, 10645–10648 CrossRef PubMed.
- Y. Wang, B. Zhang, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2018, 57, 2150–2154 CrossRef PubMed.
- M. Mutailipu, M. Zhang, H. Wu, Z. Yang, Y. Shen, J. Sun and S. Pan, Nat. Commun., 2018, 9, 3089 CrossRef PubMed.
- M. Mutailipu, M. Zhang, B. Zhang, L. Wang, Z. Yang, X. Zhou and S. Pan, Angew. Chem., Int. Ed., 2018, 57, 6095–6099 CrossRef PubMed.
- C. Chen, Z. Xu, D. Deng, J. Zhang, G. K. L. Wong, B. Wu, N. Ye and D. Tang, Appl. Phys. Lett., 1996, 68, 2930–2932 CrossRef.
- B. Wu, D. Tang, N. Ye and C. Chen, Opt. Mater., 1996, 5, 105–109 CrossRef.
- S. C. Wang, N. Ye, W. Li and D. Zhao, J. Am. Chem. Soc., 2010, 132, 8779–8786 CrossRef PubMed.
- X. X. Jiang, S. Y. Luo, L. Kang, P. F. Gong, H. W. Huang, S. C. Wang, Z. S. Lin and C. T. Chen, ACS Photonics, 2015, 2, 1183–1191 CrossRef.
- H. W. Huang, L. J. Liu, S. F. Jin, W. J. Yao, Y. H. Zhang and C. T. Chen, J. Am. Chem. Soc., 2013, 135, 18319–18322 CrossRef PubMed.
- H. Yu, W. Zhang, J. Young, J. M. Rondinelli and P. S. Halasyamani, Adv. Mater., 2015, 27, 7380–7385 CrossRef PubMed.
- C. Wu, L. Li, G. Yang, J. Song, B. Yan, M. G. Humphrey, L. Zhang, J. Shao and C. Zhang, Dalton Trans., 2017, 46, 12605–12611 RSC.
- X. Xu, C. L. Hu, F. Kong, J. H. Zhang, J. G. Mao and J. Sun, Inorg. Chem., 2013, 52, 5831–5837 CrossRef PubMed.
- J. H. Zhang, C. L. Hu, X. Xu, F. Kong and J. G. Mao, Inorg. Chem., 2011, 50, 1973–1982 CrossRef PubMed.
- F. Kong, H. L. Jiang, T. Hu and J. G. Mao, Inorg. Chem., 2008, 47, 10611–10617 CrossRef PubMed.
- H. Wu, H. Yu, S. Pan, Z. Huang, Z. Yang, X. Su and K. R. Poeppelmeier, Angew. Chem., Int. Ed., 2013, 52, 3406–3410 CrossRef PubMed.
- Z. Zhou, Y. Qiu, F. Liang, L. Palatinus, M. Poupon, T. Yang, R. Cong, Z. Lin and J. Sun, Chem. Mater., 2018, 30, 2203–2207 CrossRef.
- X. Lin, F. Zhang, S. Pan, H. Yu, F. Zhang, X. Dong, S. Han, L. Dong, C. Bai and Z. Wang, J. Mater. Chem. C, 2014, 2, 4257–4264 RSC.
- R. Pan, J. W. Cheng, B. F. Yang and G. Y. Yang, Inorg. Chem., 2017, 56, 2371–2374 CrossRef PubMed.
- B. Petermuller, L. L. Petschnig, K. Wurst, G. Heymann and H. Huppertz, Inorg. Chem., 2014, 53, 9722–9728 CrossRef PubMed.
- J. H. Zhang, F. Kong and J. G. Mao, Inorg. Chem., 2011, 50, 3037–3043 CrossRef PubMed.
- X. Xu, C. L. Hu, F. Kong, J. H. Zhang and J. G. Mao, Inorg. Chem., 2011, 50, 8861–8868 CrossRef PubMed.
- J. L. Song, C. L. Hu, X. Xu, F. Kong and J. G. Mao, Angew. Chem., Int. Ed., 2015, 54, 3679 CrossRef PubMed.
- M. Xia, X. Jiang, Z. Lin and R. Li, J. Am. Chem. Soc., 2016, 138, 14190–14193 CrossRef PubMed.
- M. Luo, F. Liang, Y. Song, D. Zhao, N. Ye and Z. Lin, J. Am. Chem. Soc., 2018, 140, 6814–6817 CrossRef PubMed.
- L. Li, Q. Jing, Z. Yang, X. Su, B.-H. Lei, S. Pan, F. Zhang and J. Zhang, J. Appl. Physiol., 2015, 118, 979–992 Search PubMed.
- Y. Shi, J. K. Liang, H. Zhang, J. L. Yang, W. D. Zhuangv and G. H. Rao, J. Alloys Compd., 1997, 259, 163–169 CrossRef.
- H. Yang, C. L. Hu, X. Xu and J. G. Mao, Inorg. Chem., 2015, 54, 7516–7523 CrossRef PubMed.
- P. Kubelka and F. Z. Munk, Tech. Phys., 1931, 12, 593–601 Search PubMed.
- J. Zhu, W. D. Cheng, D. S. Wu, H. Zhang, Y. J. Gong and H. N. Tong, J. Solid State Chem., 2006, 179, 597–604 CrossRef.
- J. Zhu, W. D. Cheng and D. S. Wu, Eur. J. Inorg. Chem., 2010, 2007, 285–290 CrossRef.
- B. P. Yang, C. L. Hu, X. Xu, C. Huang and J. G. Mao, Inorg. Chem., 2013, 52, 5378–5384 CrossRef CAS PubMed.
- D. Yan, C. L. Hu and J. G. Mao, CrystEngComm, 2016, 18, 1655–1664 RSC.
- X. Y. Cheng, M. H. Whangbo, G. C. Guo, M. C. Hong and S. Q. Deng, Angew. Chem., Int. Ed., 2018, 57, 3933–3937 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Prof. Jin-Shun Huang on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1864258. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc04342f |
| This journal is © The Royal Society of Chemistry 2019 |