Open Access Article
Open Access ArticleCrystallographic identification of Eu@C2n (2n = 88, 86 and 84): completing a transformation map for existing metallofullerenes†
Lipiao
Bao
,
Pengyuan
Yu
,
Changwang
Pan
,
Wangqiang
Shen
and
Xing
Lu
 *
*
State Key Laboratory of Materials Processing and Die & Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, 430074, China. E-mail: lux@hust.edu.cn
First published on 17th December 2018
Abstract
Revealing the transformation routes among existing fullerene isomers is key to understanding the formation mechanism of fullerenes which is still unclear now because of the absence of typical key links. Herein, we have crystallographically identified four new fullerene cages, namely, C2(27)-C88, C1(7)-C86, C2(13)-C84 and C2(11)-C84, in the form of Eu@C2n, which are important links to complete a transformation map that contains as many as 98% (176 compounds in total) of the reported metallofullerenes with clear cage structures (C2n, 2n = 86–74). Importantly, the mutual transformations between the metallofullerene isomers included in the map require only one or two well-established steps (Stone–Wales transformation and/or C2 insertion/extrusion). Moreover, structural analysis demonstrates that the unique C2(27)-C88 cage may serve as a key point in the map and is directly transformable from a graphene fragment. Thus, our work provides important insights into the formation mechanism of fullerenes.
Introduction
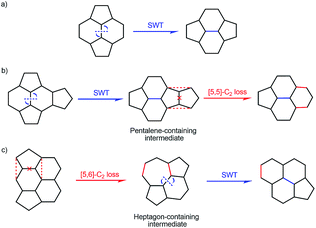
Fullerenes are a collection of spherical pure-carbon molecules consisting of exactly 12 pentagons and a variable number of hexagons.1 Although C60 and C70 are abundantly produced and have received extensive attention,2 pure isomers of higher fullerenes (C2n, 2n > 70) are rarely reported because of their low solubility in organic solvents and the tremendous number of possible isomers that may exist in reality.3 Alternatively, endohedral metal-doping has had great success in stabilizing higher fullerenes to produce a new family of hybrid molecules named endohedral metallofullerenes (EMFs).4,5 Up to now, hundreds of EMF-isomers have been isolated and structurally characterized.4–7 Their cage sizes range from C60 to even C108 and the metallic species are also diverse and can be one or two pure metal atoms or a cluster of metal carbides, nitrides, oxides, sulfides and even cyanides.4–7 It is noteworthy that although many possible cage structures are available for a given C2n,3 the experimentally obtained species are limited to a small number of compounds.4–7 For instance, as many as seven isomers that obey the ‘isolated pentagon rule’ (IPR)8 are available for C80,3 but only the Ih-C80 and D5h-C80 cages have been found for M3N@C80,4,9–11 and C2v(5)-C80 has been found for Sc2C2@C80.12,13It is certainly of special interest to understand the inter-cage transformation between isolated fullerene/EMF isomers which is key to clarifying the formation mechanism of fullerenes. In the very beginning of fullerene research, the structural transition from C60 to C70 was described like this: C60 is cut in half, and one half is rotated by 36 degrees relative to the other, and then a 10-carbon ring is added in between, and thus C70 forms.14 Obviously, this route is too complicated to happen in reality. In fact, both theoretical and experimental results suggest that the transformations between fullerene isomers may involve merely two processes: Stone–Wales transformation (SWT)15 and C2-extrusion or insertion (Fig. 1). Theoretical calculations suggest that the energy barrier of such facile transformations might be easily overcome in the very hot environments of fullerene formation16 and the reported transformation reactions are favored in terms of entropy.17 The proposed SWT and C2 elimination/addition could generate labile pentalene/heptagon containing intermediates, which has been demonstrated by the experimental identification of heptagon18,19 and fused-pentagon20–26 containing metallofullerenes with the endohedral metallic unit or exohedral functionalized moieties27–29 as stabilizers.
During recent years, more and more higher-fullerene cages have been captured and structurally identified, mainly in the form of EMFs which enables in-depth investigation of the inter-cage transformations among existing fullerene cages.4,5 For example, Balch and co-workers identified Sc2S@Cs(6)-C82 and Sc2S@C3v(8)-C82 and proposed the interconversion process between Cs(6)-C82 and C3v(8)-C82 through two SWTs30 with the commonly encountered C2v(9)-C82 as the intermediate. In another work, they found that four Sm@C90 isomers can be related pairwise to one another through sequential SWTs.31 Besides, Feng et al. revealed that Sc2O@C2v(3)-C78 and Sc2O@D3h(5)-C78 are closely related via a single SWT transformation.32 A more brilliant study was from Dorn and co-workers who captured and identified M2C2@C1(51383)-C84 (M = Y and Gd) which can transform into the other seven smaller cages, namely C1(51383)-C84, C3v(8)-C82, C2v(9)-C82, Cs(6)-C82, Cs(39663)-C82, Ih(7)-C80 and D5h(6)-C80, implying a ‘top-down formation mechanism’ for fullerenes.26 In that report, the authors also provided concrete mass spectrometric results to confirm that the formation route of empty fullerenes is the same as that of EMFs.26 Recently, our group reported that the defective C2(816)-C104 cage can change to the other three ideal cages, namely D5(450)-C100, Cs(574)-C102 and D3d(822)-C104, via multiple SWTs and C2-extrusion.33 It is clear that the transformation scheme of existing fullerenes/EMFs is rather fragmentary with the entire map remaining far from complete.
Herein, we have made a solid step towards completing the transformation map of existing metallofullerenes by capturing four key cages, namely, C2(27)-C88, C1(7)-C86, C2(13)-C84 and C2(11)-C84 in the form of europium-containing metallofullerenes. These four cages are key links in the map that covers up to 98% of known metallofullerenes with a C2n (2n = 74–86) cage through very facile transformation routes. In addition, we propose that C2(27)-C88 is a starting point of the map, and is not obtainable from any of the existing C90 isomers through facile steps, but is a possible product of self-assembly of a graphene fragment.
Results and discussion
Soot containing europium metallofullerenes was synthesized by vaporizing graphite rods containing a mixture of Eu2O3 and graphite powder (molar ratio of Eu/C = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
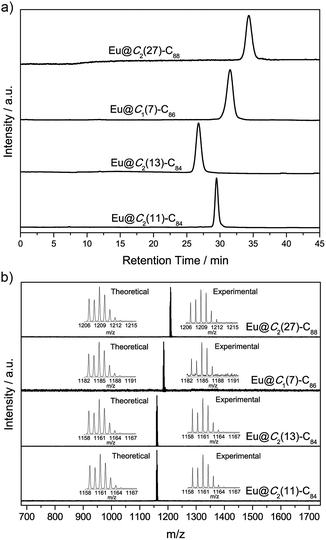
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) under 300 mbar helium in an arc-discharge chamber. The soot was collected and extracted with carbon disulfide. Upon solvent removal, the crude mixture was redissolved in toluene and subjected to multi-stage high performance liquid chromatographic (HPLC) separation (Fig. S1†). The purity of the isolated Eu@C2n (2n = 88, 86 and 84) isomers is confirmed by both HPLC (Fig. 2a) and laser desorption/ionization time-of-flight (LDI-TOF) mass spectroscopic analyses (Fig. 2b). The vis-NIR absorption spectra of the four compounds are shown in Fig. 3. All show obvious absorptions in the range from 400 to 2000 nm with relatively large onsets, suggesting small optical bandgaps (<1.0 eV) and correspondingly low thermodynamic stability. More details are listed in Fig. S2 and Table S1.†
50) under 300 mbar helium in an arc-discharge chamber. The soot was collected and extracted with carbon disulfide. Upon solvent removal, the crude mixture was redissolved in toluene and subjected to multi-stage high performance liquid chromatographic (HPLC) separation (Fig. S1†). The purity of the isolated Eu@C2n (2n = 88, 86 and 84) isomers is confirmed by both HPLC (Fig. 2a) and laser desorption/ionization time-of-flight (LDI-TOF) mass spectroscopic analyses (Fig. 2b). The vis-NIR absorption spectra of the four compounds are shown in Fig. 3. All show obvious absorptions in the range from 400 to 2000 nm with relatively large onsets, suggesting small optical bandgaps (<1.0 eV) and correspondingly low thermodynamic stability. More details are listed in Fig. S2 and Table S1.†
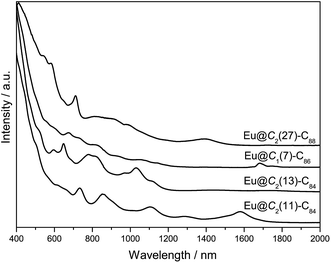 | ||
| Fig. 3 Vis-NIR absorption spectra of Eu@C2(27)-C88, Eu@C1(7)-C86, Eu@C2(13)-C84 and Eu@C2(11)-C84 dissolved in CS2. The curves are vertically shifted for the ease of comparison. | ||
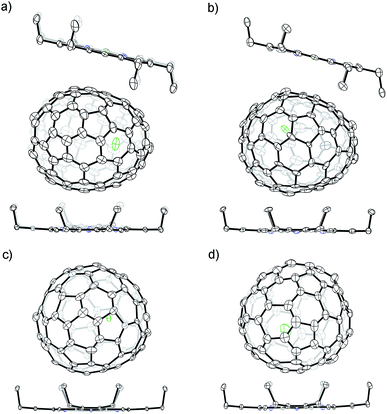
The molecular structures of the four europium-containing metallofullerenes are unambiguously determined by X-ray crystallography as Eu@C2(27)-C88, Eu@C1(7)-C86, Eu@C2(13)-C84 and Eu@C2(11)-C84. Notably, this is the first crystallographic identification of europium-containing EMFs since they were first reported over two decades ago.34 More significantly, the C2(27)-C88 and C1(7)-C86 cages are unprecedented even without theoretical prediction.
Fig. 4 shows the molecular structures of these four endohedrals co-crystallized with NiII(OEP) (OEP is the dianion of octaethylporphyrin). The porphyrin moiety faces a relatively flat region of each cage with the shortest Ni-to-cage-carbon distances ranging from 2.825 to 2.926 Å, indicative of π–π interactions. In the case of Eu@C2(27)-C88 and Eu@C1(7)-C86, each fullerene cage is surrounded by two nonparallel NiII(OEP) molecules in a sandwich-like arrangement and the ethyl groups of one NiII(OEP) molecule face towards opposite sides to enhance the π–π interactions. In contrast, only one Ni(OEP) molecule is required to assist in the crystallization of the smaller Eu@C2(13)-C84 or Eu@C2(11)-C84 molecules. These results demonstrate that the stacking mode in the co-crystals depends on the shape and size of the endohedral. The Eu atom in each compound shows some degree of disorder (Fig. S3†), suggesting a motional behavior.
Based on these four new cages, we are now able to complete a transformation map of existing metallofullerenes which includes up to 98% of the reported metallofullerenes (metal@C2n, 2n = 74–86) with clear cage structures (176 compounds in total; see Table S2† for details). More importantly, the inter-cage transformations require only one or two well-established steps (Stone–Wales transformation or C2 extrusion/insertion). Note that the transformation map matches with both the bottom-up and top-down formation mechanisms. For the ease of explanation, we follow a top-down manner in the map and context.
Fig. 5 depicts the transformation map and the detailed pathways are illustrated in the ESI (Fig. S4–S41).† The transformation from C2(27)-C88 to C1(7)-C86 is straightforward by a direct [5,6]-C2 loss and a subsequent SWT. Then a [5,6]-C2 loss and a SWT on C1(7)-C86 generate C2(13)-C84. A SWT on C2(13)-C84 affords C1(12)-C84 and a further SWT on the latter produces C2(11)-C84. Two SWTs convert C2(11)-C84 and C1(12)-C84 into D3d(19)-C84 and D2d(23)-C84, respectively. Elimination of a C2-unit from a pentalene unit generated by a SWT on C1(7)-C86 produces the non-IPR missing link C1(51383)-C84.26 A subsequent SWT on C1(51383)-C84 gives another non-IPR Cs(51365)-C84.26
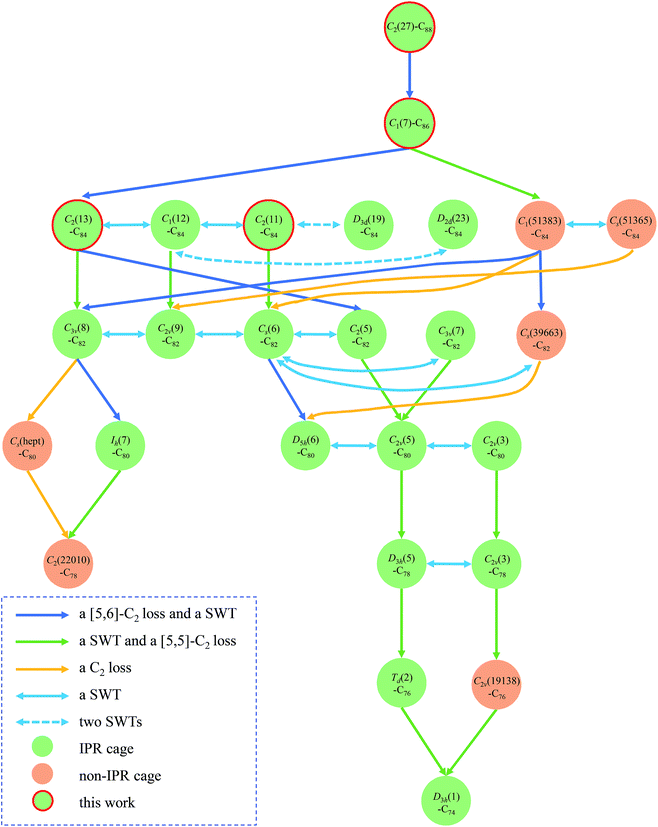 | ||
| Fig. 5 Transformations among existing metallofullerenes with C2(27)-C88 as the starting top point. As many as 98% of metallofullerenes from C86 to C74 with known structures are included in the map. The rearrangement pathways involve one or two well-established steps. IPR cages are marked in green and non-IPR cages are marked in yellow. The cages reported in this work are highlighted with red circles. The blue one-way arrow indicates a [5,6]-C2 loss with a subsequent SWT while the green one-way arrow refers to a SWT followed by a [5,5]-C2 loss. The yellow one-way arrow corresponds to merely one C2 loss while the aqua blue two-way arrow indicates a SWT and the dashed aqua blue two-way arrow refers to two SWTs. The detailed transformation pathways are illustrated in the ESI (Fig. S4–S41).† | ||
The transformations from C84 to C82 and the isomerization between C82 cages are rather clear. A [5,6]-C2 loss with a subsequent SWT on C2(13)-C84 produces C2(5)-C82 while a SWT with a [5,5]-C2 loss converts C2(13)-C84, C1(12)-C84 and C2(11)-C84 into C3v(8)-C82, C2v(9)-C82 and Cs(6)-C82, respectively. Alternatively, elimination of the remaining pentalene unit on C1(51383)-C84 and C1(51365)-C84 affords Cs(6)-C82 and C2v(9)-C82, respectively.26 A [5,6]-C2 extrusion plus an additional SWT on C1(51383)-C84 generates the non-IPR Cs(39663)-C82 as well as C3v(8)-C82 which are related with a single SWT.26
Furthermore, C2v(5)-C80 is obtainable via one SWT and one subsequent [5,5]-C2 extrusion from either C2(5)-C82 or C3v(7)-C82, while D5h(6)-C80 is generated from Cs(6)-C82 through a [5,6]-C2 loss and a subsequent SWT or from Cs(39663)-C82via merely a [5,5]-C2 elimination.26 Besides, the C3v(8)-C82 cage can shrink into the popular Ih(7)-C80 cage via a [5,6]-C2 loss plus a SWT26 or into the heptagon-containing Cs(hept)-C80 cage18via merely a [5,6]-C2 extrusion. Structural transformation can also be demonstrated among three isomeric C80 cages (D5h(6)-C80, C2v(5)-C80 and C2v(3)-C80) via merely one SWT.
In further steps, C80 can shrink to smaller fullerene cages such as C78, C76 and C74. A SWT plus a [5,5]-C2 loss converts C2v(5)-C80, C2v(3)-C80 and Ih(7)-C80 into D3h(5)-C78, C2v(3)-C78 and C2(22010)-C78, respectively. Alternatively, the non-IPR C2(22010)-C78 is also obtainable from Cs(hept)-C80via a [5,5]-C2 loss. The transformation between D3h(5)-C78 and C2v(3)-C78 can be realized by only one SWT.32 Furthermore, Td(2)-C76 and the non-IPR C2v(19138)-C76 are formed with D3h(5)-C78 (ref. 32) and C2v(3)-C78 as their respective precursors through a SWT plus a [5,5]-C2 elimination. Both Td(2)-C76 and C2v(19138)-C76 can transform into D3h(1)-C74via a SWT and a [5,5]-C2 extrusion. Thus, the transformations among up to 98% of the identified C2n (2n = 86–74) cages employed by EMFs have been uncovered with the inter-cage transformations involving merely one or two well-established steps (Fig. 1). Depending on the encapsulated species, different transformation routes can be expected.26 However, it should also be mentioned that the encaged metallic unit may change during the transformation process. For example, Y2@C84 is shown to encapsulate a C2 unit inside the cage to form Y2C2@C3v(8)-C82.35
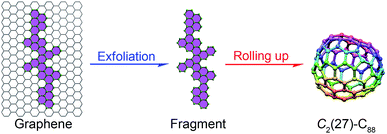
One may think that the top C2(27)-C88 cage is possibly converted from a C90 cage. However, although as many as six isomeric C90 cages (C1(21)-C90, C2(40)-C90, C2(42)-C90, C2(45)-C90, C2v(46)-C90 and C2(41)-C90) have been identified in the form of EMFs,31,36–38 none of them could transform into the C2(27)-C88 cage via the defined facile routes (Fig. 1). However, our topological analysis reveals that the C2(27)-C88 cage may be directly obtained by the self-assembly of a graphene fragment (Fig. 6), indicating the top-down formation process of fullerenes. Another argument for the unique role of the C88 cage is the templating effect of Nd3N leading to the formation of M3N@C88, while the smaller Sc3N and the larger La3N clusters adopt the C80 and C96 cages, respectively.9,39,40 This interesting C8 interval (C96–C88–C80) phenomenon is further corroborated by the fact that the defective C2(816)-C104 cage (obtained in the form of La2C2@C2(816)-C104) can change to the other three ideal cages, namely D5(450)-C100, Cs(574)-C102 and D3d(822)-C104via facile routes.33 Accordingly, we propose that the C8 interval may play an important role in determining the starting points of metallofullerene transformation. In this regard, the missing transformation between C74 and C72 in the map can be understood by considering a C8 interval between C80 and C72, which implies that C72 may be included in a different map. We acknowledge that the experimentally observed C2v(9)-C86, D3(19)-C86, D2(35)-C88 and Cs(hept)-C88 are not included in our map, which might be a sign of the presence of other transformation routes19 and hence uncovering new transformation links is still of vital importance.
Conclusions
In summary, we have captured and crystallographically identified four fullerene cages, namely, C2(27)-C88, C1(7)-C86, C2(13)-C84 and C2(11)-C84, in the form of europium-containing metallofullerenes. With their accession, a transformation map covering as many as 98% of the experimentally identified EMFs with clear C2n (2n = 86–74) cage structures is presented, in which the inter-cage transformations are limited to merely one or two very favorable steps. Structural analysis demonstrates that the unique C2(27)-C88 cage may serve as the key point in the map and is directly transformable from a graphene fragment. Completing the transformation map is key to solving the long-standing puzzle of fullerene formation and could shed light on the construction and applications of novel nano-carbon molecules.Experimental
Raw soot containing europium-metallofullerenes was synthesized by a direct-arc discharge method and extracted with carbon disulfide. After solvent removal, the mixture was dissolved in toluene and subjected to multi-stage high performance liquid chromatographic (HPLC) separation which gave the desired compounds (Fig. S1†).Black crystals were obtained by slow diffusion of a benzene solution of NiII(OEP) into a carbon disulfide solution of each metallofullerene over four weeks. Single-crystal X-ray data were collected at 100 K using synchrotron radiation (0.65250 Å) with a MarCCD detector at beamline BL17B of the Shanghai Synchrotron Radiation Facility. A multi-scan method (SADABS) was used for absorption corrections. The structures were solved with direct methods and were refined with SHELXL-2016.41
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the NSFC (No. 51472095 and 51672093) is gratefully acknowledged. We acknowledge the staff at the BL17B beamline of the National Center for Protein Sciences Shanghai (NCPSS) at the Shanghai Synchrotron Radiation Facility for assistance during data collection, and the Analytical and Testing Center in the Huazhong University of Science and Technology for all related measurements.Notes and references
- H. W. Kroto, J. R. Heath, S. C. Obrien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- K. M. Kadish and R. Ruoff, Fullerenes: Chemistry, Physics, and Technology, Wiley-VCH, New York, 2000 Search PubMed.
- P. W. Fowler and D. E. Manolopoulos, An Atlas of Fullerenes, Dover Publications, Clarendon, 2007 Search PubMed.
- A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- X. Lu, L. Echegoyen, A. L. Balch, S. Nagase and T. Akasaka, Endohedral Metallofullerenes: Basics and Applications, CRC Press, 2014 Search PubMed.
- S. Yang, T. Wei and F. Jin, Chem. Soc. Rev., 2017, 46, 5005–5058 RSC.
- X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723–7760 RSC.
- H. W. Kroto, Nature, 1987, 329, 529–531 CrossRef CAS.
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. R. Jordan, J. Craft, E. Hadju, R. Bible, M. M. Olmstead, K. Maitra, A. J. Fisher, A. L. Balch and H. C. Dorn, Nature, 1999, 401, 55–57 CrossRef CAS.
- J. C. Duchamp, A. Demortier, K. R. Fletcher, D. Dorn, E. B. Iezzi, T. Glass and H. C. Dorn, Chem. Phys. Lett., 2003, 375, 655–659 CrossRef CAS.
- T. Cai, L. S. Xu, M. R. Anderson, Z. X. Ge, T. M. Zuo, X. L. Wang, M. M. Olmstead, A. L. Balch, H. W. Gibson and H. C. Dorn, J. Am. Chem. Soc., 2006, 128, 8581–8589 CrossRef CAS PubMed.
- H. Kurihara, X. Lu, Y. Iiduka, N. Mizorogi, Z. Slanina, T. Tsuchiya, T. Akasaka and S. Nagase, J. Am. Chem. Soc., 2011, 133, 2382–2385 CrossRef CAS PubMed.
- H. Kurihara, X. Lu, Y. Iiduka, H. Nikawa, M. Hachiya, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, Inorg. Chem., 2012, 51, 746–750 CrossRef CAS PubMed.
- R. F. Curl, Angew. Chem., Int. Ed., 1997, 36, 1566–1576 CrossRef.
- A. J. Stone and D. J. Wales, Chem. Phys. Lett., 1986, 128, 501–503 CrossRef CAS.
- M. Mulet-Gas, L. Abella, P. W. Dunk, A. Rodríguez-Fortea, H. W. Kroto and J. M. Poblet, Chem. Sci., 2015, 6, 675–686 RSC.
- R. J. Cross and M. Saunders, J. Am. Chem. Soc., 2005, 127, 3044–3047 CrossRef CAS PubMed.
- Y. Zhang, K. B. Ghiassi, Q. Deng, N. A. Samoylova, M. M. Olmstead, A. L. Balch and A. A. Popov, Angew. Chem., Int. Ed., 2015, 54, 495–499 CAS.
- C.-H. Chen, L. Abella, M. R. Cerón, M. A. Guerrero-Ayala, A. Rodríguez-Fortea, M. M. Olmstead, X. B. Powers, A. L. Balch, J. M. Poblet and L. Echegoyen, J. Am. Chem. Soc., 2016, 138, 13030–13037 CrossRef CAS PubMed.
- M. Yamada, H. Kurihara, M. Suzuki, J. D. Guo, M. Waelchli, M. M. Olmstead, A. L. Balch, S. Nagase, Y. Maeda, T. Hasegawa, X. Lu and T. Akasaka, J. Am. Chem. Soc., 2014, 136, 7611–7614 CrossRef CAS PubMed.
- M. M. Olmstead, H. M. Lee, J. C. Duchamp, S. Stevenson, D. Marciu, H. C. Dorn and A. L. Balch, Angew. Chem., Int. Ed., 2003, 115, 928–931 CrossRef.
- N. Chen, M. Mulet-Gas, Y. Y. Li, R. E. Stene, C. W. Atherton, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, Chem. Sci., 2013, 4, 180–186 RSC.
- B. Q. Mercado, C. M. Beavers, M. M. Olmstead, M. N. Chaur, K. Walker, B. C. Holloway, L. Echegoyen and A. L. Balch, J. Am. Chem. Soc., 2008, 130, 7854–7855 CrossRef CAS PubMed.
- X. Lu, H. Nikawa, T. Nakahodo, T. Tsuchiya, M. O. Ishitsuka, Y. Maeda, T. Akasaka, M. Toki, H. Sawa, Z. Slanina, N. Mizorogi and S. Nagase, J. Am. Chem. Soc., 2008, 130, 9129–9136 CrossRef CAS PubMed.
- T. Wakahara, H. Nikawa, T. Kikuchi, T. Nakahodo, G. M. A. Rahman, T. Tsuchiya, Y. Maeda, T. Akasaka, K. Yoza, E. Horn, K. Yamamoto, N. Mizorogi, Z. Slanina and S. Nagase, J. Am. Chem. Soc., 2006, 128, 14228–14229 CrossRef CAS PubMed.
- J. Zhang, F. L. Bowles, D. W. Bearden, W. K. Ray, T. Fuhrer, Y. Ye, C. Dixon, K. Harich, R. F. Helm, M. M. Olmstead, A. L. Balch and H. C. Dorn, Nat. Chem., 2013, 5, 880–885 CrossRef CAS PubMed.
- P. A. Troshin, A. G. Avent, A. D. Darwish, N. Martsinovich, A. K. Abdulsada, J. M. Street and R. Taylor, Science, 2005, 309, 278–281 CrossRef CAS PubMed.
- I. N. Ioffe, C. Chen, S. Yang, L. N. Sidorov, E. Kemnitz and S. I. Troyanov, Angew. Chem., Int. Ed., 2010, 49, 4784–4787 CrossRef CAS PubMed.
- S. Yang, S. Wang, E. Kemnitz and S. I. Troyanov, Angew. Chem., Int. Ed., 2014, 53, 2460–2463 CrossRef CAS PubMed.
- B. Q. Mercado, N. Chen, A. Rodríguez-Fortea, M. A. Mackey, S. Stevenson, L. Echegoyen, J. M. Poblet, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2011, 133, 6752–6760 CrossRef CAS PubMed.
- H. Yang, H. X. Jin, H. Y. Zhen, Z. M. Wang, Z. Y. Liu, C. M. Beavers, B. Q. Mercado, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2011, 133, 6299–6306 CrossRef CAS PubMed.
- Y. Hao, Q. Tang, X. Li, M. Zhang, Y. Wan, L. Feng, N. Chen, Z. Slanina, L. Adamowicz and F. Uhlík, Inorg. Chem., 2016, 55, 11354–11361 CrossRef CAS PubMed.
- W. Cai, F.-F. Li, L. Bao, Y. Xie and X. Lu, J. Am. Chem. Soc., 2016, 138, 6670–6675 CrossRef CAS PubMed.
- P. Kuran, M. Krause, A. Bartl and L. Dunsch, Chem. Phys. Lett., 1998, 292, 580–586 CrossRef CAS.
- T. Inoue, T. Tomiyama, T. Sugai, T. Okazaki, T. Suematsu, N. Fujii, H. Utsumi, K. Nojima and H. Shinohara, J. Phys. Chem. B, 2004, 108, 7573–7579 CrossRef CAS.
- H. Yang, H. X. Jin, B. Hong, Z. Y. Liu, C. M. Beavers, H. Y. Zhen, Z. M. Wang, B. Q. Mercado, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2011, 133, 16911–16919 CrossRef CAS PubMed.
- H. Yang, C. Lu, Z. Liu, H. Jin, Y. Che, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2008, 130, 17296–17300 CrossRef CAS PubMed.
- S. Zhao, P. Zhao, W. Cai, L. Bao, M. Chen, Y. Xie, X. Zhao and X. Lu, J. Am. Chem. Soc., 2017, 139, 4724–4728 CrossRef CAS PubMed.
- F. Melin, M. N. Chaur, S. Engmann, B. Elliott, A. Kumbhar, A. J. Athans and L. Echegoyen, Angew. Chem., Int. Ed., 2007, 46, 9032–9035 CrossRef CAS PubMed.
- M. N. Chaur, F. Melin, J. Ashby, B. Elliott, A. Kumbhar, A. M. Rao and L. Echegoyen, Chem.–Eur. J., 2008, 14, 8213–8219 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Spectroscopic results, crystal structures, metallofullerenes included in the transformation map, and transformation routes. CCDC 1578319–1578322. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc04906h |
| This journal is © The Royal Society of Chemistry 2019 |