Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gold-catalyzed bicyclic annulations of 4-methoxy-1,2-dienyl-5-ynes with isoxazoles to form indolizine derivatives via an Au-π-allene intermediate†
Antony Sekar
Kulandai Raj
a,
Kuo-Chen
Tan
a,
Liang-Yu
Chen
b,
Mu-Jeng
Cheng
 *b and
Rai-Shung
Liu
*b and
Rai-Shung
Liu
 *a
*a
aFrontier Research Centers on Fundamental and Applied Science of Matters, Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, Republic of China. E-mail: rsliu@mx.nthu.edu.tw
bDepartment of Chemistry, National Cheng Kung University, Tainan 701, Taiwan. E-mail: mjcheng@mail.ncku.tw
First published on 22nd May 2019
Abstract
Gold-catalyzed bicyclic annulations of 4-methoxy-1,2-dienyl-5-ynes with isoxazoles afford indolizine derivatives with a structural rearrangement. The mechanism of these new annulations does not involve α-imino gold carbenes generated from gold π-alkyne intermediates. We postulate alkyne attack on gold π-allenes, yielding vinyl gold carbenes. These newly generated carbenes react with isoxazole derivatives to yield Z-3-imino-2-en-1-als, further enabling sequential cyclizations to deliver indolizine derivatives in two distinct classes.
Introduction
The advent of gold catalysis has greatly promoted the synthetic utility of alkynes. Apart from the functionalizations of alkynes with O, N and C based nucleophiles, gold catalysts also accelerate the development of new alkyne annulations1 with π-bond motifs. Isoxazoles are readily available aromatic heterocycles; interest in their gold-catalyzed alkyne annulations2,3 is rapidly growing because of the easy generation of α-imino gold carbenes (eqn (1)). Ye and coworkers reported the first [3 + 2]-annulations of ynamides with isoxazoles to deliver pyrrole derivatives via α-imino gold carbenes In-1 (eqn (1)).3a–c The use of electron-deficient alkynes also afforded pyrrole products with similar carbene intermediates.3d We employed 1,4-diyn-3-ols to seek other azacycles,4 but still producing pyrrole derivatives via a 1,2-alkyne migration to α-imino gold carbenes (eqn (2)). Despite intensive efforts, the strong preference toward pyrrole products limits the utility of these isoxazole/alkyne annulations. Similar π-alkyne routes were observed for the anthranil/alkyne annulations, yielding indole derivatives.5 We sought to achieve the synthesis of other azacyclic compounds beyond pyrrole or indole derivatives; generation of intermediates other than α-imino gold carbenes is a viable route. This work reports gold-catalyzed bicyclic annulations of 4-methoxy-1-allenyl-5-ynes with isoxazoles to form 8- and 7-formylindolizines 3 and 5; the structural rearrangement of products is noted here (eqn (3)). We postulate an atypical mechanism for these bicyclic annulations via a 1,4-alkyne migration, activated by a gold π-allene intermediate; the resulting vinyl gold carbene In-3 is trapped by an isoxazole to enable initial sequential cyclizations before delivering indolizine products. This new annulation rationalizes the carbon source of indolizines 3 and 5 from the two reactants well.Previous work: gold carbene via π-alkyne intermediates
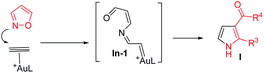 | (1) |
One example:
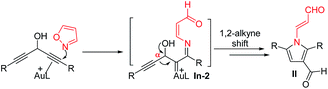 | (2) |
This work: vinyl gold carbene via π-alkyne intermediates
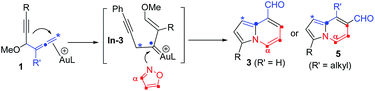 | (3) |
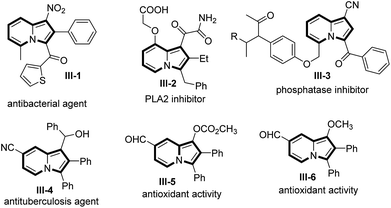
Indolizine frameworks are present in the core structures of natural products including (−)-swainsonine, (+)-castanospermine, lamellarins and camptothecin.6,7 Synthetic indolizine derivatives, such as compounds III-1–III-4, are demonstrated to be antibacterial reagents, PLA2 inhibitors, phosphatase inhibitors and antituberculosis agents8 whereas species III-5 and III-6 show antioxidant activity.9 Indolizine species III-5 and III-6 structurally match with our resulting products 5 bearing a C(7)-aldehyde (Scheme 1).
 | (4) |
Results and discussion
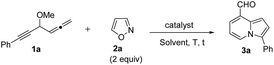
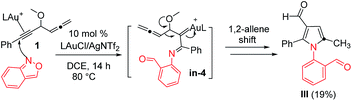
Our initial target focused on the reactions of 4-methoxy-1,2-dienyl-5-ynes 1a with anthranil using gold catalysts; the reactions gave pyrrole derivatives III again (eqn (4)).10 A mechanistic analysis indicates a typical route of the alkyne activation, involving a 1,2-allene migration to the gold carbene center. We switch our attention to isoxazole derivatives. Table 1 shows the optimizations of a new bicyclic annulation of 4-methoxy-1,2-dienyl-5-yne 1a with isoxazole 2a using various gold catalysts. Our initial tests with IPrAuCl/AgNTf2 (10 mol%) in DCE at 25 °C (27 h) led to a high recovery of the starting alkyne 1a (entry 1). IPrAuCl/AgNTf2 (10 mol%) in DCE at 45 °C (48 h) gave unreacted 1a with a 28% recovery (entry 2). To our pleasure, the reaction in a hot DCE solution (65 °C, 14 h) afforded an indolizine derivative 3a bearing a C(8)-aldehyde group; the yield was 88% (entry 3). Under these optimized conditions, P(t-Bu)2(o-biphenyl) AuCl/AgNTf2 was less efficient to yield product 3a and unreacted 1a in 62% and 21%, respectively (entry 4). Other gold phosphines such as LAuCl/AgNTf2 (L = PPh3, P(OPh)3) were catalytically inactive (entries 5 and 6). Alternations of silver salts as in IPrAuCl/AgX (X = SbF6 and OTf) rendered the reactions less efficient, giving compounds in 61% and 0% yields respectively; the reactions were only compatible with non-coordinating anions (entries 7 and 8). IPrAuCl or AgNTf2 alone (10 mol%) was entirely inactive (entries 9 and 10). IPrAuCl/AgNTf2 became inefficient in THF, MeCN and toluene (entries 11–13). The structure of compound 3a was inferred from X-ray diffraction studies of its related compounds 3c and 3d,11 as depicted in Table 2, and further verified with 1H NOE spectra.| Entry | Catalyst (mol%) | T [°C] | t [h] | Solvent | Yieldb [%] | |
|---|---|---|---|---|---|---|
| 1a | 3a | |||||
| a [1a] = 0.15 M. b Product yields are reported after separation from a silica column. c IPr = 1,3-bis(diisopropylphenyl)imidazole-2-ylidene. d L = P(t-Bu)2(o-biphenyl), DCE = 1,2-dichloroethane, DCM = dichloromethane, THF = tetrahydrofuran, MeCN = acetonitrile, Tf = trifluoromethanesulfonyl. | ||||||
| 1 | IPrAuCl/AgNTf2 (10)c | 25 | 27 | DCE | 75 | Trace |
| 2 | IPrAuCl/AgNTf2 (10) | 45 | 48 | DCE | 28 | Trace |
| 3 | IPrAuCl/AgNTf2 (10) | 65 | 14 | DCE | — | 88 |
| 4 | LAuCl/AgNTf2 (10)d | 65 | 27 | DCE | 21 | 62 |
| 5 | PPh3AuCl/AgNTf2 (10) | 65 | 35 | DCE | 94 | — |
| 6 | P(OPh)3AuCl/AgNTf2 (10) | 65 | 32 | DCE | 95 | — |
| 7 | IPrAuCl/AgSbF6 (10) | 65 | 24 | DCE | 24 | 61 |
| 8 | IPrAuCl/AgOTf (10) | 65 | 22 | DCE | — | — |
| 9 | IPrAuCl (10) | 65 | 13 | DCE | 85 | — |
| 10 | AgNTf2 (10) | 65 | 30 | DCE | 76 | — |
| 11 | IPrAuCl/AgNTf2 (10) | 65 | 25 | THF | — | — |
| 12 | IPrAuCl/AgNTf2 (10) | 80 | 21 | MeCN | 87 | — |
| 13 | IPrAuCl/AgNTf2 (10) | 100 | 21 | Toluene | — | Trace |
We assessed the generality of these bicyclic annulations with various 4-methoxy-1,2-dienyl-5-ynes and substituted isoxazoles; the results are depicted in Table 2. We tested these annulations first on 4-phenylethynyl allene substrates 1b–1e (X = Me, tert-butyl, Cl and Br), smoothly affording 8-formylindolizine derivatives 3b–3e in good yields (78–85%, entries 1–4); X-ray diffraction revealed that products 3c and 3d bear an aldehyde at their C(8)-carbons. The reactions were further compatible with alkylethynyl allenes 1f–1i (R = n-butyl, cyclopropyl, isopropyl and cyclohexyl), yielding desired indolizines 3f–3i in 76–87% (entries 5–8). For 2-napthylethynyl allene 1j, its corresponding indolizine 3j was obtained in 84% yield (entry 9). We performed the reaction on 5-methylisoxazole 2b (R2 = Me), yielding 7-methyl-8-formylindolizines 3k and 3l in 38% and 37% yields, respectively(entries 10 and 11); the yields of the two products were increased to 51% and 54% using a high loading of isoxazole 2b (3 equiv.). The molecular structure of indolizine 3l was confirmed with X-ray diffraction.11 For 3-methylisoxazole 2c (R3 = Me), its corresponding indolizines 3m and 3n were obtained in 61% and 76% yields respectively (entries 12 and 13); the proposed structure of 3m was verified by 1H NOE spectra. We tested the reactions on an alkyl-substituted allene substrate with 2c rendered desired 3o with 24% yield (entry 14). Structural analysis of these indolizine products supports a 1,4-migration of the alkynyl moiety to the C(1)-allene carbon.
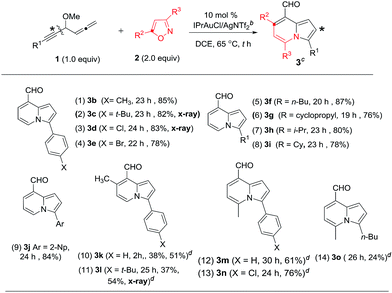
As depicted in Table 3, 3-disubstituted allene derivatives 4 gave distinct 7-formylindolizines 5 under the same conditions. We assessed the scope of this new annulation using various allenylynes bearing R1 and R2 substituents. Entries 1–3 show the applicability of this catalysis to various phenylethynyl allenes 4a–4c (X = H, Cl and Br), rendering the desired products 5a–5c in 69–76% yields (entries 1–3); the molecular structure of the chloro derivative 5b was determined with X-ray diffraction.11 For 2-napthylethynyl allene 4d, its corresponding product 5d was obtained in 71% yield (entry 4). The reaction was extensible to substrate 4e bearing 3-methylallene (R2 = Me), yielding compound 5e in 39% yield (entry 5). We tested the reactions on all alkyl-substituted 1,2-dienyl-5-allenes 4f–4j (R1, R2 = alkyl), delivering the desired 7-formylindolizines 5f–5j in satisfactory yields (76–81%, entries 6–10). The proposed structure of compound 5j was confirmed with X-ray diffraction study.11
To test the electronic effect of allenyl substituents, we prepared an allenyl ester 6 that reacted with 5-arylisoxazoles 2d (Ar = Ph) and 2e (Ar = 4-ClPh) to yield indolizine derivatives 7a and 7b (eqn (5)). The X-ray diffraction results of compound 7b confirmed its structure with no 1,4-alkyne shift; the formation of these two products arose from gold π-alkyne intermediates as described before (eqn (4)). The change of chemoselectivity is attributed to a weak coordination between gold and an allenyl ester.
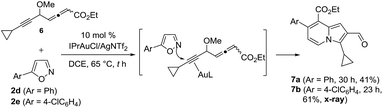 | (5) |
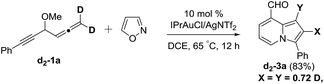
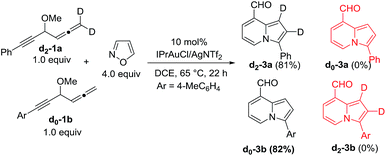
We performed a series of experiments to elucidate the mechanisms of formation of 8- and 7-formylindolizines 3 and 5. We prepared 13C-enriched 1a and 4e; each contained 10% 13C content in the CH–OMe carbon. Their resulting products 13C-3a and 13C-5e were found to have the enrichment at the aldehyde carbons (eqn (6) and (7)). We prepared d2-1a bearing ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CD2 at the allene C(1)-carbon; its resulting indolizine d2-3a comprised equal deuterium content (X = Y = 0.72 D) at the two pyrrolyl carbons. We also performed a crossover experiment involving d2-1a and d0-1b; this mixture only produced d2-3a and d0-3b according the mass analysis. The entire 1,2-dienyl-5-yne skeleton 1 remained completely on the resulting indolizine molecule.
CD2 at the allene C(1)-carbon; its resulting indolizine d2-3a comprised equal deuterium content (X = Y = 0.72 D) at the two pyrrolyl carbons. We also performed a crossover experiment involving d2-1a and d0-1b; this mixture only produced d2-3a and d0-3b according the mass analysis. The entire 1,2-dienyl-5-yne skeleton 1 remained completely on the resulting indolizine molecule.
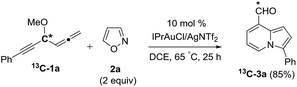 | (6) |
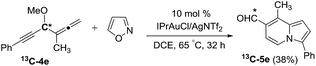 | (7) |
 | (8) |
 | (9) |
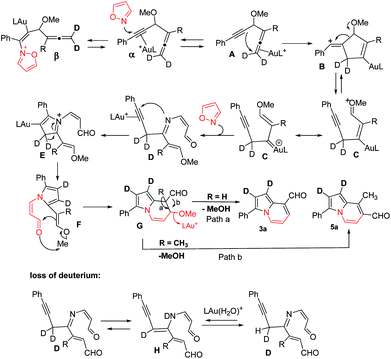
According the structural analysis of the resulting indolizines 3 and 5, we postulate a mechanism involving an allene-activation route. This mechanism rationalizes the deuterium and crossover experiments well (eqn (8) and (9)). We use d2-1a (R = H) as a tool to verify the mechanism. In the N-attack of isoxazole 2a with Au-π-alkyne α, the resulting intermediate β has a highly aromatic isoxazole ring that is difficult to cleave. We postulate an alternative path involving nucleophilic attack of an alkyne at its tethered Au-π-allene A to form vinyl cation B. An alkyne as a nucleophile to attack an electrophilic Au-π-allene is noted in gold catalysis.12 We conceive that this vinyl cation induces a subsequent C–C bond cleavage of species B to form phenylalkyne species C bearing an allyl cation C, as stabilized by the gold and methoxy group. This species has a resonance form of vinyl gold carbene that reacts smoothly with isoxazole to yield a 3-imino-2-en-1-al D with Z-configuration.13 An amination on the alkyne of species D is expected to form an azacyclic intermediate E which leads to the desired pyrrole intermediate F. For mono-substituted allenes 1 (R = H), a further carbonyl–ene reaction of species F yields pyrrole-fused six-membered species G, which loses MeOH to yield 8-formyl indolizine 3a. In the case of a 3,3-disubstituted allene 4 (R = alkyl), a 1,2-formyl shift to the neighboring carbocation occurs preferentially to give 7-formyl indolizine derivative 5a (Scheme 2).
This postulated mechanism rationalizes a small loss of deuterium content of the indolizine product d2-3a (X = Y = 0.72 D), as depicted in eqn (8). In the hot DCE solution (65 °C 12 h), an imine–enamine tautomerization, as shown by species D and H, results in a deuterium loss of species D because of an exchange with residual water. In this mechanism, a major concern is the cleavage of the sigma C–C bond of species B to yield vinyl gold carbene C.
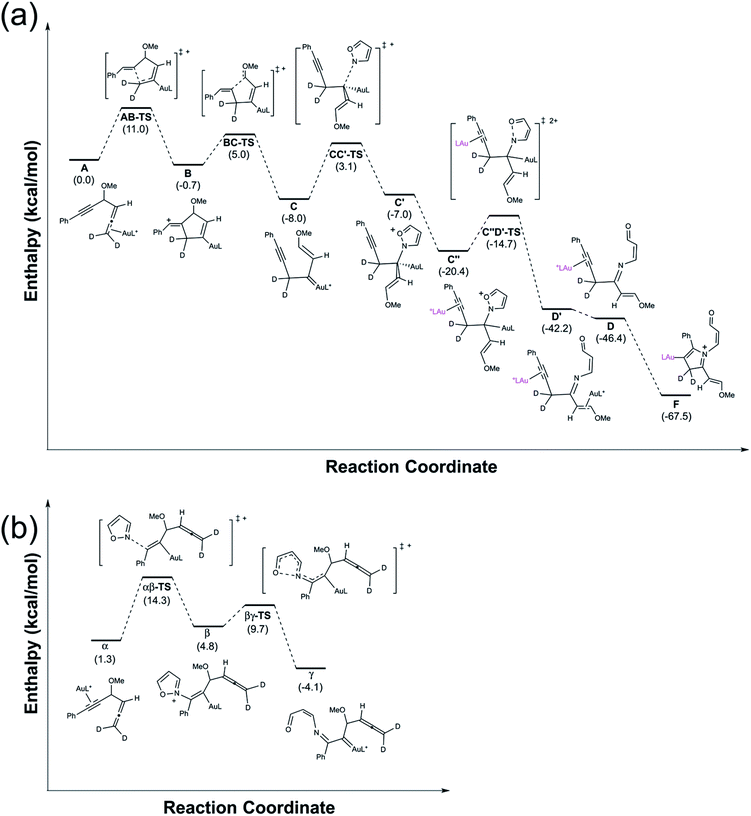
Calculations with density functional theory (B3LYP) were performed to support our proposed mechanism. Attention was paid to the transformations of the gold π-allene intermediate A (Fig. 1) to gold pyrrolium (F), since the last few steps are well known in organic reactions. 1,4-Alkyne migration of A to form C is a stepwise process: transformation A → B occurs with ΔH‡/ΔH = 11.0/−0.7 kcal mol; cleavage of the C–C bond of species B results in the formation of intermediate C with ΔH‡/ΔH = 5.7/−7.3 kcal mol−1. Species C is subsequently attacked by an isoxazole to generate C′ with ΔH‡/ΔH = 11.1/1.0 kcal mol−1. Next, the ligation of another IPrAu+ to species C′ is expected to form a digold species C′′ with ΔH = −13.4 kcal mol; this process is accompanied by a N–O cleavage of the isoxazole moiety of species C′′ to generate D′ with ΔH‡/ΔH = 5.7/−21.8 kcal mol−1. Finally, a release of IPrAu+ from species D′ eventually yields a gold-π-alkyne D with ΔH = −4.2 kcal mol; an intramolecular cyclization of species D generates gold-containing pyrrolium species F with no kinetic barrier and ΔH = −21.1 kcal mol−1. In this D → F step, the electronic barrier is 0.01 kcal mol−1, which disappears after correction for zero-point energy. Overall, all the kinetic barriers are less than 11.1 kcal mol−1 with all the steps being thermodynamically downhill except the step C → C′ (ΔH = +1.0 kcal mol−1). The entire reaction (A → F) releases an enthalpy −67.5 kcal mol−1. Our calculations thus show that the entire process is kinetically facile and thermodynamically favorable, verifying the proposed mechanism.
We also perform the calculation on a competitive reaction involving gold π-alkyne intermediates α, which has energy 1.3 kcal mol−1 greater than that of the gold π-allene (A). The attack of an isoxazole on π-alkyne α generated alkenylgold species β with ΔH‡/ΔH = 13.0/3.5 kcal mol−1. This was followed by a ring-opening reaction to form α-imino gold carbene γ with ΔH‡/ΔH = 4.9/−8.9 kcal mol−1. Notably, the barrier for formation and the energy state of intermediate β are greater than those of all intermediates in the π-allene route. We conclude that this π-alkyne route is unlikely to play an important role in the reaction.
Conclusions
In summary, we report new gold-catalyzed bicyclic annulations between 4-methoxy-1,2-dienyl-5-ynes and isoxazoles to form 7- and 8-formyl indolizine derivatives.13 This reaction process does not follow a typical π-alkyne route; α-imino gold carbenes14,15 do not form here. Instead, the mechanism involves π-allene intermediates to induce a 1,4-alkyne shift, yielding a vinyl gold carbene C that is trapped with an isoxazole to generate an α-imino-2-en-1-al. Gold-catalyzed sequential cyclizations of this imine intermediate enable the construction of an indolizine skeleton. This mechanism rationalizes the isotope labeling and crossover experiments well. New versions for these gold-catalyzed annulations will be helpful for the design of new catalysis.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the Ministry of Education (MOE 106N506CE1) and Ministry of Science and Technology (MOST 107-3017-F-007-002), Taiwan for financial support of this work.Notes and references
- For gold-catalyzed annulations or cycloaddition reactions of alkynes, see selected reviews: (a) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (b) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed; (c) S. M. Abu Sohel and R.-S. Liu, Chem. Soc. Rev., 2009, 38, 2269 RSC; (d) M. E. Muratore, A. Homs, C. Obradors and A. M. Echavarren, Chem.–Asian J., 2014, 9, 3066 CrossRef CAS PubMed.
- For catalytic reactions of isoxazoles and anthranils with alkynes, see selected reviews: (a) L. Li, T. D. Tan, Y.-Q. Zhang, X. Liu and L.-W. Ye, Org. Biomol. Chem., 2017, 5, 8483 RSC; (b) D. B. Huple, S. Ghorpade and R.-S. Liu, Adv. Synth. Catal., 2016, 358, 1348 CrossRef CAS.
- (a) A.-H. Zhou, Q. He, C. Shu, Y.-F. Yu, S. Liu, T. Zhao, W. Zhang, X. Lu and L.-W. Ye, Chem. Sci., 2015, 6, 1265 RSC; (b) X.-Y. Xiao, A.-H. Zhou, C. Shu, F. Pan, T. Li and L.-W. Ye, Chem.–Asian J., 2015, 10, 1854 CrossRef CAS PubMed; (c) W.-B. Shen, X.-Y. Xiao, Q. Sun, B. Zhou, X.-Q. Zhu, J.-Z. Yan, X. Lu and L.-W. Ye, Angew. Chem., Int. Ed., 2017, 56, 605 CrossRef CAS PubMed; (d) R. L. Sahani and R.-S. Liu, Angew. Chem., Int. Ed., 2017, 56, 1026 CrossRef CAS PubMed; (e) R. L. Sahani and R.-S. Liu, Angew. Chem., Int. Ed., 2017, 56, 12736 CrossRef CAS PubMed.
- (a) R. D. Kardile, B. S. Kale, P. Sharma and R.-S. Liu, Org. Lett., 2018, 20, 3806 CrossRef CAS PubMed; (b) Y.-C. Hsu, S.-A. Hsieh, P.-H. Li and R.-S. Liu, Chem. Commun., 2018, 54, 2114 RSC.
- (a) H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794 CrossRef CAS PubMed; (b) H. Jin, B. Tian, X. Song, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 12688 CrossRef CAS PubMed; (c) Z. Zeng, H. Jin, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2018, 57, 16549 CrossRef CAS PubMed.
- For the medicinal chemistry of indolizines, see a leading review: V. Sharma and V. Kumar, Med. Chem. Res., 2014, 23, 3593 CrossRef CAS.
- (a) D. Yang, Y. Yu, Y. Wu, H. Feng, X. Li and H. Cao, Org. Lett., 2018, 20, 2477 CrossRef CAS PubMed; (b) G. S. Singh and E. E. Mmatli, Eur. J. Med. Chem., 2011, 46, 5237 CrossRef CAS PubMed; (c) B. V. M. Teodoro, J. T. M. Correia and F. Coelho, J. Org. Chem., 2015, 80, 2529 CrossRef CAS PubMed; (d) J. P. Michael, Nat. Prod. Rep., 2008, 25, 139 RSC.
- (a) B. Shen, B. Li and B. Wang, Org. Lett., 2016, 18, 2816 CrossRef CAS PubMed; (b) T. Lepitre, R. Le Biannic, M. Othman, A. M. Lawson and A. Daïch, Org. Lett., 2017, 19, 1978 CrossRef CAS PubMed; (c) B. Sadowski, J. Klajin and D. T. Gryko, Org. Biomol. Chem., 2016, 14, 7804 RSC.
- A. I. Nasir, L.-L. Gundersen, F. Rise, O. Antonsen, T. Kristensen, B. Langhelle, A. Bast, I. Custers, G. R. M. M. Haenen and H. Wikstrom, Bioorg. Med. Chem. Lett., 1998, 8, 1829 CrossRef CAS PubMed.
- H.-C. Hsieh, K.-C. Tan, A. S. K. Raj and R.-S. Liu, Chem. Commun., 2019, 55, 1979 RSC.
- Crystallographic data of compounds 3c, 3d, 3l, 5b, 5j and 7b were deposited at Cambridge Crystallographic Data Center: 3c (CCDC 1894127), 3d (CCDC 1894128), 3l (CCDC 1894129), 5b (CCDC 1894126), 5j (CCDC 1894125) and 7b (CCDC 1913325).†.
- We recently reported nucleophilic attack of an alkyne at a gold-π-allene to yield a vinyl cation that was trapped with water; the reaction scheme is shown below. Our proposed mechanism is similar to this process. See: C.-Y. Yang, G.-Y. Lin, H.-Y. Liao, S. Datta and R.-S. Liu, J. Org. Chem., 2008, 73, 4907 CrossRef CAS PubMed
. - For the reactions of isoxazoles and gold carbenes, see a recent example: B. D. Mokar, P. D. Jadhav, Y. B. Pandit and R.-S. Liu, Chem. Sci., 2018, 9, 4488 RSC.
- (a) D. G. Hulcoop and M. Lautens, Org. Lett., 2007, 9, 1761 CrossRef CAS PubMed; (b) F.-S. Wu, H.-Y. Zhao, Y.-L. Xu, K. Hu, Y.-M. Pan and X.-L. Ma, J. Org. Chem., 2017, 82, 4289 CrossRef CAS PubMed; (c) C.-L. Ma, J.-H. Zhao, Y. Yang, M.-K. Zhang, C. Shen, R. Sheng, X.-W. Dong and Y.-Z. Hu, Sci. Rep., 2017, 7, 16640 CrossRef PubMed; (d) S. Teklu, L.-L. Gundersen, T. Larsen, K. E. Malterud and F. Rise, Bioorg. Med. Chem. Lett., 2005, 13, 3127 CrossRef CAS PubMed.
- For generation of α-imino gold carbenes with other nitrene sources, see selected examples: (a) R. J. Reddy, M. P. B. Jones and P. W. Davies, Angew. Chem., Int. Ed., 2017, 56, 13310 CrossRef CAS PubMed; (b) P. W. Davies, A. Cremonesi and L. Dumitrescu, Angew. Chem., Int. Ed., 2011, 50, 8931 CrossRef CAS PubMed; (c) E. Chatzopoulou and P. W. Davies, Chem. Commun., 2013, 49, 8617 RSC; (d) L. Zhu, Y. Yu, Z. Mao and X. Huang, Org. Lett., 2015, 17, 30 CrossRef CAS PubMed; (e) S. K. Pawar, R. L. Sahani and R. S. Liu, Chem.–Eur. J., 2015, 21, 10843 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1894125–1894129 and 1913325. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc00735k |
| This journal is © The Royal Society of Chemistry 2019 |