 Open Access Article
Open Access ArticleStrategies based on metal-based nanoparticles for hypoxic-tumor radiotherapy
Chenyang
Zhang
ac,
Liang
Yan
 a,
Zhanjun
Gu
a,
Zhanjun
Gu
 *ac and
Yuliang
Zhao
*bc
*ac and
Yuliang
Zhao
*bc
aCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China. E-mail: zjgu@ihep.ac.cn
bCAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology of China, Chinese Academy of Sciences, Beijing 100190, China. E-mail: zhaoyl@nanoctr.cn
cCollege of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing 100049, China
First published on 11th June 2019
Abstract
Radiotherapy (RT) is one of the most effective and frequent clinical cancer treatments. Nevertheless, RT can cause damage to normal tissues around tumors under high-dose ionizing radiation. Inspired by versatile metal-based nanomaterials, great efforts have been devoted to developing nanomaterials with high-Z metal elements as radiosensitizers by depositing more energy into tumors for RT enhancement. However, these metal-based nanomaterial-mediated RTs are highly O2-dependent. Unfortunately, O2 concentrations within the majority of solid tumors exhibit low levels, which seriously hampers the antitumor efficacy of these nanomaterials during RT. Therefore, the development of novel metal-based nanomaterials as radiosensitizers capable of avoiding the radioresistance induced by tumor hypoxia is highly desirable and important. Currently, the most effective approaches to reverse the radioresistance of hypoxic tumors are to introduce nanomaterials with O2-elevating ability by delivering exogenous O2, generating O2in situ, increasing intratumoral blood flow, or reducing HIF-1 expression to harness the O2 level in solid tumors. Besides these, recently, some innovative and simple strategies by employing nanoradiosensitizers with diminished oxygen dependence have also been applied to combat unmet hypoxic challenges, in which nanoradiosensitizers can target tumor hypoxia for selective RT, enhance oxygen-independent ROS generation, or combine with non-oxygen dependent cancer therapies for synergistic treatments. These approaches and strategies provide new avenues for enhanced hypoxic-tumor RT. Nevertheless, an overall review aiming specifically at these strategies is still rare. Herein, we present an overview about recent advances in metal-based nanomaterials for hypoxic-tumor RT, and give a detailed discussion about the design and working mechanisms of these strategies in their application of RT. Finally, current challenges and future perspectives are also pointed out in this field.
1. Introduction
So far, cancer-caused death has seriously threatened human health. Radiotherapy (RT), as one of the most effective and frequent tumor treatments, employs high-energy ionizing radiation such as X-rays/γ-rays to kill tumor cells via directly interacting with biomolecules and indirectly causing radiolysis of water molecules within tumor cells to generate free radicals for oxidative damage.1–4 The vast majority of patients with malignant tumors need to receive RT, either RT alone or in combination with other treatments such as chemotherapy or surgery.5–7 Despite the widespread use of RT in cancer clinical treatments, RT can cause damage to normal tissues around tumors under high-dose ionizing radiation, resulting in severe side effects. Therefore, it's crucial to develop strategies for seeking a balance between eliminating tumor cells and protecting normal tissues.One promising approach to address the obstacles is to employ radiosensitizers, which can ensure radiotherapeutic efficacy under low-dose ionizing radiation. Inspired by versatile metal-based nanomaterials, great efforts have been devoted to developing nanomaterials with high-Z metal elements as radiosensitizers for decreasing the doses of ionizing radiation and improving the radiosensitivity of tumors by depositing more high-energy photons and secondary electrons into tumor tissue or increasing reactive oxygen species (ROS) generation.8–10 However, these metal-based nanomaterial-mediated RTs are still highly O2-dependent, because O2 molecules can be used to generate a tremendous amount of destructive oxygen radicals as well as permanently immobilize ionizing radiation-induced biomolecule damage.11–13 Unfortunately, low O2 levels (hypoxia) are the unique inherent feature in the majority of malignant tumors caused by rapid cell proliferation and aberrant blood vessel formation, which promotes the survival of tumor cells from high-dose ionizing radiation and results in tumor radioresistance during RT.14–16
To overcome this challenge, in recent years, various strategies based on metal-based nanomedicines capable of avoiding the radioresistance have been proposed to overcome hypoxic-tumor RT.17,18 Of these, one of the most applied approaches to reverse the radioresistance of hypoxic tumors is to employ O2-elevated nanomaterials to directly harness the O2 level in solid tumors.19–21 Besides this, some innovative and simple strategies by employing oxygen-independent nanoradiosensitizers have also been explored for hypoxic-tumor RT.22–24 These strategies provide promising opportunities for enhancing the radiotherapeutic efficacy of hypoxic tumors. Currently, a number of interesting and significant nanomedicines based on metal-based nanomaterials that can be used to combat unmet hypoxic challenges are reported. Therefore, an overall review with future perspectives and challenges aiming specifically at the most recent advances in this field is necessary and important, which provides guidance for researchers and promotes the development of metal-based nanomedicines for RT enhancement. In this review, we summarize approaches based on metal-based nanomaterials for relieving tumor hypoxia by regulating the O2 level in tumor regions, and for the first time present an overview of innovative strategies to overcome the limitation of tumor hypoxia by introducing nanoradiosensitizers with diminished oxygen dependence. Therefore, these approaches and strategies can be roughly dived into two categories (Fig. 1), including (i) employing O2-harnessing nanoradiosensitizers to relieve tumor hypoxia by delivering exogenous O2, generating O2in situ, increasing intratumoral blood flow, or reducing hypoxia-inducible factor (HIF-1) expression; (ii) developing oxygen-independent nanoradiosensitizers to ignore tumor hypoxia via targeting tumor hypoxia for selective RT, enhancing oxygen-independent ROS generation, or combining with non-oxygen dependent cancer therapies for synergistic treatments. We also discuss the design and working mechanisms of these strategies in their application of hypoxic-tumor RT in detail. Finally, current challenges and future perspectives also are pointed out in this field.
 | ||
| Fig. 1 Scheme of strategies based on metal-based nanoparticles for hypoxic-tumor RT. Effective approaches to harness the O2 level within tumor cells: (1) delivering exogenous O2;25 reproduced with permission from ref. 25. Copyright 2016 Elsevier. (2) Generating O2in situ;21 reproduced with permission from ref. 21. Copyright 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (3) Increasing intratumoral blood flow;26 reproduced with permission from ref. 26. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (4) And reducing HIF-1 expression.27 Reproduced with permission from ref. 27. Copyright 2017 American Chemical Society. Innovative and simple strategies to realize diminished oxygen-dependence radiosensitization: (1) targeting tumor hypoxia for selective RT;28 reproduced with permission from ref. 28. Copyright 2019 Elsevier. (2) Enhancing oxygen-independent ROS generation;22 reproduced with permission from ref. 22. Copyright 2019 American Chemical Society. (3) And combining with non-oxygen dependent cancer therapy.29 Reproduced with permission from ref. 29. Copyright 2018 Springer Nature. | ||
2. Harnessing the O2 level for hypoxic-tumor RT
In traditional RT, ionizing radiation can work by the direct or indirect effect. For the direct effect, ionizing radiation causes damage to tumors by direct ionization of some cellular biomolecules, especially DNA, which can result in cell necrosis and apoptosis. For the indirect effect, free radicals, mainly involving ROS with unpaired electrons, generated from the radiolysis of water can destroy biomolecules by a series of chemical reactions such as hydrogen extraction, disproportionation, electron capture, etc.4 In normoxic tumor regions, the oxygen will bind to short-lived free radical sites of biomolecules, and then fix biomolecule damage induced by free radicals, boosting the radiotherapeutic efficacy. However, under hypoxic conditions, biomolecule damage can be repaired by –SH– containing compounds as electron donors such as glutathione via reducing free radicals, competing with the oxygen effect, which leads to radioresistance of tumor tissues.4,12,13 It is observed that intracellular oxygen determines the extent of biomolecule damage. Furthermore, oxygen can rapidly increase reactive free radical generation, further consolidating the damage of biomolecules. Therefore, regulating tumor hypoxia is very meaningful for improving the therapeutic effects of RT.Recently, direct improvement of the O2 level within tumors is the most effective approach to reverse the radioresistance of hypoxic tumors.11,25,30 Nanomaterials, especially metal-based nanomaterials with high-Z metal elements, can be used to construct an O2-elevated system for relieving tumor hypoxia and enhancing the radiotherapeutic efficacy due to their ability to integrate with the O2-evolving function and deposit more ionizing radiation within tumors.17,21 So far, various metal-based nanomaterials have been explored to harness the O2 level for hypoxic-tumor RT.
2.1 Improving tumor hypoxia by delivering exogenous O2
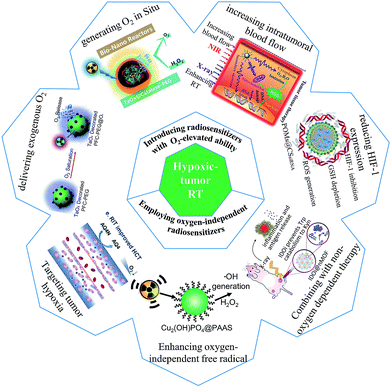
The approach to relieve tumor hypoxia by directly delivering exogenous O2 into tumors can provide sufficient O2 to optimize tumor oxygenation. The key factor for the O2 delivery system is the O2 carrier and triggered way of oxygen release. For the O2 carrier, it requires to possess high O2 capacity and long circulation half-life. Currently, perfluorocarbon (PFC) as a desirable alternative for the O2 carrier has been applied to overcome hypoxia.19,31 In order to ensure the stability and circulation half-life of the O2 carrier, nanomaterials with high loading capability as promising transporters of the O2 carrier may have the potential to solve these problems and effectively increase the delivery of the O2 carrier into tumor cells.25 More importantly, for these nanomaterials containing high-Z metal elements, the radiotherapeutic efficacy will be further enhanced, attributed to various physical processes between ionizing radiation and nanomaterials involving the photoelectric effect, Compton scattering, etc.8,32 Recently, several different types of metal-based nanomaterials as PFC carriers showed the capability of O2 delivery for radiosensitization.17,25,33 For example, tantalum oxide (TaOx) nanoparticles decorated with PFC (TaOx@PFC-PEG@O2) were employed to improve tumor hypoxia.25 The prepared TaOx@PFC-PEG@O2 was able to gradually release oxygen. Then the high-Z element tantalum (Ta) in TaOx nanoparticles and the improved O2 level within tumors synergistically enhanced the radiotherapeutic efficacy. Therefore, O2-delivery systems by employing metal-based nanomaterials with high-Z metal elements and O2 carriers have a promising potential for overcoming tumor hypoxia for enhanced RT.Despite the fact that PFC as an O2 reservoir can spontaneously release O2 within tumors, a burst oxygen release and diffusion in tumors from the O2 carrier that can fight for the maximization of the O2 level is more desired for enhancing RT. Thus, some exogenous stimuli such as the NIR laser or ultrasound have been used to controllably trigger and rapidly promote the O2 release from the O2 carrier.17,33,34 For example, Bi2Se3 nanoparticles with a hollow structure to load PFC (PEG-Bi2Se3@PFC@O2) can result in a rapid O2 release under NIR irradiation for increased tumor oxygenation, where the rapid O2 release can be attributed to the improved temperature by Bi2Se3 nanoparticles as the photothermal agent under NIR irradiation, effectively relieving the radioresistance of tumors (Fig. 2).17 Meanwhile, similar to the above-mentioned TaOx nanoparticles, Bi2Se3 nanoparticles with high-Z metal elements as radiosensitizers can also enhance the hypoxic-tumor RT. As a result, the PEG-Bi2Se3@PFC@O2 with NIR and X-ray treatment exhibited a significant antitumor effect. A burst O2 release under NIR irradiation has a remarkable advantage to obtain maximized O2 concentration in tumors within a relatively short period, however, NIR still exhibits dissatisfactory tissue penetration. In this regard, an exogenous stimulus with a larger penetration depth such as the magnetic field or X-ray may have promising potential to be used for triggering O2 release for the hypoxic RT of deep tumors.
 | ||
| Fig. 2 Hollow Bi2Se3 nanoparticles with PFC for NIR-induced oxygen delivery to enhance RT.17 (a) Scheme of a burst release of oxygen from PEG-Bi2Se3@PFC@O2 under NIR irradiation. (b) The photothermal profiles of aqueous solutions of Bi2Se3 nanoparticles with different concentrations. (c) O2 concentration changes in solutions of samples under NIR irradiation. (d) Fluorescence staining of tumor slices for hypoxia detection. (e) Tumor volume growth curves of different groups of mice with different treatments. Adapted with permission from ref. 17. Copyright 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
2.2 Improving tumor hypoxia by generating O2in situ
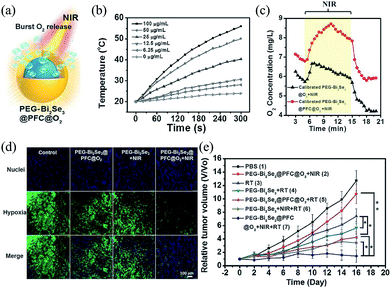
In fact, besides the above-mentioned issues in the O2-delivery system, it also still faces the problem of spontaneous O2 release from the O2 carrier before being internalized by cancer cells. Recently, given the overexpressed hydrogen peroxide (H2O2) within malignant tumors,35,36 the strategy of O2 generation in situ via catalyzing intracellular H2O2 provides a new approach to control the continuous O2 release only in tumor cells, avoiding the premature release of O2.14,21 In this regard, catalase (Cat) and inorganic catalysts are good candidates for O2 generation in situ.21,37 Catalase as a very important enzyme in avoiding ROS-induced damage of cells can catalyze H2O2 to form water molecules and O2, and therefore, delivering external Cat into tumor cells has the potential to overcome tumor hypoxia.38,39 Recently, nanomaterials with high-Z elements integrated with Cat are expected to enhance hypoxic-tumor RT. For example, Song et al. prepared a radiosensitizer by encapsulating Cat into hollow TaOx (TaOx@Cat).21 On one hand, like traditional nanomaterials with high-Z elements, TaOx can effectively enhance the radiotherapeutic efficacy by depositing more ionizing radiation energy within tumors. On the other hand, the loaded Cat in the designed TaOx@Cat can rapidly decompose the intracellular H2O2 into O2 to further enhance the X-ray-induced DNA damage.In addition to catalase, inorganic catalysts such as manganese dioxide (MnO2) and cerium oxide with catalase-like activity can also be used to decompose H2O2 into O2 within the tumor microenvironment.40,41 Biodegradable MnO2 as an O2-evolving nanomaterial has been widely studied for hypoxic-tumor treatments due to its response to acidic and H2O2-sufficient tumor microenvironments. Fan et al. reported an O2-evolving nanoplatform by anchoring upconversion nanoparticles (UCSMs) in MnO2 nanosheets for synergetic hypoxia-radio/photodynamic therapy (RT and PDT) shown in Fig. 3.37 In their work, the redox reaction of MnO2 toward acidic H2O2 not only can generate a great deal of O2 to relieve tumor hypoxia, but also recover the upconversion luminescence (UCL) signal quenched by MnO2 nanosheets because MnO2 nanosheets can be decomposed into free Mn2+ during the reaction, whereafter the ROS production was effectively increased under NIR and X-ray irradiation, which is attributed to O2 generation and recovered UCL, realizing enhanced synergetic therapy. Since these inorganic catalysts with catalase-like activity are facilely synthesized and show considerable activity, it is worth developing O2-evolving catalysts as new radiosensitizers. Apart from the above strategies for O2 release by decomposing intracellular H2O2, some nanomaterials such as photocatalysts that can trigger O2 generation by water splitting under light irradiation also exhibit enormous potential in RT. Of course, the feasibility of nanomaterial-mediated water splitting under X-ray irradiation should be further verified, and the issue of low efficiency for X-ray-induced water splitting may need to be solved.
 | ||
| Fig. 3 MnO2 nanosheets anchored with upconversion nanoparticles for oxygen-elevated synergetic therapy.37 (a) Scheme of the decomposition of MnO2 nanosheets from UCSMs attributed to the redox reaction between MnO2 nanosheets and acidic H2O2, resulting in the recovery of the upconversion luminescence and massive oxygen generation for enhanced PDT/RT. (b) Upconversion luminescence intensity for UCSMs under different conditions. (c) O2 generation in hypoxic 4T1 cells treated with UCSMs. (d) Relative tumor growth curve of different groups of mice with different treatments. Adapted with permission from ref. 37. Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
2.3 Improving tumor hypoxia by increasing intratumoral blood flow
Aberrant blood vessel formation within tumors is the main reason for tumor hypoxia.42 In many past clinical studies of RT, when the tumor temperature reaches 40–42 °C, such mild temperature hyperthermia can enhance the radiotherapeutic efficacy, attributed to the elevated tumor oxygenation induced by an enduring increase in blood flow.30,43 So increasing intratumoral blood flow induced by the thermal effect as an effective approach for improving tumor hypoxia can be used for RT enhancement. Inspired by the remarkable photothermal effect of some nanomaterials under NIR irradiation, researchers have employed these nanomaterials as heat generators to relieve tumor hypoxia.44,45 Especially for nanomaterials with high-Z elements, they can simultaneously realize reduced tumor hypoxia and enhanced ionizing radiation energy deposition within tumors for improved radiotherapeutic efficacy in a single nanoplatform without an additional and complicated O2-delivery or O2-generation system, which provides a simple and effective strategy for hypoxic-tumor RT.26,30,46 For example, Bi2Se3 nanoparticles with high photothermal conversion efficiency were employed to enhance the radiotherapeutic efficacy. Under NIR irradiation, the mild hyperthermia induced by Bi2Se3 nanoparticles can effectively improve oxygenation within tumors by increasing intratumoral blood flow, which can reduce the radioresistance of tumors.26 As a result, the as-prepared Bi2Se3 nanoparticles with a high-Z element (bismuth, Bi) exhibited significant radiosensitization of tumors pretreated with NIR. In addition, the mild heating induced by these metal-based nanomaterials can also increase the vascular permeability of tumor cells to increase the uptake of nanomedicines, further improving the radiotherapeutic efficacy.23 Furthermore, these metal-based nanomaterials with high-Z elements can also be used as X-ray computed tomography (CT)/photoacoustic (PA) imaging contrast agents for image-guided photothermal-radiation therapy, attributed to their strong X-ray attenuation ability and high NIR absorption.30,47 In addition to that, metal-based nanosystems can be labeled with radioisotope ions for single photon emission computed tomography (SPECT) imaging.48,49 Even they can integrate with nanostructures with paramagnetic properties such as gadolinium-containing nanoparticles for magnetic resonance (MR) imaging.44,50 Multimodal image-guided photothermal-radiation therapy mediated by metal-based nanomaterials has tremendous application potential in precise tumor treatment. Besides the photothermal effect caused by nanomaterials, anti-angiogenesis agents combined with nanomaterials can also increase blood-flow perfusion to reduce hypoxia via promoting transient tumour vascular normalization and strengthening blood vessel integrity.51In addition to the above strategies for increasing intratumoral blood flow to relieve hypoxia, recently, nitric oxide (NO) was also used to improve tumor oxygenation for overcoming the hypoxia-associated radioresistance by promoting vasodilation and altering blood flow under low NO concentrations.52,53 And not only that, NO can also inhibit the overexpression of hypoxia inducible factor-1 (HIF-1α) to regulate the oxygen level within tumors.54,55 In addition, with increasing concentration, NO can act as a “killer” to kill tumor cells, attributed to the oxidation or nitrosation to inhibit the DNA and mitochondria repair.18,56 Therefore, NO as an efficient hypoxic radiosensitizer has shown enormous potential and advantage in hypoxic-tumor RT.52,57 The controllable generation of NO plays a key role in tumor treatments since NO exhibits dose-dependent biological effects.58,59 Some strategies by employing exogenous stimuli such as NIR can realize controlled NO release on demand.60–62 However, NIR exhibits powerless working in deeper tumors due to its limited penetration depth. So X-rays as an exogenous stimulus with a large penetration depth can be employed to trigger NO release. For example, Shi and Bu et al. chose the X-ray to control the NO release for enhancing the radiotherapeutic efficacy of deep tumors.18 The designed NO-releasing nanoplatform (USMSs-SNO) that is composed of upconversion nanoparticles (UCNPs) and a NO donor (SNO) can realize X-ray dose-dependent NO release in normoxic and hypoxic HeLa cells. Under X-ray irradiation, the radiolysis of water can generate large amounts of ROS, which results in the structural variation of –SNO groups in the USMSs-SNO and causes the cleavage of S–N bonds for NO release. The released NO can promote the X-ray-induced apoptosis/necrosis of normoxic and hypoxic tumor cells, which is attributed to the significant radiation enhancement effects of X-ray-triggered NO. Recently, Du et al. also designed an X-ray-controlled NO-releasing system consisting of scintillating nanoparticles (SCNPs, Ce-doped LiLuF4) and Roussin's black salt (RBS) for enhancing the radiotherapeutic efficacy of deep tumors, which is attributed to the improved tumor hypoxia and highly toxic peroxynitrite (ONOO−) generation.53 In the work, the ultraviolet light (UV) emitted from Ce-doped LiLuF4 under X-ray irradiation can activate the photoactive RBS to release NO, promoting vasodilation and inhibiting HIF-1α expression to overcome the radioresistance of hypoxic tumors. Meanwhile, SCNPs with high-Z elements as radiosensitizers can enhance the production of ROS including superoxide anions (˙O2−). Then, the simultaneous release of NO and ˙O2− can result in a redox reaction between them for the generation of highly toxic ONOO−, causing damage to tumor DNA. The strategy not only can promote vasodilation and inhibit HIF-1α to overcome the radioresistance of hypoxic tumors by X-ray-controlled NO release, but also transform low toxicity NO and ˙O2− into highly toxic ONOO−. The work provided a new idea to realize X-ray-triggered NO release for deep tumor treatments and take full advantage of the functional NO for radiosensitization of hypoxic tumors.
2.4 Improving tumor hypoxia by reducing HIF-1 expression
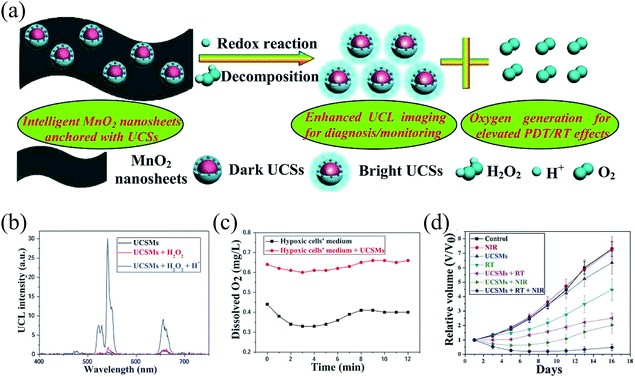
Tumor hypoxia can be mediated by regulating gene expression such as HIF-1. HIF-1 consisting of the HIF-1β subunit and HIF-1α subunit is a heterodimeric protein, which plays an important role in O2 homeostasis by increasing O2 availability and promoting adaptation of tumor cells to O2 deprivation.63 The HIF-1β subunit is constitutively expressed, while the HIF-1α subunit is O2-regulated, determining the activity of HIF-1. Under hypoxic conditions, HIF-1α is rapidly induced, and then dimerizes with HIF-1β, binds to the nucleus, and initiates gene transcription.64,65 Many studies suggest that HIF-1 is overexpressed in many cancer types, which can activate transcription enzymes and proteins associated with tumor invasiveness, resistance and metastasis under hypoxic conditions.63 Therefore, various strategies that focus on regulating HIF-1 expression for tumor treatments have been reported, especially in RT, which mainly utilize HIF-1 inhibitors to downregulate HIF-1 expression.11,27,66,67 Like other drug delivery systems, in consideration of the pharmacokinetics of these HIF-1 inhibitors, integrating them with nanomaterials is a feasible way to improve their pharmacokinetic characteristics. In recent years, nanomaterial-mediated inhibition of HIF-1α expression has drawn researchers' attention. For example, Yong et al. developed a Gd-containing polyoxometalate (GdW10 nanosphere) as a radiosensitizer to enhance the radiotherapeutic efficacy by suppressing HIF-1α expression, depleting GSH, and generating ROS.27 The obtained GdW10 nanospheres with high-Z metal elements (gadolinium and tungsten) can deposit considerable radiation dosage into tumors via strong physical interactions between X-ray and GdW10 nanospheres. A more attractive thing was that GdW10 nanospheres can deplete GSH to reduce the elimination of GSH to ROS by a redox reaction, further facilitating X-ray-triggered ROS generation. In addition, GdW10 nanospheres as HIF-1α siRNA carriers showed a good loading capacity of HIF-1α, which protected HIF-1α from degradation caused by ribonuclease (RNase), reducing the radioresistance associated with tumor hypoxia. As a result, GdW10 nanospheres can obviously improve the radiotherapeutic efficacy by versatile approaches, providing a new idea to address the hypoxic-tumor RT.As can be seen from the above example, single HIF expression inhibition is inadequate to enhance the hypoxic-tumor RT. Therefore, tumor oxygenation strategies or other collaborative approaches combined with HIF-1 inhibition are ideal to obtain effective RT. Based on this point, Meng et al. reported a nanoplatform (ACF@MnO2) consisting of MnO2 NPs and the HIF-1 functional inhibitor (acriflavine, ACF) to increase the oxygen level of tumors and inhibit HIF-1 for enhanced RT and abscopal effects (Fig. 4).11 In the work, MnO2 NPs can decompose overexpressed H2O2 within tumors into O2. Meanwhile, MnO2 NPs were degraded and the loaded ACF was released into tumor cells, which remarkably enhanced the radiotherapeutic efficiency. These results indicated that the strategy of combining tumor oxygenation with HIF-1 functional inhibition can downregulate the expression of genes associated with radioresistance (VEGF, MMP-9), thus reducing the metastatic issue of tumors. More interestingly, the strategy can provide the ACF@MnO2 NPs with a potential to act as checkpoint inhibitors to activate immune T cells because the synergetic strategy of tumor oxygenation and HIF-1 functional inhibition can decrease the PD-L1 expression. Overall, combining HIF-1 inhibition with other treatments exhibits optimal radiotherapeutic outcomes.
 | ||
| Fig. 4 Tumor oxygenation and hypoxia inducible factor-1 functional inhibition or enhancing radiation therapy and abscopal effects.11 (a) The mechanism of ACF@MnO2 for enhanced RT and abscopal effects via tumor oxygenation and HIF-1 function inhibition. (b) ACF release from ACF@MnO2 under different conditions. (c) CT26 cell spheroids stained with hypoxic detection probes after treatment with free ACF or ACF@MnO2, scale bar = 100 μm. (d) Primary tumor slices stained with the PD-L1 antibody, scale bar = 100 μm. (e) Primary and (f) distant tumor growth curves of different groups of mice with different treatments. Adapted with permission from ref. 11. Copyright 2018 American Chemical Society. | ||
3. Nanoradiosensitizers with diminished oxygen dependence for hypoxic-tumor RT
The radiotherapeutic efficacy of traditional RT seriously depends on the O2 concentration of tumors. O2-elevated nanomaterials can directly harness the O2 level to relieve radioresistance associated with tumor hypoxia. However, they still encounter many limitations, such as non-ideal stability, transient generation of O2, unsatisfactory tumor specificity and poor penetration of oxygen gas. Therefore, some innovative strategies have been proposed to avoid the hypoxia-induced radioresistance for enhancing RT by employing nanomaterials with diminished dependence on oxygen, in which these facile nanomaterials can be used to target tumor hypoxia for selective RT, enhance oxygen-independent ROS generation, or combine with non-oxygen dependent cancer therapy for synergistic treatments.22,29,683.1 Targeting tumor hypoxia for selective RT
For tumor hypoxia, opportunities and challenges exist together. On the negative side, tumor hypoxia has negative effects on the therapeutic efficacy of many clinical treatments. However, on the other hand, tumor hypoxia offers endogenous signals or targets to provide treatments with tumor selectivity, where the therapeutic effect of nanomedicines can only happen in tumor regions and thus reduce the side-effects of these treatments.69–71 Recently, some bioreductive anticancer drugs with high hypoxia-specific cytotoxicity such as Tirapazamine (TPZ), AQ4N and Mitomycin C (MMC) have been widely applied for treating hypoxic tumors since they can be only activated under hypoxic conditions, which offers an ideal strategy to selectively enhance the hypoxic-tumor RT.24,28,72 For example, Liu et al. adopted upconversion nanoparticle@mesoporous silica (UCHMs) to wrap TPZ to overcome the hypoxic RT.24 The designed radiosensitizer can effectively amplify the radiotherapeutic efficacy due to the doped high-Z rare earth elements. Furthermore, the loaded TPZ provided complementary killing to the insufficient antitumor effect of RT for cancer cells, attributed to its highly hypoxia-specific cytotoxicity. It was observed that hypoxia-specific drugs have potential advantages in various hypoxia-tumor therapy.In addition to directly utilizing the characteristics of low oxygen levels in solid tumors, an alternative strategy to realize hypoxia-targeted RT involves exploiting the unique features of the hypoxic tumor microenvironment. For example, tumor vasculature, biological responses to hypoxia or overexpressed molecules in hypoxia-specific tumors may all become the targets for precise RT.68,73 Huo et al. synthesized tungsten oxide nanoclusters (WOACC NPs) with enhanced passive tumor accumulation effect and hypoxic microenvironment-targeting ability for radiosensitization by aggregating ultrasmall tungsten oxide nanoparticles (WO NPs), where the WOACC NPs were integrated with CCL-28 chemokine-targeted ligands and cleavable peptides (MMP-2).68 Compared with ultrasmall WO NPs, the half-life of WOACC NPs was improved due to their enlarged size, which was favourable for effective tumor accumulation via the EPR effect, whereafter the overexpressed MMP-2 enzyme within the tumor microenvironment resulted in the destruction of these WOACC NPs bound by MMP-2. And then the ultrasmall WO NPs modified with CCL-28 chemokine-targeted ligands (WOAC NPs) were released, which can deeply penetrate inside the solid tumor due to their small size. More importantly, the CCL-28 chemokine-targeted ligands in WO NPs can effectively target the upregulated CCL-28 in the severely hypoxic regions of tumors.74 As a result, the strategy achieved increased therapeutic efficacy for hypoxic tumors. Therefore, taking tumor hypoxia as a therapeutic target to overcome the limitation caused by hypoxia is feasible. In addition, the development of nanomedicines with the ability of deep tumor penetration is significant for clinical treatments of solid tumors because the abnormal vasculature and the dense interstitial matrix within solid tumors seriously hinder the delivery of nanomedicines.75
3.2 Enhancing oxygen-independent free radical generation
Free radicals induced by the interaction between ionizing radiation and water molecules play a vital role in RT, which can effectively kill tumor cells by damaging biomacromolecules involving lipids, proteins, and DNA.76,77 To date, great efforts have been devoted to developing diversified nanomaterials with free radical-enhancing ability to improve the radiotherapeutic efficacy.78–80 The generated free radical mainly involving oxygen radicals are highly O2-dependent. Nevertheless, the tumor microenvironment with inherent hypoxic characteristics hampers oxygen free radical generation, and then influences the therapeutic effect seriously. Therefore, developing radiosensitizers capable of generating oxygen-independent free radicals to overcome the limitation of hypoxia is proposed. Recently, some semiconductor photocatalytic nanomaterials were employed to promote the decomposition of H2O to generate free radicals with diminished dependence on oxygen via an X-ray-triggered catalytic reaction.23,81 For example, Zhang et al. utilized the holes generated by semiconductors for non-oxygen dependent hydroxyl radical (˙OH) generation via decomposing H2O molecules during RT.81 They prepared a CeIII-doped LiYF4 nanoscintillator loaded with ZnO nanoparticles, in which the nanoscintillator can emit ultraviolet light under X-ray irradiation. And then the emitted ultraviolet light can activate ZnO semiconductor nanoparticles to generate abundant electron–hole pairs, which results in highly toxic ˙OH generation, and thus enhances the antitumor therapeutic efficacy.In the process of H2O decomposition mediated by semiconductor nanomaterials, the effective separation of electron–hole pairs is the key for ROS generation. Therefore, in order to further elevate the performance of oxygen-independent ROS generation by H2O decomposition, Guo et al. designed semiconductor heterojunction nanoparticles consisting of two different semiconductors that are BiOI and Bi2S3 (BSA-coated BiOI@Bi2S3) to enhance free radical generation by suppressing the recombination of electron–hole pairs (Fig. 5).23 In the work, BSA-coated BiOI@Bi2S3 under X-ray irradiation can effectively generate electron–hole pairs compared to single BiOI due to their matching energy level structure, which provided an efficient way to inhibit the recombination of electron–hole pairs in BiOI and Bi2S3, and then resulted in highly efficient catalytic performance. The results indicated that the two half reactions of free radical generation happened in the conduction band (CB) of BiOI and the valence band (VB) of Bi2S3, where the electrons in the CB of Bi2S3 can migrate to the CB of BiOI for ˙O2− generation by transferring electrons to oxygen molecules and the holes in the VB of BiOI can move to the VB of Bi2S3 for ˙OH generation by decomposing the adsorbed H2O in the surface of Bi2S3. The strategy by constructing semiconductor heterojunction nanoparticles maximally amplified their free radical generation ability under hypoxic conditions. Besides constructing semiconductor heterostructures, some sacrificial agents that can capture electrons or holes are also ideal to promote the separation of electron–hole pairs. In this regard, Wang et al. designed a composite consisting of scintillators and Ag3PO4 with a cisplatin prodrug (Pt(IV)), where the cisplatin prodrug as the electron acceptor can effectively capture the photogenerated electrons to effectively generate holes for enhancing oxygen-independent ˙OH generation.82 In the study, the scintillators under X-ray irradiation can activate the Ag3PO4 to generate electron–hole pairs. Then the photogenerated electrons can react with the loaded cisplatin prodrug, which not only prevented the recombination of photogenerated electron–hole pairs, but also transformed cisplatin prodrug (Pt(IV)) into highly toxic cisplatin (Pt(II)). The effective separation between electrons and holes increased the generation of ˙OH by boosting the reaction between holes and H2O. Meanwhile, the photocatalysis-induced cisplatin further enhanced the damage to tumor cells. It was observed that the semiconductor nanomaterial activated by ionizing radiation for non-oxygen dependent free radical generation innovated the mechanism of traditional radiosensitization mediated by nanomaterials with high-Z elements.
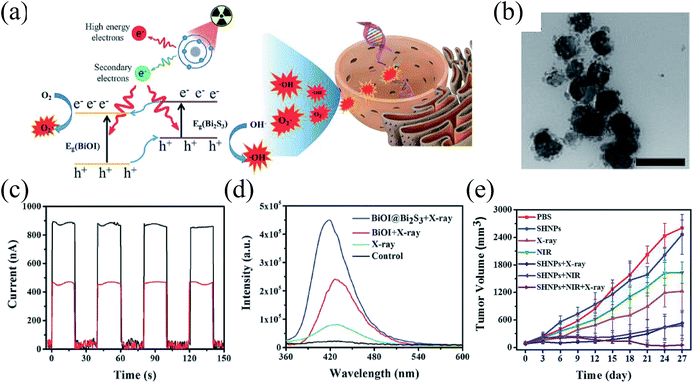 | ||
| Fig. 5 Semiconductor heterojunction nanoparticles for synergistic therapy of tumors.23 (a) The mechanism of X-ray-induced ROS generation by BiOI@Bi2S3. (b) TEM image of as-prepared heterojunction nanoparticles. (c) Photocurrent of as-prepared samples under X-ray irradiation. (d) X-ray-induced ROS generated by as-prepared samples. (e) Tumor growth curves of different groups of mice with different treatments. Adapted with permission from ref. 23. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In addition to decomposing H2O, H2O2 can also be used to generate oxygen-independent ROS. Recently, encouraged by overexpressed H2O2 within malignant solid tumors as well as a burst of additional H2O2 caused by ionizing radiation, many nanomaterials mainly including Fenton's reagent and catalytic nanomaterials have been used to enhance radiotherapeutic efficacy via catalyzing sufficient endogenous H2O2 within tumors into oxygen-independent and highly toxic ˙OH.22,76,83–85 More importantly, the catalytic reaction can only be effectively executed in tumors but not in normal cells because the H2O2 is overexpressed within tumor cells relative to normal cells, which can enhance the selective killing of tumors and reduce the damage to surrounding healthy tissues. For example, Zhang et al. reported a simple and smart radiosensitizer based on Cu2(OH)PO4 nanocrystals. These nanocrystals can generate CuI active sites under X-ray irradiation, which can decompose intracellular H2O2 into ˙OH via a Fenton-like reaction for enhanced RT.22 Compared to traditional spontaneous Fe-based Fenton's reaction, the Cu2(OH)PO4 nanocrystal-mediated Fenton-like reaction possessed considerable catalytic performance in a broader pH range. In addition, the X-ray-induced Fenton-like reaction showed an obvious advantage in controllability because the Fenton-like reaction can only be triggered in the presence of exogenous X-rays and endogenous H2O2. More importantly, they found that the X-ray-triggered Fenton-like reaction failed to effectively occur in normal cells due to insufficient H2O2 within normal cells. Moreover, the normal oxygen within normal cells may also limit the Fenton-like reaction because the X-ray-triggered CuI active sites can be oxidized under oxygen-rich conditions. The limited ˙OH generated in normal cells implied that Cu2(OH)PO4 nanocrystal-mediated RT had a potential to reduce the damage to normal tissues, while the hypoxic environment in tumor cells would favor the Fenton-like performance of Cu2(OH)PO4 nanocrystals, resulting in enhanced RT with tumor selectivity. The strategy was greatly helpful to provide new activation approaches to realize controllable and selective hypoxic-tumor RT.
3.3 Combination with non-oxygen dependent cancer therapies
In clinics, it is usual to combine RT with other treatments to enhance the therapeutic effect by taking advantage of both and avoid the disadvantages of each other. Therefore, assistant therapies that can be used to complement the killing of tumor cells in RT have shown a promising potential for ideal therapeutic efficacy. The most attractive candidates for assistant therapies are treatments that can ignore tumor hypoxia. In general, since gene therapy, photothermal therapy (PTT) and immunotherapy exhibit less dependence on oxygen relative to RT, a combination of RT and gene therapy, PTT, or immunotherapy may be a promising strategy to overcome the hypoxic limitation during RT. For the synergistic therapy of RT and gene therapy mediated by metal-based nanomaterials, exogenous genes can change the radiotherapeutic efficacy by improving tumor radiosensitivity, in which metal-based nanomaterials can be used as nanocarriers to deliver genes and radiosensitizers to enhance the therapeutic efficacy.27,86,87 In addition to genes, some anticancer drugs with the ability to adjust tumor radiosensitivity such as 7-ethyl-10-hydroxycamptothecin, paclitaxel and docetaxel are also applied to enhance hypoxic-tumor RT by combining with metal-based nanomaterials, where the loaded anticancer drugs can arrest tumor cells in radiosensitive phases such as G1 and G2/M phases, further increasing the tumor damage caused by ionizing radiation.88–90 Meanwhile, some of them exhibited a good antitumor effect. So the strategy provides a remarkable synergistic therapeutic outcome in chemoradiotherapy. For the combination of RT and PTT, in general, employing a single nanosystem with high-Z elements and good photothermal conversion efficiency can effectively realize two treatments.47,49,91,92 Meanwhile, the accumulation and penetration of nanosystems within tumors may be enhanced, attributed to improved transmembrane permeability induced by the thermal effect.23,30Recently, RT combined with cancer immunotherapy as an important treatment modality has shown enormous potential in tumor ablation, where the immunotherapy can cause complementary damage to tumors in RT-mediated local therapy and also enhance the radiotherapeutic efficacy to distant tumors. Immunotherapy is a type of cancer treatment that helps the immune system fight cancer. Under normal physiological conditions, the immune system can monitor and eliminate tumor cells. However, tumor cells can escape from T-cell recognition and killing by a series of regulatory mechanisms, resulting in immune resistance.93,94 In checkpoint blockade immunotherapy which is one of the most promising approaches to activate antitumour immunity, the dysregulated expression of immune-checkpoint proteins is the key immune resistance mechanism, inhibiting T cell activity within tumors.95,96 Some immunomodulatory adjuvant treatments are desired to fight the immune resistance and enhance the antitumor immunity. Recent evidence has indicated that high-dose ionizing radiation as immunomodulatory adjuvant treatment can result in an immunomodulatory effect.12,97 However, it still suffers from problems such as that high-dose ionizing radiation can result in severe injury to normal tissue and the RT-induced immunomodulatory effect makes it difficult to obtain systemic tumour rejection. Recently, heavy metal-based nanomaterials with antibodies and inhibitors have been employed to overcome the above problems, which not only can reduce X-ray doses while ensuring damage to tumors, but also enhance the checkpoint blockade immunotherapy for systemic antitumor immunity.29,98 Ni et al. reported Hf-based nanoscale metal–organic frameworks (nMOF) with the anti-programmed death-ligand 1 (anti-PD-L1) antibody, which can be used as radiosensitizers to effectively enhance the local RT and transfer the local radiotherapeutic efficacy to distant tumors via abscopal effects (Fig. 6).98 As a local therapy, Hf-based nMOFs (Hf6-DBA and Hf12-DBA) were more efficient radiosensitizers than HfO2. The evaluation of cell-surface expression of calreticulin (CRT) in vitro and in vivo certified that Hf12-DBA can result in stronger immunogenic cell death, which was consistent with the examined HMGB1 excretion from cells, indicating that Hf12-DBA-mediated RT may possess the killing activity of immune. In addition, the results of antitumor immunity indicated that nMOF-mediated RT with PD-L1 checkpoint blockade therapy relative to other groups can effectively induce the maximum antitumor efficacy for distant tumors, which is likely attributed to the increased CD8+ T cells and NK cells in distant tumors. In addition to combination with the anti-PD-L1 antibody, they also designed an immune checkpoint molecule indoleamine 2,3-dioxygenase (IDOi)-loaded nMOFs for local low-dose RT and systemic tumour rejection.29 The above strategies based on metal-based nanomaterials by combining RT and immunotherapy presented a strong synergy to enhance the local radiotherapeutic efficacy and minimize collateral damage to normal tissues. Meanwhile, it can realize effective antitumor efficacy for distant tumors.
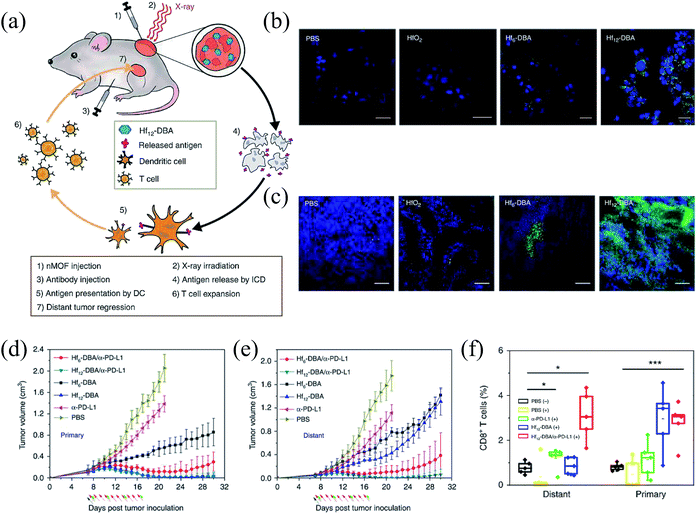 | ||
| Fig. 6 Metal–organic frameworks for enhanced RT and checkpoint blockade immunotherapy.98 (a) Abscopal effect of nMOF-mediated RT and immune checkpoint blockade. The expression of calreticulin (CRT) both in vitro (b) and in vivo (c). Tumor growth curves of (d) primary tumors and (e) distant tumors of CT26 bilateral tumor-bearing mice with different treatments. (f) Tumor-infiltrating CD8+ T cells in both the primary tumors and the distant tumors. | ||
4. Conclusions
Tumor hypoxia seriously inhibits the radiotherapeutic efficacy, and therefore, it's crucial to seek effective strategies for realizing hypoxic-tumor RT enhancement. Inspired by versatile properties, various metal-based nanomaterials have been developed to overcome the radioresistance of hypoxic tumor tissue and enhance the radiotherapeutic efficacy. In this review, we made a summary of the strategies of hypoxic-tumor RT including employing metal-based nanomaterials as oxygen generators to relieve tumor hypoxia and introducing nanoradiosensitizers with diminished oxygen dependence to escape tumor hypoxia. Recent strategies for addressing hypoxia-associated radioresistance bring a promising opportunity for hypoxic-tumor RT. However, most of them are still in the preliminary stage and there still exist challenges in clinical translation.For O2-elevated nanosystems based on metal-based nanomaterials, they have been successfully applied to improve the O2 level of tumors for hypoxic-tumor RT. However, these nanomaterials may be rapidly eliminated by the reticuloendothelial system, which reduces the efficiency of tumor oxygenation. Therefore, O2-elevated nanomaterials with good selectivity and targeting ability for tumors should be pursued to further enhance the radiotherapeutic efficacy and reduce the radiotoxicity to normal tissues. In addition, the NIR-triggered O2-delivery system can avoid the premature release of O2 and maximize the O2 level within tumors. Nevertheless, NIR with the limited penetration depth fails to deal with deep tumors. An exogenous stimulus with a larger penetration depth such as the magnetic field or X-ray may have a promising potential to be used for controllable O2-delivery systems in hypoxic-tumor RT. Besides this, the functional levels of O2 in O2-elevated nanomaterial-mediated RT should be quantified, which is important to provide the standard for constructing an effective O2-elevated nanosystem.
For nanomaterials with diminished oxygen dependence, they contribute a new avenue for hypoxic-tumor RT. However, the efficiency of X-ray-induced free radicals generated by oxygen-independent nanomaterials should be further improved. In addition, due to the complexity of organisms, the existing mechanisms of radiosensitization mediated by these nanomaterials are inadequate. Therefore, deep investigations about mechanisms at molecular levels are necessary. Furthermore, the clinical applications for tumors in situ of nanoradiosensitizers with diminished oxygen dependence should be explored. In this regard, some ultrasmall nanoparticles or these nanomaterials integrated with targeting ligands may be used to deeply penetrate inside solid tumors. Besides, despite being relatively safe, these nanomaterials need to be further evaluated for much higher biosafety before their application in clinics.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Basic Research Program of China (2016YFA2021600), the National Natural Science Foundation of China (51822207, 51772292, 31571015 and 11621505), Chinese Academy of Sciences Youth Innovation Promotion Association (2013007), and CAS Key Research Program of Frontier Sciences (QYZDJ-SSW-SLH022).References
- G. Song, L. Cheng, Y. Chao, K. Yang and Z. Liu, Adv. Mater., 2017, 29, 1700996 CrossRef PubMed
.
- J. Xie, L. Gong, S. Zhu, Y. Yong, Z. Gu and Y. Zhao, Adv. Mater., 2019, 31, 1802244 CrossRef PubMed
.
- H. Chen, Z. Gu, H. An, C. Chen, J. Chen, R. Cui, S. Chen, W. Chen, X. Chen, X. Chen, Z. Chen, B. Ding, Q. Dong, Q. Fan, T. Fu, D. Hou, Q. Jiang, H. Ke, X. Jiang, G. Liu, S. Li, T. Li, Z. Liu, G. Nie, M. Ovais, D. Pang, N. Qiu, Y. Shen, H. Tian, C. Wang, H. Wang, Z. Wang, H. Xu, J.-F. Xu, X. Yang, S. Zhu, X. Zheng, X. Zhang, Y. Zhao, W. Tan, X. Zhang and Y. Zhao, Sci. China: Chem., 2018, 61, 1503–1552 CrossRef CAS
.
- H. Wang, X. Mu, H. He and X.-D. Zhang, Trends Pharmacol. Sci., 2018, 39, 24–48 CrossRef CAS PubMed
.
- K. Haume, S. Rosa, S. Grellet, M. A. Śmiałek, K. T. Butterworth, A. V. Solov'yov, K. M. Prise, J. Golding and N. J. Mason, Cancer Nanotechnol., 2016, 7, 8 CrossRef PubMed
.
- Y. Wang, R. Liang and F. Fang, J. Nanosci. Nanotechnol., 2015, 15, 5487–5500 CrossRef CAS PubMed
.
- G. Le Duc, I. Miladi, C. Alric, P. Mowat, E. Bräuer-Krisch, A. Bouchet, E. Khalil, C. Billotey, M. Janier, F. Lux, T. Epicier, P. Perriat, S. Roux and O. Tillement, ACS Nano, 2011, 5, 9566–9574 CrossRef CAS PubMed
.
- D. Kwatra, A. Venugopal and S. Anant, Transl. Cancer Res., 2013, 2, 330–342 CAS
.
- Y.-S. Yang, R. P. Carney, F. Stellacci and D. J. Irvine, ACS Nano, 2014, 8, 8992–9002 CrossRef CAS PubMed
.
- N. Li, L. Yu, J. Wang, X. Gao, Y. Chen, W. Pan and B. Tang, Chem. Sci., 2018, 9, 3159–3164 RSC
.
- L. Meng, Y. Cheng, X. Tong, S. Gan, Y. Ding, Y. Zhang, C. Wang, L. Xu, Y. Zhu, J. Wu, Y. Hu and A. Yuan, ACS Nano, 2018, 12, 8308–8322 CrossRef CAS PubMed
.
- H. E. Barker, J. T. E. Paget, A. A. Khan and K. J. Harrington, Nat. Rev. Cancer, 2015, 15, 409 CrossRef CAS PubMed
.
- C. Borek, J. Nutr., 2004, 134, 3207S–3209S CrossRef CAS PubMed
.
- P. Prasad, C. R. Gordijo, A. Z. Abbasi, A. Maeda, A. Ip, A. M. Rauth, R. S. DaCosta and X. Y. Wu, ACS Nano, 2014, 8, 3202–3212 CrossRef CAS PubMed
.
- R. G. Bristow and R. P. Hill, Nat. Rev. Cancer, 2008, 8, 180 CrossRef CAS PubMed
.
- O. Jens, J. Clin. Oncol., 2007, 25, 4066 CrossRef PubMed
.
- G. Song, C. Liang, X. Yi, Q. Zhao, L. Cheng, K. Yang and Z. Liu, Adv. Mater., 2016, 28, 2716–2723 CrossRef CAS PubMed
.
- W. Fan, W. Bu, Z. Zhang, B. Shen, H. Zhang, Q. He, D. Ni, Z. Cui, K. Zhao, J. Bu, J. Du, J. Liu and J. Shi, Angew. Chem., 2015, 127, 14232–14236 CrossRef
.
- M. Gao, C. Liang, X. Song, Q. Chen, Q. Jin, C. Wang and Z. Liu, Adv. Mater., 2017, 29, 1701429 CrossRef PubMed
.
- M. H. Cho, E.-S. Choi, S. Kim, S.-H. Goh and Y. Choi, Front. Chem., 2017, 5, 109 CrossRef PubMed
.
- G. Song, Y. Chen, C. Liang, X. Yi, J. Liu, X. Sun, S. Shen, K. Yang and Z. Liu, Adv. Mater., 2016, 28, 7143–7148 CrossRef CAS PubMed
.
- C. Zhang, L. Yan, X. Wang, X. Dong, R. Zhou, Z. Gu and Y. Zhao, Nano Lett., 2019, 19, 1749–1757 CrossRef CAS PubMed
.
- Z. Guo, S. Zhu, Y. Yong, X. Zhang, X. Dong, J. Du, J. Xie, Q. Wang, Z. Gu and Y. Zhao, Adv. Mater., 2017, 29, 1704136 CrossRef PubMed
.
- Y. Liu, Y. Liu, W. Bu, Q. Xiao, Y. Sun, K. Zhao, W. Fan, J. Liu and J. Shi, Biomaterials, 2015, 49, 1–8 CrossRef CAS PubMed
.
- G. Song, C. Ji, C. Liang, X. Song, X. Yi, Z. Dong, K. Yang and Z. Liu, Biomaterials, 2017, 112, 257–263 CrossRef CAS PubMed
.
- J. Du, Z. Gu, L. Yan, Y. Yong, X. Yi, X. Zhang, J. Liu, R. Wu, C. Ge, C. Chen and Y. Zhao, Adv. Mater., 2017, 29, 1701268 CrossRef PubMed
.
- Y. Yong, C. Zhang, Z. Gu, J. Du, Z. Guo, X. Dong, J. Xie, G. Zhang, X. Liu and Y. Zhao, ACS Nano, 2017, 11, 7164–7176 CrossRef CAS PubMed
.
- C. Liang, Y. Chao, X. Yi, J. Xu, L. Feng, Q. Zhao, K. Yang and Z. Liu, Biomaterials, 2019, 197, 368–379 CrossRef CAS PubMed
.
- K. Lu, C. He, N. Guo, C. Chan, K. Ni, G. Lan, H. Tang, C. Pelizzari, Y.-X. Fu, M. T. Spiotto, R. R. Weichselbaum and W. Lin, Nat. Biomed. Eng., 2018, 2, 600–610 CrossRef CAS PubMed
.
- Y. Yong, X. Cheng, T. Bao, M. Zu, L. Yan, W. Yin, C. Ge, D. Wang, Z. Gu and Y. Zhao, ACS Nano, 2015, 9, 12451–12463 CrossRef CAS PubMed
.
- Y. Cheng, H. Cheng, C. Jiang, X. Qiu, K. Wang, W. Huan, A. Yuan, J. Wu and Y. Hu, Nat. Commun., 2015, 6, 8785 CrossRef CAS PubMed
.
- Y. Dou, Y. Guo, X. Li, X. Li, S. Wang, L. Wang, G. Lv, X. Zhang, H. Wang, X. Gong and J. Chang, ACS Nano, 2016, 10, 2536–2548 CrossRef CAS PubMed
.
- N. Lu, W. Fan, X. Yi, S. Wang, Z. Wang, R. Tian, O. Jacobson, Y. Liu, B. C. Yung, G. Zhang, Z. Teng, K. Yang, M. Zhang, G. Niu, G. Lu and X. Chen, ACS Nano, 2018, 12, 1580–1591 CrossRef CAS PubMed
.
- X. Song, L. Feng, C. Liang, K. Yang and Z. Liu, Nano Lett., 2016, 16, 6145–6153 CrossRef CAS PubMed
.
- P. Zhu, Y. Chen and J. Shi, ACS Nano, 2018, 12, 3780–3795 CrossRef CAS PubMed
.
- J. Kim, H. R. Cho, H. Jeon, D. Kim, C. Song, N. Lee, S. H. Choi and T. Hyeon, J. Am. Chem. Soc., 2017, 139, 10992–10995 CrossRef CAS PubMed
.
- W. Fan, W. Bu, B. Shen, Q. He, Z. Cui, Y. Liu, X. Zheng, K. Zhao and J. Shi, Adv. Mater., 2015, 27, 4155–4161 CrossRef CAS PubMed
.
- X. Song, J. Xu, C. Liang, Y. Chao, Q. Jin, C. Wang, M. Chen and Z. Liu, Nano Lett., 2018, 18, 6360–6368 CrossRef CAS PubMed
.
- R. Zhang, X. Song, C. Liang, X. Yi, G. Song, Y. Chao, Y. Yang, K. Yang, L. Feng and Z. Liu, Biomaterials, 2017, 138, 13–21 CrossRef CAS PubMed
.
- W. Zhu, Z. Dong, T. Fu, J. Liu, Q. Chen, Y. Li, R. Zhu, L. Xu and Z. Liu, Adv. Funct. Mater., 2016, 26, 5490–5498 CrossRef CAS
.
- C. Yao, W. Wang, P. Wang, M. Zhao, X. Li and F. Zhang, Adv. Mater., 2018, 30, 1704833 CrossRef PubMed
.
- A. L. Harris, Nat. Rev. Cancer, 2002, 2, 38 CrossRef CAS PubMed
.
- C. W. Song, H. J. Park, C. K. Lee and R. Griffin, Int. J. Hyperthermia, 2005, 21, 761–767 CrossRef CAS PubMed
.
- Q. Xiao, X. Zheng, W. Bu, W. Ge, S. Zhang, F. Chen, H. Xing, Q. Ren, W. Fan, K. Zhao, Y. Hua and J. Shi, J. Am. Chem. Soc., 2013, 135, 13041–13048 CrossRef CAS PubMed
.
- X. Yi, K. Yang, C. Liang, X. Zhong, P. Ning, G. Song, D. Wang, C. Ge, C. Chen, Z. Chai and Z. Liu, Adv. Funct. Mater., 2015, 25, 4689–4699 CrossRef CAS
.
- G. Song, C. Liang, H. Gong, M. Li, X. Zheng, L. Cheng, K. Yang, X. Jiang and Z. Liu, Adv. Mater., 2015, 27, 6110–6117 CrossRef CAS PubMed
.
- J. Wang, X. Tan, X. Pang, L. Liu, F. Tan and N. Li, ACS Appl. Mater. Interfaces, 2016, 8, 24331–24338 CrossRef CAS PubMed
.
- L. Cheng, S. Shen, S. Shi, Y. Yi, X. Wang, G. Song, K. Yang, G. Liu, T. E. Barnhart, W. Cai and Z. Liu, Adv. Funct. Mater., 2016, 26, 2185–2197 CrossRef CAS PubMed
.
- F. Mao, L. Wen, C. Sun, S. Zhang, G. Wang, J. Zeng, Y. Wang, J. Ma, M. Gao and Z. Li, ACS Nano, 2016, 10, 11145–11155 CrossRef CAS PubMed
.
- A. Detappe, E. Thomas, M. W. Tibbitt, S. Kunjachan, O. Zavidij, N. Parnandi, E. Reznichenko, F. Lux, O. Tillement and R. Berbeco, Nano Lett., 2017, 17, 1733–1740 CrossRef CAS PubMed
.
- W. Li, X. Zhao, B. Du, X. Li, S. Liu, X.-Y. Yang, H. Ding, W. Yang, F. Pan, X. Wu, L. Qin and Y. Pan, Sci. Rep., 2016, 6, 30619 CrossRef CAS PubMed
.
- B. F. Jordan, P. Sonveaux, O. Feron, V. Grégoire, N. Beghein, C. Dessy and B. Gallez, Int. J. Cancer, 2004, 109, 768–773 CrossRef CAS PubMed
.
- Z. Du, X. Zhang, Z. Guo, J. Xie, X. Dong, S. Zhu, J. Du, Z. Gu and Y. Zhao, Adv. Mater., 2018, 30, 1804046 CrossRef PubMed
.
- F. H. Agani, M. Puchowicz, J. C. Chavez, P. Pichiule and J. LaManna, Am. J. Physiol.: Cell Physiol., 2002, 283, 178–186 CrossRef PubMed
.
- U. Berchner-Pfannschmidt, H. Yamac, B. Trinidad and J. Fandrey, J. Biol. Chem., 2007, 282, 1788–1796 CrossRef CAS PubMed
.
- T. Cook, Z. Wang, S. Alber, K. Liu, S. C. Watkins, Y. Vodovotz, T. R. Billiar and D. Blumberg, Cancer Res., 2004, 64, 8015 CrossRef CAS PubMed
.
- M. De Ridder, D. Verellen, V. Verovski and G. Storme, Nitric Oxide, 2008, 19, 164–169 CrossRef CAS PubMed
.
- A. W. Carpenter and M. H. Schoenfisch, Chem. Soc. Rev., 2012, 41, 3742–3752 RSC
.
- D. A. Wink, F. Laval, J. Laval, J. B. Mitchell, M. W. Dewhirst and Y. Vodovotz, Carcinogenesis, 1998, 19, 711–721 CrossRef CAS PubMed
.
- X. Zhang, G. Tian, W. Yin, L. Wang, X. Zheng, L. Yan, J. Li, H. Su, C. Chen, Z. Gu and Y. Zhao, Adv. Funct. Mater., 2015, 25, 3049–3056 CrossRef CAS
.
- X. Zhang, Z. Guo, J. Liu, G. Tian, K. Chen, S. Yu and Z. Gu, Sci. Bull., 2017, 62, 985–996 CrossRef CAS
.
- X. Zhang, J. Du, Z. Guo, J. Yu, Q. Gao, W. Yin, S. Zhu, Z. Gu and Y. Zhao, Adv. Sci., 2019, 6, 1801122 CrossRef PubMed
.
- H. Zhong, A. M. De Marzo, E. Laughner, M. Lim, D. A. Hilton, D. Zagzag, P. Buechler, W. B. Isaacs, G. L. Semenza and J. W. Simons, Cancer Res., 1999, 59, 5830 CAS
.
- S. Kizaka-Kondoh, M. Inoue, H. Harada and M. Hiraoka, Cancer Sci., 2003, 94, 1021–1028 CrossRef CAS PubMed
.
- G. L. Semenza, Oncogene, 2009, 29, 625 CrossRef PubMed
.
- W.-H. Chen, R. L. G. Lecaros, Y.-C. Tseng, L. Huang and Y.-C. Hsu, Cancer Lett., 2015, 359, 65–74 CrossRef CAS PubMed
.
- M. I. Koukourakis, A. Giatromanolaki, J. Skarlatos, L. Corti, S. Blandamura, M. Piazza, K. C. Gatter and A. L. Harris, Cancer Res., 2001, 61, 1830 CAS
.
- D. Huo, S. Liu, C. Zhang, J. He, Z. Zhou, H. Zhang and Y. Hu, ACS Nano, 2017, 11, 10159–10174 CrossRef CAS PubMed
.
- W. R. Wilson and M. P. Hay, Nat. Rev. Cancer, 2011, 11, 393 CrossRef CAS PubMed
.
- T. Thambi, V. G. Deepagan, H. Y. Yoon, H. S. Han, S.-H. Kim, S. Son, D.-G. Jo, C.-H. Ahn, Y. D. Suh, K. Kim, I. Chan Kwon, D. S. Lee and J. H. Park, Biomaterials, 2014, 35, 1735–1743 CrossRef CAS PubMed
.
- Y. Wang, Y. Xie, J. Li, Z.-H. Peng, Y. Sheinin, J. Zhou and D. Oupický, ACS Nano, 2017, 11, 2227–2238 CrossRef CAS PubMed
.
- W. Fan, B. Shen, W. Bu, X. Zheng, Q. He, Z. Cui, K. Zhao, S. Zhang and J. Shi, Chem. Sci., 2015, 6, 1747–1753 RSC
.
- B. G. Wouters, S. A. Weppler, M. Koritzinsky, W. Landuyt, S. Nuyts, J. Theys, R. K. Chiu and P. Lambin, Eur. J. Cancer, 2002, 38, 240–257 CrossRef CAS PubMed
.
- A. Facciabene, X. Peng, I. S. Hagemann, K. Balint, A. Barchetti, L.-P. Wang, P. A. Gimotty, C. B. Gilks, P. Lal, L. Zhang and G. Coukos, Nature, 2011, 475, 226 CrossRef CAS PubMed
.
- C. Wong, T. Stylianopoulos, J. Cui, J. Martin, V. P. Chauhan, W. Jiang, Z. Popović, R. K. Jain, M. G. Bawendi and D. Fukumura, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2426 CrossRef CAS
.
- E. I. Azzam, J.-P. Jay-Gerin and D. Pain, Cancer Lett., 2012, 327, 48–60 CrossRef CAS
.
- M. E. Lomax, L. K. Folkes and P. O'Neill, Clin. Oncol., 2013, 25, 578–585 CrossRef CAS PubMed
.
- Y. Chang, L. He, Z. Li, L. Zeng, Z. Song, P. Li, L. Chan, Y. You, X.-F. Yu, P. K. Chu and T. Chen, ACS Nano, 2017, 11, 4848–4858 CrossRef CAS PubMed
.
- W. Fan, B. Shen, W. Bu, X. Zheng, Q. He, Z. Cui, D. Ni, K. Zhao, S. Zhang and J. Shi, Biomaterials, 2015, 69, 89–98 CrossRef CAS PubMed
.
- L. Wen, L. Chen, S. Zheng, J. Zeng, G. Duan, Y. Wang, G. Wang, Z. Chai, Z. Li and M. Gao, Adv. Mater., 2016, 28, 5072–5079 CrossRef CAS
.
- C. Zhang, K. Zhao, W. Bu, D. Ni, Y. Liu, J. Feng and J. Shi, Angew. Chem., Int. Ed., 2015, 54, 1770–1774 CrossRef CAS PubMed
.
- H. Wang, B. Lv, Z. Tang, M. Zhang, W. Ge, Y. Liu, X. He, K. Zhao, X. Zheng, M. He and W. Bu, Nano Lett., 2018, 18, 5768–5774 CrossRef CAS PubMed
.
- R. Zhou, H. Wang, Y. Yang, C. Zhang, X. Dong, J. Du, L. Yan, G. Zhang, Z. Gu and Y. Zhao, Biomaterials, 2019, 189, 11–22 CrossRef CAS PubMed
.
- A. K. Hauser, M. I. Mitov, E. F. Daley, R. C. McGarry, K. W. Anderson and J. Z. Hilt, Biomaterials, 2016, 105, 127–135 CrossRef CAS PubMed
.
- S. Klein, M. Kızaloğlu, L. Portilla, H. Park, T. Rejek, J. Hümmer, K. Meyer, R. Hock, L. V. R. Distel, M. Halik and C. Kryschi, Small, 2018, 14, 1704111 CrossRef PubMed
.
- F. M. Kievit, K. Wang, T. Ozawa, A. W. Tarudji, J. R. Silber, E. C. Holland, R. G. Ellenbogen and M. Zhang, J. Nanomed. Nanotechnol., 2017, 13, 2131–2139 CrossRef CAS PubMed
.
- J. Chen, S. Zhu, L. Tong, J. Li, F. Chen, Y. Han, M. Zhao and W. Xiong, BMC Cancer, 2014, 14, 114 CrossRef
.
- C. Jin, H. Wu, J. Liu, L. Bai and G. Guo, J. Clin. Pharm. Ther., 2007, 32, 41–47 CrossRef CAS PubMed
.
- C. Mirjolet, J. Boudon, A. Loiseau, S. Chevrier, R. Boidot, A. Oudot, B. Collin, E. Martin, P. A. Joy, N. Millot and G. Créhange, Int. J. Nanomed., 2017, 12, 6357–6364 CrossRef CAS PubMed
.
- J. Liu, H. Wang, X. Yi, Y. Chao, Y. Geng, L. Xu, K. Yang and Z. Liu, Adv. Funct. Mater., 2017, 27, 1703832 CrossRef
.
- X. Liu, X. Zhang, M. Zhu, G. Lin, J. Liu, Z. Zhou, X. Tian and Y. Pan, ACS Appl. Mater. Interfaces, 2017, 9, 279–285 CrossRef CAS PubMed
.
- M. Chen, Z. Guo, Q. Chen, J. Wei, J. Li, C. Shi, D. Xu, D. Zhou, X. Zhang and N. Zheng, Chem. Sci., 2018, 9, 4268–4274 RSC
.
- G. P. Dunn, A. T. Bruce, H. Ikeda, L. J. Old and R. D. Schreiber, Nat. Immunol., 2002, 3, 991 CrossRef CAS
.
- M. J. Smyth, D. I. Godfrey and J. A. Trapani, Nat. Immunol., 2001, 2, 293 CrossRef CAS PubMed
.
- D. M. Pardoll, Nat. Rev. Cancer, 2012, 12, 252 CrossRef CAS PubMed
.
- P. C. Tumeh, C. L. Harview, J. H. Yearley, I. P. Shintaku, E. J. M. Taylor, L. Robert, B. Chmielowski, M. Spasic, G. Henry, V. Ciobanu, A. N. West, M. Carmona, C. Kivork, E. Seja, G. Cherry, A. J. Gutierrez, T. R. Grogan, C. Mateus, G. Tomasic, J. A. Glaspy, R. O. Emerson, H. Robins, R. H. Pierce, D. A. Elashoff, C. Robert and A. Ribas, Nature, 2014, 515, 568 CrossRef CAS
.
- D. Schaue, Front. Immunol., 2017, 8, 431 Search PubMed
.
- K. Ni, G. Lan, C. Chan, B. Quigley, K. Lu, T. Aung, N. Guo, P. La Riviere, R. R. Weichselbaum and W. Lin, Nat. Commun., 2018, 9, 2351 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2019 |
