Open Access Article
Open Access ArticleSpin-crossover modulation via single-crystal to single-crystal photochemical [2 + 2] reaction in Hofmann-type frameworks†
Long-Fei
Wang
,
Wei-Man
Zhuang
,
Guo-Zhang
Huang
 ,
Yan-Cong
Chen
,
Yan-Cong
Chen
 ,
Jiang-Zhen
Qiu
,
Zhao-Ping
Ni
and
Ming-Liang
Tong
,
Jiang-Zhen
Qiu
,
Zhao-Ping
Ni
and
Ming-Liang
Tong
 *
*
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of ChemistrySun, Yat-Sen University, Guangzhou, 510275 P. R. China. E-mail: tongml@mail.sysu.edu.cn; Fax: +86 20 8411 2245
First published on 3rd July 2019
Abstract
This study reports the first modulation of spin-crossover (SCO) behavior via a photochemical [2 + 2] cycloaddition reaction. Here we construct two no-solvent Fe(II)–Ag(I) bimetallic Hofmann-type frameworks, [Fe(4-spy)2{Ag(CN)2}2] (1) and [Fe(2,4-bpe)2{Ag(CN)2}2] (2) (4-spy = 4-styrylpyridine, 2,4-bpe = trans-1-(2-pyridyl)-2-(4-pyridyl)ethylene). For 1, the dimerization of 4-spy results in a single-crystal to single-crystal (SCSC) transformation from 2D interdigitated layers to a 3D interpenetrated structure. Additionally, a 3D → 3D structural transformation accompanied with Ag(I)–N bond breaking is achieved via the photochemical cycloaddition reaction of 2,4-bpe in 2. More importantly, both the spin transition temperatures and the SCO character are effectively modulated; thus, this approach provides a new strategy for constructing photo-responsive SCO magnetic materials.
Introduction
Scientists have focused on making advanced materials with functionalities that respond to external stimuli.1 A promising method that has been rapidly developed is the post-synthetic modification (PSM) of coordination polymers.2–5 One particularly interesting type of PSM is the single-crystal to single-crystal (SCSC) structural transformation via a photochemical [2 + 2] cycloaddition reaction.6–11 The stereoselective photodimerization of olefins can proceed in the solid-state if the reactive C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are parallel and separated by less than 4.2 Å.12,13 This results in the modulation of physical properties, such as magnetism,14–17 photoluminescence,18 and conductivity,19,20 which has been previously reported.
C bonds are parallel and separated by less than 4.2 Å.12,13 This results in the modulation of physical properties, such as magnetism,14–17 photoluminescence,18 and conductivity,19,20 which has been previously reported.
Spin-crossover (SCO) compounds are fascinating advanced materials that can switch between high-spin (HS) and low-spin (LS) states under external stimuli (temperature, light, or pressure).21,22 More importantly, the population of SCO behavior in crystal lattice is highly dependent on the presenting elastic cooperativity between metal centers.23 As a result, slight structural perturbations, such as π-stacking, hydrogen bonding and van der Waals contacts, in the crystal lattice can significantly affect the cooperativity of SCO behavior such as abruptness, hysteresis and steps. For example, SCO properties are highly sensitive to anions, solvents or guests.24–26 As a result, porous SCO metal–organic frameworks (MOFs) have been actively explored. Their SCO behaviors can be adjusted by guest adsorption and desorption.27–32 Moreover, successful covalent PSMs have been obtained through chemical reaction of an active guest,33 redox intercalation of a coordinatively unsaturated metal site,34,35 and chemical modification of a bridging ligand.36 However, the unreacted chemical reagents and solvents left in the pores can also affect SCO properties, which hinders the analysis on the modulation mechanism. PSM within the framework itself, without introducing other reagents, can prevent this problem. For example, some photochromic building units have been introduced into SCO systems to develop novel photo-responsive magnetic materials,37,38 such as the cis–trans photoisomerization of stilbenes39–41 or azobenzenes,42 and the intramolecular photocyclization of dithienylethene.43–46 The photochemical [2 + 2] cycloaddition reaction, which can induce drastic changes of structural dimensionality, the electronic conjugation and the supramolecular interaction, shall shows promising impacts on the SCO properties, but such challenging integration has not been reported.
In the present study, a regioselective photochemical [2 + 2] cycloaddition reaction was incorporated into the SCO framework for the first time (Scheme 1). After UV irradiation, two solvent-free SCO frameworks, [Fe(4-spy)2{Ag(CN)2}2] (1, 4-spy = 4-styrylpyridine) and [Fe(2,4-bpe)2{Ag(CN)2}2] (2, 2,4-bpe = trans-1-(2-pyridyl)-2-(4-pyridyl)ethylene), undergo quantitative SCSC photodimerization to produce [Fe(rctt-4-ppcb){Ag(CN)2}2] (1′, rctt-4-ppcb = rctt-1,3-bis(4-pyridyl)-2,4-bis(phenyl)cyclobutane, r = reference group, c = cis and t = trans) and [Fe(rctt-2,4-tpcb-ht){Ag(CN)2}2] (2′, rctt-2,4-tpcb-ht = rctt-1,3-bis(2-pyridyl)-2,4-bis(4-pyridyl)cyclobutane, ht = head-to-tail). A comprehensive study and discussion on the variations of structures and magnetic dynamics convincingly demonstrate the effectiveness of photochemical [2 + 2] reaction on tuning spin crossover magnetic responses.
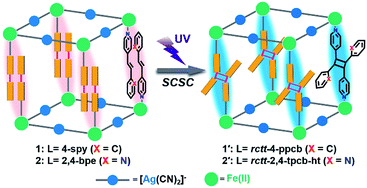 | ||
| Scheme 1 Strategy for the incorporation of a photochemical [2 + 2] cycloaddition into SCO Hofmann-type frameworks [Fe(L)2{Ag(CN)2}2]. | ||
Results and discussion
Compound 1 was obtained as orange block singe crystals through the slow diffusion of Fe(ClO4)2·6H2O, KAg(CN)2 and 4-spy ligand in EtOH solution. Replacing of 4-spy by 2,4-bpe ligand in the similar synthetic route gave the block single crystals of 2 with deeper color. Magnetic susceptibility measurements reveal hysteretic one-step and two-step SCO behaviors for 1 and 2, respectively (Fig. 1). For 1, χMT values decrease gradually from 3.71 cm3 mol−1 K−1 at 300 K to 3.61 cm3 mol−1 K−1 at 250 K then decrease suddenly to 0.10 cm3 mol−1 K−1 at 180 K, indicating a complete SCO behavior (Fig. 1a and S1a†). The critical temperatures in the cooling and warming modes are 212 and 215 K (Table S1†), respectively, revealing a 3 K thermal hysteresis loop. Such hysteretic SCO property is further confirmed by the differential scanning calorimetry (DSC) measurement with exothermic and endothermic peaks at 207 and 210 K, respectively (Fig. S2a†). For 2, obvious plateaus are observed in the range of 149–158 K (cooling mode) and 153–160 K (warming mode) (Fig. 1b and S3a†), corresponding to the HS0.5LS0.5 state. The critical temperatures are Tc1↓ = 164 K, Tc1↑ = 167 K, Tc2↓ = 141 K and Tc2↑ = 146 K, defining 3 and 5 K thermal hysteresis loops, respectively (Table S1†). Furthermore, DSC measurements (Fig. S4†) reveal peaks at Tc1↓ = 161 K, Tc1↑ = 166 K and Tc2↓ = 138 K, Tc2↑ = 146 K, which are consistent with the Tc values from the magnetic data.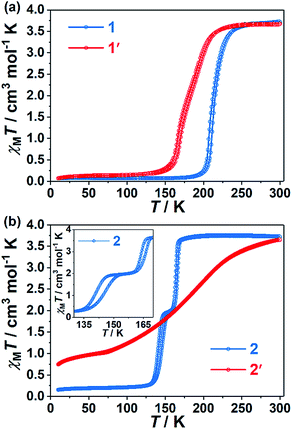 | ||
| Fig. 1 Magnetic susceptibility data for 1 (a, blue) and 2 (b, blue) and their photochemical [2 + 2] cycloaddition products 1′ (a, red) and 2′ (b, red). | ||
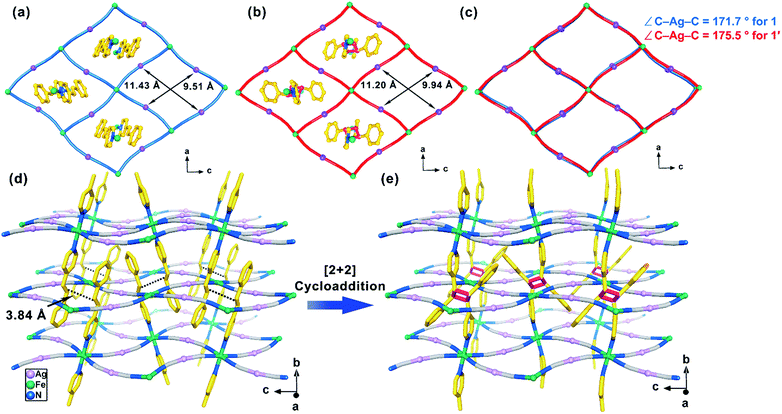
Variable-temperature single-crystal X-ray diffraction data were collected at 120, 212, and 260 K for 1 and 120, 155, and 185 K for 2 (Tables S3 and S4†). Compound 1 remains in the orthorhombic space group Pbac, and all the Fe(II) ions are crystallographically equivalent at all the measured temperatures. The average distance of Fe–N bond contracts from 2.157 Å at 260 K to 1.956 Å at 120 K (Table S5†), corresponding to a complete interconversion between HS and LS of the Fe(II) ions and matches well with the magnetic data. The structure at 260 K is described representatively in the following. Its asymmetric unit contains a half-occupied Fe(II) ion located at the inversion center, one [Ag(CN)2]− ion and one 4-spy ligand (Fig. S5a†). The octahedral Fe(II) ions are bridged equatorially by four separate [Ag(CN)2]− linkers to form rhombic [Fe4{Ag(CN)2}4] grids and then generate corrugated 2D [Fe{Ag(CN)2}2]∞ layers (Fig. 2a). Each Fe(II) ion is coordinated axially by two monodentate 4-spy ligands, resulting in a 2D Hofmann-type coordination polymer (Fig. 2d). The 4-spy ligands are disordered in two positions with a ratio of 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34, suggesting the pendulum movement of the benzene rings.
34, suggesting the pendulum movement of the benzene rings.
Moreover, it should be noted each [Fe4{Ag(CN)2}4] grid in the 2D layer possesses a rhombic window and is further threaded by two 4-spy ligands from adjacent upper and lower layers, respectively, giving the interdigitation of layers (Fig. S6a–d†). The shortest interlayer Ag(I)⋯Ag(I) distance is 7.40 Å, indicating the absent of argyrophilic interaction which is usually observed in 2D or 3D Hofmann-type SCO compounds. Offset face-to-face π⋯π interactions are observed between the benzene and pyridine rings, with dihedral angles of 13.6° and 9.2° for the two disordered ligands and a centroid-to-centroid distance of 4.01 Å at 260 K (Fig. S7a and Table S6†). Most importantly, the center-to-center distances between the parallel ethylene groups of the two disordered 4-spy ligands are 3.97 and 3.84 Å, which satisfies Schmidt's photochemical [2 + 2] cycloaddition reaction criteria (<4.2 Å).12,13
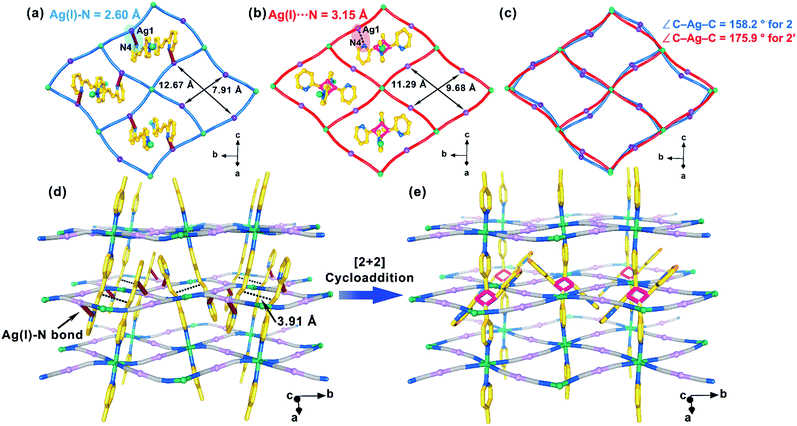
Compound 2 crystalizes in the monoclinic space group P21/c, and only one crystallographically independent Fe(II) ion is observed at 120 and 185 K. The coordination environment around the Fe(II) ion is similar to that in 1 (Fig. S8a†). Instead of 4-spy in 1, the 4-pyridyl unit of 2,4-bpe ligand is axially coordinated to the Fe(II) ion in 2. The average Fe–N distances (Table S7†) are 1.967 Å (120 K) and 2.174 Å (185 K), corresponding to the LS and HS states, respectively. Moreover, each [Fe4{Ag(CN)2}4] grid is threaded by two 2,4-bpe ligands from neighboring layers with offset π⋯π interactions between the pyridine rings (Fig. S9a†). The distance between the centers of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of the 2,4-bpe ligands is 3.91 Å at 185 K, indicating the possibility of photo-dimerization reaction. Notably, the 2-pyridyl groups of the two trapped 2,4-bpe ligands further coordinate to Ag(I) ions on the opposite edges of the [Fe4{Ag(CN)2}4] grid (Fig. 3a), generating a unique 3D Hofmann-type coordination polymer (Fig. 3d). A topological analysis with TOPOS 4.0 (ref. 47 and 48) yields a (3,6)-connected rtl topological net (Fig. S10†). Due to the additional Ag(I)–Npy bond, the 2,4-bpe ligand is fixed, and no structural pendulum movement is observed. Moreover, the Ag(CN)2− unit with a C–Ag(I)–C angle of 158.2(3)° in 2 at 185 K is more bent49 than that in 1. When the temperature decreases from 185 to 155 K, symmetry breaking from P21/c to P
C groups of the 2,4-bpe ligands is 3.91 Å at 185 K, indicating the possibility of photo-dimerization reaction. Notably, the 2-pyridyl groups of the two trapped 2,4-bpe ligands further coordinate to Ag(I) ions on the opposite edges of the [Fe4{Ag(CN)2}4] grid (Fig. 3a), generating a unique 3D Hofmann-type coordination polymer (Fig. 3d). A topological analysis with TOPOS 4.0 (ref. 47 and 48) yields a (3,6)-connected rtl topological net (Fig. S10†). Due to the additional Ag(I)–Npy bond, the 2,4-bpe ligand is fixed, and no structural pendulum movement is observed. Moreover, the Ag(CN)2− unit with a C–Ag(I)–C angle of 158.2(3)° in 2 at 185 K is more bent49 than that in 1. When the temperature decreases from 185 to 155 K, symmetry breaking from P21/c to P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) is observed (Table S4†), together with a conversion from one to two crystallographically independent Fe(II) ions (Fig. S11 and S12†). The average Fe–N distances of the Fe1 and Fe2 ions are 1.960 and 2.165 Å, respectively, which is in good agreement with the intermediate HS0.5LS0.5 state observed in the magnetic data.
is observed (Table S4†), together with a conversion from one to two crystallographically independent Fe(II) ions (Fig. S11 and S12†). The average Fe–N distances of the Fe1 and Fe2 ions are 1.960 and 2.165 Å, respectively, which is in good agreement with the intermediate HS0.5LS0.5 state observed in the magnetic data.
Photochemical [2 + 2] cycloaddition reactions were performed by irradiating single crystals of 1 and 2 for three and four days, respectively, under an high-pressure mercury lamp (P = 500 W), which quantitatively yielded the photoproducts 1′ and 2′, respectively, via a SCSC transformation. The single crystals of 1 remained intact during the whole irradiation process. The photochemical reaction process can be directly monitored via the crystal color change from orange to light gray (Fig. 4a). In contrast, the single crystals of 2 are cracked into pieces with color change from orange to light gray (Fig. S13†) after 4 days, in which some pieces still keep the single crystal properties. In consistent with the observed photochromic effect of the crystals after irradiation, both photoproducts show the obvious decrease of absorption in the visible region (Fig. S14†).
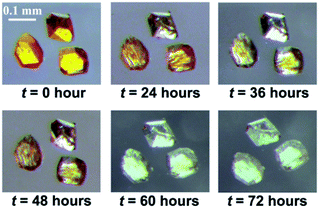 | ||
| Fig. 4 Single crystal pictures of 1 under the exposure of high-pressure Hg lamp (P = 500 W) at different irradiation times, showing obvious color change from orange to light gray. | ||
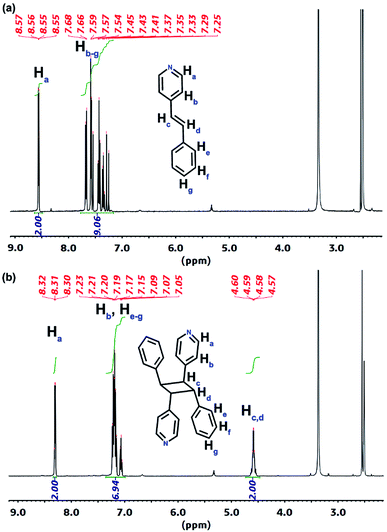
Further 1H NMR spectra measurement of the digestion products of 1′ and 2′ in D6-DMSO reveal 99% and 95% conversions of the 4-spy ligands to rctt-4-ppcb and the 2,4-bpe ligands to rctt-2,4-tpcb-ht. The signal at 4.60 ppm corresponds to the C–H protons of the cyclobutane ring in rctt-4-ppcb (Fig. 5). Meanwhile, the signals at 4.78 and 4.84 ppm are the characteristic signals of the cyclobutane protons of rctt-2,4-tpcb-ht, indicating a regioselective ht [2 + 2] photocycloaddition (Fig. S15†). The IR spectra (Fig. S16†) also show the disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H out-of-plane bending vibrations at 967 cm−1 (978 cm−1) for 1 (2) and appearance of the saturated C–H stretching vibrations at 2934 cm−1 (2928 cm−1) for 1′ (2′), respectively. The PSM processes are further supported by the powder X-ray diffraction patterns (Fig. S17 and S18†). To the best of our knowledge, a photochemical [2 + 2] cycloaddition reaction based on an Fe(II) coordination compound has not been previously reported.
C–H out-of-plane bending vibrations at 967 cm−1 (978 cm−1) for 1 (2) and appearance of the saturated C–H stretching vibrations at 2934 cm−1 (2928 cm−1) for 1′ (2′), respectively. The PSM processes are further supported by the powder X-ray diffraction patterns (Fig. S17 and S18†). To the best of our knowledge, a photochemical [2 + 2] cycloaddition reaction based on an Fe(II) coordination compound has not been previously reported.
The regioselective [2 + 2] photocycloaddition is clearly confirmed by the single-crystal analysis (at 120, 178, and 260 K for 1′ and 90, 185, and 300 K for 2′). After the UV irradiation of 1, the space group Pbac is retained with comparable unit cell dimensions. The main change is that the two 4-spy ligands trapped in each [Fe4{Ag(CN)2}4] grid are dimerized to form a bis-monodentate rctt-4-ppcb ligand, which bridges the upper and lower layers to form a twofold-interpenetrated 3D framework with pcu topology (Fig. 2e and S6e–h†). Therefore, a 2D → 3D SCSC photochemical transformation has been achieved. Accordingly, due to the geometric restrictions of the cyclobutane ring with sp3 hybridization, the dihedral angle between the benzene and the pyridine rings changes from 13.6° to 41.5° at 260 K (Table S6†). Meanwhile, offset face-to-face π⋯π interactions between the trapped 4-spy ligands in 1 are replaced by edge-to-face π⋯π interactions between the intermolecular rctt-ht substituted cyclobutanes in 1′ (Fig. S7b†). The comparison of 2D fingerprint plots between 1 and 1′ further confirm the different supramolecular interaction (Fig. 6).50 Obviously, as revealed by the 2D fingerprint plots of C⋯C contacts, the blue region around de ≈ di ≈ 1.8 Å in 1 (Fig. 6a) is absent in 1′ (Fig. 6c), corresponding to the losing of π⋯π stacking between the aromatic rings.51 Meanwhile, the positions of the ‘wings’ in the fingerprint plots, which correspond to C–H⋯π interactions become more visually dominant in 1′(Fig. 6b) than 1 (Fig. 6d), suggesting an additional contribution from edge-to-face π⋯π interactions.
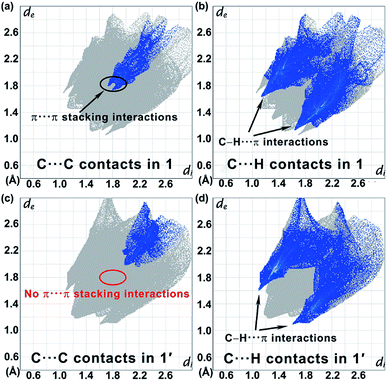 | ||
| Fig. 6 The 2D fingerprint plots of C⋯C contacts (a, c) and C⋯H contacts (b, d) for 1 (a, b) and its photoproduct 1′ (c, d). | ||
A regiospecific [2 + 2] photodimerization is also achieved during the 2 → 2′ process, which forms the rctt-2,4-tpcb-ht link. Interestingly, the Ag(I)–Npy bond between the Ag(I) ion and the 2-pyridyl unit in 2 is broken in 2′ (Ag⋯N = 3.15 Å at 300 K) (Fig. 3b), which should be resulted from the geometric restriction of rctt-2,4-tpcb-ht ligand. Moreover, it is found the pyridine rings of the bridging ligand is disordered with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio (Fig. S8b†). Similar to rctt-4-ppcb ligand in 1′, the dihedral angles between two adjacent pyridine units are changed to 46.9 and 45.1° at 300 K for two disordered parts (Table S6†), leading to edge-to-face π⋯π interactions between rctt-4-ppcb ligand (Fig. S9b†). Thus, a twofold-interpenetrated 3D framework with a pcu net is also formed in 2′ (Fig. S10e–h†). As far as we know, this represents the first example of a 3D → 3D SCSC photochemical transformation associated with bond breaking.
50 ratio (Fig. S8b†). Similar to rctt-4-ppcb ligand in 1′, the dihedral angles between two adjacent pyridine units are changed to 46.9 and 45.1° at 300 K for two disordered parts (Table S6†), leading to edge-to-face π⋯π interactions between rctt-4-ppcb ligand (Fig. S9b†). Thus, a twofold-interpenetrated 3D framework with a pcu net is also formed in 2′ (Fig. S10e–h†). As far as we know, this represents the first example of a 3D → 3D SCSC photochemical transformation associated with bond breaking.
Magnetic susceptibility measurements reveal that the SCO behaviors are dramatically modulated by the photochemical [2 + 2] cycloaddition reaction. The spin transition curve of 1′ is more gradual and occurs at a lower temperature compared to that of 1 (Fig. 1a). In contrast to the one-step hysteresis of 1, two-step hysteretic SCO (Tc1↓ = 190 K, Tc1↑ = 194 K, Tc2↓ = 166 K and Tc2↑ = 169 K) without an obvious plateau is observed in 1′. This is further confirmed by the DSC measurements (Tc1↓ = 193 K, Tc1↑ = 198 K, Tc2↓ = 165 K and Tc2↑ = 167 K, Fig. S2b†). For 2′, an incomplete, gradual and one-step SCO (Tc = 162 K) without a hysteretic loop is observed (Fig. 1b), which is dramatically different from the complete, abrupt, and two-step SCO with a hysteretic loop in 2. Therefore, not only the spin transition temperatures but also the character of the SCO can be effectively adjusted by the photochemical [2 + 2] cycloaddition reaction.
The average Fe–N distances of 1′ and 2′ at different temperatures are consistent with their magnetic states. Furthermore, the variation in the unit cell parameters of 1′ and 2′ are consistent with the magnetic curves (Fig. S20–S23†). Thus, the different SCO properties after the cycloaddition reaction are intrinsic to the electronic and structural properties of the materials rather than to the crystal lattice defects.52 At first glance, the Tc values of 1′ are shifted to lower temperatures. By considering the structural aspect, 1′ possesses the longer Fe–Nav distance (2.181 Å) and smaller Fe–N–C angle (164.4°) at 260 K than those in 1 (2.157 Å and 165.4°, Table S5†), which provide a smaller ligand field and tend to decrease the T1/2 value. By considering the electronic aspect, the π-accepting ability is decreased in 1′ when changing from an ethylene group to a cyclobutane ring, which results in a reduced ligand field to decrease the T1/2 value. Therefore, both the structural and electronic effects support the lower spin transition temperature in 1′. Meanwhile, the contribution to the SCO cooperativity from weaker π⋯π interactions after the cycloaddition reaction (Fig. 6 and Table S6†) outcompetes the formation of the flexible linker and results in the gradual SCO in 1′.24,53
For 2 → 2′, the average Fe–N distance decrease from 2.174 Å (185 K) to 2.145 Å (300 K) in the HS state, which hint a higher T1/2 value in 2′. Moreover, the breaking of the Ag–Npy bond after the cycloaddition reaction results in a more regular rhombic [Fe4{Ag(CN)2}4] grid (Fig. 3a–c). The Fe–N–C and C–Ag–C angles change from 164.7° and 158.2° in 2 at 185 K (HS state) to 168.8° and 175.9° in 2′ at 300 K (HS state) (Table S7†), respectively, which tend to increase the T1/2 value. However, the decreased π-accepting ability in 2′ when changing from an ethylene group to a cyclobutane ring results in a weaker ligand field and tend to decrease the T1/2 value. Therefore, competition between the electronic and structural effects results in the small change of T1/2 from 155 K (2) to 162 K (2′). As for the SCO cooperativity, the contribution from weaker π⋯π interactions outcompetes the formation of the flexible linker after the cycloaddition reaction. Moreover, the loss of the Ag–Npy bond also decreases the SCO cooperativity for 2 → 2′. Therefore, only an incomplete and gradual SCO behavior is observed in 2′.
Conclusions
In summary, for the first time, we successfully integrate photochemical [2 + 2] cycloaddition reaction into the SCO magnetic systems. After UV irradiation, a SCSC transformation from 2D interdigitated layers to a 3D interpenetrated structure is achieved in 1 → 1′. Meanwhile, a 3D → 3D photochemical transformation associated with Ag–Npy bonds breaking during the conversion from 2 to 2′ is firstly observed. Moreover, it is found the spin transition temperatures and characters, such as abruptness, hysteresis and steps, were obviously changed due to a series of structural and electronic perturbations caused by the dimerization reaction of photoreactive ligand, demonstrating the effective modulation of SCO behaviors. This work develops a new photochemical modulation mechanism for spin crossover materials by the [2 + 2] cycloaddition reaction, with high quantum efficiency, stable photoproducts and intact crystallinity. Such strategy not only extends the potential application of photochemical reactions in multifunction materials but also open up a new area in the designing of photo-responsive magnetic materials. Further work focused on improving performance, e.g. response speed and reversibility, of such photo-responsive SCO materials is on progress.Experimental
Materials and methods
All the reagents were commercially available and used as received. The solid UV-visible absorption data were collected in the range of 4000–400 nm on a Shimadzu UV-3600Plus UV/VIS/NIR spectrometer. The FT-IR spectra were recorded in KBr tablets in the range of 4000–400 cm−1 on a Thermo Nicolet AVATAR 330 FT-IR spectrometer. The powder XRD patterns were recorded on a Rigaku Smartlab X-ray diffractometer with CuKα (λ = 1.54178 Å) radiation. Thermogravimetric (TG) analyses were performed on a PerkinElmer TGA7 thermogravimetric analyzer in a N2 flow with a heating rate of 10 K min−1 from ambient temperature to 800 °C, with an empty Al2O3 crucible as reference. The C, H, and N microanalyses were performed for the dry crystals on an Elementar Vario-ELCHNS elemental analyzer. Differential Scanning Calorimetry (DSC) measurement was performed by cooling and heating the microcrystals sample in an aluminium crucible using a NETZSCH5, which was carried out at sweeping rate of 10 K min−1 under nitrogen. The 1H NMR spectra were recorded at ambient temperature on a Bruker advance III HD 400 spectrometer. 1H NMR chemical shifts were referenced to the solvent signal in DMSO-d6. The UV-irradiation experiments were conducted with a high-pressure mercury lamp with P = 500 W. Magnetic susceptibility measurements for the microcrystals samples (20–30 mg) were performed on a Quantum Design PPMS instrument operating in the range of 5–300 K and under a field of 1000 Oe. Diamagnetic correction was performed based on the Pascal's coefficients.1H NMR experiments
For the 1H NMR spectra measurement, the organic ligands in compounds 1, 2 and their photoproducts are extracted through a reported framework digestion approach36 with slight difference.20 mg samples were digested in 2 mL DMSO overnight to give a dark orange solution. The solution was layered onto CH2Cl2 (10 mL) and then washed with H2O for more than 5 times. The organic phase was separated and evaporated to give the solid, which was taken up in DMSO-d6 for analysis.
Synthesis
[Fe(4-spy)2{Ag(CN)2}2] (1) was prepared through a vial-in-vial slow diffusion technique. A solution of Fe(ClO4)2·6H2O (0.05 mmol, 13 mg) in EtOH (1 mL) was placed in a 2 ml vial and a mixture of spy (0.1 mmol, 18 mg) and KAg(CN)2 (0.1 mmol, 20 mg) in EtOH (1 mL) were placed in a 15 ml vial. The small vial was placed in the large one and then both of them were slowly filled with ethanol. Orange block crystals were collected after about two weeks. Yield: 63%. Anal. calcd for C30H22Ag2FeN6: C, 48.82; H, 3.00; N, 11.39. Found: C, 48.73; H, 3.11; N, 11.42.[Fe(2,4-bpe)2{Ag(CN)2}2] (2) was prepared in the same way as described in 1 except that 4-spy ligand was replaced by the 2,4-bpe ligand. Orange block crystals were collected after about two weeks. Yield: 76%. Anal. calcd for C28H20Ag2FeN8: C, 45.44; H, 2.72; N, 15.14. Found: C, 45.36; H, 2.78; N, 15.04.
[Fe(rctt-4-ppcb){Ag(CN)2}2] (1′) was obtained through the UV irradiation of 1 for 3 days under a high-pressure mercury lamp with P = 500 W, which was further confirmed by the SC-XRD structural analyses, PXRD patterns, IR spectra and 1H NMR spectra measurements. Anal. calcd for C30H22Ag2FeN6: C, 48.82; H, 3.00; N, 11.39. Found: C, 48.67; H, 2.93; N, 11.43.
[Fe(rctt-2,4-tpcb-ht){Ag(CN)2}2] (2′) was obtained through the UV irradiation of 2 for 4 days under a high-pressure mercury lamp with P = 500 W, which was further confirmed by the SC-XRD structural analyses, PXRD patterns, IR spectra and 1H NMR spectra measurements. Anal. calcd for C28H20Ag2FeN8: C, 45.44; H, 2.72; N, 15.14. Found: C, 45.56; H, 2.81; N, 15.22.
X-ray crystallography
Single-crystal diffraction data were recorded on a Bruker D8 QUEST diffractometer with MoKα (λ = 0.71073 Å) radiation in sequence at 260 K, 212 K, 120 K for 1, 260 K, 178 K, 120 K for 1′ and 300 K, 185 K, 90 K for 2′. Single-crystal diffraction data of 2 were recorded on a Rigaku XtaLAB Synergy R diffractometer with CuKα (λ = 1.54178 Å) radiation in sequence at 185 K, 155 K and 120 K. The crystal structures were solved by direct methods, and all non-hydrogen atoms were refined anisotropically by least-squares on F2 using the SHELXTL 2014/7 program.54 Hydrogen atoms on organic ligands were generated by the riding mode. The responses to the alerts from checkCIF are quoted within the validation response form. CCDC 1897816 (260 K), 1897817 (212 K), 1897818 (120 K), for 1, 1897822 (260 K), 1897823 (178 K), 1897824 (120 K), for 1′, 1897819 (185 K), 1897820 (155 K), 1897821 (120 K) for 2 and 1897825 (300 K), 1897826 (185 K), 1897827 (90 K) for 2′ contain the supplementary crystallographic data for this paper.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0306001), the NSFC (21773316, 21801258, 21821003, and 21771200), and the Pearl River Talent Plan of Guangdong (2017BT01C161).Notes and references
- O. Sato, Nat. Chem., 2016, 8, 644–656 CrossRef CAS.
- S. M. Cohen, Chem. Sci., 2010, 1, 32–36 RSC.
- Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC.
- S. M. Cohen, Chem. Rev., 2012, 112, 970–1000 CrossRef CAS.
- P. Deria, J. E. Mondloch, O. Karagiaridi, W. Bury, J. T. Hupp and O. K. Farha, Chem. Soc. Rev., 2014, 43, 5896–5912 RSC.
- I. G. Georgiev and L. R. MacGillivray, Chem. Soc. Rev., 2007, 36, 1239–1248 RSC.
- M. Nagarathinam, A. M. P. Peedikakkal and J. J. Vittal, Chem. Commun., 2008, 5277–5288 RSC.
- R. Medishetty, I.-H. Park, S. S. Lee and J. J. Vittal, Chem. Commun., 2016, 52, 3989–4001 RSC.
- Y.-C. Ou, D.-S. Zhi, W.-T. Liu, Z.-P. Ni and M.-L. Tong, Chem.–Eur. J., 2012, 18, 7357–7361 CrossRef CAS.
- Y.-C. Ou, W.-T. Liu, J.-Y. Li, G.-G. Zhang, J. Wang and M.-L. Tong, Chem. Commun., 2011, 47, 9384–9386 RSC.
- F.-L. Hu, Y. Mi, C. Zhu, B. F. Abrahams, P. Braunstein and J.-P. Lang, Angew. Chem., Int. Ed., 2018, 57, 12696–12701 CrossRef CAS.
- M. D. Cohen, G. M. J. Schmidt and F. I. Sonntag, J. Chem. Soc., 1964, 2000–2013 RSC.
- G. M. J. Schmidt, Pure Appl. Chem., 1971, 27, 647–678 CAS.
- A. Chanthapally, G. K. Kole, K. Qian, G. K. Tan, S. Gao and J. J. Vittal, Chem.–Eur. J., 2012, 18, 7869–7877 CrossRef CAS.
- A. Hazra, S. Bonakala, K. K. Bejagam, S. Balasubramanian and T. K. Maji, Chem.–Eur. J., 2016, 22, 7792–7799 CrossRef CAS.
- L.-F. Wang, J.-Z. Qiu, J.-L. Liu, Y.-C. Chen, J.-H. Jia, J. Jover, E. Ruiz and M.-L. Tong, Chem. Commun., 2015, 51, 15358–15361 RSC.
- L.-F. Wang, J.-Z. Qiu, Y.-C. Chen, J.-L. Liu, Q.-W. Li, J.-H. Jia and M.-L. Tong, Inorg. Chem. Front., 2017, 4, 1311–1318 RSC.
- G. S. Papaefstathiou, Z. Zhong, L. Geng and L. R. MacGillivray, J. Am. Chem. Soc., 2004, 126, 9158–9159 CrossRef CAS.
- K. M. Hutchins, T. P. Rupasinghe, L. R. Ditzler, D. C. Swenson, J. R. G. Sander, J. Baltrusaitis, A. V. Tivanski and L. R. MacGillivray, J. Am. Chem. Soc., 2014, 136, 6778–6781 CrossRef CAS.
- B. Dutta, A. Dey, C. Sinha, P. P. Ray and M. H. Mir, Inorg. Chem., 2018, 57, 8029–8032 CrossRef CAS PubMed.
- P. Gütlich, Y. Garciaa and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419–427 RSC.
- E. Konig, G. Ritter and S. K. Kulshreshtha, Chem. Rev., 1985, 85, 219–234 CrossRef CAS.
- H. Spiering, E. Meissner, H. Köppen, E. W. Müller and P. Gütlich, Chem. Phys., 1982, 68, 65–71 CrossRef CAS.
- M. A. Halcrow, Chem. Soc. Rev., 2011, 40, 4119–4142 RSC.
- M. C. Muñoza and J. A. Real, Coord. Chem. Rev., 2011, 255, 2068–2093 CrossRef.
- Z.-P. Ni, J.-L. Liu, M. N. Hoque, W. Liu, J.-Y. Li, Y.-C. Chen and M.-L. Tong, Coord. Chem. Rev., 2017, 335, 28–43 CrossRef CAS.
- W. Liu, Y.-Y. Peng, S.-G. Wu, Y.-C. Chen, M. N. Hoque, Z.-P. Ni, X.-M. Chen and M.-L. Tong, Angew. Chem., Int. Ed., 2017, 56, 14982–14986 CrossRef CAS.
- M. Ohba, K. Yoneda, G. Agustí, M. C. Muñoz, A. B. Gaspar, J. A. Real, M. Yamasaki, H. Ando, Y. Nakao, S. Sakaki and S. Kitagawa, Angew. Chem., Int. Ed., 2009, 48, 4767–4771 CrossRef CAS.
- P. D. Southon, L. Liu, E. A. Fellows, D. J. Price, G. J. Halder, K. W. Chapman, B. Moubaraki, K. S. Murray, J.-F. Létard and C. J. Kepert, J. Am. Chem. Soc., 2009, 131, 10998–11009 CrossRef CAS.
- E. Coronado, M. Giménez-Marqus, G. M. Espallargas, F. Rey and I. J. Vitórica-Yrezábal, J. Am. Chem. Soc., 2013, 135, 15986–15989 CrossRef CAS.
- G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray and J. D. Cashion, Science, 2002, 298, 1762–1765 CrossRef CAS.
- J.-Y. Ge, Z. Chen, L. Zhang, X. Liang, J. Su, M. Kurmoo and J.-L. Zuo, Angew. Chem., Int. Ed., 2019, 58, 8789–8793 CrossRef CAS.
- X. Bao, H. J. Shepherd, L. Salmon, G. Molnar, M. L. Tong and A. Bousseksou, Angew. Chem., Int. Ed., 2013, 52, 1198–1202 CrossRef CAS.
- G. Agustí, R. Ohtani, K. Yoneda, A. B. Gaspar, M. Ohba, J. F. Sánchez-Royo, M. C. Muñoz, S. Kitagawa and J. A. Real, Angew. Chem., Int. Ed., 2009, 48, 8944–8947 CrossRef.
- R. Ohtani, K. Yoneda, S. Furukawa, N. Horike, S. Kitagawa, A. B. Gaspar, M. C. Muñoz, J. A. Real and M. Ohba, J. Am. Chem. Soc., 2011, 133, 8600–8605 CrossRef CAS.
- J. E. Clements, J. R. Price, S. M. Neville and C. J. Kepert, Angew. Chem., Int. Ed., 2014, 53, 10164–10168 CrossRef CAS PubMed.
- M. M. Khusniyarov, Chem.–Eur. J., 2016, 22, 15178–15191 CrossRef CAS.
- B. Brachňaková and I. Šalitroš, Chem. Pap., 2018, 72, 773–798 CrossRef.
- M.-L. Boillot, C. Roux, J.-P. Audière, A. Dausse and J. Zarembowitch, Inorg. Chem., 1996, 35, 3975–3980 CrossRef CAS.
- M.-L. Boillot, S. Pillet, A. Tissot, E. Rivière, N. Claiser and C. Lecomte, Inorg. Chem., 2009, 48, 4729–4736 CrossRef CAS.
- Y. Hasegawa, K. Takahashi, S. Kume and H. Nishihara, Chem. Commun., 2011, 47, 6846–6848 RSC.
- Y. Hasegawa, S. Kume and H. Nishihar, Dalton Trans., 2009, 280–284 RSC.
- B. Rçsner, M. Milek, A. Witt, B. Gobaut, P. Torelli, R. H. Fink and M. M. Khusniyarov, Angew. Chem., Int. Ed., 2015, 54, 12976–12980 CrossRef.
- M. Estrader, J. S. Uber, L. A. Barrios, J. Garcia, P. Lloyd-Williams, O. Roubeau, S. J. Teat and G. A. Aromí, Angew. Chem., Int. Ed., 2017, 56, 15622–15627 CrossRef CAS.
- M. Nihei, Y. Suzuki, N. Kimura, Y. Kera and H. Oshio, Chem.–Eur. J., 2013, 19, 6946–6949 CrossRef CAS.
- Z.-Y. Li, J.-W. Dai, M. Damjanović, T. Shiga, J.-H. Wang, J. Zhao, H. Oshio, M. Yamashita and X.-H. Bu, Angew. Chem., Int. Ed., 2019, 58, 4339–4344 CrossRef CAS.
- V. A. Blatov, Struct. Chem., 2012, 23, 955–963 CrossRef CAS.
- E. V. Alexandrov, V. A. Blatov, A. V. Kochetkova and D. M. Proserpio, CrystEngComm, 2011, 13, 3947–3958 RSC.
- G. Agustí, A. B. Gaspar, M. C. Muñoz, P. G. Lacroix and J. A. Real, Aust. J. Chem., 2009, 62, 1155–1165 CrossRef.
- M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, P. R. Spackman, D. Jayatilaka and M. A. Spackman, Crystal Explorer 17, University of Western Australia, 2017 Search PubMed.
- J. J. McKinnon, M. A. Spackmana and A. S. Mitchell, Acta Crystallogr., Sect. B: Struct. Sci., 2004, 60, 627–668 CrossRef.
- M. S. Haddad, W. D. Federer, M. W. Lynch and D. N. Hendrickson, Inorg. Chem., 1981, 20, 131–139 CrossRef CAS.
- J. A. Real, A. B. Gaspar, V. Niel and M. C. Muñoz, Coord. Chem. Rev., 2003, 236, 121–141 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1897816–1897818, 1897822–1897824, 1897819–1897821 and 1897825–1897827. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc02274k |
| This journal is © The Royal Society of Chemistry 2019 |