Na3V2(PO4)2F3–SWCNT: a high voltage cathode for non-aqueous and aqueous sodium-ion batteries†
Shuang
Liu
a,
Liubin
Wang
a,
Jian
Liu
a,
Meng
Zhou
a,
Qingshun
Nian
a,
Yazhi
Feng
a,
Zhanliang
Tao
 *a and
Lianyi
Shao
*b
*a and
Lianyi
Shao
*b
aKey Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin, 300071, P. R. China. E-mail: taozhl@nankai.edu.cn
bSchool of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, Guangdong, P. R. China. E-mail: shaolianyi@gdut.edu.cn
First published on 22nd November 2018
Abstract
Due to the merits of low cost, safety, environmental friendliness, and abundant sodium reserves, non-aqueous and aqueous sodium-ion batteries are wonderful alternatives for large-scale energy storage. Na+ super ionic conductor structured Na3V2(PO4)2F3 is considered as a potential high-capacity cathode material for Na-ion batteries. However, its insufficient cyclability remains a challenge for battery applications. In addition, the narrow electrochemical stability window (1.23 V) of aqueous electrolytes does not allow us to make the best use of it. All of these pose obstacles to the practical voltage and energy output. Therefore, we designed and synthesized a Na3V2(PO4)2F3–SWCNT composite, which demonstrated superior Na+-storage performance with a high reversible capacity of 117 mA h g−1 at a moderate current of 0.5C in non-aqueous electrolyte. In addition, we developed an aqueous rechargeable sodium ion battery using Na3V2(PO4)2F3–SWCNT as the cathode and NaTi2(PO4)3–MWCNT as the anode, and this battery can deliver a high energy density of 150 W h kg−1 with an operating voltage of up to 2.0 V by using the high concentration electrolyte, 17 m NaClO4. The outstanding electrochemical performance makes Na3V2(PO4)2F3–SWCNT a promising cathode for Na+ storage and encourages more investigations into practical sodium-ion battery applications.
Introduction
Despite the great success of lithium-ion batteries (LIBs) in portable electronics and electric vehicles in recent years, the insufficient reserves, unbalanced distribution as well as increasing cost of lithium resources restrict their practical application in large-scale electrical energy storage systems.1–3 Sodium-ion batteries (SIBs) are considered as an excellent alternative to LIBs due to the natural abundant reserves of sodium.4–6 However, Na+ is about 42% larger in ionic radius than Li+, which makes it difficult to find suitable host materials that are capable of accommodating Na+ and allowing reversible and rapid Na+ insertion and extraction.7,8 Three-dimensional (3D) host framework structured materials could provide enough interstitial channels for Na+ transit and permit the reversible intercalation/de-intercalation of large Na+, and have attracted great interest for SIBs in the past years.9–13 Among them, Na+ super ionic conductor (NASICON) structured Na3V2(PO4)2F3 has attracted extensive attention for its high potential plateaus (3.6 V/4.1 V),13 good specific capacity (128 mA h g−1), and high thermal stability. However, its poor electronic conductivity (∼10−12 S cm−1) usually causes low utilization of active material and restricts high-rate performance. Constructing nanosized particles or conductive surface coatings, such as by nanocrystallization, particle formation from liquid dispersion systems, and particle coating, can effectively shorten the ionic transfer pathway and increase electronic conductivity, hence realizing a high electrochemical performance.14–16 In addition, compared with expensive and flammable organic liquid electrolytes, aqueous electrolytes have advantages such as low cost, safety, and environmental friendliness. Therefore, aqueous rechargeable sodium-ion batteries (ARSBs) have received increasing attention in the field of electrochemical energy storage (EES) technology and represent a promising alternative in future power grids.17,18In recent years, various ARSBs have been reported. Unfortunately, they are limited by the narrow electrochemical stability window (1.23 V) of the aqueous electrolyte, which restricts the output voltage of ARSBs to under 1.50 V and leads to compromised energy densities,19,20 for instance, Na0.66[Mn0.66Ti0.34]O2//NaTi2(PO4)3 (1.0 V),21 Alizarin//PPy (1.06 V),22 Na0.44MnO2//NaTi2(PO4)3 (1.1 V),23 NaVPO4F//Polyimide (1.3 V),24 Na3MnTi(PO4)3//Na3MnTi(PO4)3 (1.4 V),25 Na2NiFe(CN)6//NaTi2(PO4)3 (1.4 V),26 Na3V2O2(PO4)2F–SWCNT//NaTi2(PO4)3–MWCNT (1.5 V),27etc. For ARSBs, increasing the voltage is more effective than increasing the capacity of the electrode material in terms of enhancing the energy density, since the electrode materials only account for about 30% of the total weight of the assembled complete cell.28 Therefore, expanding the electrochemical stability window of the aqueous electrolyte, eliminating the O2 in it and suppressing the water activity are of vital importance. Several approaches have been developed to resolve these problems; for example, increasing the concentration of the electrolyte enlarges the potential window, such as 9.26 m sodium trifluoromethane sulfonate aq. (NaCF3SO3, or NaOTf, 2.5 V),21 and 17 m NaClO4 aq. (2.8 V),29 where m represents molality (molality [m] = moles of solute/weight of solvent [mol kg−1]). Modification with a surfactant such as by the addition of sodium dodecyl sulfate (SDS) to the dilute aqueous electrolyte expanded the window to about 2.5 V.30 These strategies, especially the use of high-concentration electrolytes, can significantly improve the performance of ARSBs.
In this work, we synthesize Na3V2(PO4)2F3–single walled carbon nanotube (Na3V2(PO4)2F3–SWCNT) composites through a simple solvothermal method at low temperature, and the composites demonstrate superior Na+-storage performance with a high reversible capacity of 117 mA h g−1 at a moderate current of 0.5C in non-aqueous electrolyte. In addition, a high voltage 2.0 V ARSB full-cell is assembled using Na3V2(PO4)2F3–SWCNT composites as the cathode, highly stable NaTi2(PO4)3 microblock-multi walled carbon nanotube (NaTi2(PO4)3–MWCNT) composites as the anode, and 17 m NaClO4 aq. as the electrolyte, which demonstrates the great potential of the ARSBs for grid-scale energy storage systems.
Experimental
Preparation of the Na3V2(PO4)2F3–SWCNT composites
For the preparation of Na3V2(PO4)2F3–SWCNT composites, 0.434 mmol vanadium(III) 2,4-pentanedionate (C15H21O6V) (98%, energy) is added to 12 mL ethanol solution and stirred for 1 h. At the same time, 0.651 mmol sodium dihydrogen phosphate (NaH2PO4·2H2O) (99%, energy) is dissolved in 4 mL water, and then 0.72 mmol sodium fluoride (NaF) (99%, Meryer) is added into it. After they are simultaneously stirred at room temperature for 0.5 h, a certain amount of SWCNT (0.5 mg mL−1) aqueous solution is added to the latter aqueous solution and stirred for 0.5 h. The two solutions are transferred to a 100 mL polytetrafluoroethylene-lined hydrothermal autoclave and reacted at 120 °C for 10 h, and then the product is filtered and washed with ethanol and water repeatedly, dried in a vacuum oven at 80 °C, and collected. To synthesize products with different SWCNT content, different amounts of SWCNT aqueous solution (0.5 mg mL−1), where the quantities of SWCNTs are 5 wt%, 12.5 wt% and 20 wt% based on the yield of bare Na3V2(PO4)2F3, are added into the mixed solution of NaH2PO4·2H2O and NaF; all other operations are the same as above. A slight excess of NaF is employed instead of the stoichiometric amount in order to guarantee that the vanadium precursor can react completely. The procedure for preparing bare Na3V2(PO4)2F3 is the same except for the addition of SWCNTs. The formation of Na3V2(PO4)2F3 can be explained according to the following chemical reaction (formula (1)):| 2V(C5H7O2)3 + 3NaH2PO4 + 3NaF → Na3V2(PO4)2F3 + 6C5H8O2 + Na3PO4 | (1) |
Preparation of the NaTi2(PO4)3–MWCNT composites
NaTi2(PO4)3–MWCNT composites are prepared by the solid state method. In a typical synthesis, stoichiometric amounts of NaH2PO4·2H2O (1.02 mmol, Aladdin), TiO2 (2 mmol, 99.8% metals basis, 5–10 nm, Aladdin), and (NH4)2HPO4 (1.96 mmol, 99.9%, Aladdin), mixed with 10 wt% multi-walled carbon nanotubes (MWCNTs, CNano Technology Ltd.) based on the theoretical yield of NaTi2(PO4)3 are ball-milled in a planetary ball mill (PM 200) using zirconia milling media in ethanol at 400 rpm for 12 h, followed by drying at 80 °C in a vacuum. The precursor is pre-sintered at 400 °C for 2 h in a flowing argon atmosphere, and then reground in an agate mortar. A final heat treatment is done at 850 °C for 12 h in a flowing argon atmosphere as well.Characterization
The structure of the as-prepared samples is characterized by powder X-ray diffraction (XRD, Rigaku Mini Flex 600 X-ray generator, Cu Kα radiation, λ = 1.5406 Å) at a scanning rate of 4° min−1. The morphology of the as-prepared products is examined by field-emission scanning electron microscopy (SEM, JEOL JSM-6700F Field Emission, operating at 5 kV) and high-resolution transmission electron microscopy (HRTEM, JEOL JEM-2010 FEF TEM, operating at 200 kV) coupled with energy dispersive spectroscopy (EDS). The crystal structure is obtained using a confocal Raman microscope (DXR, Thermo-Fisher Scientific, 532 nm excitation from an argon-ion laser), and a Fourier transform infrared spectrometer (FTIR, Bruker Tensor II). The electronic states of the samples are investigated by X-ray photoelectron spectroscopy (XPS, Perkin Elmer PHI 1600 ESCA). The content of carbon is measured using an elemental analyzer (EA, Vario EL CUBE). The specific surface area is measured using Brunauer–Emmett–Teller (BET) nitrogen adsorption–desorption measurements (BELSORP-mini instrument) at 77 K. The porosity is estimated from the adsorption isotherms by the Barrett–Joyner–Halenda (BJH) method.Electrochemical measurements
The cathode, anode and SWCNT electrodes are all fabricated by mixing 70 wt% of the active material with 20 wt% of Super P and 10 wt% polyvinylidene difluoride (PVDF) in a N-methylpyrrolidone (NMP) solvent to make a slurry.For the non-aqueous electrolyte tests, the obtained Na3V2(PO4)2F3–SWCNT slurry is coated on Al foil, dried at 80 °C for 12 h in a vacuum oven and cut into circular electrodes with diameters of 10 mm. The loading is ∼0.3 mg cm−2. CR2032 coin cells are assembled in an argon-filled dry glove box (with less than 0.1 ppm of H2O and O2) using sodium metal as the counter electrode and reference electrode, borosilicate glass fibers (Whatman GF/D) as the separator, and 1 M NaPF6 in propylene carbonate (PC) with 5 vol% of fluoroethylene carbonate (FEC) as the electrolyte.
For the aqueous electrolyte tests, the prepared Na3V2(PO4)2F3–SWCNT and NaTi2(PO4)3–MWCNT slurries are respectively coated on carbon paper and titanium foil that are 10 mm in diameter. The loading for the Na3V2(PO4)2F3–SWCNT and NaTi2(PO4)3–MWCNT electrodes is ∼0.52 and ∼0.38 mg cm−2, respectively. Both the as-prepared electrodes are dried at 80 °C for 12 h in a vacuum oven. The electrochemical properties of the electrodes are obtained using a three electrode system, where platinum foil is used as the counter electrode, 17 m NaClO4 aq. (purged with Ar before use) is used as the electrolyte, and an Ag/AgCl electrode (0.2412 V vs. standard hydrogen electrode) is used as the reference electrode. The performance of the Na3V2(PO4)2F3–SWCNT//NaTi2(PO4)3–MWCNT full-cell is tested using CR2032 coin cells, which are assembled in an argon-filled dry glove box (with less than 0.1 ppm of O2).
Cyclic voltammograms (CVs) are recorded using a Parstat 263A electrochemical workstation (AMETEK). Galvanostatic charge/discharge tests are performed on a LAND battery-testing instrument (CT2001A). Electrochemical impedance spectroscopy (EIS) measurements are carried out using a Parstat 2273 electrochemical workstation over a frequency range from 2 Hz to 100 kHz and at an AC amplitude of 5 mV. All electrochemical tests are performed at room temperature.
Results and discussion
The Na3V2(PO4)2F3–SWCNT composites are synthesized by a simple solvothermal route. By adjusting the mass ratio of SWCNTs, three products with different additive amounts of SWCNTs are obtained. The elemental analysis results (Fig. 1a) suggest that the carbon content is 9.56, 13.45, and 54.61 wt%, respectively. The three samples with different carbon content are denoted as Na3V2(PO4)2F3–SWCNT-1, Na3V2(PO4)2F3–SWCNT-2, and Na3V2(PO4)2F3–SWCNT-3, respectively.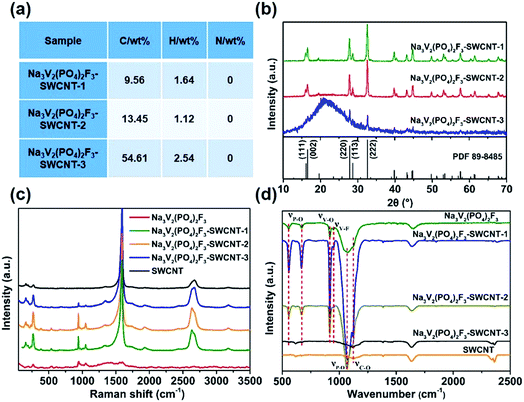 | ||
| Fig. 1 (a) CHN content, (b) XRD spectra, (c) Raman spectra, and (d) FTIR spectra of Na3V2(PO4)2F3, Na3V2(PO4)2F3–SWCNT serial samples, and SWCNTs. | ||
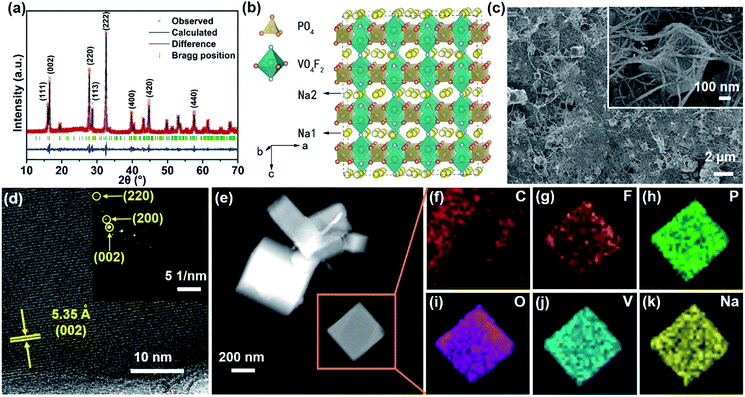
The structure of Na3V2(PO4)2F3–SWCNT is confirmed by the XRD technique. As shown in Fig. 1b, all the peaks are readily assigned to the space group P42/mnm (no. 136), being consistent with the standard values of the tetragonal Na3V2(PO4)2F3 (JCPDS no. 89-8485). Five main peaks appearing at 16.12°, 16.52°, 27.76°, 28.68° and 32.54° can be perfectly matched to the (111), (002), (220), (113) and (222) crystal planes, respectively.31,32 Furthermore, it is worth noting that the (111), (002), (220) and (113) characteristic peaks are obviously sharp for Na3V2(PO4)2F3–SWCNT-1 and Na3V2(PO4)2F3–SWCNT-2, compared with those of the bare Na3V2(PO4)2F3 (Fig. S1a†), indicating that the SWCNTs significantly increase the crystallinity of the materials. In addition, the intensities of the (111), (002), (220) and (113) characteristic peaks of Na3V2(PO4)2F3–SWCNT-3 decrease evidently, indicating a decreased crystallinity and the negative impact of excessive SWCNTs on Na3V2(PO4)2F3. All these results indicate that SWCNTs can not only contribute to the formation of a pure phase, but can also be used as seed crystals to improve the crystallinity of the material.
This phenomenon can also be verified from the SEM results. Bare Na3V2(PO4)2F3 exhibits low crystallinity with the agglomeration of particles (Fig. S1b†). However, things are quite different when it is combined with SWCNTs. In Na3V2(PO4)2F3–SWCNT-1 (Fig. S2a and b†), there are hexahedral blocks which are connected by a small amount of threadlike SWCNTs, while in Na3V2(PO4)2F3–SWCNT-2 (Fig. S2c and d†), large sized cuboids appear. Moreover, the cuboids are wrapped and linked with each other by a SWCNT network, which would be quite helpful in increasing the electronic conductivity; however, for Na3V2(PO4)2F3–SWCNT-3 (Fig. S2e and f†), individual SWCNT bundles can be observed due to the poor dispersibility of the excessive SWCNTs. The particles and SWCNTs are randomly mixed without strong interactions, and deposited separately from each other. As a result, the aggregation of Na3V2(PO4)2F3 particles could not be avoided.33 All of these results demonstrate the importance of the SWCNT content in crystal formation. Considering its regular structure and Na3V2(PO4)2F3 content, the Na3V2(PO4)2F3–SWCNT-2 composite is chosen for further study.
The Raman spectra are analyzed to further characterize the as-prepared samples. Fig. 1c displays the Raman spectra of the three samples. The band at 945 cm−1 is assigned to the P–O symmetrical stretching vibration of PO4, and the band at 1050 cm−1 is attributed to the P–O asymmetrical stretching vibration of PO4.34–37 The bands observed below 400 cm−1 (269 cm−1 and 192 cm−1) are the result of the external modes of PO4.34,35 Besides, the characteristic bands of SWCNTs (Radial Breathing Mode (RBM) band of 158.8 cm−1, D band of 1345 cm−1, G band of 1590 cm−1 and 2D band of 2632 cm−1) can be observed. Similar results can be verified from the FTIR spectra of Na3V2(PO4)2F3–SWCNT (Fig. 1d). The band at 1072 cm−1 belongs to the asymmetrical stretching vibration of the PO4 tetrahedron. The bands at 674 cm−1 and 560 cm−1 are due to the stretching vibration mode and symmetrical bending vibration of P–O, respectively. Bands appearing at 912 cm−1 and 955 cm−1 correspond to the stretching vibration mode of V–O and V–F.38–41 On the FTIR curve of SWCNTs, the band at 1128 cm−1 represents the tensile vibration of the C–O bond.42–44 The above characteristic FTIR peaks can be observed on all the FTIR curves of the Na3V2(PO4)2F3–SWCNT series products. Combining the results of Raman and FTIR spectroscopy, it can be concluded that all the as-prepared samples possess the structure of Na3V2(PO4)2F3, and Na3V2(PO4)2F3 and SWCNTs have been well combined in the solvothermal process.
In order to further explore the structural properties of Na3V2(PO4)2F3–SWCNT, refinement of Na3V2(PO4)2F3–SWCNT-2 is performed as shown in Fig. 2a. All the diffraction peaks can be readily indexed to the NASICON structure in the single phase P42/mnm (no. 136) space group, which indicates the high purity of the prepared material. The lattice parameters are calculated as a = b = 9.047 Å and c = 10.705 Å (Rp = 12.96%, Rwp = 9.28%). The crystal structure of Na3V2(PO4)2F3–SWCNT-2 is illustrated in Fig. 2b. The 3D framework structure of Na3V2(PO4)2F3 can be described in terms of [V2O8F3] bi-octahedral and [PO4] tetrahedral units as illustrated in Fig. 2b.45 The [V2O8F3] bi-octahedron is bridged with two [VO4F2] octahedral units by one fluorine atom, whereas the oxygen atoms are all interconnected through the [PO4] units. Two different interstitial sites (Na1 and Na2) are occupied by sodium ions. The sodium ions are statistically distributed in the network where the NASICON framework grants Na ions significant mobility. This property confers to Na3V2(PO4)2F3 the ability to intercalate/deintercalate Na+ reversibly, making it a favorable positive electrode material choice for SIBs.45–47
Fig. 2c shows the SEM images of the Na3V2(PO4)2F3–SWCNT-2 composites. The prepared materials have a nanocube-like morphology with a height of 0.5–1 μm and width and length of 300–500 nm, and these cubes are well dispersed in the network of SWCNTs. The typical HRTEM image of Na3V2(PO4)2F3–SWCNT-2 in Fig. 2d displays clear and continuous lattices with a d-spacing of 5.35 Å corresponding to the (002) facets, indicating the high crystallinity of the Na3V2(PO4)2F3–SWCNT-2 sample. The SAED pattern in the inset of Fig. 2d can be indexed to the (002), (200), and (220) planes, which are attributed to the tetragonal phase of Na3V2(PO4)2F3–SWCNT-2. The elemental maps (Fig. 2e–k) show the uniform distribution of C, F, P, O, V, and Na through the whole nanocube. Furthermore, the N2 adsorption/desorption isotherms of Na3V2(PO4)2F3–SWCNT-2 show typical type-IV isotherms in Fig. S3.† The specific surface area of Na3V2(PO4)2F3–SWCNT-2 is calculated to be 69.3 m2 g−1 based on the BET method. The pore size distribution derived via the BJH method is in the range of 7–22 nm, as shown in the inset of Fig. S3.†
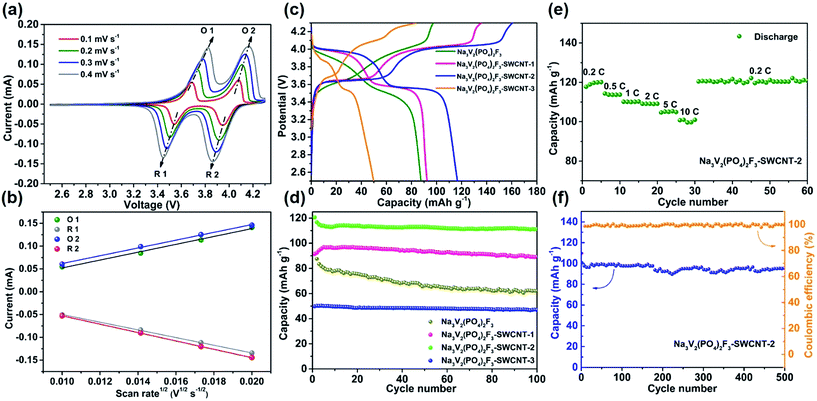
To evaluate the electrochemical properties of Na3V2(PO4)2F3–SWCNT, a series of half cells (Na3V2(PO4)2F3–SWCNT-2//Na) have been tested. All the capacities in this work are calculated based on the mass of the Na3V2(PO4)2F3–SWCNT-2 composites, although the SWCNTs have a limited capacity (ca. 12 mA h g−1) (Fig. S4†). Fig. 3a shows the CV results at different scan rates from 0.1 to 0.4 mV s−1 in the potential range between 2.5 and 4.3 V vs. Na+/Na. There are two cathodic peaks (located at 3.54 and 3.95 V of 0.1 mV s−1) and two anodic peaks (near 4.08 and 3.7 V of 0.1 mV s−1), corresponding to the redox reaction of V3+/V4+ with extraction/insertion of two sodium ions. As the scan rate increases, the height and the area of the CV curve increase due to the constant capacity of the electrode. The peak current ratio of the anodic and cathodic peak appears to be unity, i.e., |ipc/ipa| = 1, indicating the excellent reversibility of the sodium insertion and extraction. The profiles between ip and ν1/2 based on the data in Fig. 3a are shown in Fig. 3b. The excellent linear fitting results of ipvs. ν1/2 confirm the diffusion-controlled behavior of the electrode reactions in Na3V2(PO4)2F3–SWCNT-2.48,49 The sodium ion diffusion coefficient can be calculated from the Randles–Sevcik formula:50,51
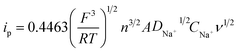 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), R is the molar gas constant (8.314 J K−1 mol−1), T is the temperature in our experiment (298 K), n is the number of electrons per molecule taking part in the electron transfer reaction (n = 2), A is the electrode area (here the geometric area of the electrode, 0.785 × 10−4 m2, is used for simplicity48), CNa+ is the concentration of Na+ in the electrode (9.1 mol L−1), DNa+ is the diffusion coefficient, and ν is the scan rate referring to peaks O1, R1, O2, and R2, respectively. Therefore, the apparent Na+ diffusion coefficient (DNa+) can be calculated to be 2.52 × 10−12, 2.40 × 10−12, 2.48 × 10−12, and 2.86 × 10−12 cm2 s−1, respectively, according to eqn (2). It is worth noting that the presently calculated DNa+ values of Na3V2(PO4)2F3–SWCNT are higher than those of other polyanion type phosphate cathodes for SIBs,32,52,53 and are significantly higher than those of LiFePO4 measured by Prosini et al. (10−16 to 10−14 cm2 s−1)54 and Franger et al. (10−14 to 10−13 cm2 s−1).55 This high DNa+ indicates the excellent diffusivity of the NASICON-type structure of Na3V2(PO4)2F3, allowing Na+ ion transport in an open 3D framework and resulting in a high ion diffusion capability.
485 C mol−1), R is the molar gas constant (8.314 J K−1 mol−1), T is the temperature in our experiment (298 K), n is the number of electrons per molecule taking part in the electron transfer reaction (n = 2), A is the electrode area (here the geometric area of the electrode, 0.785 × 10−4 m2, is used for simplicity48), CNa+ is the concentration of Na+ in the electrode (9.1 mol L−1), DNa+ is the diffusion coefficient, and ν is the scan rate referring to peaks O1, R1, O2, and R2, respectively. Therefore, the apparent Na+ diffusion coefficient (DNa+) can be calculated to be 2.52 × 10−12, 2.40 × 10−12, 2.48 × 10−12, and 2.86 × 10−12 cm2 s−1, respectively, according to eqn (2). It is worth noting that the presently calculated DNa+ values of Na3V2(PO4)2F3–SWCNT are higher than those of other polyanion type phosphate cathodes for SIBs,32,52,53 and are significantly higher than those of LiFePO4 measured by Prosini et al. (10−16 to 10−14 cm2 s−1)54 and Franger et al. (10−14 to 10−13 cm2 s−1).55 This high DNa+ indicates the excellent diffusivity of the NASICON-type structure of Na3V2(PO4)2F3, allowing Na+ ion transport in an open 3D framework and resulting in a high ion diffusion capability.
To clarify the Na intercalation/extraction processes of Na3V2(PO4)2F3–SWCNT as a cathode material for SIBs, the galvanostatic charge/discharge curves and cycle life curves are investigated. Fig. 3c indicates the typical charge/discharge profiles of the Na3V2(PO4)2F3, Na3V2(PO4)2F3–SWCNT-1, Na3V2(PO4)2F3–SWCNT-2, and Na3V2(PO4)2F3–SWCNT-3 electrodes at a current rate of 0.5C (1C = 128 mA g−1) between 2.5 and 4.3 V vs. Na+/Na in the first cycle. The cells exhibit charge/discharge capacities of 97.4/87.6, 135.9/92.4, 160.6/116.7 and 83.5/49.8 mA h g−1, respectively. It can be concluded that the specific capacity of the materials composited with a certain amount of SWCNTs is greatly improved compared with that of the Na3V2(PO4)2F3 electrode under the same conditions. Note that the discharge capacity of Na3V2(PO4)2F3–SWCNT-2 reached 117 mA h g−1, which is very close to the theoretical capacity of Na3V2(PO4)2F3 (128 mA h g−1), indicating a full use of the material for the Na+-storage reaction. The cycling performance of the Na3V2(PO4)2F3–SWCNT serial samples is given in Fig. 3d. It is found that Na3V2(PO4)2F3–SWCNT-2 has the highest specific capacity and good cycling stability. At the 100th cycle, the capacity can be maintained at 111.4 mA h g−1 with a capacity retention of 92.4%, demonstrating the good stability of this material. Therefore, the following electrochemical properties are studied using Na3V2(PO4)2F3–SWCNT-2. In addition, the rate performances are further investigated at different current rates ranging from 0.2 to 10C. As shown in Fig. 3e, Na3V2(PO4)2F3–SWCNT-2 delivers reversible capacities of 117.7, 114.3, 110.2, 109.1, 104.7, 100.7 and 120 mA h g−1 at current densities of 0.2, 0.5, 1, 2, 5, and 10C, respectively. In particular, when the current density returns to 0.2C after 30 cycles, the specific capacity nearly recovers to the same extent as observed in the initial five cycles, indicating an excellent reversibility even at high-rate charge/discharge. The cycle stability of Na3V2(PO4)2F3–SWCNT-2 is also recorded for 500 cycles and shown in Fig. 3f with a capacity retention of 96% at 10C. Additionally, when the loading mass of Na3V2(PO4)2F3–SWCNT-2 is more than 1 mg cm−2, the electrochemical performance is still comparable with that of lower loading mass (∼0.3 mg cm−2). The excellent capability of the Na3V2(PO4)2F3–SWCNT-2 composites can be attributed to their 3D framework with large tunnels as well as nanoscale morphology, which enables rapid sodium-ion extraction/insertion.46,49
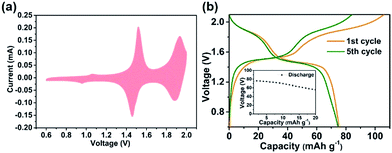
It has been reported that Na3V2(PO4)2F3 only allows one Na+ insertion/extraction in aqueous solution.22,56–58 In theory, when the number of sodium ions that can be stored doubles, the full battery's energy density can be increased by 2.67 times (based on the mass of cathode). Therefore, it is especially important to make full use of the high potential platform in aqueous electrolyte for the development of EES. Fig. 4 shows the electrochemical properties of the Na3V2(PO4)2F3–SWCNT-2 composites in 17 m NaClO4 aq. The CV plot for the Na3V2(PO4)2F3–SWCNT composites is shown in Fig. 4a. Two pairs of charge/discharge plateaus appearing at 1.21/1.1 V and 0.82/0.71 V vs. Ag/AgCl are clearly observed in the first cycle, which are reversible Na+ ion extraction and insertion from/into the structure. Moreover, the oxygen evolution potential which is located at 1.38 V vs. Ag/AgCl is completely separated from the redox potential, which implies the possibility of high potential in 17 m NaClO4 aq. The charge/discharge voltage profiles for different cycles are shown in Fig. 4b. The initial charge and discharge capacities are 92.4 and 81.3 mA h g−1, corresponding to the first coulombic efficiency of 87.98%. This low coulombic efficiency is attributed to the formation of a solid-state interface (SEI) film caused by the irreversible interfacial storage. In addition, after 60 cycles, the capacity decreases to 58 mA h g−1 with a retention of 71.3% of the capacity in the first cycle (Fig. S5†). The XRD patterns (Fig. S6†) and SEM images (Fig. S7a and b†) for the pristine Na3V2(PO4)2F3–SWCNT-2 electrode before cycling and the Na3V2(PO4)2F3–SWCNT-2 electrode after 60 cycles show the structural degradation of Na3V2(PO4)2F3–SWCNT. Details are discussed in the ESI.† The redox reaction in the cathode and anode, and the formation of the SEI in the cathode can be verified by XPS.
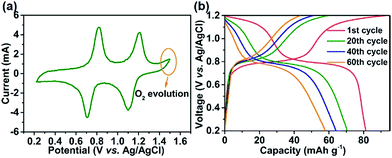 | ||
| Fig. 4 (a) CVs obtained at 2 mV s−1, and (b) charge/discharge curves at 1C (1C = 128 mA g−1) for the Na3V2(PO4)2F3–SWCNT-2 composite in 17 m NaClO4 aq. | ||
Fig. S8† shows the XPS spectra of the V element of the Na3V2(PO4)2F3–SWCNT electrode and the Ti element of the NaTi2(PO4)3–MWCNT electrode at selected pristine, charged and discharged states during the initial cycle. These valence changes indicate a normal redox process. Details are discussed in the ESI.† It's generally known that the SEI film is mainly composed of C and F elements. To investigate the surface change of Na3V2(PO4)2F3–SWCNT after cycling, comparisons of the XPS spectra of C1s and F1s at pristine, fully charged 1.2 V vs. Ag/AgCl and fully discharged 0.2 V vs. Ag/AgCl states are drawn. As shown in Fig. 5a, the C1s spectra show a new peak at 288.3 eV for the fully charged and discharged electrodes compared with the that for the pristine (electrolyte-soaked Na3V2(PO4)2F3–SWCNT) electrode, which can be attributed to the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionality of the SEI film.58 Similarly, in comparison with that of the pristine electrode, the new signal occurring at 684.2 eV in the F1s spectra (Fig. 5b) of the cycled Na3V2(PO4)2F3–SWCNT electrode originates from NaF due to the formation of the SEI film.59 Therefore, the main composition of the SEI film on the cathode may be Na2CO3 and NaF. The SEI film on the cathode favors the suppression of cathode dissolution and reduction of water splitting, which occurs in aqueous electrolyte, according to the theory proposed by Chen,60 Wang,61 and Wang and Jiang.58
O functionality of the SEI film.58 Similarly, in comparison with that of the pristine electrode, the new signal occurring at 684.2 eV in the F1s spectra (Fig. 5b) of the cycled Na3V2(PO4)2F3–SWCNT electrode originates from NaF due to the formation of the SEI film.59 Therefore, the main composition of the SEI film on the cathode may be Na2CO3 and NaF. The SEI film on the cathode favors the suppression of cathode dissolution and reduction of water splitting, which occurs in aqueous electrolyte, according to the theory proposed by Chen,60 Wang,61 and Wang and Jiang.58
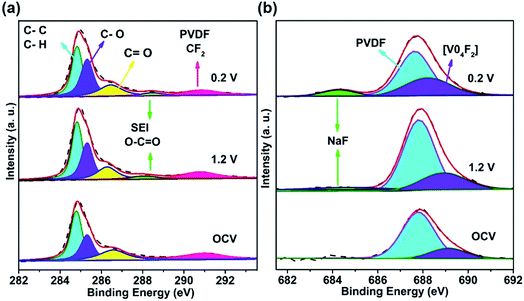 | ||
| Fig. 5 XPS spectra of (a) C1s and (b) F1s, of the Na3V2(PO4)2F3–SWCNT electrodes at different charge–discharge stages in 17 m NaClO4 aq. | ||
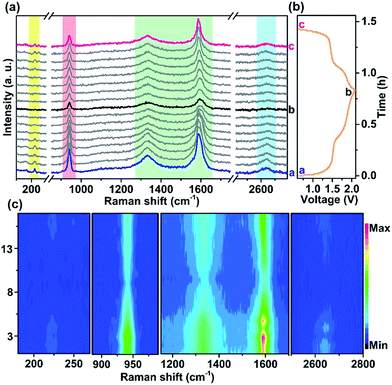
To look into the functioning of Na3V2(PO4)2F3–SWCNT in aqueous electrolyte during charging and discharging, we performed ex situ XRD at various states of charge and discharge in the first cycle in 17 m NaClO4 aq. (Fig. S9a and b†). All these patterns present almost coincident characteristic peaks. As shown in the enlarged patterns within the 2θ ranges of 27–34°, 39–46°, 48–51° and 56–60° (Fig. S9c–f†), when the Na3V2(PO4)2F3–SWCNT electrodes are charged to 0.8 V, the characteristic peaks belonging to the (220), (222), (400), (333), and (440) planes shift to higher angles, corresponding to the transformation from Na3V2(PO4)2F3 to Na2V2(PO4)2F3 with the extraction of Na+ at fully occupied positions. As the voltage reaches 1.2 V vs. Ag/AgCl, the positions of these peaks change even more, indicating that another Na+ is extracted from Na2V2(PO4)2F3 to give NaV2(PO4)2F3.47,49,62 The locations of diffraction peaks can be fully recovered by discharging to 0.8 V and further to 0.2 V. These results demonstrate that the electrochemical behavior of the Na3V2(PO4)2F3–SWCNT electrodes is a reversible process of Na+ extraction/insertion (Na3V2(PO4)2F3 ↔ NaV2(PO4)2F3 + 2Na+ + 2e−), which is consistent with the mechanism in non-aqueous electrolyte.47,49 Moreover, a full battery is assembled for the convenience of the in situ Raman test on the Na3V2(PO4)2F3–SWCNT electrode. The full cell uses NaTi2(PO4)3–SWCNT as the anode, which can accommodate 2 Na+ per molecular unit.63 The performance characterization is shown in Fig. S10† and is also discussed in detail in the ESI.†Fig. 6a shows the in situ Raman results between 0.6 and 2.1 V. The corresponding charge/discharge profiles are shown in Fig. 6b. At open circuit voltage, one major distinct peak in the Raman spectrum is observed at 941.3 cm−1 (P–O symmetrical stretching vibration of PO4) for Na3V2(PO4)2F3–SWCNT, and four major peaks for the SWCNTs are observed at 222 (RBM), 1330 (D band), 1586 (G band), and 2641 cm−1 (2D band). As the voltage increases, the band at 941.3 cm−1 weakens quickly, which can be attributed to the effect of changes in local symmetry on the ν(PO6) vibration mode. As sodium ions are deintercalated from the bulk, the V electronic configurations change to balance the charge. Because each phosphate ion is surrounded by four vanadium ions, the removal of Na+ and the associated oxidation of V3+ ion to V4+ will affect the four adjacent phosphate groups.64 In addition, the intensity of each Raman characteristic peak of SWCNTs decreases, which can be attributed to some Na+ being captured by the SWCNTs (poor but existing capacity, ca. 25 mA h g−1 Fig. S11†), which leads to a modification of the nanotube electronic structure and causes changes (reduction in intensity) in the resonance Raman signal.65,66 The in situ Raman spectra of the Na3V2(PO4)2F3–SWCNT electrode in the discharge process show a recovery along the original path. This excellent electrochemical reversibility in the range of 0.6–2.1 V makes this material a highly rechargeable positive electrode for Na3V2(PO4)2F3–SWCNT//NaTi2(PO4)3–MWCNT batteries.
Based on the above mentioned results, we investigate the electrochemical performance of the Na3V2(PO4)2F3–SWCNT//NaTi2(PO4)3–MWCNT aqueous full-cell. All the specific capacities of the full cell are calculated based on the mass of Na3V2(PO4)2F3–SWCNT-2. Fig. 7a gives the CVs and the charge/discharge performances of the Na3V2(PO4)2F3–SWCNT//NaTi2(PO4)3–MWCNT aqueous full-cell in a voltage range of 0.6 V to 2.1 V. Three obvious pairs of symmetric redox bands at 1.06/0.96, 1.52/1.46, and 1.94/1.89 V can be observed. They are attributed to extraction/insertion reactions of the sodium ions.67,68 The voltage for the Na3V2(PO4)2F3–MWCNT//NaTi2(PO4)3–MWCNT full-cell is 1.92 V, which is one of the highest voltage obtained by using intercalation anode and cathode materials in aqueous electrolyte for ARSBs. EIS measurements of the full cell are also performed (as shown in Fig. S12†). The EIS curve is composed of a semicircle in the high-frequency region and a sloping line in the low-frequency region. The low impedance of 4.23 Ω benefits from the high conductivity of this aqueous electrolyte. The charge/discharge voltage profiles for each cycle are shown in Fig. 7b. A discharge capacity of 75.2 mA h g−1 is achieved at a 1C rate (128 mA g−1), with an excellent coulombic efficiency of 99%. Although the capacitance retention (ca. 74% for the 20th cycle), depicted in the inset of Fig. 7b, is not very superior due to the unwanted decomposition of water and the variation of the structure of the electrode material during ion intercalation/de-intercalation,69 the significance of this work is that it at least verified the possibility of such a high voltage. In our opinion, we believe that with the modification of Na3V2(PO4)2F3 and the electrolyte, there will be remarkably excellent performance.
Conclusions
We have synthesized Na3V2(PO4)2F3–SWCNT by a low temperature solvothermal method, and the Na3V2(PO4)2F3–SWCNT cathode in a sodium ion battery exhibits a specific capacity of 117 mA h g−1 with a voltage plateau of 4.1 V. When measured at 0.5C, Na3V2(PO4)2F3–SWCNT could produce 114 mA h g−1 and retain 92.4% after 100 cycles. In addition, the Na3V2(PO4)2F3–SWCNT//NaTi2(PO4)3–MWCNT aqueous full-cell can deliver a high voltage of 1.92 V, and an average voltage of 1.7 V, achieving a high energy density of 150 W h kg−1 in 17 m NaClO4 aq. These encouraging results may accelerate the development of ARSBs with a high concentration electrolyte, high potential cathode, and low potential anode.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFB0901500, and 2016YFB0101201), the National Natural Science Foundation of China (51771094), the Ministry of Education of China (B12015 and IRT13R30) and the Fundamental Research Funds for the Central Universities.Notes and references
- D. Kundu, E. Talaie, V. Duffort and L. F. Nazar, Angew. Chem., Int. Ed., 2015, 54, 3431 CrossRef CAS PubMed.
- L. M. Suo, Y. S. Hu, H. Li, M. Armand and L. Q. Chen, Nat. Commun., 2013, 4, 2513 CrossRef PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167 CrossRef CAS PubMed.
- W. Luo, F. Shen, C. Bommier, H. L. Zhu, X. L. Ji and L. B. Hu, Acc. Chem. Res., 2016, 49, 231 CrossRef CAS PubMed.
- Y. Y. Lu, P. F. Zhou, K. X. Lei, Q. Zhao, Z. L. Tao and J. Chen, Adv. Energy Mater., 2017, 7, 1601973 CrossRef.
- X. D. Xiang, K. Zhang and J. Chen, Adv. Mater., 2015, 27, 5343 CrossRef CAS PubMed.
- S. Liu, L. Y. Shao, X. J. Zhang, M. Zhou, Z. L. Tao and J. Chen, J. Alloys Compd., 2018, 754, 147 CrossRef CAS.
- L. Y. Shao, Q. Zhao and J. Chen, Chem. Commun., 2017, 53, 435 Search PubMed.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Energy Environ. Sci., 2012, 5, 5884 RSC.
- J. F. Mao, C. Luo, T. Gao, X. L. Fan and C. S. Wang, J. Mater. Chem. A, 2015, 3, 10378 RSC.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636 CrossRef CAS PubMed.
- Y. Y. Huang, X. Lia, J. S. Wang, L. Miao, C. Li, J. T. Han and Y. H. Huang, Energy Storage Materials, 2018, 15, 108 CrossRef.
- Y. Y. Huang, Y. H. Zheng, X. Li, F. Adams, W. Luo, Y. H. Huang and L. B. Hu, ACS Energy Lett., 2018, 3, 1604 CrossRef CAS.
- J. Zhao, Y. Gao, Q. Liu, X. Meng, N. Chen, C. Z. Wang, F. Du and G. Chen, Chem.–Eur. J., 2018, 24, 2913 CrossRef CAS PubMed.
- W. X. Song and S. Q. Liu, Solid State Sci., 2013, 15, 1 CrossRef CAS.
- B. L. Ellis, K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 691 CrossRef CAS.
- Z. Li, D. Young, K. Xiang, W. C. Carter and Y. M. Chiang, Adv. Energy Mater., 2013, 3, 290 CrossRef CAS.
- Z. Chang, Y. Q. Yang, M. X. Li, X. W. Wang and Y. P. Wu, J. Mater. Chem. A, 2014, 2, 10739 RSC.
- Y. S. Wang, J. Liu, B. Lee, R. M. Qiao, Z. Z. Yang, S. Y. Xu, X. Q. Yu, L. Gu, Y. S. Hu, W. L. Yang, K. Kang, H. Li, X. Q. Yang, L. Q. Chen and X. J. Huang, Nat. Commun., 2015, 6, 6401 CrossRef CAS PubMed.
- Y. S. Wang, L. Q. Mu, J. Liu, Z. Z. Yang, X. Q. Yu, L. Gu, Y. S. Hu, H. Li, X. Q. Yang, L. Q. Chen and X. J. Huang, Adv. Energy Mater., 2015, 5, 1501005 CrossRef.
- L. M. Suo, O. Borodin, Y. S. Wang, X. H. Rong, W. Sun, X. L. Fan, S. Y. Xu, M. A. Schroeder, A. V. Cresce, F. Wang, C. Y. Yang, Y. S. Hu, K. Xu and C. S. Wang, Adv. Energy Mater., 2017, 7, 1701189 CrossRef.
- H. W. Long, W. Zeng, H. Wang, M. M. Qian, Y. H. Liang and Z. C. Wang, Adv. Sci., 2018, 1700634 CrossRef.
- Z. W. Guo, Y. Zhao, Y. X. Ding, Y. Y. Xia, H. S. Peng and Y. G. Wang, Chem, 2017, 3, 348 CAS.
- H. Qin, Z. P. Song, H. Zhan and Y. H. Zhou, J. Power Sources, 2014, 249, 367 CrossRef CAS.
- H. C. Gao and J. B. Goodenough, Angew. Chem., Int. Ed., 2016, 128, 12960 CrossRef.
- X. Y. Wu, M. Y. Sun, Y. F. Shen, J. F. Qian, Y. L. Cao, X. P. Ai and H. X. Yang, ChemElectroChem, 2014, 7, 407 CAS.
- P. R. Kumar, Y. H. Jung, J. E. Wang and D. K. Kim, J. Power Sources, 2016, 324, 421 CrossRef CAS.
- Q. Zhang, C. Y. Liao, T. Y. Zhai and H. Q. Li, Electrochim. Acta, 2016, 196, 470 CrossRef CAS.
- K. Nakamoto, R. Sakamoto, M. Ito, A. Kitajou and S. Okada, Electrochemistry, 2017, 85, 179 CrossRef CAS.
- Z. G. Hou, X. Q. Zhang, X. N. Li, Y. C. Zhu, J. W. Liang and Y. T. Qian, J. Mater. Chem. A, 2017, 5, 730 RSC.
- Y. Yao, L. Zhang, Y. Gao, G. Chen, C. Z. Wang and F. Du, RSC Adv., 2018, 8, 2958 RSC.
- W. X. Song, X. B. Ji, Z. P. Wu, Y. C. Yang, Z. Zhou, F. Q. Li and C. E. Banks, J. Power Sources, 2014, 256, 258 CrossRef CAS.
- X. Q. Xie, M.-Q. Zhao, B. Anasori, K. Maleski, C. E. Ren, J. W. Li, B. W. Byles, E. Pomerantseva, G. X. Wang and Y. Gogotsi, Nano Energy, 2016, 26, 513 CrossRef CAS.
- G. L. Polles, J. J. Videau, R. Olazcuaga and M. Couzi, J. Solid State Chem., 1996, 127, 341 CrossRef.
- Z. L. Jian, W. Z. Han, X. Lu, H. X. Yang, Y. S. Hu, J. Zhou, Z. B. Zhou, J. Q. Li, W. Chen, D. F. Chen and L. Q. Chen, Adv. Energy Mater., 2013, 3, 156 CrossRef CAS.
- P. R. Kumar, Y. H. Jung and D. K. Kim, J. Solid State Electrochem., 2017, 21, 223 CrossRef CAS.
- Y. Zhang, S. R. Guo and H. Y. Xu, J. Mater. Chem. A, 2018, 6, 4525 RSC.
- L. Li, Y. L. Xu, X. F. Sun, S. N. He and L. Li, Chem. Eng. J., 2018, 331, 712 CrossRef CAS.
- J. Z. Guo, P. F. Wang, X. L. Wu, X. H. Zhang, Q. Y. Yan, H. Chen, J. P. Zhang and Y. G. Guo, Adv. Mater., 2017, 29, 1701968 CrossRef.
- F. Sauvage, E. Quarez, J. M. Tarascon and E. Baudrin, Solid State Sci., 2006, 8, 1215 CrossRef CAS.
- Y. R. Qi, L. Q. Mu, J. M. Zhao, Y. S. Hu, H. Z. Liu and S. Dai, Angew. Chem., Int. Ed., 2015, 54, 9911 CrossRef CAS.
- S. Yu, X. Wang, R. Zhang, T. Yang, Y. Ai, T. Wen, W. Huang, T. Hayat, A. Alsaedi and X. Wang, Sci. Rep., 2017, 7, 39625 CrossRef CAS PubMed.
- J. M. Shim, D. G. Kim, H. J. Kim, J. H. Lee, J. H. Baika and J. C. Lee, J. Mater. Chem. A, 2014, 2, 13873 RSC.
- L. Shi, X. X. Fu, C. Y. Fan, S. Q. Yu, G. D. Qian and Z. Y. Wang, RSC Adv., 2015, 5, 85179 RSC.
- R. A. Shakoor, D. H. Seo, H. Kim, Y. U. Park, J. Kim, S. W. Kim, H. Gwon, S. Leec and K. Kang, J. Mater. Chem., 2012, 22, 20535 RSC.
- C. B. Zhu, C. Wu, C. C. Chen, P. Kopold, P. A. van Aken, J. Maier and Y. Yu, Chem. Mater., 2017, 29, 5207 CrossRef CAS.
- W. X. Song, X. Y. Cao, Z. P. Wu, J. Chen, Y. R. Zhu, H. S. Hou, Q. Lan and X. B. Ji, Langmuir, 2014, 30, 12438 CrossRef CAS PubMed.
- H. M. Yi, M. X. Ling, W. B. Xu, X. F. Li, Q. Zheng and H. M. Zhang, Nano Energy, 2018, 47, 340 CrossRef CAS.
- Y. S. Cai, X. X. Cao, Z. G. Luo, G. Z. Fang, F. Liu, J. Zhou, A. Q. Pan and S. Q. Liang, Adv. Sci., 2018, 1800680 CrossRef PubMed.
- Y. N. Xu, Q. L. Wei, C. Xu, Q. D. Li, Q. Y. An, P. F. Zhang, J. Z. Sheng, L. Zhou and L. Q. Mai, Adv. Energy Mater., 2016, 6, 1600389 CrossRef.
- D. Wang, X. Bie, Q. Fu, D. Dixon, N. Bramnik, Y. S. Hu, F. Fauth, Y. Wei, H. Ehrenberg, G. Chen and F. Du, Nat. Commun., 2017, 8, 15888 CrossRef CAS PubMed.
- Y. B. Niu, M. W. Xu, Y. Zhang, J. Han, Y. Wang and C. M. Li, RSC Adv., 2016, 6, 45605 RSC.
- Q. Y. Wang, B. D. Zhao, S. Zhang, X. H. Gao and C. Deng, J. Mater. Chem. A, 2015, 3, 7732 RSC.
- P. P. Prosini, M. Lisi, D. Zane and M. Pasquali, Solid State Ionics, 2002, 148, 45 CrossRef CAS.
- S. Franger, F. L. Cras, C. Bourbon and H. Rouault, Electrochem. Solid-State Lett., 2002, 5, A231 CrossRef CAS.
- P. R. Kumar, Y. H. Jung, B. Moorthy and D. K. Kim, J. Electrochem. Soc., 2016, 163, A1484 CrossRef CAS.
- P. R. Kumar, Y. H. Jung, C. H. Lim and D. K. Kim, J. Mater. Chem. A, 2015, 3, 6271 RSC.
- W. Lia, K. L. Wang, S. J. Cheng and K. Jiang, Energy Storage Materials, 2018, 15, 14 CrossRef.
- M. A. Muñoz-Marquez, M. Zarrabeitia, E. Castillo-Martínez, A. Eguía-Barrio, T. Rojo and M. Casas-Cabanas, ACS Appl. Mater. Interfaces, 2015, 7, 7801 CrossRef PubMed.
- N. Zhang, F. Y. Cheng, J. X. Liu, L. B. Wang, X. H. Long, X. S. Liu, F. J. Li and J. Chen, Nat. Commun., 2017, 8, 405 CrossRef PubMed.
- F. Wang, Y. X. Lin, L. M. Suo, X. L. Fan, T. Gao, C. Y. Yang, F. D. Han, Y. Qi, K. Xu and C. S. Wang, Energy Environ. Sci., 2016, 9, 3666 RSC.
- M. Bianchini, F. Fauth, N. Brisset, F. Weill, E. Suard, C. Masquelier and L. Croguennec, Chem. Mater., 2015, 27, 3009 CrossRef CAS.
- S. I. I. Park, I. Gocheva, S. Okada and J. Yamaki, J. Electrochem. Soc., 2011, 158, A1067 CrossRef CAS.
- Z. G. Liu, Y. Y. Hu, M. T. Dunstan, H. Huo, X. G. Hao, H. Zou, G. M. Zhong, Y. Yang and C. P. Grey, Chem. Mater., 2014, 26, 2513 CrossRef CAS.
- Y. A. Kim, M. Kojima, H. Muramatsu, S. Umemoto, T. Watanabe, K. Yoshida, K. Sato, T. Ikeda, T. Hayashi, M. Endo, M. Terrones and M. S. Dresselhaus, Small, 2006, 2, 667 CrossRef CAS.
- M. Kalbac, Y. P. Hsieh, H. Farhat, L. Kavan, M. Hofmann, J. Kong and M. S. Dresselhaus, Nano Lett., 2010, 10, 4619 CrossRef CAS.
- C. Shen, H. Long, G. C. Wang, W. Lu, L. Shao and K. Y. Xie, J. Mater. Chem. A, 2018, 6, 6007 RSC.
- I. L. Matts, S. Dacek, T. K. Pietrzak, R. Malik and G. Ceder, Chem. Mater., 2015, 27, 6008 CrossRef CAS.
- W. X. Song, X. B. Ji, Y. R. Zhu, H. J. Zhu, F. Lu, Y. P. Yao and C. E. Ban, ChemElectroChem, 2014, 1, 871 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional results are included. See DOI: 10.1039/c8ta09194c |
| This journal is © The Royal Society of Chemistry 2019 |