Mesoporous NiS2 nanospheres as a hydrophobic anticancer drug delivery vehicle for synergistic photothermal–chemotherapy†
Gang
He‡
a,
Yan
Ma‡
a,
Hu
Zhou‡
b,
Siyuan
Sun
a,
Xianwen
Wang
*a,
Haisheng
Qian
 a,
Yan
Xu
a,
Zhaohua
Miao
*a and
Zhengbao
Zha
a,
Yan
Xu
a,
Zhaohua
Miao
*a and
Zhengbao
Zha
 *a
*a
aSchool of Food and Biological Engineering, Hefei University of Technology, Hefei, Anhui 230009, P. R. China. E-mail: xianwenwang@mail.hfut.edu.cn; zhaohua_miao@hfut.edu.cn; zbzha@hfut.edu.cn; Tel: +86 551 62901285
bThe First Affiliated Hospital of University of Science and Technology of China, Anhui Provincial Cancer Hospital, Hefei, Anhui 230001, P. R. China
First published on 21st November 2018
Abstract
To overcome the unfavorable effects of the hydrophobicity of drugs and cancer resistance, mesoporous NiS2 nanospheres (mNiS2 NSs) have been successfully developed here to package hydrophobic camptothecin (CPT) and realize the synergistic photothermal–chemotherapy of cancer. The mNiS2 NSs which were prepared through a facile solvothermal method here exhibited not only considerable near-infrared (NIR) absorption and good photothermal conversion efficiency as high as 44.6%, but also achieved a NIR light induced CPT release property which were both beneficial for improving the cancer cell-killing efficacy. After a short period of NIR light illumination, a significant intensified cell killing efficacy was observed when 4T1 or HepG2 cancer cells were incubated with CPT@mNiS2 NSs. Thus, mNiS2 NSs have been demonstrated here to have potential as a novel NIR light-responsive hydrophobic drug delivery nanoplatform for realizing synergistic cancer treatment.
Introduction
Cancer, a disease with a high mortality rate, has become one of the most challenging problems in the world.1–3 Although chemotherapy has occupied an indispensable position in the adjuvant treatment of cancer, its efficacy still needs to be further improved owing to some barriers, including the hydrophobic properties of chemical substances and various physiological barriers for drug delivery as well as drug resistance.4,5 Currently, nearly 40% of the produced chemical anticancer drugs suffer from poor water solubility and consequently result in a significant barrier for intravenous administration.6 For instance, camptothecin (CPT), an effective chemical substance in the treatment of various carcinomas, has limited clinical applications due to its poor dispersity in a physiological medium.7–10 A universal strategy is to use a water-soluble salt formulation of CPT with different physicochemical characteristics for intravenous injection, but these chemical modifications may lead to unexpected changes in either the antitumor activity or toxicological profile.11–13 To date, nanoparticle technology has gained considerable attention in oncology due to its potential of delivering hydrophobic anticancer drugs into tumor lesions with enhanced therapeutic outcomes and reduced adverse effects.14–16 To be realistic, the currently developed nanocarrier formulations still have room for improvement in increasing the drug loading capacity and their responsiveness to some specific stimuli for realizing controlled drug release.17–19 Thus, developing novel stimuli-responsive hydrophobic drug delivery systems to realize controlled and sustained drug release profiles is highly desirable.It is generally accepted that single chemotherapy cannot erase cancer completely due to the complicated and interconnected disease pathways of cancer, resulting in the emergence of drug resistance and tumor recurrence.20–22 Encouraged by the fact that hyperthermia could facilitate the cellular uptake of chemotherapeutic drugs, combined thermo-chemo therapy would result in an enhanced therapeutic outcome and reduced side effects.23–25 Recently, near-infrared (NIR) light induced photothermal therapy (PTT), which can utilize light-absorbing agents to directly convert the energy of incident light into hyperthermia to locally “boil” cancer cells, has attracted great interest in combating cancer due to its minimal invasiveness and high selectivity.26–29 Thus, there would be unique advantages to integrate photothermal agents and chemotherapeutic drugs into a single nanoplatform. Up to now, although various nanoplatforms have been successfully constructed for combined photothermal–chemotherapy,30–33 the non-biodegradable characteristic of these reported inorganic nanoplatforms has hampered their clinical translation due to long-term safety concerns. Therefore, still there is an enormous demand for the development of novel degradable photothermal nanoplatforms for hydrophobic drug delivery to achieve efficient cancer cell-killing.
Recently, due to their unique physicochemical properties as well as low cost, nickel-containing nanomaterials have received great attention in various fields, including batteries, catalysis, biomedical sensors and solar cells.34–37 For instance, Ni-integrated CuS nanoparticles were developed as a novel photoacoustic (PA)/magnetic resonance imaging (MRI) contrast agent by a doping method.38 PEGylated nickel carbide nanocrystals (Ni3C NCs) were demonstrated as an effective PTT agent for cancer therapy in vivo.39 Another paradigm is that nickel oxide nanoparticles (NiO NPs) were reported for combined photodynamic therapy and chemotherapy.40 In our previous work, polyacrylic acid-functionalized Ni0.85Se nanoparticles were successfully constructed to realize PA imaging guided photothermal–chemo treatment of cancer.23 Very recently, monodispersed mesoporous NiS2 nanospheres (mNiS2 NSs) have been developed via a facile solvothermal process for producing high-rate and long-life sodium-ion batteries.36 To the best of our knowledge, developing mNiS2 NSs as a hydrophobic drug delivery nanoplatform for combined photothermal–chemotherapy owing to their good mesoporous structure has not been reported yet.
In this study, monodispersed mNiS2 NSs were prepared via a facile template-free solvothermal process according to a reported method with minor modifications (Scheme 1a).36 The as-prepared mNiS2 NSs exhibited considerable NIR absorption, gradual degradation and high photothermal conversion efficiency. Moreover, the mesoporous nanostructure could render mNiS2 NSs the ability for loading hydrophobic CPT owing to their high specific surface area. Upon NIR laser irradiation, the mNiS2 NSs could achieve not only thermo-responsive CPT release, but also synergistic photothermal–chemotherapy for killing cancer cells (Scheme 1b).
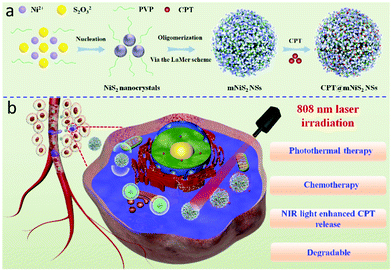 | ||
| Scheme 1 Schematic illustration of CPT@mNiS2 NSs, (a) preparation process and (b) synergistic photothermal–chemotherapy. | ||
Experimental section
Materials
Nickel nitrate hexahydrate (Ni(NO3)2·6H2O) was bought from Aladdin Chemical Reagent Co., Ltd. Ethylene glycol (EG), sodium thiosulfate pentahydrate (Na2S2O3·5H2O) and polyvinylpyrrolidone (PVP, K30) were obtained from Sinopharm Chemical Reagent Co., Ltd. Anticancer drug CPT was obtained from Beijing Huafeng United Technology Co., Ltd. Deionized water (DI water) with a resistivity of 18.2 MΩ cm was used for all the experiments.Synthesis of mNiS2 NSs
mNiS2 NSs were prepared via a reported solvothermal method with minor modifications.36 Typically, 0.6 g PVP, 2 mmol Ni(NO3)2·6H2O and 6 mmoL Na2S2O3·5H2O were completely dissolved in a mixed solvent of 80 mL EG and DI water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v:v) under magnetic stirring for 1 h. Thereafter, the mixed solution was sealed in an autoclave and stewed at 180 °C for 12 h. The final product (mNiS2 NSs) was obtained through standard centrifugation/washing cycles.
1 v:v) under magnetic stirring for 1 h. Thereafter, the mixed solution was sealed in an autoclave and stewed at 180 °C for 12 h. The final product (mNiS2 NSs) was obtained through standard centrifugation/washing cycles.
Characterizations of mNiS2 NSs
The surface chemical composition and crystallographic structure of the as-prepared mNiS2 NSs were examined by X-ray photoelectron spectroscopy (XPS, ESCALab 250Xi, USA) and X-ray diffraction (XRD, PANalytical B. V., Holland). Scanning electron microscopy (SEM, Hitachi S-4800, Japan) and transmission electron microscopy (TEM, JEM-2100F, Japan) were used to characterize the morphology of the mNiS2 NSs. The hydrodynamic diameter and size distribution were recorded using a NanoBrook-90 Plus instrument. The Brunauer–Emmett–Teller (BET) surface area was measured by using an Autosorb IQ3 instrument.Photothermal effect of mNiS2 NSs under NIR irradiation
To investigate the photothermal conversion effect, mNiS2 NS aqueous solutions with gradient concentrations were illuminated with NIR light (808 nm, 2.0 W cm−2) for 10 min. In consideration of the demand of repeated cancer treatments during PTT, five laser on/off cycles were also used to evaluate the photo-stability of the mNiS2 NSs. Moreover, the photothermal conversion efficiency of the mNiS2 NSs was calculated according to the methods previously described.41,42In vitro CPT loading and releasing
For CPT loading, 5 mg mNiS2 NSs and 20 mg CPT were dispersed and stirred in 3 mL dimethyl sulfoxide (DMSO) at ambient atmosphere. After 24 h, the CPT loaded mNiS2 NSs (CPT@mNiS2 NSs) were obtained through centrifugation and washed with phosphate buffer solution (PBS, pH = 7.4) three times. The concentration of CPT contained in the supernatant was calculated by determining the absorbance at 367 nm. The loading capacity (LC) was estimated as follows: LC (%) = (weight of whole CPT − weight of CPT in the discard solution)/(weight of NSs + weight of CPT loaded into the NSs) × 100%.The in vitro CPT release profile from CPT@mNiS2 NSs was investigated under different conditions. In detail, 1 mg CPT@mNiS2 NSs was first suspended in 1 mL DI water and then sealed in a dialysis bag (Mw = 3500) which was immersed in a centrifuge tube containing 30 mL PBS (pH = 7.4, 6.0 or 5.0) at 37 °C or 50 °C. At predetermined time intervals, typical sampling and replenish cycles were performed to monitor the release profile of CPT. Moreover, NIR laser illumination was further executed to assess the influence of NIR light/heat on the CPT release properties from CPT@mNiS2 NSs.
Cell study
The biocompatibility of the as-prepared mNiS2 NSs was assessed through a typical MTT assay by using human cervical vein endothelial cells (HUVECs). A hemolysis test was also carried out to evaluate the hematotoxicity of the mNiS2 NSs by using the blood of healthy mice according to a previously reported method.43The localized photothermal cancer cell ablation ability of the mNiS2 NSs was further evaluated by irradiating 4T1 breast cancer cells with a NIR laser (808 nm, 2.0 W) and standard live/dead cell staining assay (green fluorescence from calcein-AM for live cells and red fluorescence from propidium iodide (PI) for dead cells). Furthermore, the intensified cell-killing effect from combined photothermal–chemotherapy was quantitatively investigated by a standard MTT assay.
Results and discussion
Characterization of mNiS2 NSs
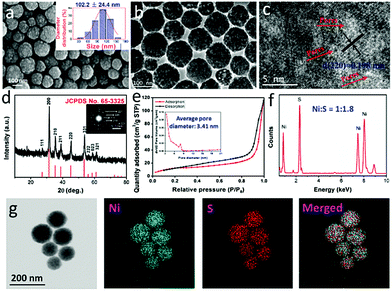
According to a reported study,36 the formation mechanism for mNiS2 NSs is that NiS2 is solvothermally synthesized by reaction of S2O32− and Ni2+, in which S2O32− is used as both a sulfur source and a reducing agent. Solvent EG would be converted into acetaldehyde at high temperature, which serves as an oxidizing agent in the formation of NiS2 nuclei. Due to the amphipathic nature of PVP stabilizers, the NiS2 nuclei tend to absorb on the carbonyl groups of PVP, and then gradually become more tightly packed. With further processing, hierarchical spherical shaped mNiS2 NSs form by oligomerization of tiny nanocrystals via the LaMer scheme.44 As shown in Fig. 1a, the SEM image of the as-prepared mNiS2 NSs clearly showed a regular spherical morphology and good monodispersity with an average diameter of 102.2 ± 24.4 nm. Moreover, each mNiS2 NS was composed of tightly piled NiS2 nanocrystals with ultrasmall size, confirming the reasonability of the deductive formation mechanism of mNiS2 NSs. In addition, the TEM characterization further revealed that mNiS2 NSs have a highly ordered pore structure, due to the spherical packing of NiS2 nanocrystals (Fig. 1b and c). The lattice fringes of NiS2 display an interplanar spacing of 0.198 nm, which is in good agreement with the (220) planes of the cubic NiS2.Moreover, the phase structure of the as-prepared product was further characterized by XRD analysis (Fig. 1d), showing that all diffraction peaks containing the (111), (200), (210), (211), (220), (331), (222), (023) and (321) faces could be clearly assigned to the cubic NiS2 phase (JCPDS no. 65-3325), was confirmed by the results of the SAED pattern (inset of Fig. 1d). A relatively high specific BET surface area (34.0 m2 g−1) was observed (Fig. 1e), and the average pore size was calculated to be 3.4 nm (inset of Fig. 1e), indicating the mesoporous structure of the as-prepared mNiS2 NSs which is good for encapsulating anticancer drugs. In addition, as shown in Fig. 1f, except the signals of Cu, O, and C elements which were mainly from air or the sample-support substrate for the measurement, the EDS results further verify the presence of Ni and S at a molar ratio of around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8, indicating the composition of NiS2 which is further confirmed by the results of elemental mapping showed that both Ni and S elements were evenly distributed throughout the whole nanospheres (Fig. 1g). Further confirmed by the results of inductively coupled plasma atomic emission spectrometry (ICP-AES, as 100 μg mL−1 of NPs consisted of 43.3 and 47.1 μg mL−1 of nickel and sulfur elements, respectively), a Ni/S stoichiometry was calculated to be 1
1.8, indicating the composition of NiS2 which is further confirmed by the results of elemental mapping showed that both Ni and S elements were evenly distributed throughout the whole nanospheres (Fig. 1g). Further confirmed by the results of inductively coupled plasma atomic emission spectrometry (ICP-AES, as 100 μg mL−1 of NPs consisted of 43.3 and 47.1 μg mL−1 of nickel and sulfur elements, respectively), a Ni/S stoichiometry was calculated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 which demonstrated the formation of the NiS2 phase.
2 which demonstrated the formation of the NiS2 phase.
Moreover, XPS measurements were also performed to further analyze the surface electronic states and elemental composition of mNiS2 NSs (Fig. 2a–d). In the Ni 2p spectra (Fig. 2a), the binding energies of Ni 2p1/2 and Ni 2p3/2 for the mNiS2 NSs are observed at about 870.68 and 853.58 eV, respectively, along with two satellite peaks (abbreviated as Sat.) at about 876.38 and 859.68 eV.45 In the S 2p spectra (Fig. 2b), the peaks at about 162.28 and 163.47 eV are assigned to S 2p1/2 and S 2p3/2 of S22−, respectively, indicating the presence of S–S bonds in the mNiS2 NSs. Besides, the peaks at 168.64 eV can be attributed to S–O bonding owing to the surface oxidation of the mNiS2 NSs.46 In the C 1s spectra (Fig. 2c), the strong peak at 284.83 eV is attributed to C 1s, and it may mainly come from the PVP molecules on the surface of the mNiS2 NSs. All the binding energies are closer to the previously reported values. In addition, XPS survey spectra show the presence of O 1s due to its exposure to oxygen present in air (Fig. 2d). The XPS analysis further confirmed the formation of cubic NiS2, which is consistent with the XRD data of the mNiS2 NSs.
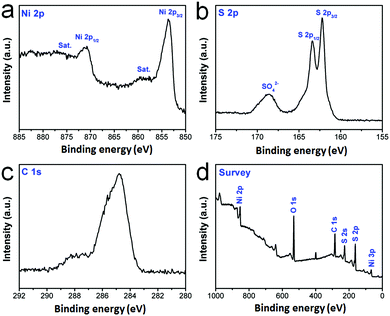 | ||
| Fig. 2 XPS characterization. The core level spectrum of (a) Ni 2p; (b) S 2p; (c) C 1s; (d) the survey spectrum. | ||
Photothermal conversion performance and in vitro CPT release
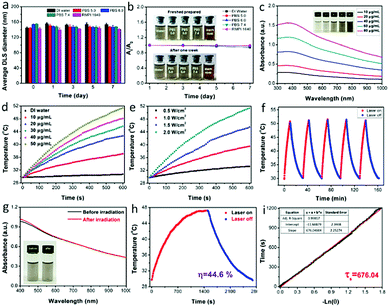
Due to the importance of the storage stability of nanomaterials for biomedical applications to avoid any adverse effect, the stabilities of the mNiS2 NSs in various media including DI water, PBS with different pH and DMEM cell culture medium were investigated. As shown in Fig. 3a, owing to the inherent hydrophilicity of PVP stabilizers, no significant diameter change of mNiS2 NSs was observed in various media for one week (4 °C and room temperature) and there were no significant absorbance decrease and observable macroscopic aggregates (Fig. 3b and Fig. S1, ESI†), indicating their good storage stability for biomedical applications. Thereafter, the UV-vis-NIR spectrum of the mNiS2 NS aqueous solution exhibited a strong and broad absorption in the whole range from 300 to 1000 nm (Fig. 3c), with a calculated mass extinction coefficient of 10.9 L g−1 cm−1 at 808 nm based on the Lambert–Beer law,47 which is comparable to those of some reported inorganic nanomaterials, such as Au nanorods (13.9 L g−1 cm−1 at 808 nm),47 graphene oxide (7.9 L g−1 cm−1 at 808 nm),48 and platinum–copper alloy nanoparticles (6.3 L g−1 cm−1 at 808 nm).49Next, the temperatures of 3.0 mL mNiS2 NS aqueous solutions with various concentrations (10, 20, 30, 40 and 50 μg mL−1) were recorded under NIR light illumination (808 nm, 2.0 W cm−2). After continuously being illuminated for 10 min, the temperature was increased from 29.7 °C to 37.1 °C, 42.7 °C, 45.7 °C, 48.1 °C, and 51.4 °C, respectively, indicating an obvious concentration-dependent manner (Fig. 3d). In contrast, the temperature of DI water was increased by only 0.9 °C with the same treatment. Similarly, the temperature of the mNiS2 NS aqueous solution (50 μg mL−1) was monitored under 808 nm laser irradiation with various power, and a distinct incident-energy dependent performance was also observed (Fig. 3e). Moreover, to assess the photo-stability of the as-prepared mNiS2 NSs for the potential repeated PTT and on-demand drug release, five laser on/off cycles were used by irradiating mNiS2 NSs aqueous solution (50 μg mL−1) with an NIR laser, followed by natural cooling to ambient temperature without illumination. No significant change in temperature elevation and UV-vis-NIR absorption spectra was observed before and after five irradiation cycles, demonstrating the good photo-stability of the mNiS2 NSs (Fig. 3f and g). In addition, according to a reported method,50 the photothermal conversion efficiency of the mNiS2 NSs was calculated to be 44.6% (Fig. 3h and i), which is higher than those of other reported inorganic nanomaterials, such as Cu3BiS3 hollow nanospheres (27.5% at 980 nm),51 Cu2−xSe nanocrystals (22% at 800 nm),52 MoO3−x hollow nanospheres (22.64% at 808 nm),53 Ni3C nanocrystals (16.9% at 800 nm)39 and Prussian blue nanoparticles (36.7% at 808 nm).54 Therefore, these data indicated that mNiS2 NSs could act as a potential photothermal agent for cancer treatment.
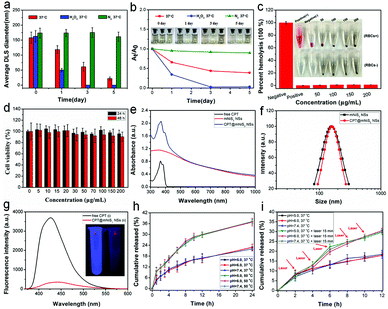
The non-biodegradable characteristic of many reported inorganic nanomaterials hampered their further clinical translation due to long-term toxicological concerns. In this study, mNiS2 NS aqueous solutions were selectively exposed to dissolved oxygen, H2O2 (50 μM, mimic the tumor microenvironment) and a nitrogen atmosphere at 37 °C to investigate the oxidation and degradation performance of the as-prepared mNiS2 NSs. As shown in Fig. 4a and b, a gradual decrease in the hydrodynamic diameter and absorbance and the solution color change from brown to transparent were observed when mNiS2 NSs came into contact with dissolved oxygen and H2O2, indicating the occurrence of the oxidation-induced degradation process of the mNiS2 NSs. In contrast, the mNiS2 NS solutions kept in the nitrogen atmosphere showed no significant change in color, hydrodynamic diameter and absorbance, suggesting that oxygen/H2O2 should be a vital factor in the degradation process of the mNiS2 NSs. The gradual oxidation and degradation performance of the mNiS2 NSs when exposed to dissolved oxygen and H2O2 would allow them to be used as a degradable nanoplatform due to the relatively high concentration of dissolved oxygen in blood and H2O2 in the tumor microenvironment. Thereafter, typical hemolysis and MTT assays were both performed to investigate the cytotoxicity of the mNiS2 NSs. As shown in Fig. 4c, the mNiS2 NSs showed no observable hemolysis effect with a concentration as high as 200 μg mL−1, demonstrating their safety when circulated in the blood pool. Additionally, a typical MTT assay was used to characterize the cell viability of HUVECs when incubated with mNiS2 NSs as long as 48 h. As expected, the as-prepared mNiS2 NSs were largely biocompatible as the relative cell viability remains exceeding 90% in comparison to negative groups as incubated with 200 μg mL−1 of mNiS2 NSs for 48 h (Fig. 4d).
Afterwards, inspired by the good photothermal conversion efficiency and biocompatibility of mNiS2 NSs, the potential of the mNiS2 NSs as a hydrophobic drug delivery vehicle has been revealed due to the amphipathic nature of PVP stabilizers which distributed on all of the pore structures. Compared to free CPT and mNiS2 NSs, the obtained CPT@mNiS2 NSs acquired characteristic absorption of both free CPT and mNiS2 NSs (Fig. 4e), while no significant change of morphology (Fig. S2, ESI†) and hydrodynamic diameter (Fig. 4f) occurred between mNiS2 NSs and CPT@mNiS2 NSs. Unlike the results of the UV-vis-NIR spectra, the fluorescence intensity of CPT in CPT@mNiS2 NSs nearly disappeared when compared to that of free CPT with an equivalent amount, which could be visualized more easily from the fluorescence image (Fig. 4g). This phenomenon was ascribed to the aggregated state of CPT in CPT@mNiS2 NSs, demonstrating the successful CPT loading in mNiS2 NSs (CPT loading capacity: 15.6%). The mesoporous structure of the mNiS2 NSs (BET surface area of 25.2 m2 g−1) and pore size (3.1 nm) were still preserved after CPT loading (Fig. S3, ESI†), guaranteeing the successful release of loaded CPT. Thereafter, the in vitro CPT release profile was investigated by using a typical dialysis method. As seen in Fig. 4h, unlike the temperature-responsive CPT release profile, no obvious pH-responsive CPT release behavior was observed which may be due to the fact that no acidic protonation or degradation of either CPT or mNiS2 NSs occurred in a mild acidic environment. Different from the influence of the acidic environment, irradiation with an NIR laser would increase the accumulative release amount of CPT from about 18.0% to 30% during 12 h when incubated with neutral PBS owing to the accelerated molecular movement in a heated solution (Fig. 4i).
Synergistic cell-killing effect of CPT@mNiS2 NSs
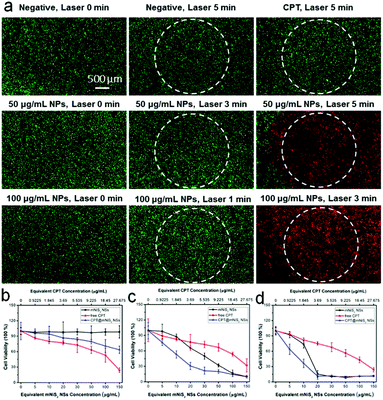
Encouraged by the good photothermal conversion efficiency and ability to deliver hydrophobic CPT, the synergistic cell-killing effect of the as-prepared CPT@mNiS2 NSs was further evaluated. Firstly, localized photothermal destruction ability was qualitatively investigated by using 4T1 breast cancer cells. After various treatments with mNiS2 NSs/free CPT or NIR laser irradiation, 4T1 cells were co-stained with calcein AM and PI to visualize live and dead cells. As seen in Fig. 5a, strong red fluorescence was seen only in the presence of both mNiS2 NSs and NIR laser irradiation, and the region of dead cells (red fluorescence) expanded as the concentration of mNiS2 NSs and irradiation time increased. No acute cancer death was observed when 4T1 cells were treated with single NIR laser irradiation, free CPT or mNiS2 NSs, similar to the negative group (largely green fluorescence). These data confirm that mNiS2 NSs plus appropriate NIR laser irradiation would realize localized tumor ablation.A standard MTT assay was further used to quantitatively investigate the synergistic cancer cell-killing effect of combined photothermal–chemotherapy. As depicted in Fig. 5b, without NIR light illumination, mNiS2 NSs showed low toxicity as the cell viability of 4T1 cancer cells remained exceeding 90% even after incubation with mNiS2 NSs (150 μg mL−1) for 24 h. Due to the sustained CPT release from CPT@mNiS2 NSs, CPT@mNiS2 NSs exhibited relatively weak cell-killing efficiency in comparison to free CPT with an equivalent concentration. In contrast, significant cell death was observed when 4T1 cancer cells were treated with mNiS2 NSs or CPT@mNiS2 NSs plus NIR light irradiation due to the generation of hyperthermia, exhibiting a concentration/irradiation energy dependent manner. Importantly, the CPT@mNiS2 NSs plus NIR laser irradiation exhibited a synergistic cell-killing effect in comparison to single chemotherapy (free CPT) or PTT (mNiS2 NSs plus NIR laser irradiation). For instance, with 1 min of NIR laser irradiation (808 nm, 2.0 W cm−2), the 4T1 cancer cell viability was 65.8% or 76.8% when treated with mNiS2 NSs or free CPT (the equivalent NS concentration was 20 μg mL−1), respectively. Satisfyingly, an obvious lower cell survival (30.7%) was observed when 4T1 cells were subjected to combined treatment of CPT@mNiS2 NSs and NIR light illumination. In addition, a similar enhanced cell-killing effect was realized when HepG2 cancer cells were treated with CPT@mNiS2 NSs plus NIR light illumination (Fig. S4, ESI†), indicating the universality of the as-prepared CPT@mNiS2 NSs for cancer cell elimination. Furthermore, the heat generated from CPT@mNiS2 NSs under NIR laser irradiation could promote the intracellular uptake of CPT due to the accelerated metabolism and fluidity of the cell membrane (Fig. 6a). For instance, upon treatment with 100 μg mL−1 of CPT@mNiS2 NSs and 5 min NIR laser irradiation, the generated heat could nearly double the amount of intracellular CPT in comparison to the group treated with free CPT (Fig. 6b). Therefore, these data demonstrated that the mNiS2 NSs prepared here were able to serve as a vehicle for loading hydrophobic cancer drugs to achieve a synergistic cancer cell-killing effect.
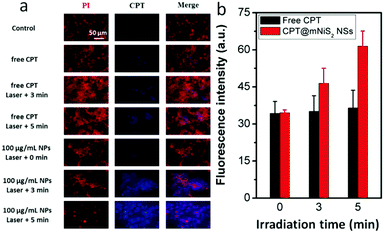 | ||
| Fig. 6 Characterization of heat-promoted CPT uptake. (a) Fluorescence image of 4T1 cells; (b) quantitative analysis of the fluorescence intensity of intracellular CPT. | ||
Conclusions
In summary, degradable and monodispersed mNiS2 NSs have been successfully prepared via a facile solvothermal method. Owing to considerable absorption in the NIR region, the as-prepared mNiS2 NSs could be used as a potential PTT agent. Interestingly, a gradual oxidation and degradation performance could be observed when mNiS2 NSs were exposed to a relatively high concentration of dissolved oxygen and H2O2, implying biosafety without long-term toxicological concerns. In addition, the mNiS2 NSs possess large pore volume and high surface area which are beneficial for the efficient encapsulation of hydrophobic CPT, thus realizing an NIR laser responsive CPT release. Importantly, a synergistic cancer cell killing effect has been observed when 4T1 or HepG2 cancer cells were treated with CPT@mNiS2 NSs and NIR laser irradiation simultaneously. Thus, our data have demonstrated the good potential of mNiS2 NSs to act as a hydrophobic drug delivery vehicle for enhanced cell-killing efficiency through combined photothermal–chemotherapy.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Natural Science Foundation of China (no. 31500808; 81501590) and the Fundamental Research Funds for the Central Universities (no. JZ2018HGPA0273; JZ2018HGTB0247; JZ2018HGTA0202; and JZ2018HGBZ0156).Notes and references
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2016, 66, 7–30 CrossRef.
- A. Rider, S. Simpson, B. Bennett, K. Byrne, P. Hallworth, T. Desai and K. Cocks, Ann. Oncol., 2017, 28 Search PubMed.
- V. Lambert-Obry, A. Gouault-Laliberte, A. Castonguay, G. Zanotti, T. Tran, M. Mates, J. Lemieux, P. Chabot, C. Prady, F. Couture and J. Lachaine, Curr. Oncol., 2018, 25, E282–E290 CAS.
- D. P. Ferris, J. Lu, C. Gothard, R. Yanes, C. R. Thomas, J. C. Olsen, J. F. Stoddart, F. Tamanoi and J. I. Zink, Small, 2011, 7, 1816–1826 CrossRef CAS.
- V. Wagner, A. Dullaart, A. K. Bock and A. Zweck, Nat. Biotechnol., 2006, 24, 1211–1217 CrossRef CAS.
- J. Lu, M. Liong, J. I. Zink and F. Tamanoi, Small, 2007, 3, 1341–1346 CrossRef CAS.
- J. Liu, Z. Jiang, S. Zhang and W. M. Saltzman, Biomaterials, 2009, 30, 5707–5719 CrossRef CAS PubMed.
- M. T. Morgan, Y. Nakanishi, D. J. Kroll, A. P. Griset, M. A. Carnahan, M. Wathier, N. H. Oberlies, G. Manikumar, M. C. Wani and M. W. Grinstaff, Cancer Res., 2006, 66, 11913–11921 CrossRef CAS PubMed.
- M. Loredo-Tovias, A. Duran-Meza, M. Villagrana-Escareño, R. Vega-Acosta, E. Reynaga-Hernández, L. M. Flores-Tandy, O. Valdes-Resendiz, R. Cadena-Nava, E. Alvizo-Paez and J. Ruiz-Garcia, Nanoscale, 2017, 9, 11625–11631 RSC.
- F. Zhang, G. Zhu, O. Jacobson, Y. Liu, K. Chen, G. Yu, Q. Ni, J. Fan, Z. Yang and F. Xu, ACS Nano, 2017, 11, 8838–8848 CrossRef CAS.
- C. Fuchs, E. P. Mitchell and P. M. Hoff, Cancer Treat. Rev., 2006, 32, 491–503 CrossRef CAS PubMed.
- K. S. Cunha, M. L. Reguly, U. Graf and H. H. Rodrigues de Andrade, Mutagenesis, 2002, 17, 141–147 CrossRef CAS.
- A. N. C. Sortibrán, M. G. O. Téllez and R. Rodríguez-Arnaiz, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2006, 604, 83–90 CrossRef.
- P. Zhang, A. G. Cheetham, Y.-A. Lin and H. Cui, ACS Nano, 2013, 7, 5965–5977 CrossRef CAS.
- H. Meng, M. Wang, H. Liu, X. Liu, A. Situ, B. Wu, Z. Ji, C. H. Chang and A. E. Nel, ACS Nano, 2015, 9, 3540–3557 CrossRef CAS.
- D. Liu, W. Wu, X. Chen, S. Wen, X. Zhang, Q. Ding, G. Teng and N. Gu, Nanoscale, 2012, 4, 2306–2310 RSC.
- K. Dong, Z. Liu, Z. H. Li, J. S. Ren and X. G. Qu, Adv. Mater., 2013, 25, 4452–4458 CrossRef CAS.
- S. P. Hudson, R. F. Padera, R. Langer and D. S. Kohane, Biomaterials, 2008, 29, 4045–4055 CrossRef CAS PubMed.
- H. Wang, K. Wang, B. Tian, R. Revia, Q. Mu, M. Jeon, F. C. Chang and M. Zhang, Small, 2016, 12, 6388–6397 CrossRef CAS.
- H. P. Gerber and N. Ferrara, Cancer Res., 2005, 65, 671–680 CAS.
- N. Ibrahim, Y. Yu, W. R. Walsh and J. L. Yang, Oncol. Rep., 2012, 27, 1303–1311 CAS.
- C.-M. J. Hu and L. Zhang, Biochem. Pharmacol., 2012, 83, 1104–1111 CrossRef CAS PubMed.
- X. Wang, F. Li, X. Yan, Y. Ma, Z.-H. Miao, L. Dong, H. Chen, Y. Lu and Z. Zha, ACS Appl. Mater. Interfaces, 2017, 9, 41782–41793 CrossRef CAS.
- H. Chen, Y. Ma, X. Wang and Z. Zha, J. Mater. Chem. B, 2017, 5, 7051–7058 RSC.
- W. Chen, K. Zeng, H. Liu, J. Ouyang, L. Wang, Y. Liu, H. Wang, L. Deng and Y. N. Liu, Adv. Funct. Mater., 2017, 27, 1605795 CrossRef.
- X. Huang, W. Zhang, G. Guan, G. Song, R. Zou and J. Hu, Acc. Chem. Res., 2017, 50, 2529–2538 CrossRef CAS.
- X. Huang, G. Deng, L. Liao, W. Zhang, G. Guan, F. Zhou, Z. Xiao, R. Zou, Q. Wang and J. Hu, Nanoscale, 2017, 9, 2626–2632 RSC.
- S. Shen, Y. Chao, Z. Dong, G. Wang, X. Yi, G. Song, K. Yang, Z. Liu and L. Cheng, Adv. Funct. Mater., 2017, 27, 1700250 CrossRef.
- Z. Zha, X. Yue, Q. Ren and Z. Dai, Adv. Mater., 2013, 25, 777–782 CrossRef CAS.
- W. Chen, J. Ouyang, H. Liu, M. Chen, K. Zeng, J. Sheng, Z. Liu, Y. Han, L. Wang and J. Li, Adv. Mater., 2017, 29, 1603864 CrossRef.
- Y. Zhang, D. Yang, H. Chen, W. Q. Lim, F. S. Z. Phua, G. An, P. Yang and Y. Zhao, Biomaterials, 2018, 163, 14–24 CrossRef CAS PubMed.
- A. Li Volsi, C. Scialabba, V. Vetri, G. Cavallaro, M. Licciardi and G. Giammona, ACS Appl. Mater. Interfaces, 2017, 9, 14453–14469 CrossRef CAS.
- W. Tian, Y. Su, Y. Tian, S. Wang, X. Su, Y. Liu, Y. Zhang, Y. Tang, Q. Ni and W. Liu, Adv. Sci., 2017, 4, 1600356 CrossRef.
- K. Mebrouk, M. Ciancone, T. Vives, S. Cammas-Marion, T. Benvegnu, C. Le Goff-Gaillard, Y. Arlot-Bonnemains, M. Fourmigué and F. Camerel, ChemMedChem, 2017, 12, 1753–1758 CrossRef CAS.
- K. Jayaramulu, J. Masa, O. Tomanec, D. Peeters, V. Ranc, A. Schneemann, R. Zboril, W. Schuhmann and R. A. Fischer, Adv. Funct. Mater., 2017, 27 Search PubMed.
- R. Sun, S. Liu, Q. Wei, J. Sheng, S. Zhu, Q. An and L. Mai, Small, 2017, 13, 1701744 CrossRef.
- H. Zhu, Z. Lai, Y. Fang, X. Zhen, C. Tan, X. Qi, D. Ding, P. Chen, H. Zhang and K. Pu, Small, 2017, 13, 1604139 CrossRef.
- D. Gao, P. Zhang, C. Liu, C. Chen, G. Gao, Y. Wu, Z. Sheng, L. Song and L. Cai, Nanoscale, 2015, 7, 17631–17636 RSC.
- Z. Zhou, J. Wang, W. Liu, C. Yu, B. Kong, Y. Sun, H. Yang, S. Yang and W. Wang, Nanoscale, 2014, 6, 12591–12600 RSC.
- S. Bano, S. Nazir, S. Munir, M. F. AlAjmi, M. Afzal and K. Mazhar, Int. J. Nanomed., 2016, 11, 3159 CrossRef CAS PubMed.
- Q. Tian, F. Jiang, R. Zou, Q. Liu, Z. Chen, M. Zhu, S. Yang, J. Wang, J. Wang and J. Hu, ACS Nano, 2011, 5, 9761–9771 CrossRef CAS.
- Q. Tian, J. Hu, Y. Zhu, R. Zou, Z. Chen, S. Yang, R. Li, Q. Su, Y. Han and X. Liu, J. Am. Chem. Soc., 2013, 135, 8571–8577 CrossRef CAS.
- K. Liu, X. Yan, Y.-J. Xu, L. Dong, L.-N. Hao, Y.-H. Song, F. Li, Y. Su, Y.-D. Wu and H.-S. Qian, Biomater. Sci., 2017, 5, 2403–2415 RSC.
- Y. Cui, X. Lai, L. Li, Z. Hu, S. Wang, J. E. Halpert, R. Yu and D. Wang, ChemPhysChem, 2012, 13, 2610–2614 CrossRef CAS.
- Z. Wan, C. Jia and Y. Wang, Nanoscale, 2015, 7, 12737–12742 RSC.
- T. Tian, L. Huang, L. Ai and J. Jiang, J. Mater. Chem. A, 2017, 5, 20985–20992 RSC.
- G. Song, J. Hao, C. Liang, T. Liu, M. Gao, L. Cheng, J. Hu and Z. Liu, Angew. Chem., Int. Ed., 2016, 55, 2122–2126 CrossRef CAS.
- L. Feng, K. Li, X. Shi, M. Gao, J. Liu and Z. Liu, Adv. Healthcare Mater., 2014, 3, 1261–1271 CrossRef CAS.
- Y. Wang, J. Yang, H. Liu, X. Wang, Z. Zhou, Q. Huang, D. Song, X. Cai, L. Li, K. Lin, J. Xiao, P. Liu, Q. Zhang and Y. Cheng, Biomaterials, 2017, 114, 97–105 CrossRef CAS.
- D. K. Roper, W. Ahn and M. Hoepfner, J. Phys. Chem. C, 2007, 111, 3636–3641 CrossRef CAS.
- S.-M. Zhou, D.-K. Ma, S.-H. Zhang, W. Wang, W. Chen, S.-M. Huang and K. Yu, Nanoscale, 2016, 8, 1374–1382 RSC.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560–2566 CrossRef CAS PubMed.
- T. Bao, W. Yin, X. Zheng, X. Zhang, J. Yu, X. Dong, Y. Yong, F. Gao, L. Yan and Z. Gu, Biomaterials, 2016, 76, 11–24 CrossRef CAS PubMed.
- H. Chen, Y. Ma, X. Wang, X. Wu and Z. Zha, RSC Adv., 2017, 7, 248–255 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Morphology of CPT@mNiS2 NSs, the cell viability of HepG2 cancer cells, etc. See DOI: 10.1039/c8tb02473a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |