A facile random copolymer strategy to achieve highly conductive polymer gel electrolytes for electrochemical applications†
Yong Min
Kim
,
Dong Gyu
Seo
,
Hwan
Oh
and
Hong Chul
Moon
 *
*
Department of Chemical Engineering, University of Seoul, Seoul 02504, Republic of Korea. E-mail: hcmoon@uos.ac.kr
First published on 30th November 2018
Abstract
A facile random copolymer strategy based on poly(styrene-ran-methyl methacrylate) (PS-r-PMMA) is proposed for the preparation of highly conductive and mechanically elastic solid-state gel electrolytes. In contrast to previous random copolymers serving as polymer hosts, PS-r-PMMA can be readily synthesized by one-pot reversible additional–fragmentation chain transfer (RAFT) polymerization. PS-r-PMMA and the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMI][TFSI]) are blended to fabricate physically cross-linked ion gel electrolytes. We systematically investigate the dependence of gel properties on experimental variables, such as the styrene fraction and molecular weight of PS-r-PMMAs, and the ion gel composition. The physical properties of the gels are optimized to simultaneously exhibit good ionic conductivity (∼0.98 mS cm−1) and mechanical resilience (∼7.2 × 104 Pa) at room temperature. The versatility of the PS-r-PMMA-based gels as a solid-state electrolyte platform is successfully demonstrated by applying it in two types of electrochemical devices, electrochemiluminescent (ECL) and electrochromic (EC) displays. These results imply that PS-r-PMMAs can be easily synthesized without post reactions and are a simple and effective polymer host for high-performance ion gel electrolytes for diverse electrochemical applications.
1. Introduction
Electrochemical devices have found diverse applications that include energy storage devices (e.g. battery and capacitor),1–7 electrical skins,8–11 and displays.12–20 The paradigm of electrolytes has been shifting from liquid- to solid-state as researchers have pursued faster, low-voltage, and bendable/wearable devices. However, a fully solid electrolyte cannot provide high ionic conductivity at room temperature, which limits its application spectrum.21,22 Polymer gel electrolytes (PGEs), particularly ion gels consisting of copolymers and room temperature ionic liquids (RTILs), are considered to be promising electrolyte platforms, because they are satisfactory with regard to both ionic conductivity and mechanical robustness.23–26 Also, ion gels do not dry even under reduced pressure or high temperature, in contrast to conventional gel-type electrolytes containing organic solvents. Therefore, issues related to the deterioration of device performance arising from concentration variation can be excluded.The most representative ion gels consist of a RTIL such as 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMI][TFSI]) and ABA triblock copolymers in which A blocks are designed not to be dissolved in the RTIL but the B mid-block is RTIL-soluble (e.g. polystyrene-block-poly(methyl methacrylate)-block-polystyrene, SMS).17,27,28 When these materials are blended, self-assembly into spherical A domains and a continuous ion conductive channel (i.e. B domains + RTIL) occurs to minimize the thermodynamic energetic state.24 As a result, physically cross-linked ion gels are obtained.29–31 In general, there is an inevitable trade-off between two important metrics to characterize electrolyte performance, ionic conductivity and mechanical robustness. The introduction of the chemical cross-linking of IL-insoluble A blocks enhanced the mechanical property of ion gels without ionic conductivity loss.32 However, complicated copolymer synthesis and additional post-processing, such as UV irradiation or thermal annealing, cannot be avoided.
To address this issue, much simpler systems such as homopolymer-based ion gels have been developed. Yang et al. fabricated free-standing solid polymer electrolytes based on poly(vinylidene fluoride) (PVDF) homopolymer and [EMI][TFSI], in which crystalline PVDF domains served as a RTIL-soluble part and amorphous domains swollen with [EMI][TFSI] provided the ion conductive channel.33 Although the resulting electrolytes exhibited outstanding mechanical properties with high ionic conductivity, the precise control of PVDF crystals is not trivial. Recently, a random copolymer, poly[styrene-ran-1-(4-vinylbenzyl)-3-methylimidazolium hexafluorophosphate], (P[S-r-VBMI][PF6]), was developed as a polymer host of ion gels.23 Even with a small portion (e.g. mole fraction ∼0.22) of the P[VBMI][PF6] block, a larger amount of ionic liquid could be fully incorporated and good ionic conductivity (σ > 1 mS cm−1 at RT) could be induced. This means that more PS segments can be present in the random copolymers, simultaneously providing larger mechanical resilience. Nonetheless, the synthesis of P[S-r-VBMI][PF6] requires two-step post reactions in addition to polymerization.
In this work, we present a very convenient strategy to prepare high-performance ion gel electrolytes based on one of the simplest random copolymers, poly(styrene-ran-methyl methacrylate) (PS-r-PMMA). Although PS-r-PMMAs have been extensively employed in diverse applications (e.g. as a neutral brush for balancing interfacial energy to control the orientation of block copolymer self-assembly structures),34–36 there is no report on their use in gel electrolytes as a polymer matrix. Moreover, PS-r-PMMA can be readily prepared by one-step reversible addition–fragmentation chain transfer (RAFT) polymerization. Thus, we investigated the feasibility of PS-r-PMMAs as a polymer host of gel electrolytes, and determined that styrene (Sty) components must be included at less than 29 mol%. Otherwise, homogeneous ion gels could not be achieved when incorporated with [EMI][TFSI]. Accordingly, we fixed the composition of Sty in PS-r-PMMA as 29 mol%. The variation in electrochemical and mechanical properties of gels composed of PS-r-PMMA and [EMI][TFSI] was systematically examined by varying the gel composition and total molecular weight of PS-r-PMMA. As a result, we were able to realize mechanically robust (elastic modulus ∼7.2 × 104 Pa) and highly conductive (σ ∼ 0.98 mS cm−1) solid-state electrolytes. The gels were successfully functionalized and applied in electrochemiluminescent (ECL) and electrochromic (EC) displays. Overall, the random copolymer strategy with PS-r-PMMAs that can be readily polymerized by a one-step reaction offers a simple and directional methodology to prepare high-performance electrolytes for electrochemical applications.
2. Experimental section
Materials
All chemicals were purchased from Sigma-Aldrich, except a red ECL luminophore of tris(2,2′-bipyridyl)-dichlororuthenium(II) hexahydrate (Ru(bpy)3Cl2) (Tokyo Chemical Industry Co. Ltd), and were used as received. A green ECL luminophore, 2,2′-bipyridylbis[2-(2′,4′difluorophenyl)pyridine]iridium(III) hexafluorophosphate (Ir(diFppy)2(bpy)PF6), was synthesized via a two-step reaction according to the literature.37,38 2,2′-Azobisisobutyro-nitrile (AIBN) was purified by recrystallization from a methanol solution. A room temperature ionic liquid (IL), 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMI][TFSI]), was prepared via an anion exchange reaction between 1-ethyl-3-methylimidazolium bromide ([EMI][Br]) and excess lithium bis(trifluoromethyl-sulfonyl)imide (LiTFSI) in deionized (DI)-water. To increase solubility in the ion gels, we exchanged halogen anions (Cl− or Br−) of ionic species (e.g. ECL luminophores and EC chromophores) with PF6−, with which homogeneous ECL or EC gels could be prepared. ITO-coated glass (sheet resistance: 10 Ω sq−1, Asahi Glass Co.) was washed with acetone (5 min), methanol (5 min), and isopropanol (5 min) under sonication and was employed as a transparent conducting electrode. The resulting electrodes were exposed to UV/ozone for 10 min before use. All random copolymers were prepared by a one-pot reversible addition–fragmentation chain transfer (RAFT) (Fig. S1a, ESI†). The detailed synthetic process, SEC traces (Fig. S1b, ESI†), and 1H NMR spectra (Fig. S2–S6, ESI†) of the prepared PS-r-PMMAs are provided in the ESI.† The molecular characteristics of the synthesized PS-r-PMMAs are summarized in Table 1. Three PS-r-PMMAs containing ∼29 mol% Sty, which can produce homogeneous ion gels with [EMI][TFSI], were classified by molecular weight (MW): PS-r-PMMA-L, PS-r-PMMA-M, and PS-r-PMMA-H where -L, -M, and -H refer to low, medium, and high MW, respectively (see Table 1).| Code | M n (g mol−1) | M w (g mol−1) | Đ | Styb (mol%) |
|---|---|---|---|---|
| a Measured by SEC calibrated with PS standards. b Determined by 1H NMR spectroscopy. | ||||
| 39.0-PS-r-PMMA | 113![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.19 | 39.0 |
| 33.5-PS-r-PMMA | 119![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.17 | 33.5 |
| PS-r-PMMA-L | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.11 | 29.1 |
| PS-r-PMMA-M | 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.17 | 29.0 |
| PS-r-PMMA-H | 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
271![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.23 | 29.4 |
Characterization
The molecular weight and polydispersity index (Đ) of the prepared PS-r-PMMA were measured by size exclusion chromatography (SEC) (LC-20AT, Shimadzu) using tetrahydrofuran (THF) (eluent) at 30 °C. The SEC was equipped with two PLgel 5 μm mixed-C 300 × 7.5 mm columns (Agilent Technologies) and a refractive index detector (Shimadzu, RID-20A). The calibration was carried out based on polystyrene standards. 1H NMR spectra were recorded using a Bruker digital Avance III 400 instrument with chloroform-d (CDCl3) as a solvent. The randomness of the prepared PS-r-PMMAs was confirmed by differential scanning calorimetry (DSC) (DSC 4000, PerkinElmer) under a N2 atmosphere. The sample (∼6.5 mg) was first heated to 200 °C and held for 20 min to remove thermal history, followed by quenching to 0 °C. Then, DSC thermograms were recorded upon the second heating at a rate of 10 °C min−1. The mechanical properties of the ion gels were evaluated using dynamic mechanical analysis (DMA) (Q800, TA instruments, USA), in which circular-shaped samples (thickness: 5.0 mm and diameter: 9.0 mm) were employed for compression tests. Stress–strain curves were recorded from 0% to 20% strain at a scan rate of 3 N min−1, and the elastic moduli were extracted from the slope of the line fitted to the linear regime. The ionic conductivities of the ion gels were determined by electrochemical impedance spectrometry (EIS) (IM6, ZAHNER), and the frequency range and AC amplitude were 10−1 to 106 Hz and 10 mV, respectively. The ion gels for EIS and DMA experiments were prepared by drop-casting of an acetone solution onto a slide glass. After fully drying the acetone, the resultant gels were filled into poly(tetrafluoroethylene) molds and annealed at 80 °C for 1 h. Then, the ion gels with desired size and shape were separated from the molds.To characterize the ECL emissive devices, the emission spectra, luminance, and CIE color coordinates of emitted light were obtained by using a spectroradiometer (CS-2000, Konica Minolta). Transient applied voltage and device current profiles were recorded using an oscilloscope (TDS2024C, Tektronix), but ECL profiles were collected using a photodetector (PDA36A, Thorlabs). Applied AC voltages were supplied by a function/arbitrary waveform generator (33210A, Keysight). To evaluate the ECD performances based on PS-r-PMMA gels, the changes in optical properties upon the application of external voltages were recorded using a UV-vis spectrometer (V-730, Jasco), from which several characteristics were extracted, including CIELAB color coordinates, dynamics, and coloration efficiency. A potentiostat (Wave Driver 10, Pine Instrument) was employed to investigate the redox behaviors of EC chromophores, where dmFc was used as an internal standard. The scan rate was 20 mV s−1.
Fabrication of electrochemical devices
Ion gels applied to electrochemical devices were based on PS-r-PMMA and ionic liquid ([EMI][TFSI]) with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 weight ratio, because both the ionic conductivity and the mechanical resilience were optimal at this composition. To make ECL gels, ECL luminophores, Ru(bpy)3(PF6)2 or Ir(diFppy)2(bpy)PF6 (∼10 wt% relative to [EMI][TFSI]), were added. On the other hand, equimolar [MHV(PF6)] (EC chromophore) and dmFc (anodic species) were introduced (typically, the amount of EC chromophore was ∼5 wt% relative to [EMI][TFSI]) to impart the functionality of electrochromism. All components (PS-r-PMMA, [EMI][TFSI]), and ECL/EC materials were dissolved in acetone. Then, the solution was drop-cast onto the substrate (e.g. ITO-coated glass), resulting in homogeneous functional polymer gel electrolytes. Then, another electrode was assembled on top of the gel, where 88 μm-thick double-sided tape served as an adhesive and spacer.
7 weight ratio, because both the ionic conductivity and the mechanical resilience were optimal at this composition. To make ECL gels, ECL luminophores, Ru(bpy)3(PF6)2 or Ir(diFppy)2(bpy)PF6 (∼10 wt% relative to [EMI][TFSI]), were added. On the other hand, equimolar [MHV(PF6)] (EC chromophore) and dmFc (anodic species) were introduced (typically, the amount of EC chromophore was ∼5 wt% relative to [EMI][TFSI]) to impart the functionality of electrochromism. All components (PS-r-PMMA, [EMI][TFSI]), and ECL/EC materials were dissolved in acetone. Then, the solution was drop-cast onto the substrate (e.g. ITO-coated glass), resulting in homogeneous functional polymer gel electrolytes. Then, another electrode was assembled on top of the gel, where 88 μm-thick double-sided tape served as an adhesive and spacer.
3. Results and discussion
The polymer host of PS-r-PMMA in this work was prepared via one-step RAFT polymerization without further modifications. The constituents of PS-r-PMMAs, the IL-insoluble PS and IL-soluble PMMA, impart mechanical strength and ion conductivity, respectively, when copolymers and [EMI][TFSI] are blended. Fig. S1b (ESI†) shows the SEC chromatograms of the three PS-r-PMMAs employed in this study, in which the molecular weight of PS-r-PMMA was controlled by adjusting the amount of monomer/RAFT agent feed ratio or polymerization time. All copolymers showed a sharp and single molecular distribution (see Fig. S1b in the ESI†). The randomness of the PS-r-PMMAs was revealed by thermo-analytical experiments. For example, the DSC thermogram of PS-r-PMMA-M exhibited only a single glass transition temperature (Tg) at ∼113.5 °C (Fig. S7, ESI†), which implies that the synthesized PS-r-PMMA-M corresponds to a random copolymer and not a block or gradient copolymer. Moreover, we calculated a weight fraction of Sty (wSty) in the PS-r-PMMA-M using the Fox equation (see eqn (1)) with the Tg of PS (∼100 °C)39 and PMMA (∼120 °C),40 giving wSty ∼ 31.3.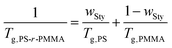 | (1) |
It is noted that the PS mole fraction of the resulting PS-r-PMMA determined by 1H NMR spectroscopy or DSC is slightly larger than the feed monomer mole fraction of styrene, 0.20. Thus, we estimated the Sty mole fraction in the random copolymer using the Mayo–Lewis equation:41
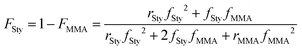 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MMA was 0.2
MMA was 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8.
0.8.
The composition of PS-r-PMMA is a critical factor to determine the physical properties of the resulting ion gel electrolytes. As the IL-insoluble Sty fraction increases, more mechanically robust gels can be made, but there is also an increase in the possibility of obtaining heterogeneous gels that cannot be applied in electrochemical electronics. When we employed PS-r-PMMA with 33.5 mol% Sty or higher, a non-uniform and turbid ion gel was obtained (see Fig. 1a and b). On the other hand, homogeneous gel electrolytes were realized with PS-r-PMMA including 29.0 mol% Sty (Fig. 1c). Therefore, in subsequent studies, we employed a PS-r-PMMA with 29.0 mol% Sty.
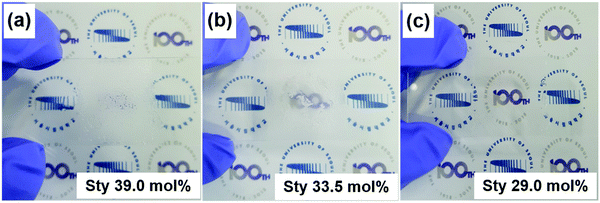 | ||
| Fig. 1 Photographs of ion gels based on PS-r-PMMAs with various Sty mol% contents: (a) 39.0, (b) 33.5, and (c) 29.0 mol%, in which 70 wt% ionic liquid ([EMI][TFSI]) was included in the gels. | ||
The fundamental metrics for evaluating ion gel performance are mechanical strength and ionic conductivity, which are estimated using dynamic mechanical analysis (DMA) and electrochemical impedance spectroscopy (EIS), respectively. Fig. 2a shows the stress–strain curves of ion gels consisting of PS-r-PMMA-H and [EMI][TFSI], in which five different gel compositions were investigated. Elastic moduli were extracted from the slope of a line fitted to the linear regime. As the portion of copolymer increases, larger mechanical resilience is observed. For example, an elastic modulus of ∼6.7 × 105 Pa was achieved at a weight ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (PS-r-PMMA-H
60 (PS-r-PMMA-H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EMI][TFSI]), whereas a much lower value, ∼2.3 × 104 Pa, was measured at a weight ratio of 20
[EMI][TFSI]), whereas a much lower value, ∼2.3 × 104 Pa, was measured at a weight ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 (PS-r-PMMA-H
80 (PS-r-PMMA-H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EMI][TFSI]). As anticipated from the trade-off relation between the mechanical properties and ionic conductivity, the highest ionic conductivity was observed from the gel containing 20 wt% PS-r-PMMAs. Fig. 2b displays the dependence of Z′ on the frequency with various gel compositions. The conductivity value (σ) was calculated using σ = h/AR where h, A, and R correspond to the gel thickness, area, and bulk resistance, respectively.14,23 The plateau Z′ at high frequency was selected to evaluate ionic conductivity, because the resistance of bulk gels mainly contributes to the impedance. A relatively low ionic conductivity of ∼0.16 mS cm−1 was measured at 40 wt% PS-r-PMMA-H, whereas a dramatic increase in conductivity (∼2.4 mS cm−1) was observed when the content of PS-r-PMMA-H in the gel was reduced to 20 wt%.
[EMI][TFSI]). As anticipated from the trade-off relation between the mechanical properties and ionic conductivity, the highest ionic conductivity was observed from the gel containing 20 wt% PS-r-PMMAs. Fig. 2b displays the dependence of Z′ on the frequency with various gel compositions. The conductivity value (σ) was calculated using σ = h/AR where h, A, and R correspond to the gel thickness, area, and bulk resistance, respectively.14,23 The plateau Z′ at high frequency was selected to evaluate ionic conductivity, because the resistance of bulk gels mainly contributes to the impedance. A relatively low ionic conductivity of ∼0.16 mS cm−1 was measured at 40 wt% PS-r-PMMA-H, whereas a dramatic increase in conductivity (∼2.4 mS cm−1) was observed when the content of PS-r-PMMA-H in the gel was reduced to 20 wt%.
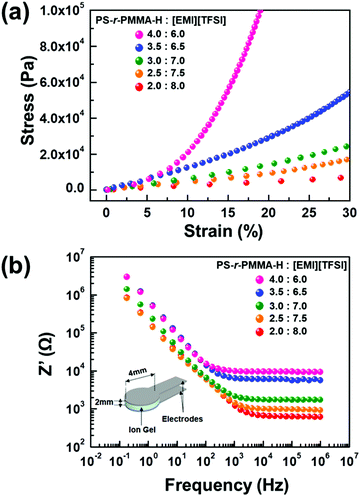 | ||
| Fig. 2 (a) Stress–strain curves, and (b) frequency dependence of resistance (Z′) for ion gels, in which five different weight ratios of PS-r-PMMA-H and [EMI][TFSI] were tested. | ||
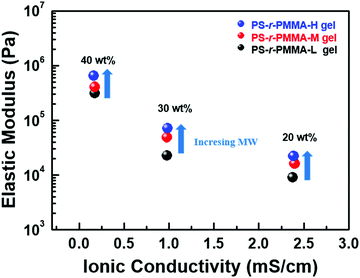
We also controlled the chain length of PS-r-PMMA, for which two molecular weights, Mn = 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (PS-r-PMMA-L) and 117
000 (PS-r-PMMA-L) and 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (PS-r-PMMA-M), were prepared (see Table 1 and Fig. S1b, ESI†) and applied to the gels. Fig. 3 summarizes the effect of molecular weights on the gel performance (see also detailed DMA and EIS experimental results given in Fig. S9 and S10 of the ESI†). As the molecular weight increased, mechanical resilience was enhanced, but ionic motions were not significantly affected (namely, a similar level of conductivity was measured). The gel optimized with PS-r-PMMA-H (30 wt%) and [EMI][TFSI] (70 wt%) showed both acceptable ionic conductivity (∼0.98 mS cm−1) and elastic modulus (∼7.2 × 104 Pa).
000 (PS-r-PMMA-M), were prepared (see Table 1 and Fig. S1b, ESI†) and applied to the gels. Fig. 3 summarizes the effect of molecular weights on the gel performance (see also detailed DMA and EIS experimental results given in Fig. S9 and S10 of the ESI†). As the molecular weight increased, mechanical resilience was enhanced, but ionic motions were not significantly affected (namely, a similar level of conductivity was measured). The gel optimized with PS-r-PMMA-H (30 wt%) and [EMI][TFSI] (70 wt%) showed both acceptable ionic conductivity (∼0.98 mS cm−1) and elastic modulus (∼7.2 × 104 Pa).
We compared the ionic conductivity and mechanical modulus of PS-r-PMMA-based ion gels and those of other ion gels including the same ionic liquid, [EMI][TFSI] (see Table 2). The direct comparison is not fair due to the different gel composition. Namely, the trade-off between conductivity and mechanical resilience was observed. For instance, a smaller polymer content (e.g. 10 wt%) resulted in high ionic conductivity, but the mechanical properties were not good. Nonetheless, we could conclude that the properties of the PS-r-PMMA-based gels in this work are comparable to those of the previously reported systems. When considering the relatively simple synthetic process, PS-r-PMMA is a simple and effective polymer host for high performance gel electrolytes.
| Ref. | Polymer | Ionic liquid (IL) | Polymer/IL (wt/wt) | Temperature (°C) | Ionic conductivity (mS cm−1) | Modulus (Pa) |
|---|---|---|---|---|---|---|
| a Moduli measured by compression stress test. b Moduli measured by shear stress test. c P[S-r-VBMI][PF6]: poly[styrene-ran-1-(4-vinylbenzyl)-3-methylimidazolium hexafluorophosphate]. d P(VDF-co-HFP): poly(vinylidene fluoride-co-hexafluoropropylene). e SMS: poly(styrene-b-methyl methacrylate-b-styrene). f SOS: poly(styrene-b-ethylene oxide-b-styrene). g PNIPAm-b-PEO-b-PNIPAm: poly(N-isopropyl acrylamide-b-ethylene oxide-b-N-isopropyl acrylamide). | ||||||
| This work | PS-r-PMMA | [EMI][TFSI] | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
25 | ∼0.98 | ∼7.2 × 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
∼7.5 × 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
||||||
| Ref. 23 | P[S-r-VBMI][PF6]c | [EMI][TFSI] | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
25 | ∼1.15 | ∼1.0 × 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Ref. 14 | P(VDF-co-HFP)d | [EMI][TFSI] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
20 | ∼6.7 | ∼1.8 × 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Ref. 31 | SMSe | [EMI][TFSI] | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
30 | ∼0.4 | ∼2.0 × 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Ref. 32 | SOS-N3f (after crosslinking) | [EMI][TFSI] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
25 | ∼6.0 | ∼8.0 × 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Ref. 47 | PNIPAm-b-PEO-b-PNIPAmg | [EMI][TFSI] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
25 | ∼5.0 | ∼1.0 × 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
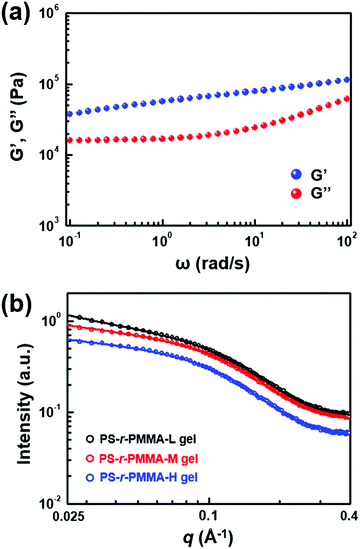
To understand the structural stability of the gels, dynamic isothermal frequency sweeps of storage (G′) and loss (G′′) moduli were conducted. Fig. 4a shows the variations in G′ and G′′ of the optimized gel based on 30 wt% PS-r-PMMA-H, in which G′ values were higher than G′′ irrespective of the applied frequency. This result implies that the gel corresponds to an elastic solid with a stable network structure. Small angle X-ray scattering (SAXS) experiments provide further insights into the ion gel formation with PS-r-PMMAs (Fig. 4b). Featureless scattering profiles were detected for neat PS-r-PMMA copolymers (see Fig. S11 in the ESI†), implying a homogeneous phase. In contrast, the SAXS profiles of the gels presented the Guinier regime in the low q region. By fitting the scattering profiles using the unified model in the Irena program developed by Jan Ilavky,43–46 radius of gyration (Rg) values were extracted as 1.25, 1.31, and 1.38 nm for the gels based on PS-r-PMMA-L, PS-r-PMMA-M, and PS-r-PMMA-H, respectively. When we take into account undissolved PS within the PMMA matrix largely swollen with [EMI][TFSI], the estimated Rg is likely to be that of the PS domains.
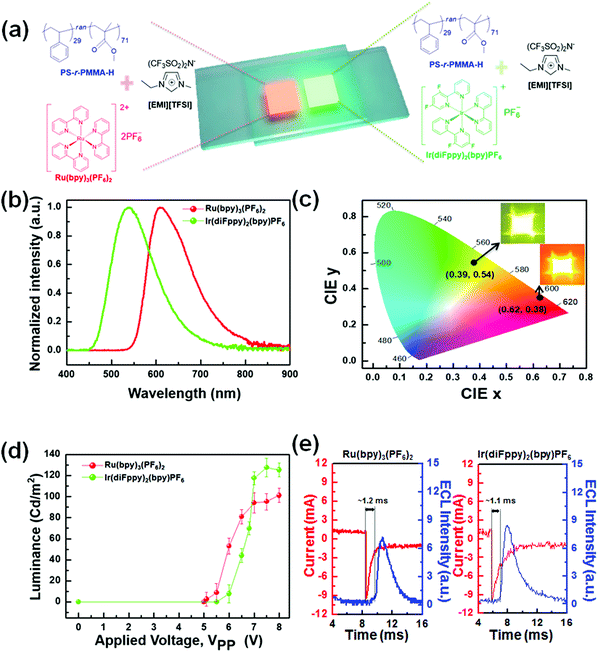
In order to demonstrate the successful applicability to electrochemical devices, we fabricated two types of displays: emissive electrochemiluminescent (ECL) displays and reflective electrochromic (EC) displays. Fig. 5a depicts a schematic illustration of the ECL devices based on the gels with PS-r-PMMA-H. ECL devices utilize electrochemical redox reactions of luminophores to produce excited species that emit light. Therefore, a device structure can be very simple, consisting of just two electrodes and an ECL electrolyte layer (Fig. 5a). Ru(bpy)32+ and Ir(diFppy)2(bpy)+ were chosen as ECL luminophores due to their good solubility in the gel and ability to form stable redox species that can participate in the annihilation reactions.48 An example of the series of annihilation reactions is given for Ru(bpy)32+ (see eqn (3)–(6)).49,50 From the emission spectra (Fig. 5b), the wavelength at maximum intensity (λmax) values for the ECL devices including Ru(bpy)32+ and Ir(diFppy)2(bpy)+ were determined to be ∼610 and ∼540 nm, respectively. Namely, Ru(bpy)32+ successfully served as a red-orange light emitter (CIE coordinates: (0.62, 0.38)), while yellow-green colored (CIE coordinates: (0.39, 0.54)) light was emitted from Ir(diFppy)2(bpy)+ (Fig. 5c).
| Ru(bpy)32+ + e− → Ru(bpy)3˙1+ | (3) |
| Ru(bpy)32+ − e− → Ru(bpy)3˙3+ | (4) |
| Ru(bpy)3˙1+ + Ru(bpy)3˙3+ → Ru(bpy)32+* + Ru(bpy)32+ | (5) |
| Ru(bpy)32+* → Ru(bpy)32+ + hν | (6) |
Notably, to minimize the diffusion distance of reduced and oxidized species and to facilitate the electron-transfer reaction of eqn (5), we supplied AC square voltages characterized as peak-to-peak voltage (VPP). When we investigated the voltage dependence of luminance, the turn-on voltage (∼5.1 VPP) of Ru(bpy)32+-based devices was slightly lower than that (∼6.0 VPP) of the devices containing Ir(diFppy)2(bpy)+ (Fig. 5d). In addition, the luminance was saturated at a certain voltage. Redox species were consumed more rapidly and depleted near the electrode when a higher voltage was applied. However, fresh redox sources cannot be supplied from the bulk by diffusion as fast as the electrochemical reaction. Thus, the observation of luminance saturation can be rationalized. Compared to the luminance of other gel electrolyte systems (e.g. ∼60 and ∼80 Cd m−2 of the PS-b-PMMA-b-PS gel including Ru(bpy)32+17 and Ir(diFppy)2(bpy)+,17 and ∼90 and ∼50 Cd m−2 of the P(VDF-co-HFP) gel including Ru(bpy)32+51 and Ir(diFppy)2(bpy)+,52 respectively), the devices with PS-r-PMMA-based gels showed favorably comparable brightness values, ∼100 and ∼130 Cd m−2 for red-orange and yellow-green colors, respectively. We also examined the dynamic response of the devices, for which we measured a time interval (namely, response time) between the transient current and ECL profiles. Both showed a response of milliseconds (Fig. 5e). These results indicate that PS-r-PMMA-based gels can be successfully used as an electrolyte platform for ECL devices.
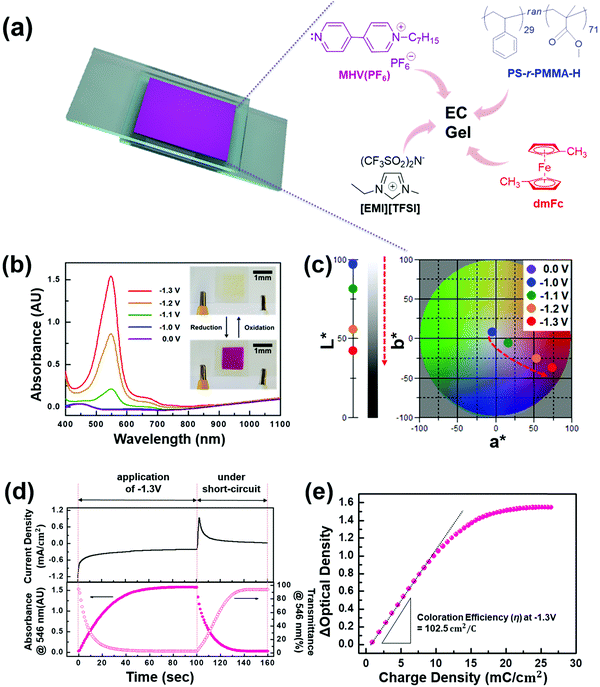
To verify the broad application spectrum of PS-r-PMMA-based gels, we prepared EC gels by adding mono-heptyl viologen (MHV+) and dimethyl ferrocene (dmFc). A quite simple device configuration of electrode/functional gel/electrode trilayers is similar to that of the ECL devices, except that it utilizes EC gel (Fig. 6a). Although dmFc (anodic species) causes yellowish bleached states when incorporated, it must be included for low-voltage and stable device operation.12–14 The change in optical properties of the EC devices was investigated with various applied voltages. Fig. 6b displays the UV-vis absorption spectra at various voltages, in which no significant absorption was detected below −1.0 V except a weak peak at ∼440 nm arising from the dissolved dmFc. As the applied voltage increased, a distinct peak at ∼546 nm appeared at −1.1 V and became more intense at higher voltages. As shown in the inset of Fig. 6b, the difference in color between bleached and colored states could be clearly detected even by the naked eye. The variation in color was quantitatively analyzed using the CIELAB color coordinates (L*, a*, b*) extracted from the UV-vis spectra in Fig. 6b. Before coloration, the L*, a*, and b* values were 95.5, −2.9, and 8.0, respectively, corresponding to a slightly yellowish color, as anticipated. A great shift of color coordinates commenced from the application of −1.1 V, and they eventually reached the coordinates corresponding to a strong magenta color (48.6, 76.2, −37.0) at −1.3 V (Fig. 6c).
We also studied other device characteristics such as the coloration/bleaching time (Fig. 6d) and coloration efficiency (Fig. 6e). Fig. 6d displays the transient current density and corresponding temporal change in absorbance at ∼546 nm (λmax), in which coloration and bleaching were carried out at −1.3 V and under short-circuit conditions, respectively. The time (Δt90%) required to achieve 90% change of maximum transmittance contrast for coloration was determined to be ∼20 s, but a slightly longer (∼29 s) duration was necessary for recovery (i.e. bleaching), which is similar to the behavior of previous gel-based EC devices.13,53 We also evaluated the coloration efficiency (η) using the expression: η = ΔOD/ΔQ = log(Tb/Tc)/ΔQ, where ΔOD is the optical density change defined by the logarithm of the ratio between the transmittance at the bleached state (Tb) and colored state (Tc) and ΔQ is the amount of charge required to induce the corresponding ΔOD. The linear regime in the plots of ΔOD versus ΔQ (Fig. 6e) was fitted with a line, and the slope of the line was extracted as η. The resulting η, ∼102.5 cm2 C−1 at −1.3 V (Fig. 6e), was similar to those (∼87.5,14 and ∼94.5 cm2 C−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53) of previously reported EC devices based on poly(vinylidene fluoride-co-hexafluoropropylene) (P(VDF-co-HFP)) gel containing MHV+.
53) of previously reported EC devices based on poly(vinylidene fluoride-co-hexafluoropropylene) (P(VDF-co-HFP)) gel containing MHV+.
4. Conclusion
In this work, we present a facile methodology to prepare balanced ion gel electrolytes based on PS-r-PMMA copolymers. We carried out systematic studies on the correlation between the resulting gel performance and experimentally adjustable parameters such as PS mol% and the molecular weight of PS-r-PMMA, and gel composition. As a result, we concluded that (1) to make homogeneous ion gels, PS contents in the copolymer should be less than ∼29 mol%, (2) increasing molecular weight was an efficient strategy for enhancing the mechanical resilience, and (3) the gels containing 30 wt% PS-r-PMMA-H exhibited the optimized properties of an ionic conductivity of ∼0.98 mS cm−1 and elastic modulus of ∼7.2 × 104 Pa at RT. To demonstrate the versatility, PS-r-PMMA-based gels were functionalized to include either ECL or EC. Then, those functionalized materials were applied to electrochemical emissive (i.e. ECL) and reflective (i.e. EC) displays, in which the gel successfully served as a highly conductive solid-state electrolyte. We comment that the ion gels based on PS-r-PMMAs are readily available because PS-r-PMMAs do not require complex synthesis and are even commercially available. However, the performance of the resulting gels and their electrochemical devices are comparable to those of other gel electrolytes. Therefore, PS-r-PMMA is a promising polymer host for high performance gel electrolytes, although the trade-off between ionic conductivity and mechanical robustness was also observed, similar to previously reported systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2016R1C1B2006296). The SAXS experiments were performed at the 9A beamline of Pohang Accelerator Laboratory (PAL) (Korea). The authors thank Dr Hyungju Ahn for assistance and helpful discussion on SAXS experiments.References
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326–1330 CrossRef CAS.
- L. Qie, W.-M. Chen, Z.-H. Wang, Q.-G. Shao, X. Li, L.-X. Yuan, X.-L. Hu, W.-X. Zhang and Y.-H. Huang, Adv. Mater., 2012, 24, 2047–2050 CrossRef.
- A. Vlad, N. Singh, J. Rolland, S. Melinte, P. M. Ajayan and J.-F. Gohy, Sci. Rep., 2014, 4, 4315 CrossRef CAS.
- J.-S. Zheng, L. Zhang, A. Shellikeri, W. Cao, Q. Wu and J. P. Zheng, Sci. Rep., 2017, 7, 41910 CrossRef CAS PubMed.
- G. Yu, L. Hu, M. Vosgueritchian, H. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 2905–2911 CrossRef CAS.
- H. Qin and M. J. Panzer, ChemElectroChem, 2017, 4, 2556–2562 CrossRef CAS.
- E.-H. Kil, K.-H. Choi, H.-J. Ha, S. Xu, J. A. Rogers, M. R. Kim, Y.-G. Lee, K. M. Kim, K. Y. Cho and S.-Y. Lee, Adv. Mater., 2013, 25, 1395–1400 CrossRef CAS.
- H.-H. Chou, A. Nguyen, A. Chortos, J. W. F. To, C. Lu, J. Mei, T. Kurosawa, W.-G. Bae, J. B.-H. Tok and Z. Bao, Nat. Commun., 2015, 6, 8011 CrossRef CAS.
- S. Y. Oh, S. Y. Hong, Y. R. Jeong, J. Y. Yun, H. Park, S. W. Jin, G. Lee, J. H. Oh, H. Lee, S.-S. Lee and J. S. Ha, ACS Appl. Mater. Interfaces, 2018, 10, 13729–13740 CrossRef CAS.
- B. C.-K. Tee, C. Wang, R. Allen and Z. Bao, Nat. Nanotechnol., 2012, 7, 825–832 CrossRef CAS.
- X. Wang, Y. Gu, Z. Xiong, Z. Cui and T. Zhang, Adv. Mater., 2014, 26, 1336–1342 CrossRef CAS.
- H. C. Moon, C.-H. Kim, T. P. Lodge and C. D. Frisbie, ACS Appl. Mater. Interfaces, 2016, 8, 6252–6260 CrossRef CAS PubMed.
- H. C. Moon, T. P. Lodge and C. D. Frisbie, Chem. Mater., 2015, 27, 1420–1425 CrossRef CAS.
- H. Oh, D. G. Seo, T. Y. Yun, C. Y. Kim and H. C. Moon, ACS Appl. Mater. Interfaces, 2017, 9, 7658–7665 CrossRef CAS PubMed.
- T. Nobeshima, T. Morimoto, K. Nakamura and N. Kobayashi, J. Mater. Chem., 2010, 20, 10630–10633 RSC.
- T. Nobeshima, M. Nakakomi, K. Nakamura and N. Kobayashi, Adv. Opt. Mater., 2013, 1, 144–149 CrossRef.
- H. C. Moon, T. P. Lodge and C. D. Frisbie, J. Am. Chem. Soc., 2014, 136, 3705–3712 CrossRef CAS.
- H. C. Moon, T. P. Lodge and C. D. Frisbie, Chem. Mater., 2014, 26, 5358–5364 CrossRef CAS.
- K. Hong, M.-G. Kim, H. M. Yang, D. C. Lim, J. Y. Lee, S. J. Kim, I. Lee, K. H. Lee and J.-L. Lee, Adv. Energy Mater., 2016, 6, 1600651 CrossRef.
- H. Hwang, J. K. Kim and H. C. Moon, J. Mater. Chem. C, 2017, 5, 12513–12519 RSC.
- T. Caruso, S. Capoleoni, E. Cazzanelli, R. G. Agostino, P. Villano and S. Passerini, Ionics, 2012, 8, 36–43 CrossRef.
- D. F. Miranda, C. Versek, M. T. Tuominen, T. P. Russell and J. J. Watkins, Macromolecules, 2013, 46, 9313–9323 CrossRef CAS.
- D. G. Seo and H. C. Moon, Adv. Funct. Mater., 2018, 28, 1706948 CrossRef.
- T. P. Lodge, Science, 2008, 321, 50–51 CrossRef CAS PubMed.
- S. Zhang, K. H. Lee, C. D. Frisbie and T. P. Lodge, Macromolecules, 2011, 44, 940–949 CrossRef CAS.
- F. Lind, L. Rebollar, P. Bengani-Lutz, A. Asatekin and M. J. Panzer, Chem. Mater., 2016, 28, 8480–8483 CrossRef CAS.
- H. Yang, M. Huang, J. Wu, Z. Lan, S. Hao and J. Lin, Mater. Chem. Phys., 2008, 110, 38–42 CrossRef CAS.
- S. Imaizumi, H. Kokubo and M. Watanabe, Macromolecules, 2012, 45, 401–409 CrossRef CAS.
- Y. Kitazawa, T. Ueki, L. D. McIntosh, S. Tamura, K. Niitsuma, S. Imaizumi, T. P. Lodge and M. Watanabe, Macromolecules, 2016, 49, 1414–1423 CrossRef CAS.
- C. C. Hall, C. Zhou, S. P. O. Danielsen and T. P. Lodge, Macromolecules, 2016, 49, 2298–2306 CrossRef CAS.
- B. Tang, S. P. White, C. D. Frisbie and T. P. Lodge, Macromolecules, 2015, 48, 4942–4950 CrossRef CAS.
- Y. Gu, S. Zhang, L. Martinetti, K. H. Lee, L. D. Mclntosh, C. D. Frisbie and T. P. Lodge, J. Am. Chem. Soc., 2013, 135, 9652–9655 CrossRef CAS PubMed.
- H. M. Yang, Y. K. Kwon, S. B. Lee, S. Kim, K. Hong and K. H. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 8813–8818 CrossRef CAS PubMed.
- P. Mansky, Y. Liu, E. Huang, T. P. Russell and C. Hawker, Science, 1997, 275, 1458–1460 CrossRef CAS.
- P. Mansky, T. P. Russell, C. J. Hawker, M. Pitsikalis and J. Mays, Macromolecules, 1997, 30, 6810–6813 CrossRef CAS.
- E. Huang, T. P. Russell, C. Harrison, P. M. Chaikin, R. A. Register, C. J. Hawker and J. Mays, Macromolecules, 1998, 31, 7641–7650 CrossRef CAS.
- D. Tordera, M. Delgado, E. Ortí, H. J. Bolink, J. Frey, Md. K. Nazeeruddin and E. Baranoff, Chem. Mater., 2012, 24, 1896–1903 CrossRef CAS.
- S. Sprouse, K. A. King, P. J. Spellane and R. J. Watts, J. Am. Chem. Soc., 1984, 106, 6647–6653 CrossRef CAS.
- I. Manners, Synthetic Metal-Containing Polymers, Wiley-VCH, Weinheim, Germany, 2003 Search PubMed.
- J. H. Park, D. K. Hwang, J. Lee, S. Im and E. Kim, Thin Solid Films, 2007, 515, 4041–4044 CrossRef CAS.
- F. R. Mayo and F. M. Lewis, J. Am. Chem. Soc., 1944, 66, 1594–1601 CrossRef CAS.
- Z. Guo, G. Yang, D. Wan and J. Huang, J. Appl. Polym. Sci., 2001, 82, 1474–1482 CrossRef CAS.
- S. J. Lou, J. M. Szarko, T. Xu, L. Yu, T. J. Marks and L. X. Chen, J. Am. Chem. Soc., 2011, 133, 20661–20663 CrossRef CAS PubMed.
- G. Beaucage, J. Appl. Crystallogr., 1995, 28, 717–728 CrossRef CAS.
- G. Beaucage, J. Appl. Crystallogr., 1996, 29, 134–146 CrossRef CAS.
- B. Hammouda, J. Appl. Crystallogr., 2010, 43, 716 CrossRef CAS.
- Y. He and T. P. Lodge, Chem. Commun., 2007, 2732–2734 RSC.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, P. S. Francis, N. W. Barnett and C. F. Hogan, Chem. Sci., 2013, 4, 977–982 RSC.
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed.
- R. J. Forster, P. Bertoncello and T. E. Keyes, Annu. Rev. Anal. Chem., 2009, 2, 359–385 CrossRef CAS PubMed.
- K. Hong, Y. K. Kwon, J. Ryu, J. Y. Lee, S. H. Lee and K. H. Lee, Sci. Rep., 2016, 6, 29805 CrossRef CAS PubMed.
- H. C. Moon, T. P. Lodge and C. D. Frisbie, J. Mater. Chem. C, 2016, 4, 8448–8453 RSC.
- T. Y. Yun and H. C. Moon, Org. Electron., 2018, 56, 178–185 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedure, SEC traces, 1H NMR spectra, and DSC thermograms of employed PS-r-PMMAs, dimensional stability test, stress–strain curves and frequency dependence of the resistance (Z′) for the gels with PS-r-PMMA-L and PS-r-PMMA-M, SAXS profiles of neat PS-r-PMMA copolymers. See DOI: 10.1039/c8tc05092a |
| This journal is © The Royal Society of Chemistry 2019 |