Red phosphorus decorated graphene oxide nanosheets: label-free DNA detection†
Tapas Kumar
Mandal‡
 *a,
Yong Rok
Lee
*a,
Yong Rok
Lee
 *a and
Nargish
Parvin‡
*a and
Nargish
Parvin‡
 *b
*b
aSchool of Chemical Engineering, Yeungnam University, Gyeongsan 38541, Republic of Korea. E-mail: tps.mndl@gmail.com; yrlee@yu.ac.kr
bState Key Laboratory of Biochemical Engineering, CAS Centre for Excellence in Nanoscience, Institute of Process Engineering, Chinese Academy of Sciences, No. 1 Beiertiao, Zhongguancun, Beijing 100190, P. R. China. E-mail: parvin@ipe.ac.cn; nargish.parvin@gmail.com
First published on 22nd November 2019
Abstract
A straightforward synthetic strategy is developed in this study to synthesize highly fluorescent red phosphorus on nitrogen-doped reduced graphene oxide (f-RP@N-rGO) nanosheets in an aqueous medium; this is used as a novel detection platform for the label-free real-time sensing of nucleic acids with low background noise and a high signal-to-noise ratio.
At an early stage, the non-invasive diagnosis of disease is crucial as a therapeutic intervention, and it is mostly easy to carry out and effective. Therefore, it is necessary to detect changes in biological compounds in body fluids while avoiding biopsies. A blood test is the best health screening process, because many biomolecules accumulate as by-products from unhealthy parts of organisms.1–3
2D (two-dimensional) graphene, consisting of sp2 hybridized carbon atoms, is becoming a popular nanosystem due to its unique multi-functional characteristics, such as good stability, a large surface area,4 and high electrical5 and thermal conductivity.6 These features make graphene a favourable candidate for biological applications, such as in drug delivery and therapeutics.7 As graphene demonstrates significant amphiphilicity and plasmonic properties, it has emerged as a reliable candidate for biosensor applications. Several methods have been developed to detect deoxyribonucleic acid (DNA) based on GO or rGO, such as electrochemical biosensors8–13 and field-effect transistor biosensors.14,15 Moreover, current 2D nanomaterial-based fluorescence sensors for DNA and RNA require pre-labelling, which is a highly expensive and lengthy process.15,16 With regard to sensors, label-free biosensors have been gaining a lot of attention recently, as they do not require any fluorescent or chemical tags and they can still achieve high selectivity and sensitivity, which is considered challenging. In this regard, the potential use of red phosphorus particles on reduced graphene oxide nanosheets (NSs) to enhance fluorescence has been explored extensively for target DNA sensors with low background noise and high signal-to-noise ratios, increasing the selectivity and sensitivity of NS sensors.
In this study, we have synthesized highly fluorescing red phosphorus on nitrogen-doped reduced graphene oxide (f-RP@N-rGO) NSs using a straightforward thermochemical method. The synthesized f-RP@N-rGO NSs serve as a fluorescent platform to identify target DNA strands. To our knowledge, this is the first example of the usage of f-RP@N-rGO as a 2D material with fluorescence modulation leading to the label-free detection of target DNA molecules.
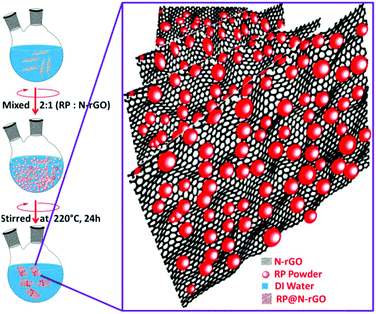
The synthetic protocol for obtaining fluorescent red phosphorus on nitrogen-doped reduced graphene oxide (f-RP@N-rGO) is illustrated in Fig. 1. f-RP@N-rGO showed good dispersion for several days without the addition of a surfactant. Even after a few months, the f-RP@N-rGO suspension did not show any precipitation. N-rGO provides highly reactive functional groups that can hold oxidized red phosphorus (RP) particles through hydrogen bonding, as previously described by other research groups.17–19 The fluorescent N-rGO layers not only electrostatically interact with the RP particles, but they also prevent these particles from aggregating. For loading RP onto the fluorescent N-rGO, ground RP powder was mixed with a N-rGO dispersion at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using a typical procedure based on the literature.20,21 Then 0.05 mmol of hexadecyltrimethylammonium bromide was immediately added and the mixture was magnetically stirred for 24 h at 220 °C. The as-prepared f-RP@N-rGO composite was then centrifuged at 7000g for 30 min with deionized (DI) water until it was nearly neutral. The product was then dried in a vacuum oven overnight at 60 °C. After the centrifugation and overnight vacuum drying of f-RP@N-rGO, the surface morphology was analyzed using transmission electron microscopy (TEM) and other characterization techniques.
1 using a typical procedure based on the literature.20,21 Then 0.05 mmol of hexadecyltrimethylammonium bromide was immediately added and the mixture was magnetically stirred for 24 h at 220 °C. The as-prepared f-RP@N-rGO composite was then centrifuged at 7000g for 30 min with deionized (DI) water until it was nearly neutral. The product was then dried in a vacuum oven overnight at 60 °C. After the centrifugation and overnight vacuum drying of f-RP@N-rGO, the surface morphology was analyzed using transmission electron microscopy (TEM) and other characterization techniques.
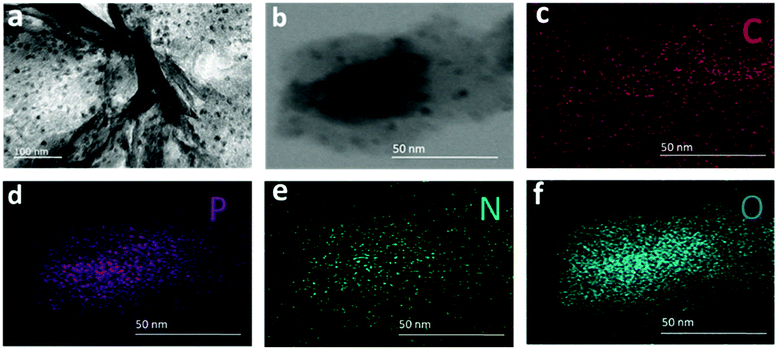
In the TEM image of the synthesized f-RP@N-rGO shown in Fig. 2a, most of the N-rGO nanosheets (NSs) exhibit a planar surface area (Fig. S1a in the ESI†) and the phosphorus particles (black spots) are deposited onto the N-rGO nanosheets densely and uniformly (Fig. S1b and S1c in the ESI†) after loading. Also, the amorphous nature of the particles can be seen in the enhanced TEM images of f-RP@N-rGO shown in Fig. S1b and S1c in the ESI.† The corresponding EDS (energy-dispersive X-ray spectroscopy) profiles for red phosphorus element of the N-rGO sheets and the f-RP@N-rGO NSs, in which the RP peak distinctly appears after loading, are shown in Fig. S2a and S2b, respectively, in the ESI.† STEM imaging of the f-RP@N-rGO NSs and the corresponding EDS element mapping profiles are shown in Fig. 2b–f. The deep black spots on the N-rGO sheets shown in the TEM and STEM images can be confirmed as phosphorus particles. From the TEM and EDS results, it can be concluded that the solid dark dots with diameters of 2–15 nm in the TEM image relate to small RP particles and the shiny components represent N-rGO.
Fluorescence images of the as-prepared materials obtained using white light and different fluorescence microscopy bandpass filters are depicted in Fig. S3 in the ESI,† which confirm the fluorescence properties of the f-RP@N-rGO NSs.
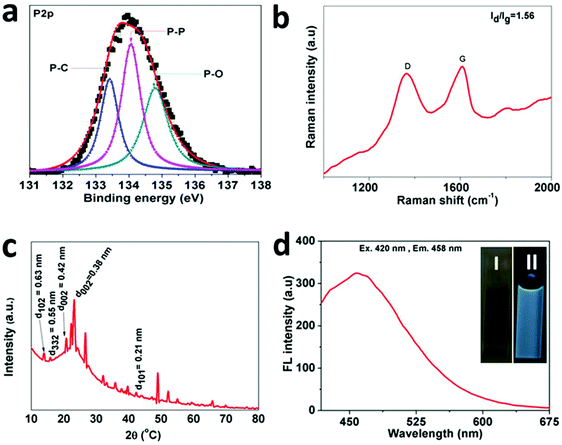
The synthesized NSs were also analysed using XPS (X-ray photoelectron spectroscopy) to advance the study of the chemical bonding between RP and the N-rGO nanosheets. The respective XPS results are shown in Fig. 3 and Fig. S4 (in the ESI†). In Fig. S4a,† the survey spectrum of the f-RP@N-rGO sample demonstrates both P 2p and P 2s peaks simultaneously, along with the nitrogen, oxygen and carbon peaks. The P 2p high-resolution spectrum in Fig. 3a can be fitted to three Gaussian–Lorentzian peaks at 133.40, 134.06, and 134.79 eV, which can be assigned to P–C, P–P, and P–O bonds, respectively.22–24 This indicates a reaction between phosphorus and oxygen originating from water or an oxygen-containing functional group of the reduced graphene oxide. Fig. S4b† exhibits the C 1s high-resolution spectrum, which can be fitted to five peaks: C–P bonding at 284.30 eV, which can be allocated to C–P sp2 bonds, as previously reported,25 demonstrating chemical bond formation between phosphorus and carbon in the experimental samples; and four other distinct peaks from C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (284.77 eV), C–O bonds (285.24 eV), C
C bonds (284.77 eV), C–O bonds (285.24 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (286.07 eV), and O–C
O bonds (286.07 eV), and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (288.77 eV ).
O bonds (288.77 eV ).
Meanwhile, Fig. S4c† shows the high-resolution N 1s spectrum results after deconvolution. This indicates that the prepared f-RP@N-rGO NSs include four types of N species corresponding to oxidized N (∼402.09 eV), graphitic N (∼401.52 eV), pyrrolic N (∼400.84 eV), and pyridinic N (∼400.16 eV).26 Further structural conformation of the f-RP@N-rGO NSs was acquired from Raman spectroscopy. As shown in Fig. 3b, similar to all sp2-carbon, two distinct peaks appear at 1364.69 and 1609.75 cm−1, corresponding to the D and G bands, respectively. The D band results from disordered carbon atoms, while the G band results from sp2-hybridized graphitic carbon atoms. The intensity ratio (ID/IG) of the D and G bands generally provides a measurement for the level of disorder; according to this, the ID/IG value of ∼1.56 for f-RP@N-rGO is higher than that of normal rGO due to the greater involvement of phosphorus atoms.27
As shown in Fig. 3c, the f-RP@N-rGO X-ray powder diffraction (XRD) spectrum exhibits one broadened characteristic peak at 15–60° with three distinct sub peaks at 23.01°, 26.71°, and 49.93°, which are consistent with the characteristic peaks of N-rGO and surface RP. Also, two broad diffraction peaks were observed at 15° and 18°, which correspond to the (102) and (332) planes of RP according to previous reports.21 These results indicate that f-RP@N-rGO NSs were successfully fabricated.
To further verify the chemical bond formation between RP and N-rGO, Fourier transform infrared spectroscopy (FTIR) analysis was conducted. As shown in Fig. S4d,† the presence of absorption bands at ∼885 and ∼997 cm−1, which are assigned to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending from H3PO4, suggest the presence of phosphoric acid, while the absorption band at ∼3413 cm−1 is associated with the stretching vibration of N–H from ethylenediamine (EDA). The absorption band at 1637 cm−1 is attributed to C–X (X = C, N) stretching vibrations, while the peak at 1161 cm−1 can possibly be assigned to the stretching of P–O or P
O bending from H3PO4, suggest the presence of phosphoric acid, while the absorption band at ∼3413 cm−1 is associated with the stretching vibration of N–H from ethylenediamine (EDA). The absorption band at 1637 cm−1 is attributed to C–X (X = C, N) stretching vibrations, while the peak at 1161 cm−1 can possibly be assigned to the stretching of P–O or P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups. These results confirm that RP is embedded in N-rGO. Fig. S4e† shows the absorption spectrum of the f-RP@N-rGO NSs, where absorption bands at ∼318 nm and ∼527 nm can be assigned to the π–π* and n–π* transitions of f-RP@N-rGO NSs. Also, the absorption band at 446 nm can be ascribed to the nitrogen-doping construction, which shifts the absorption band of graphene oxide in the red direction.28,29 A water-dispersed f-RP@N-rGO NS solution exhibited distinct green fluorescence under a 365 nm handheld UV light (inset of Fig. 3d). Moreover, the f-RP@N-rGO NSs exhibit broad emission peaking at 458 nm upon excitation at 420 nm (Fig. 3d). These results support the observations shown in Fig. S3.† Moreover, the emission of f-RP@N-rGO NSs was investigated in solutions of different ionic strength (in NaCl) and pH. There were no significant changes in emission at different ionic strengths from 0–4 M (Fig. S5†). The emission of f-RP@N-rGO NSs is unaltered over a very large pH range from 3 to 12, as shown in Fig. S6 in the ESI.† These two results are very important for biological applications.
N groups. These results confirm that RP is embedded in N-rGO. Fig. S4e† shows the absorption spectrum of the f-RP@N-rGO NSs, where absorption bands at ∼318 nm and ∼527 nm can be assigned to the π–π* and n–π* transitions of f-RP@N-rGO NSs. Also, the absorption band at 446 nm can be ascribed to the nitrogen-doping construction, which shifts the absorption band of graphene oxide in the red direction.28,29 A water-dispersed f-RP@N-rGO NS solution exhibited distinct green fluorescence under a 365 nm handheld UV light (inset of Fig. 3d). Moreover, the f-RP@N-rGO NSs exhibit broad emission peaking at 458 nm upon excitation at 420 nm (Fig. 3d). These results support the observations shown in Fig. S3.† Moreover, the emission of f-RP@N-rGO NSs was investigated in solutions of different ionic strength (in NaCl) and pH. There were no significant changes in emission at different ionic strengths from 0–4 M (Fig. S5†). The emission of f-RP@N-rGO NSs is unaltered over a very large pH range from 3 to 12, as shown in Fig. S6 in the ESI.† These two results are very important for biological applications.
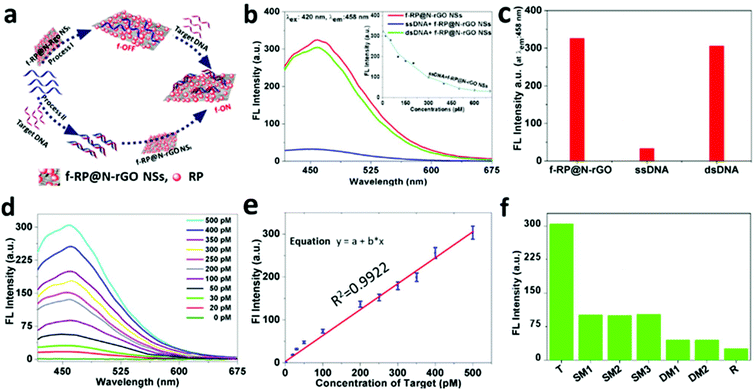
Based on previous studies, 2D NSs, such as those based on graphene,30–34 graphitic carbon nitride (g-C3N4),35 transition metal dichalcogenides,36–39 metal organic frameworks,40 and MoS2,41 have been considered as promising for fluorescence sensing applications. As a proof-of-concept, the f-RP@N-rGO NSs were used as a label-free DNA detection platform. The proposed technique is demonstrated in Fig. 4a. Principally, label-free single-stranded DNA (ssDNA) absorbed on the surface of the f-RP@N-rGO NSs via van der Waals forces between the NS basal plane and the nucleic acid bases resulted in significant fluorescence quenching of the NSs.30–34,40–42 In contrast, the nucleobases were successfully shielded within the densely negatively charged phosphate backbone of dsDNA after the ssDNA probe was hybridized with the complementary target DNA (T) to build double-stranded DNA (dsDNA). Thus, the label-free probe moves away from the surface of the NSs due to the very weak interactions between the dsDNA and NSs, ensuring the recovery of the fluorescence of the NSs upon the appearance of the target DNA. Therefore, we expected the fluorescence intensity of the NSs to assist the quantitative measurement of the target DNA. The experimental DNA sequences are shown in Table S1 (ESI†).
To assay the fluorescence change capabilities and affinities toward ssDNA and dsDNA of f-RP@N-rGO NSs, labeled free ssDNA (500 pM) and the Ebola virus subtype gene (T, 500 pM) were used in this study. Fig. 4b shows the fluorescence spectra of f-RP@N-rGO NSs under different assay conditions. The f-RP@N-rGO NSs exhibit strong fluorescence emission at 458 nm (red curve in Fig. 4b) upon excitation at 420 nm. However, the fluorescence intensity is quenched by close to 90% after the addition of ssDNA at an optimal concentration of 600 pM (as seen from the blue curve in Fig. 4b and the kinetics of quenching shown in the inset), revealing the strong interaction between ssDNA and the f-RP@N-rGO NSs and the high fluorescence quenching. The hybridization of ssDNA with an equal amount of complementary target DNA can form dsDNA. The green curve (dsDNA + f-RP@N-rGO NSs) in Fig. 4b shows the fluorescence spectrum of f-RP@N-rGO with dsDNA. The fluorescence intensity of the f-RP@N-rGO NSs is largely recovered after forming the dsDNA.
These results suggest that the interaction between dsDNA and f-RP@N-rGO NSs is weaker than that between ssDNA and f-RP@N-rGO NSs, which is in agreement with what is mentioned in some recent studies.41,42,47Fig. 4c shows the fluorescence changes in the f-RP@N-rGO NSs (10 μg mL−1) after introducing ssDNA and dsDNA at optimal concentrations (500 pM each). These results indicate that the f-RP@N-rGO NSs display various trends in relation to ssDNA and dsDNA. Because of the high quenching efficiency of ssDNA and the fluorescence signal recovery in the presence of dsDNA, we speculate that the NS based nanosensor exhibits excellent performance for achieving the quantitative detection of target DNA. To study the sensitivity of the nanosheets, various T concentrations from 0 to 500 pM were hybridized with ssDNA (500 pM) for 1 min and then mixed with the f-RP@N-rGO NSs (10 μg mL−1). According to Fig. 4d, the intensity of fluorescence increases apparently along with an increase in the concentration of T and exhibits a linear relationship over the range of 0–500 pM (Fig. 4e). Importantly, the f-RP@N-rGO NS sensor shows excellent sensitivity for detection at lower concentrations (20 pM), and the theoretical limit of detection (LOD) is estimated to be as low as 0.5 pM based on our calculations (ESI, Fig. S7†). Importantly, this LOD is lower than those of earlier reported nanomaterial-based DNA sensors (ESI, Table S2†).43–48 Moreover, such detection can be performed in less than a minute. The rapid, cost-effective, selective, and sensitive analysis of biological molecules is necessary for clinical diagnostics, disease prevention, treatment and environmental monitoring. Thus, based on the above results, the f-RP@N-rGO NS-based nanosensor is considered a good alternative biomolecule detection method. In this study, for the first time, we developed a multiplexed sensor based on f-RP@N-rGO NSs for the detection of the Ebola virus, with subtype SudZai Ebola virus DNA as the target, using label-free ssDNA.49
To evaluate the specificity of the f-RP@N-rGO NS sensing platform (Fig. 4f), the interactions with complementary target DNA (T), single-base mismatched DNA at different locations (SM1, SM2, and SM3), double-base mismatched DNA at different locations (DM1 and DM3) and random DNA (R) at similar concentrations of 500 pM were investigated. The fluorescent RP@N-rGO NS probes were excited at 420 nm.
Most of the nucleoside bases in the single and double base mismatched DNA samples were analogous to the target DNA, which is why they gave slight signals, but their signal intensities were unaltered regardless of the mismatch locations. The probes only responded significantly to the specific target DNA, and emission is observed at a wavelength of 458 nm. All these observations imply that the f-RP@N-rGO NS sensing platform demonstrates higher selectivity towards the target DNA.
Experimental section
Materials
Oligonucleotides were purchased from Shanghai Sangon Biological Engineering Technology & Services Co., Ltd (Shanghai, China). Hexadecyltrimethylammonium bromide, RP powder (Sigma-Aldrich), and all other reagents were of analytical grade and used without further purification. Ultrapure water was used throughout the research.Synthesis of f-RP@N-rGO NSs
The GO sheets were prepared using our previously reported method with small modifications.29 Briefly, 2 g of citric acid was transferred into a 50 mL beaker. 5 mL of DI water and 10 mL of H3PO4 were added to the beaker and dissolved under continuous stirring at 90 °C. After 15 min, 10 mL of 1 M NaOH solution was added dropwise to the above reaction mixture. After 15 min, 1 mL of EDA was added, and the temperature was raised to 120 °C and maintained at this level for 3 h. Subsequently, the color of the liquid transformed from colorless to pale yellow and, later, deep brown, suggesting the formation of fluorescent N-rGO. The resulting solution was simultaneously washed with DI water, HCl, and ethanol, twice. For each successive wash, the product was centrifuged at 7000g for 30 min. The deep brown N-rGO product obtained was vacuum-dried overnight at 60 °C.In a typical procedure for the loading of RP onto the N-rGO,20,21 the as-synthesized fluorescent N-rGO was suspended in water and ultrasonicated for 10 min to obtain a homogeneous aqueous dispersion at a concentration of 2.5 mg mL−1. The RP powder was milled for 10 min in an agate mortar before mixing it with the N-rGO suspension. Then, the ground RP powder was mixed with 30 mL of the N-rGO dispersion at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. To enhance the dispersion of RP, 0.07 mmol of hexadecyltrimethylammonium bromide was added. This mixture was subject to ultrasound irradiation for 30 min, followed by magnetic stirring for 24 h at 220 °C. Then, the as-prepared f-RP@N-rGO composite was centrifuged at 7000g for 30 min with DI water until it was nearly neutral, and the product was dried in a vacuum oven overnight.
1. To enhance the dispersion of RP, 0.07 mmol of hexadecyltrimethylammonium bromide was added. This mixture was subject to ultrasound irradiation for 30 min, followed by magnetic stirring for 24 h at 220 °C. Then, the as-prepared f-RP@N-rGO composite was centrifuged at 7000g for 30 min with DI water until it was nearly neutral, and the product was dried in a vacuum oven overnight.
Characterization
High-resolution TEM images were obtained using an FEI Tecnai G2 F20 TEM, with an installed charge-coupled device camera, operating at an acceleration voltage of 300 kV. Samples were prepared by placing a drop of sample solution onto a non-coated copper grid and allowing the solvent to evaporate in air at room temperature. The XRD patterns were recorded using a PANalytical X'Pert diffractometer operating at 40 kV and 30 mA using Cu Kα radiation (λ = 1.5406 Å) over a 2θ angle range of 10°–80° with a scan rate of 0.0262° s−1. The XRD samples were made via pressing the powder onto a glass substrate. The samples were analyzed using XPS [ESCALAB 250 spectrometer with a mono-X-ray source using Al Kα excitation (1486.6 eV)]. The spectra were background-corrected using the Shirley method, and the surface alignment of the samples was determined via measuring the C 1s, N 1s, O 1s, and P 2p intensity ratios (integrated peak region) normalized to the respective sensitivities. Fluorescence spectra were measured using a fluorescence spectrometer (CARY Eclipse 5,5) using a 4 mL glass cuvette. UV absorption spectra were acquired using a UV-vis spectrometer (CARY 50 Conc). IR spectra were acquired using an FTIR spectrophotometer (Nicolet Nexus Aligent 1100 series). Fluorescence images were acquired via fluorescence microscopy (Carl Zeiss vert. A1 microscope, Carl Zeiss Microimaging GmbH Department, 07740, Jena, Germany). The band-pass filter excitation wavelengths used were 488 nm, 561 nm, and 633 nm.Fluorescence detection of DNA
To study the detection of DNA with the probe, 500 pM (5 μL) ssDNA was hybridized with 500 pM (5 μL) target DNA in 990 μL of phosphate buffer (0.1 M, pH 7.4) in the presence of f-RP@N-rGO NSs (10 μg mL−1). Fluorescence spectra were observed at room temperature along with the f-RP@N-rGO NSs after immediate mixing. To optimize the sensitivity performance, 500 pM (5 μL) ssDNA was hybridized with various concentrations of target DNA (0–500 pM, 5 μL) in the presence of RP@N-rGO NSs (10 μg mL−1). The fluorescence spectra were obtained at constant excitation and emission wavelengths of 420 nm and 458 nm, respectively.To study the selectivity of the f-RP@N-rGO NS-based sensor, 500 pM (5 μL) ssDNA was incubated with 500 pM (5 μL) T, 500 pM (5 μL) single-base mismatched DNA (SM), and 500 pM (5 μL) random DNA (R), individually, in 990 μL of phosphate buffer for a few minutes to form dsDNA. Then 10 μg mL−1 f-RP@N-rGO NS solution was added into each of the aforementioned mixtures. Immediately after incubation, fluorescence measurements were performed. The excitation and emission wavelengths were 420 and 458 nm, respectively.
Conclusions
In conclusion, for the first time, we have synthesized fluorescent red phosphorus on nitrogen-doped reduced graphene oxide. It is evident that the 2D NSs possess promising fluorescence quenching abilities with good selectivity, as demonstrated by their varied affinities toward ssDNA and dsDNA. These excellent properties of the RP@N-rGO NSs can be used to develop a new biosensing technique for the multiplexed real-time fluorescent label-free detection of DNA in a very sensitive way, with a detection limit as low as 0.5 pM. The ability to prepare these f-RP@N-rGO NSs on a large scale enables them to act as a fascinating platform for the detection of multiple DNA sequences. Therefore, this assay can demonstrate the biomolecular applications of f-RP@N-rGO and may provide novel insights into the design of nanomaterial biosensors for the identification of multiplexes of biomolecules without the need for fluorescent tagging.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2018R1A2B2004432), the Priority Research Centers Program (2014R1A6A1031189) and the Nano Material Technology Development Program of the Korean National Research Foundation (NRF) funded by the Korean Ministry of education, Science, and Technology (2012M3A7B4049675). T. K. M. and N. P. contributed equally to this work.Notes and references
- D. M. Good, V. Thongboonkerd, J. Novak, J. L. Bascands, J. P. Schanstra, J. J. Coon, A. Dominiczak and H. Mischak, J. Proteome Res., 2007, 6(12), 4549–4555 CrossRef CAS PubMed.
- F. Gentile, G. Das, M. L. Coluccio, F. Mecarini, A. Accardo, L. Tirinato, R. Tallerico, G. Cojoc, C. Liberale, P. Candeloro, P. Decuzzi, F. De Angelis and E. Di Fabrizio, Microelectron. Eng., 2010, 87(5–8), 798–780 CrossRef CAS.
- A. Sahu, K. Dalal, S. Naglot, P. Aggarwal and C. Murali Krishna, PLoS One, 2013, 8(11), e78921 CrossRef CAS PubMed.
- C. H. Lu, H. H. Yang, C. L. Zhu, X. Chen and G. N. Chen, Angew. Chem., Int. Ed., 2009, 48(26), 4785–4787 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306(5696), 666–669 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438(7065), 197–200 CrossRef CAS PubMed.
- H. Shen, L. Zhang, M. Liu and Z. Zhang, Theranostics, 2012, 2(3), 283–294 CrossRef CAS PubMed.
- S. Guo, D. Du, L. Tang, Y. Ning, Q. Yao and G. J. Zhang, Analyst, 2013, 138, 3216–3220 RSC.
- B. Liu, Z. Sun, X. Zhang and J. Liu, Anal. Chem., 2013, 85, 7987–7993 CrossRef CAS PubMed.
- C. H. Lu, H. H. Yang, C. L. Zhu, X. Chen and G. N. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785–4787 CrossRef CAS PubMed.
- O. Panke, A. Kirbs and F. Lisdat, Biosens. Bioelectron., 2007, 22, 2656–2662 CrossRef PubMed.
- Z. Wang, J. Zhang, Z. Yin, S. Wu, D. Mandler and H. Zhang, Nanoscale, 2012, 4, 2728–2733 RSC.
- D. Du, S. Guo, L. Tang, Y. Ning, Q. Yao and G.-J. Zhang, Sens. Actuators, B, 2013, 186, 563–570 CrossRef CAS.
- G. J. Zhang, G. Zhang, J. H. Chua, R. E. Chee, E. H. Wong, A. Agarwal, K. D. Buddharaju, N. Singh, Z. Gao and N. Balasubramanian, Nano Lett., 2008, 8, 1066–1070 CrossRef CAS PubMed.
- X. Dong, Y. Shi, W. Huang, P. Chen and L. J. Li, Adv. Mater., 2010, 22, 1649–1653 CrossRef CAS PubMed.
- A. Star, E. Tu, J. Niemann, J. C. Gabriel, C. S. Joiner and C. Valcke, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 921–926 CrossRef CAS PubMed.
- G. H. Lee, M. R. Jo, K. Zhang and Y.-M. Kang, J. Mater. Chem. A, 2017, 5, 3683–3690 RSC.
- X. Ji and L. F. Nazar, J. Mater. Chem., 2010, 20, 9821–9826 RSC.
- L. Ji, M. Rao, H. Zheng, L. Zhang, Y. Li, W. Duan, J. Guo, E. J. Cairns and Y. Zhang, J. Am. Chem. Soc., 2011, 133, 18522–18525 CrossRef CAS PubMed.
- M. Khalid and H. Varela, J. Mater. Chem. A, 2018, 6(7), 3141–3150, 10.1039/c7ta11342k..
- Z. Xing, Z. Yuan, X. Wang, G. Jiang, J. Xiong and S. Yuan, Appl. Surf. Sci., 2018, 433, 125–132 CrossRef.
- R. Li, Z. Wei and X. Gou, ACS Catal., 2015, 5, 4133–4142 CrossRef CAS.
- D. S. Yang, D. Bhattacharjya, S. Inamdar, J. Park and J. S. Yu, J. Am. Chem. Soc., 2012, 134, 16127–16130 CrossRef CAS PubMed.
- M. Latorre-Sánchez, A. Primo and H. García, Angew. Chem., Int. Ed., 2013, 52, 11813–11816 CrossRef PubMed.
- J. Sun, G. Zheng, H. W. Lee, N. Liu, H. Wang, H. Yao, W. Yang and Y. Cui, Nano Lett., 2014, 14, 4573–4580 CrossRef CAS PubMed.
- X. Qiao, H. Peng, C. You, F. Liu, R. Zheng, D. Xu, X. Li and S. Liao, J. Power Sources, 2015, 288, 253–260 CrossRef CAS.
- X. Qiao, S. Liao, C. You and R. Chen, Catalysts, 2015, 5, 981–991, DOI:10.3390/catal5020981.
- T. Balaji and H. Matsunaga, Anal. Sci., 2005, 21, 973 CrossRef CAS PubMed.
- T. K. Mandal, Y. Hou, Z. Gao, H. Ning, W. Yang and M. Gao, Adv. Sci., 2016, 3(12), 1600217, DOI:10.1002/advs.201600217.
- Y. Wen, F. Xing, S. He, S. Song, L. Wang, Y. Long, D. Li and C. Fan, Chem. Commun., 2010, 46, 2596–2598, 10.1039/b924832c.
- S. He, B. Song, D. Li, C. Zhu, W. Qi, Y. Wen, L. Wang, S. Song, H. Fang and C. Fan, Adv. Funct. Mater., 2010, 20, 453–459 CrossRef CAS.
- X. Liu, R. Aizen, R. Freeman, O. Yehezkeli and I. Willner, ACS Nano, 2012, 6, 3553–3563 CrossRef CAS PubMed.
- X. Liu, F. Wang, R. Aizen, O. Yehezkeli and I. Willner, J. Am. Chem. Soc., 2013, 135, 11832–11839 CrossRef CAS PubMed.
- Z. Qian, X. Shan, L. Chai, J. Ma, J. Chen and H. Feng, Nanoscale, 2014, 6, 5671–5674 RSC.
- Q. Wang, W. Wang, J. Lei, N. Xu, F. Gao and H. Ju, Anal. Chem., 2013, 85, 12182–12188 CrossRef CAS PubMed.
- C. Zhu, Z. Zeng, H. Li, F. Li, C. Fan and H. Zhang, J. Am. Chem. Soc., 2013, 135, 5998–6001 CrossRef CAS PubMed.
- J. Huang, L. Ye, X. Gao, H. Li, J. Xu and Z. Li, J. Mater. Chem. B, 2015, 3, 2395–2401 RSC.
- Y. Yuan, R. Li and Z. Liu, Anal. Chem., 2014, 86, 3610–3615 CrossRef CAS PubMed.
- Y. Zhang, B. Zheng, C. Zhu, X. Zhang, C. Tan, H. Li, B. Chen, J. Yang, J. Chen, Y. Huang, L. Wang and H. Zhang, Adv. Mater., 2015, 27, 935–939 CrossRef CAS PubMed.
- M. Zhao, Y. Wang, Q. Ma, Y. Huang, X. Zhang, J. Ping, Z. Zhang, Q. Lu, Y. Yu, H. Xu, Y. Zhao and H. Zhang, Adv. Mater., 2015, 27, 7372–7378 CrossRef CAS PubMed.
- C. F. Zhu, Z. Y. Zeng, H. Li, F. Li, C. H. Fan and H. Zhang, J. Am. Chem. Soc., 2013, 135, 5998 CrossRef CAS PubMed.
- N. Parvin, Q. Jin, Y. Wei, R. Yu, B. Zheng, L. Huang, Y. Zhang, L. Wang, H. Zhang, M. Gao, H. Zhao, W. Hu, Y. Li and D. Wang, Adv. Mater., 2017, 29, 1606755 CrossRef PubMed.
- C. H. Lu, H. H. Yang, C. L. Zhu, X. Chen and G. N. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785–4787 CrossRef CAS PubMed.
- C. X. Wang, P. Yu, S. Y. Guo, L. Q. Mao, H. B. Liu and Y. L. Li, Chem. Commun., 2016, 52, 5629–5632 RSC.
- B. Dubertret, M. Calame and A. J. Libchaber, Nat. Biotechnol., 2001, 19(4), 365–370 CrossRef CAS PubMed.
- R. H. Yang, J. Y. Jin, Y. Chen, N. Shao, H. Z. Kang, Z. Xiao, Z. W. Tang, Y. R. Wu, Z. Zhu and W. H. Tan, J. Am. Chem. Soc., 2008, 130, 8351–8358, DOI:10.1021/ja800604z.
- S. J. He, B. Song, D. Li, C. F. Zhu, W. P. Qi, Y. Q. Wen, L. H. Wang, S. P. Song, H. P. Fang and C. H. Fan, Adv. Funct. Mater., 2010, 20(3), 453–459 CrossRef CAS.
- Y. Zhang, B. Zheng, C. Zhu, X. Zhang, C. Tan, H. Li, B. Chen, J. Yang, J. Chen, Y. Huang, L. Wang and H. Zhang, Adv. Mater., 2015, 27, 935–939, DOI:10.1002/adma.201404568.
- J. S. Towner, P. E. Rollin, D. G. Bausch, A. Sanchez, S. M. Crary, M. Vincent, W. F. Lee, C. F. Spiropoulou, T. G. Ksiazek, M. Lukwiya, F. Kaducu, R. Downing and S. T. Nichol, J. Virol., 2004, 78, 4330–4341, DOI:10.1128/JVI.78.8.4330-4341.2004.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9bm01341e |
| ‡ Equal contributing authors. |
| This journal is © The Royal Society of Chemistry 2020 |