Microenvironment-activated nanoparticles for oxygen self-supplemented photodynamic cancer therapy†
Hang
Liu‡
a,
Wei
Jiang‡
b,
Qin
Wang
b,
Jinxing
Xia
c,
Wenhao
Yu
a,
Yucai
Wang
 *b and
Yanmei
Wang
*b and
Yanmei
Wang
 *a
*a
aCAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: yucaiwang@ustc.edu.cn
bDivision of Molecular Medicine, Hefei National Laboratory for Physical Sciences at Microscale, CAS Key Laboratory of Innate Immunity and Chronic Disease, School of Life Sciences, University of Science and Technology of China, Hefei 230027, P. R. China. E-mail: wangyanm@ustc.edu.cn
cThe First Affiliated Hospital of Anhui Medical University, Hefei 230022, China
First published on 30th October 2019
Abstract
Tumor hypoxia, as a hallmark of most solid tumors, poses a serious impediment to O2-dependent anticancer therapies, such as photodynamic therapy (PDT). Although utilizing nanocarriers to load and transport O2 to tumor tissues has been proved effective, the therapeutic outcomes have been impeded by the low O2 capacity and limited tumor penetration of the nanocarriers. To address these problems, we incorporated perfluorooctyl moieties into nanocarriers to improve the encapsulation of perfluorooctyl bromide via fluorophilic interactions, leading to elevated O2 capacity of the nanocarriers. Meanwhile, to enhance the tumor cell penetrating ability as well as reduce reticuloendothelial system recognition, the nanocarrier was further decorated with a cell-penetrating peptide, which was masked with a protecting group via an acid-labile amide bond for prolonged circulation time and acid-activated cell penetration. The in vitro study demonstrated that, apart from remarkably boosting the photocytoxicity of chlorin 6 (Ce6) at a low dosage, the rationally designed O2@DANPCe6+PFOB could even alleviate the pre-existing tumor hypoxia. After intravenous injection, O2@DANPCe6+PFOB exhibited significant tumor accumulation and retention, and potent tumor growth inhibition compared to traditional PDT. Overall, the O2@DANPCe6+PFOB mediated O2 self-supplemented PDT with tumor acidic microenviornment-activated cell penetration provides a promising strategy in anticancer treatment.
Introduction
Photodynamic therapy (PDT), a noninvasive and spatiotemporally controlled therapeutic modality, has been approved for clinical use in treating skin, esophageal, and non-small-cell lung cancers.1–3 PDT utilizes photosensitizers to transfer light energy of a designated wavelength to tumor-dissolved oxygen (O2) for generating cytotoxic reactive oxygen species (ROS), such as singlet oxygen (1O2) to induce cell death. Therefore, sufficient O2 supply in tumor tissues is essential for continuous ROS generation, which ensures an uncompromised therapeutic outcome. However, as a hallmark of most solid tumors, hypoxia caused by rapid tumor cell proliferation and distorted tumor vasculature prevents PDT from exerting its full photocytotoxicity.4,5 Moreover, the continuous O2 depletion during PDT treatment in turn worsens tumor hypoxia, which further impairs the PDT efficacy and aggravates tumor progression and metastasis.6–8 Therefore, tumor hypoxia as a promising target for improved cancer therapy has been intensively explored.6,9–11To oxygenate tumor tissues for improved PDT outcomes, a number of strategies have been developed,12 including hyperbaric O2 inhalation,13 tumor vascular normalization,14,15 increased tumor blood perfusion via mild hypothermia effect,16,17 direct delivery of O2 by natural O2 carriers (e.g., red blood cells, hemoglobin),18–21 and in situ O2 generation by decomposing endogenous hydrogen peroxide (H2O2) and H2O with catalase or manganese dioxide (MnO2),22–26 and C3N4 or graphdiyne oxide nanosheets,16,27,28 respectively. In particular, perfluorocarbons (PFCs), a class of completely fluorinated carbon compounds, have been widely explored as O2 carriers in treating lung injury, hemorrhage, cerebral hypoxia, and carbon monoxide poisoning, due to their high O2 solubility, chemical inertness and excellent biocompatibility.29–31 Recently, the applications of PFC nanodroplets have been extended to alleviate tumor hypoxia and enhance PDT.32–34 In this regard, increasing the loading efficiency of PFCs could be an effective approach to elevate the overall O2 capacity, leading to enhanced PDT efficacy.35–38 A recent study demonstrated that polyesters with perfluorinated groups could improve PFC encapsulation, which can be attributed to the fluorophilic interactions between PFC and the perfluorinated groups.39,40 Moreover, apart from efficient O2 capacity, the therapeutic outcome of PDT has been partly limited by tumor penetration of the nanocarriers, in areas where more severe hypoxia exists.41 Tumor penetrating peptides can mediate the extravasation transport and enhance the penetration of drugs into tumors via a pathway, dubbed the C-end Rule (CendR) pathway.42–44
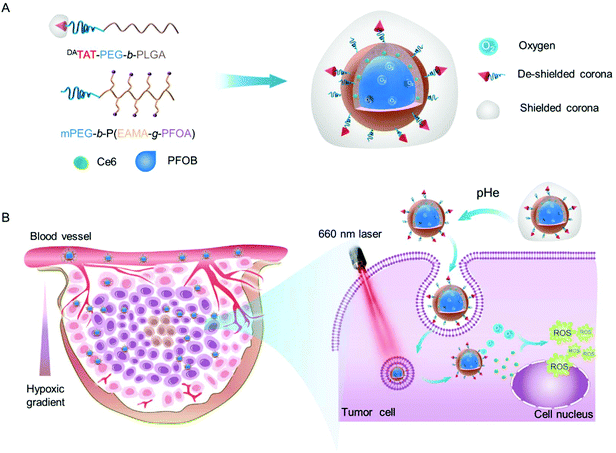
Inspired by this, we fabricated an O2 self-supplemented nanoplatform to enhance PDT efficacy by simultaneously enhancing O2 capacity and tumor penetration of photosensitizers. In particular, perfluoroalkyl chains were incorporated into the inner wall of the nanoplatforms, in an effort to improve the encapsulation of perfluorooctyl bromide (PFOB) and ultimately O2 delivery into tumors. After being co-loaded with a photosensitizer chlorin e6 (Ce6) and saturated with O2, the O2 self-supplemented nanoplatform could serve as an O2 reservoir to replenish O2 consumed during ROS production, eventually leading to alleviated tumor hypoxia and amplified PDT. Moreover, in order to improve the penetration of photosensitizers into the tumor interior, where more severe hypoxia exists, a tumor acidity-triggered TAT peptide-presenting poly(ethylene glycol)-b-poly(d, L-lactide-co-glycolide) (DAPEG-b-PLGA)45 was incorporated into the O2 self-supplemented nanoplatform (donated as O2@DANPCe6+PFOB). The protecting group 2,3-dimethylmaleic anhydride (DA) masked the TAT peptide on the surface of O2@DANPCe6+PFOBvia an acid-labile amide bond for prolonged circulation time and further acid-activated tumor penetration.45,47
After i.v. injection, the as-prepared O2@DANPCe6+PFOB could accumulate at the tumor tissues by the enhanced permeability and retention (EPR) effect.46,47 The acidic tumoral microenvironment would induce the cleavage of the acid-labile linkage and restore the cell-penetrating ability of the TAT peptide on the surface of O2@DANPCe6+PFOB, resulting in enhanced tumor accumulation and penetration into the tumor interior. The loaded O2 could gradually release from O2@DANPCe6+PFOB for efficiently modulating the tumor hypoxia microenvironment, as well as supplying O2 for ROS generation upon laser irradiation. Therefore, by employing an O2 self-supplemented nanoplatform with full biocompatibility, we are able to combine this with PDT to realize antitumor therapeutic outcomes, which holds great promise in preventing tumor relapse and to further clinical PDT applications (Scheme 1).
To boost the encapsulation of PFOB via fluorophilic interactions, we synthesized an amphiphilic diblock copolymer with perfluoroalkyl side chains by reversible addition–fragmentation chain transfer (RAFT) polymerization using a methyl poly(ethylene glycol) with an ending group 4-(4-cyanopentanoic acid) dithiobenzoate (mPEG-CPAD) as the macromolecular chain transfer agent and 2,2′-azobis(2-methylpropionitrile) (AIBN) as the initiator (Scheme S1†). Monomer N-(tert-butoxycarbonyl)-aminoethyl methacrylate (EABoc-MA) was synthesized by coupling N-(tert-butoxycarbonyl) ethanolamine (EABoc) with methacryloyl chloride, and the structure was confirmed by 1H NMR spectra (Fig. S1 and S2†). The successful synthesis of the copolymer mPEG-b-P(EABoc-MA) was verified by a shorter retention time compared to that of mPEG-CPAD in gel permeation chromatography (GPC) (Fig. S3†), along with the good agreement in chemical shifts of the 1H NMR spectrum (Fig. S4†). After deprotection of the amino groups, the perfluorooctyl side chains were grafted to the backbone via the amidation reaction to yield the final product mPEG-b-P(EAMA-g-PFOA) (Fig. S5†), which was then characterized by 19F NMR and Fourier transform infrared spectroscopy (FTIR) (Fig. S6 and S7†). Moreover, in order to potentiate the cell membrane penetrating ability, the nanoparticles were incorporated with TAT peptide end-capped amphiphilic copolymer TAT-PEG-b-PLGA. The amino groups from the lysine residue of the TAT peptide was further protected by 2,3-dimethylmaleic anhydride (DA) via an acid-labile amide bond, denoted as DATAT-PEG-b-PLGA. A detailed synthetic and well-characterized procedure can be found in our previous work.45
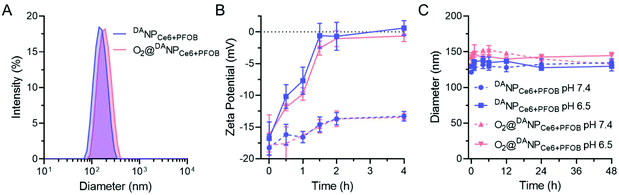
The as-prepared amphiphilic polymers mPEG-b-P(EAMA-g-PFOA) and DATAT-PEG-b-PLGA were blended with Ce6 and PFOB via the emulsion–evaporation method to yield DANPCe6+PFOB, which was then saturated with O2 to obtain O2@DANPCe6+PFOB. The dynamic light scattering (DLS) results showed that the hydrodynamic diameter of DANPCe6+PFOB increased by approximately 20 nm after O2 loading (O2@DANPCe6+PFOB), suggesting its high O2 capacity (Fig. 1A). Cryogenic transmission electron microscopy (Cryo-TEM) observations revealed that both DANPCe6+PFOB and O2@DANPCe6+PFOB have well-defined spherical morphologies,39 with a size and polydispersity in agreement with DLS measurements (Fig. S8†). According to our design, the amide bond between DA and the TAT peptide could be cleaved at mild acidic pH, which can be attributed to the intramolecular nucleophilic attack of the carbonyl group on amides by the neighboring carboxylate group, resulting in the exposure of the amino group in the TAT peptide.45 To test the acid-sensitivity of the amide bond, DANPCe6+PFOB and DANPCe6+PFOB were incubated in phosphate buffers at pH 6.5 or 7.4 for 4 h, respectively, and the zeta potential changes of NPs were recorded at preset time points. As shown in Fig. 1B, the zeta potential increased from ∼−16.8 mV to ∼0 mV for NPs incubated at pH 6.5, whereas only slight increases were observed when they were incubated at pH 7.4, which could be attributed to the recovery of the positively charged amino groups under acidic pH. Besides, DANPCe6+PFOB and DANPCe6+PFOB exhibited great stability at different pH values up to 48 h, indicating that the DA detachment had a minimal effect on the stability of the nanoparticles (Fig. 1C).
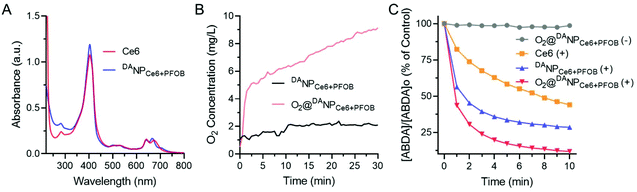
The characteristic peaks overlapped at 404 nm in the UV-Vis absorbance spectra, indicating the successful loading of Ce6 into DANPCe6+PFOB, and the drug loading content of Ce6 was 4.87 ± 0.32% (Fig. 2A). Due to the presence of favorable fluorophilic interactions between PFOB and the perfluoroalkyl segments of mPEG-b-P(EAMA-g-PFOA), the encapsulation efficiency of PFOB in DANPCe6+PFOB was determined to be ∼50%. We then investigated the O2 capability of DANPCe6+PFOB in deoxygenated water (pretreated by bubbling N2 to eliminate O2) via the Foxy Fospor-R O2 sensor. O2@DANPCe6+PFOB exhibited an efficient O2 loading as well as a gradual O2 release under hypoxia conditions, as evidenced by a dramatic increase of O2 concentration in deoxygenated water (Fig. 2B).
Then, we investigated whether the O2-enriched O2@DANPCe6+PFOB would potentiate ROS generation upon laser irradiation using 9,10-anthracenediyl-bis(methylene) dimalonic acid (ABDA) as an indicator. As expected, a sharp decline in the absorbance of ABDA was observed for O2@DANPCe6+PFOB, indicating its superior ROS generating ability compared to free Ce6 and DANPCe6+PFOB (traditional PDT regent), which is attributed to the continuous O2 supplementation during the ROS producing process (Fig. 2C).
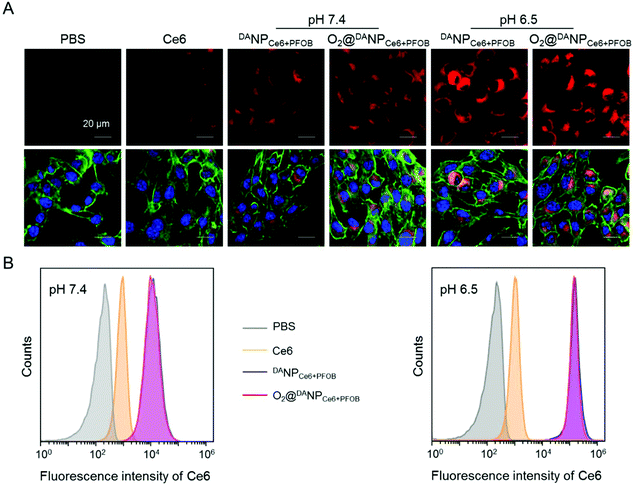
On the basis of our previous results that the TAT peptide shielded by DA should be exposed to enhance the internalization of the nano-formulations,45 we next examined the acid-activated cell penetrating ability of DANPCe6+PFOB and O2@DANPCe6+PFOB by assessing the cellular uptake using confocal laser scanning microscopy (CLSM) (Fig. 3A) and flow cytometry (Fig. 3B) in vitro, respectively. To this end, murine breast cancer 4T1 cells were incubated with different nano-formulations in acidic (pH 6.5) or neutral (pH 7.4) medium for 4 h, and then visualized with CLSM after fluorescent staining. The intracellular fluorescence of cells treated with DANPCe6+PFOB and O2@DANPCe6+PFOB in phosphate buffer (pH 6.5) was much stronger than in phosphate buffer (pH 7.4). In particular, the fluorescence intensity of DANPCe6+PFOB was 23.4-fold higher at pH 6.5 than at pH 7.4, as determined by the quantitative analysis of flow cytometry. The enhanced cellular uptake at pH 6.5 could be attributed to the DA detachment and recovery of the cell-penetrating ability of the TAT peptide at pH 6.5, which in turn facilitated the cell internalization of the TAT-decorated nano-formulations. Moreover, comparable cellular uptakes were observed for DANPCe6+PFOB and O2@DANPCe6+PFOB at different pH values, indicating that O2 loading had a minimal impact on the internalization of the nano-formulations.
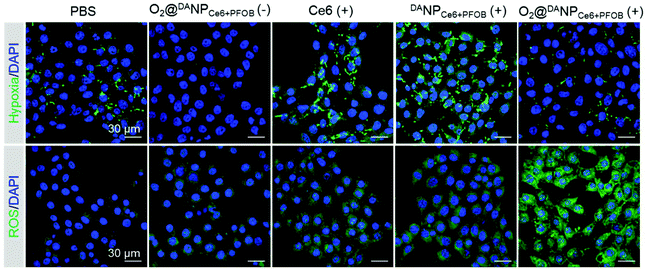
Increased cellular internalization of O2@DANPCe6+PFOB at pH 6.5 should be accompanied by increased intracellular O2 concentration and the enhanced ROS level upon laser irradiation. We next evaluated the ability of O2@DANPCe6+PFOB in intracellular hypoxia alleviation and ROS generation. In particular, 4T1 cells were incubated with different formulations (pH 6.5) in a hypoxia chamber (with 1% O2, 5% CO2 and 94% N2), which mimicked the hypoxia microenvironment in tumors. After being subjected to 660 nm laser irradiation, the cells were stained with a hypoxia probe (pimonidazole hydrochloride) and a ROS probe (dichlorodihydrofluorescein diacetate, DCFH-DA) to indicate hypoxia and ROS regions, respectively (Fig. 4). Compared to PBS treated cells, a sharp decrease in the hypoxia fluorescence signal was observed for cells treated with O2@DANPCe6+PFOB, indicating the excellent O2 capacity of O2@DANPCe6+PFOB. In contrast, a significantly enhanced hypoxia fluorescence signal was observed for the free Ce6 and DANPCe6+PFOB treated cell plus laser irradiation group compared to the PBS group, revealing that O2 was the pivotal limitation factor in PDT. It is noteworthy that the O2@DANPCe6+PFOB treated cells, even with laser irradiation, exhibited relatively dim hypoxia signals in comparison with the PBS group. The results indicated that apart from compensating for the O2 consumption in the production of ROS, the O2-enriched O2@DANPCe6+PFOB could further alleviate the pre-existing hypoxia. In line with the hypoxia results, the strongest ROS signal was observed in O2@DANPCe6+PFOB treated cells, since sufficient O2 supplementation in O2@DANPCe6+PFOB continuously fueled the generation of ROS under laser irradiation.
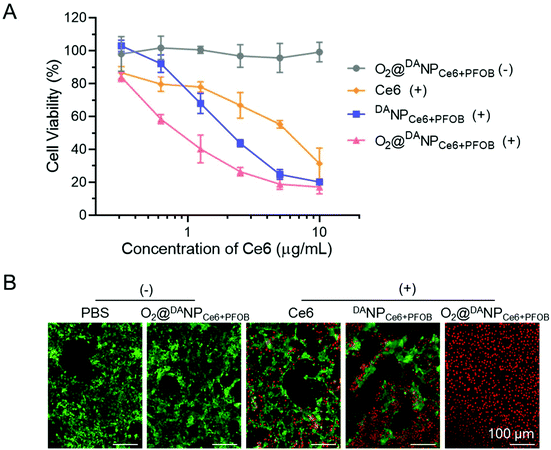
The PDT induced cytotoxicity of O2@DANPCe6+PFOB was measured by MTT assays. As a negative control, O2@DANPCe6+PFOB without laser irradiation showed negligible cytotoxicity with the Ce6 concentration even up to 10 μg mL−1, demonstrating excellent biocompatibility in potential applications in the clinic (Fig. 5A). The cytotoxicity of free Ce6, DANPCe6+PFOB and O2 self-supplemented O2@DANPCe6+PFOB exhibited a significant Ce6 concentration in a dose-dependent manner. Notably, the O2@DANPCe6+PFOB mediated PDT showed a higher cytotoxicity than that of free Ce6 and DANPCe6+PFOB at lower Ce6 concentrations (e.g. 5 μg mL−1), suggesting the distinct superiority of O2@DANPCe6+PFOB in PDT therapy. This efficient cytotoxicity of O2@DANPCe6+PFOB with laser irradiation was further confirmed by live (green fluorescence)/dead (red fluorescence) assays (Fig. 5B). The results suggested that the O2 self-supplemented strategy could reduce the dose of Ce6 and the possible side effects without compromising PDT efficacy.
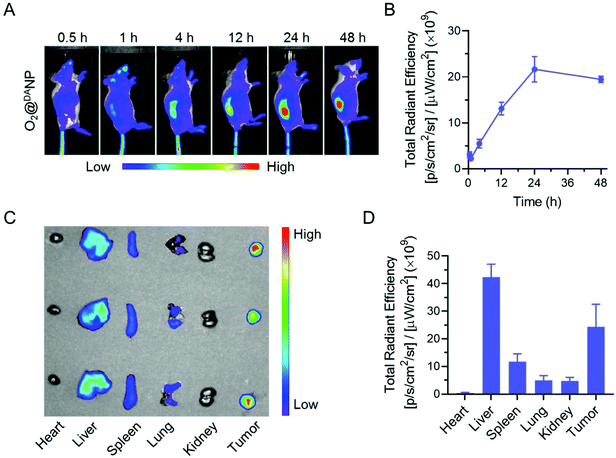
Intrigued by the superior PDT efficacy of O2@DANPCe6+PFOBin vitro, we explored the biodistribution and tumor accumulation of O2@DANP labeled with a fluorescent dye DiD using the Xenogen IVIS Lumina system. As shown in Fig. 6A and B, the accumulation of O2@DANP in tumors gradually increased and the highest fluorescence intensity was observed at 24 h post-injection, and sustained at high levels for up to 48 h. Consistent with real-time whole body fluorescence imaging, the ex vivo distribution investigation of major organs (heart, liver, spleen, lungs and kidneys) and tumors collected 48 h post-injection (Fig. 6C and D) further confirmed the prominent tumor-specific accumulation of O2@DANP. The distribution of O2@DANP indicated that O2@DANP could efficiently accumulate at the tumor site, which may be attributed to a prolonged circulation time and reduced systemic clearance under DA protection. Once reaching the tumor site, the exposure of the TAT peptide on the surface of O2@DANP would further enhance accumulation and extend residence time at the tumor tissues. Consistent with our assumption, after 24 h, i.v. injection with O2@DANPCe6+PFOB caused a significant enhancement of Ce6 accumulation in tumors and deeper penetration in the tumor mass when compared to injecting free Ce6 alone (Fig. S9†), indicating the superior penetration of O2@DANPCe6+PFOB into the tumor interior.
Moreover, the hypoxia level of tumors after the O2@DANPCe6+PFOB treatment was detected using the hypoxia sensor pimonidazole hydrochloride. Notably, the tumor treated with O2@DANPCe6+PFOB plus irradiation showed less hypoxic areas and weaker FITC hypoxia signal intensities (green), compared with those treated with free Ce6 plus irradiation and DANPCe6+PFOB plus irradiation, under which conditions more severe hypoxic areas remained (Fig. S10†), indicating that tumor hypoxia was significantly alleviated by the O2 supplied by O2@DANPCe6+PFOB. The above results confirmed that the O2 self-supplemented PDT strategy with O2@DANPCe6+PFOB as the O2 carrier could be a rather effective method to overcome tumor hypoxia and enhance PDT efficacy.
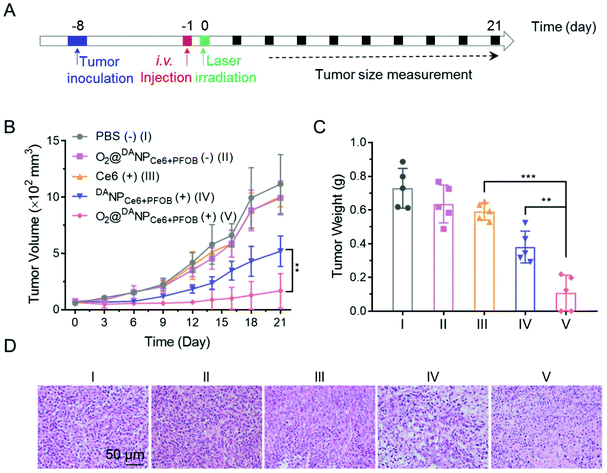
To evaluate the systemic antitumor therapeutic effects of O2@DANPCe6+PFOBin vivo, 4T1 tumor-bearing mice were randomly divided into five groups and i.v. injected with (Group I) PBS (−), (Group II) O2@DANPCe6+PFOB(−), (Group III) Ce6 (+), (Group IV) DANPCe6+PFOB (+), and (Group V) O2@DANPCe6+PFOB (+) at an equivalent dose of 2.0 mg Ce6 per kg mouse weight (n = 5 for each group), and were either subjected to (+) or not subjected to (−) laser irradiation (Fig. 7A). As shown in Fig. 7B, the administration of O2@DANPCe6+PFOB without laser irradiation (Group II) or free Ce6 plus laser irradiation (Group III) failed to control tumor growth. Meanwhile, limited by the hypoxia microenvironment in tumor tissues, PDT mediated by DANPCe6+PFOB plus laser irradiation (Group IV) only exhibited moderate tumor growth suppression. However, with a single treatment of O2@DANPCe6+PFOB (+) (Group V), the tumor growth was significantly inhibited (85.0% inhibition of tumor growth). The enhanced PDT efficacy could be attributed to the high O2 capacity and effective O2 transport into the tumor cells, which continuously supplemented the O2 consumption during the PDT treatment. The tumor weight measurements were in good accordance with the tumor volume at day 21, indicating the efficient PDT outcome of O2@DANPCe6+PFOB (Fig. 7C). Besides, no body weight loss was observed for O2@DANPCe6+PFOB, indicating excellent biocompatibility (Fig. S11†). Moreover, the improved therapeutic outcomes of O2@DANPCe6+PFOB were further confirmed by H&E staining of tumor tissues, evidenced by obvious nuclear shrinkage and severe tissue necrosis after O2@DANPCe6+PFOB (+) treatment (Fig. 7D). In addition, hematoxylin & eosin (H&E) histological analysis of major organs (heart, liver, spleen, lungs and kidneys) after different nanoformulations showed negligible tissue damage compared to the PBS group, indicating excellent biocompatibility for all formulations (Fig. 8). In addition, there were no obvious changes in blood cell counts after the treatment with O2@DANPCe6+PFOB, confirming its biosafety (Fig. S12†). Collectively, the O2 self-supplemented PDT strategy exhibited superior antitumor efficacy over traditional PDT with negligible systemic toxicity.
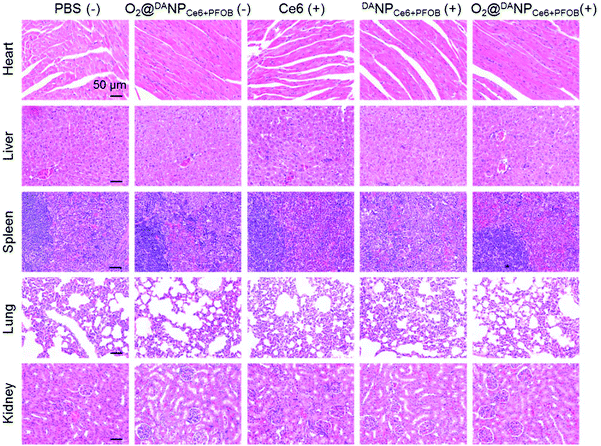 | ||
| Fig. 8 H&E staining of the heart, liver, spleen, lungs and kidneys of 4T1 tumor-bearing mice after 21 days of treatment with the five formulations (scale bar: 50 μm). | ||
Conclusions
In summary, we reported an O2 self-supplemented PDT strategy with acid-activated cell penetrating ability to overcome hypoxia-limited therapeutic efficacy. By integrating with a perfluoroalkyl moiety and end-capping with a TAT peptide, O2@DANPCe6+PFOB exhibited dramatically improved O2 capacity and cellular internalization. The in vitro hypoxia and ROS fluorescence imaging demonstrated that in addition to compensating for the O2 consumption during PDT, O2@DANPCe6+PFOB could partially relieve intrinsic hypoxia. Moreover, O2@DANPCe6+PFOB achieved enhanced tumor accumulation and retention, owing to the DA corona protection during circulation and acid-activated tumor penetration. Enhanced tumor growth inhibition compared to traditional PDT was observed in the in vivo antitumor study. Taken together, combining O2 self-supplemented PDT with acid-activated tumor penetration is a new approach in antitumor PDT.Conflicts of interest
The authors declare that there are no conflicts of interest for this work.Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFA0205600), the National Natural Science Foundation of China (51773191, 51633008, 51573176, and 21604079), the Fundamental Research Funds for the Central Universities (WK3520000009 and WK2090050042), the Open Project of Key Laboratory of Biomedical Engineering of Guangdong Province (KLBEMGD201701) and the China Postdoctoral Science Foundation (2019TQ0400). This work was partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication.References
- R. R. Allison and C. H. Sibata, Photodiagn. Photodyn. Ther., 2010, 7, 61–75 CrossRef CAS.
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Ca-Cancer J. Clin., 2011, 61, 250–281 CrossRef PubMed.
- W. P. Fan, P. Huang and X. Y. Chen, Chem. Soc. Rev., 2016, 45, 6488–6519 RSC.
- W. R. Wilson and M. P. Hay, Nat. Rev. Cancer, 2011, 11, 393–410 CrossRef CAS.
- D. M. Gilkes, G. L. Semenza and D. Wirtz, Nat. Rev. Cancer, 2014, 14, 430–439 CrossRef CAS.
- J. Dang, H. He, D. Chen and L. Yin, Biomater. Sci., 2017, 5, 1500–1511 RSC.
- R. Jahanban-Esfahlan, M. de la Guardia, D. Ahmadi and B. Yousefi, J. Cell Physiol., 2018, 233, 2019–2031 CrossRef CAS.
- Z. Zhou, J. Song, L. Nie and X. Chen, Chem. Soc. Rev., 2016, 45, 6597–6626 RSC.
- J. M. Brown and W. R. William, Nat. Rev. Cancer, 2004, 4, 437–447 CrossRef CAS.
- M. R. Horsman, L. S. Mortensen, J. B. Petersen, M. Busk and J. Overgaard, Nat. Rev. Clin. Oncol., 2012, 9, 674–687 CrossRef CAS.
- E. B. Rankin and A. J. Giaccia, Science, 2016, 352, 175–180 CrossRef CAS.
- X. Li, N. Kwon, T. Guo, Z. Liu and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 11522–11531 CrossRef CAS.
- N. Kunugita, K. Kohshi, Y. Kinoshita, T. Katoh, H. Abe, T. Tosaki, T. Kawamoto and T. Norimura, Cancer Lett., 2001, 164, 149–154 CrossRef CAS.
- P. Carmeliet and R. K. Jain, Nat. Rev. Drug Discovery, 2011, 10, 417–427 CrossRef CAS.
- A. Parodi, S. G. Haddix, N. Taghipour, S. Scaria, F. Taraballi, A. Cevenini, I. K. Yazdi, C. Corbo, R. Palomba, S. Z. Khaled, J. O. Martinez, B. S. Brown, L. Isenhart and E. Tasciotti, ACS Nano, 2014, 8, 9874–9883 CrossRef CAS PubMed.
- W. Jiang, Z. Zhang, Q. Wang, J. Dou, Y. Zhao, Y. Ma, H. Liu, H. Xu and Y. Wang, Nano Lett., 2019, 19, 4060–4067 CrossRef CAS.
- Q. Chen, Q. Hu, E. Dukhovlinova, G. Chen, S. Ahn, C. Wang, E. A. Ogunnaike, F. S. Ligler, G. Dotti and Z. Gu, Adv. Mater., 2019, 1900192 CrossRef.
- W. Tang, Z. Zhen, M. Wang, H. Wang, Y. J. Chuang, W. Zhang, G. D. Wang, T. Todd, T. Cowger and H. Chen, Adv. Funct. Mater., 2016, 26, 1757–1768 CrossRef CAS.
- L. Zhang, D. Wang, K. Yang, D. Sheng, B. Tan, Z. Wang, H. Ran, H. Yi, Y. Zhong, H. Lin and Y. Chen, Adv. Sci., 2018, 5, 1800049 CrossRef.
- Y. Jia, L. Duan and J. B. Li, Adv. Mater., 2016, 28, 1312–1318 CrossRef CAS.
- J. Yang, W. Li, L. Luo, M. Jiang, C. Zhu, B. Qin, H. Yin, X. Yuan, X. Yin, J. Zhang, Z. Luo, Y. Du and J. You, Biomaterials, 2018, 182, 145–156 CrossRef CAS.
- H. Chen, J. Tian, W. He and Z. Guo, J. Am. Chem. Soc., 2015, 137, 1539–1547 CrossRef CAS.
- W. Fan, W. Bu, B. Shen, Q. He, Z. Cui, Y. Liu, X. Zheng, K. Zhao and J. Shi, Adv. Mater., 2015, 27, 4155–4161 CrossRef CAS.
- Z. Wang, Y. Zhang, E. Ju, Z. Liu, F. Cao, Z. Chen, J. Ren and X. Qu, Nat. Commun., 2018, 9, 3334 CrossRef.
- Q. Jia, J. Ge, W. Liu, X. Zheng, S. Chen, Y. Wen, H. Zhang and P. Wang, Adv. Mater., 2018, 30, 1706090 CrossRef.
- W. Zhang, S. Li, X. Liu, C. Yang, N. Hu, L. Dou, B. Zhao, Q. Zhang, Y. Suo and J. Wang, Adv. Funct. Mater., 2018, 28, 1706375 CrossRef.
- D. W. Zheng, B. Li, C. X. Li, J. X. Fan, Q. Lei, C. Li, Z. S. Xu and X. Z. Zhang, ACS Nano, 2016, 10, 8715–8722 CrossRef CAS.
- S. T. Xu, X. Y. Zhu, C. Zhang, W. Huang, Y. F. Zhou and D. Y. Yan, Nat. Commun., 2018, 9, 2053 CrossRef.
- E. Wilhelm and R. Battino, Chem. Rev., 1973, 73, 1–9 CrossRef CAS.
- A. Scheer, M. Kirsch and K. B. Ferenz, J. Nanosci. Nanomed., 2017, 1, 21–27 Search PubMed.
- W. Wang, Y. Cheng, P. Yu, H. Wang, Y. Zhang, H. Xu, Q. Ye, A. Yuan, Y. Hu and J. Wu, Nat. Commun., 2019, 10, 1580 CrossRef.
- Y. Cheng, H. Cheng, C. Jiang, X. Qiu, K. Wang, W. Huan, A. Yuan, J. Wu and Y. Hu, Nat. Commun., 2015, 6, 8785 CrossRef CAS.
- X. Song, L. Feng, C. Liang, K. Yang and Z. Liu, Nano Lett., 2016, 16, 6145–6153 CrossRef CAS.
- W. Jiang, Q. Li, L. Xiao, J. X. Dou, Y. Liu, W. H. Yu, Y. C. Ma, X. Q. Li, Y. Z. You, Z. T. Tong, H. Liu, H. Liang, L. G. Lu, X. D. Xu, Y. D. Yao, G. Q. Zhang, Y. C. Wang and J. Wang, ACS Nano, 2018, 12, 5684–5698 CrossRef CAS.
- A. L. Lee, C. T. Gee, B. P. Weegman, S. A. Einstein, A. R. Juelfs, H. L. Ring, K. R. Hurley, S. M. Egger, G. Swindlehurst, M. Garwood, W. C. K. Pomerantz and C. L. Haynes, ACS Nano, 2017, 11, 5623–5632 CrossRef CAS.
- H. Y. Lee, H. W. Kim, J. H. Lee and S. H. Oh, Biomaterials, 2015, 53, 583–591 CrossRef CAS.
- G. Song, C. Ji, C. Liang, X. Song, X. Yi, Z. Dong, K. Yang and Z. Liu, Biomaterials, 2017, 112, 257–263 CrossRef CAS.
- G. Song, C. Liang, X. Yi, Q. Zhao, L. Cheng, K. Yang and Z. Liu, Adv. Mater., 2016, 28, 2716–2723 CrossRef CAS PubMed.
- S. Houvenagel, G. Picheth, C. Dejean, A. Brûlet, A. Chennevière, O. Couture, N. Huang, L. Moine and N. Tsapis, Polym. Chem., 2017, 8, 2559–2570 RSC.
- P. Yuan, Z. Ruan, W. Jiang, L. Liu, J. Dou, T. Li and L. Yan, J. Mater. Chem. B, 2018, 6, 2323–2331 RSC.
- Y. Wang, Y. Xie, J. Li, Z. H. Peng, Y. Sheinin, J. Zhou and D. Oupicky, ACS Nano, 2017, 11, 2227–2238 CrossRef CAS.
- E. Ruoslahti, Adv. Drug Delivery Rev., 2017, 110, 3–12 CrossRef.
- S. Silva, A. J. Almeida and N. Vale, Biomolecules, 2019, 9, 22 CrossRef.
- Q. Zhou, S. Q. Shao, J. Q. Wang, C. H. Xu, J. J. Xiang, Y. Piao, Z. X. Zhou, Q. S. Yu, J. B. Tang, X. R. Liu, Z. H. Gan, R. Mo, Z. Gu and Y. Q. Shen, Nat. Nanotechnol., 2019, 14, 799–809 CrossRef CAS.
- W. Jiang, Q. Li, Z. Zhu, Q. Wang, J. Dou, Y. Zhao, W. Lv, F. Zhong, Y. Yao and G. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 35734–35744 CrossRef CAS.
- T. J. Ji, Y. P. Ding, Y. Zhao, J. Wang, H. Qin, X. M. Liu, J. Y. Lang, R. F. Zhao, Y. L. Zhang, J. Shi, N. Tao, Z. H. Qin and G. J. Nie, Adv. Mater., 2015, 27, 1865–1873 CrossRef CAS.
- J.-Z. Du, H.-J. Li and J. Wang, Acc. Chem. Res., 2018, 51, 2848–2856 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental details and data include: the syntheses of monomer N-Boc-aminoethyl methacrylate, and block copolymer mPEG-b-P(EABoc-MA); cell culture; cellular uptake; MTT assay; animals and tumor model; biodistribution and antitumor study of O2@NPCe6+PFOB. 1H NMR spectra of monomer N-Boc-aminoethyl methacrylate, block copolymer mPEG-b-P(EABoc-MA), 19F NMR and Fourier transform infrared spectroscopy (FT-IR) analysis of mPEG-b-P(EABoc-MA); body weight variation of tumor bearing mice during antitumor treatment. See DOI: 10.1039/c9bm01537j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |