A novel mesoporous hydrogen-bonded organic framework with high porosity and stability†
Bin
Wang
 ab,
Xiu-Liang
Lv
c,
Jie
Lv
ab,
Xiu-Liang
Lv
c,
Jie
Lv
 c,
Li
Ma
b,
Rui-Biao
Lin
c,
Li
Ma
b,
Rui-Biao
Lin
 b,
Hui
Cui
b,
Hui
Cui
 b,
Jian
Zhang
b,
Jian
Zhang
 d,
Zhangjing
Zhang
d,
Zhangjing
Zhang
 a,
Shengchang
Xiang
a,
Shengchang
Xiang
 *a and
Banglin
Chen
*a and
Banglin
Chen
 *b
*b
aFujian Provincial Key Laboratory of Polymer Materials, College of Chemistry and Materials Science, Fujian Normal University, 32 Shangsan Road, Fuzhou 350007, P. R. China. E-mail: scxiang@fjnu.edu.cn
bDepartment of Chemistry, University of Texas at San Antonio, One UTSA Circle, San Antonio, Texas 78249-0698, USA. E-mail: banglin.chen@utsa.edu
cBeijing Key Laboratory for Green Catalysis and Separation and Department of Chemistry and Chemical Engineering, College of Environmental and Energy Engineering, Beijing University of Technology, Beijing 100124, P. R. China
dMolecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA
First published on 2nd December 2019
Abstract
A highly stable hydrogen-bonded organic framework, HOF-14, has been successfully constructed and structurally characterized. It possesses a permanent three dimensional (3D) porous structure. The activated HOF-14 has a high BET surface area of 2573 m2 g−1 and a record large pore volume of 1.36 cm3 g−1 among HOF materials. In addition, HOF-14 also exhibits high chemical and thermal stability and is capable of highly selective separation of light hydrocarbons under ambient conditions.
As a class of newly developed porous materials, hydrogen-bonded organic frameworks (HOFs) constructed from the self-assembly of organic building units (OBUs) through intermolecular hydrogen-bonding interactions have attracted significant attention.1,2 Similar to other famous porous materials of covalent organic frameworks (COFs)3 and metal–organic frameworks (MOFs),4,5 HOFs also have a large surface area, high porosity and a tailored pore size/shape and can be potentially used in gas storage and separation,6–12 sensing,13 biomolecule encapsulation,14 pollutant removal,15 proton conduction,16 heterogeneous catalysis17,18 and so on. Compared with the porous materials mentioned above, however, HOFs possess some unique features such as mild synthetic conditions, solution processability and easy regeneration. Specifically, the metal free nature of HOFs makes them good candidates for biological applications.19 For practical applications of HOFs, however, one of the problems that needs to be addressed is their poor chemical and thermal stability, which is ascribed to the weak hydrogen bonding nature. Since the first report of the HOF material with permanent porosity, only few HOFs can survive harsh conditions.1 Thus, the construction of stable HOFs is one of the most important challenges in this field.
Generally, there are two strategies to stabilize the framework of HOFs. One strategy is to create multiple hydrogen bonds. HOFs are constructed by hydrogen bonds between OBUs, and therefore more number of intermolecular hydrogen bonds will strengthen the entire structure. Under the guidance of this strategy, our group designed and synthesized a series of 2,4-diaminotriazinyl (DAT)-based OBUs featuring multiple amino groups. Several HOFs with permanent porosity were successfully constructed using these OBUs.7–11,20–23 The cooperation of hydrogen bonding interactions with other weak interactions such as π–π stacking is another strategy to stabilize the HOF structure. Recently, this strategy has been used to construct highly stable HOFs with a large surface area such as HOF-TCBP,24 CPHAT-1a,25 and PFC-119 using carboxylic-based OBUs. The structure of the three HOFs is similar. In the three HOFs, the discrete OBUs connect with each other through intermolecular hydrogen bonds between carboxylic groups to form a single layer, and different layers are packed together through strong face to face π–π stacking interactions to form a 3D framework with a one-dimensional (1D) channel. In particular, for PFC-1, different layers are packed together in an AA packing mode, similar to those in the well-studied stable 2D COFs.26 By selecting building units with different lengths, the pore size of 2D COF can be rationally tailored. We thus realized that the pore size of PFC-1 can also be tailored through the selection of OBUs with different lengths.
Herein, we synthesized an organic building unit with a larger size than that of H4TBAPy which is used for the construction of PFC-1 (Scheme 1). Using this OBU, a robust one-component porous mesoporous hydrogen-bonded organic framework, HOF-14 isostructural to PCF-1 but with a larger surface area and pore size, was successfully constructed.27 The Brunauer–Emmett–Teller (BET) surface area of HOF-14 is 2573 m2 g−1. In addition, HOF-14 also has a record large pore volume of 1.36 cm3 g−1 among HOF materials. HOF-14 also exhibits high chemical and thermal stability and can easily be prepared and regenerated by simple addition of tetrahydrofuran (THF) to the dimethyl sulfoxide (DMSO) solution of H4PTTNA under stirring. The activated HOF-14 turned out to be a promising candidate as to highly selectively separate CH4 from C3 and C2 hydrocarbons under ambient conditions.
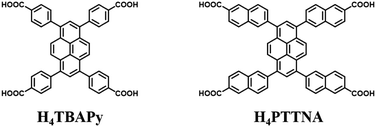 | ||
| Scheme 1 Organic building units of H4TBAPy and H4PTTNA for the construction of PFC-1 and HOF-14, respectively. | ||
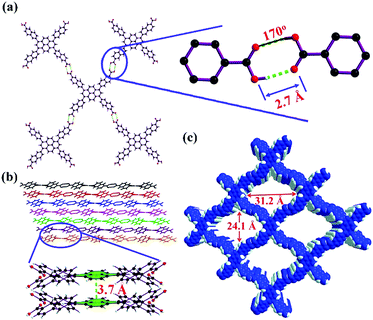
6,6′,6′′,6′′′-(Pyrene-1,3,6,8-tetrayl)tetrakis(2-naphthoic acid) (H4PTTNA) was synthesized through a Suzuki coupling reaction (see the Supporting Information). Diffusion of THF vapor to DMSO solution where H4PTTNA was dissolved at room temperature for about three weeks afforded yellow stick-shaped crystals suitable for X-ray analysis. HOF-14 can also be obtained in large scale by simple addition of THF to the DMSO solution of H4PTTNA under stirring. Single crystal X-ray diffraction analysis reveals that HOF-14 is isostructural to PFC-1 and crystallizes in a monoclinic C2/m space group. In its crystal structure, each H4PTTNA OBU interacts with four adjacent OBUs through intermolecular O–H⋯O hydrogen bonds to form a two-dimensional (2D) layer (Fig. 1a). The O–H⋯O distance and the angle are 2.7 Å and 170°, respectively, falling into a strong hydrogen bond range. From a topological view point, the H4PTTNA ligands can be seen as a 4-connected node, and the 2D layer mentioned above can thus be simplified as a 4-connected uninodal net with a point symbol of {44·62} which corresponds to the sql topology according to Topos software. The 2D square layers are further packed together in an AA mode along the c axis through strong face-to-face π–π stacking interactions (the interlayer distance is 3.7 Å) to form a 3D structure with one-dimensional square channels (Fig. 1b and c). The channel size of HOF-14 is 31.2 × 24.1 Å, larger than that of PFC-1 (18 × 23 Å).
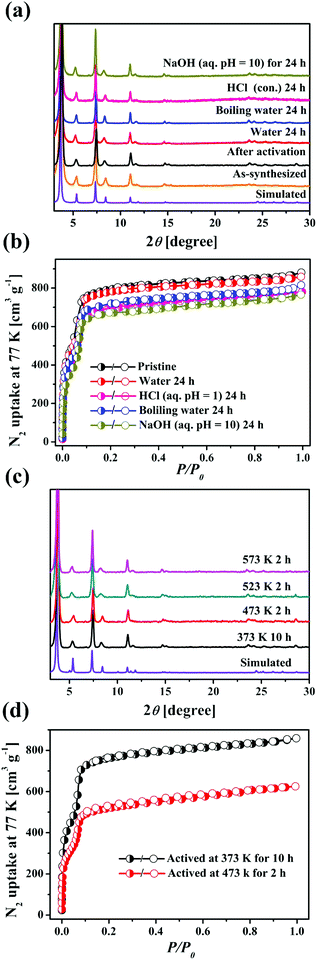
The permanent porosity of HOF-14 has been confirmed by N2 sorption at 77 K (Fig. 2a). Saturated N2 uptakes of 881 cm3 (STP) g−1 as to HOF-14 are achieved, and its evaluated BET and Langmuir surface areas are 2573 and 3204 m2 g−1, respectively (Fig. 2a). The experimental total pore volume is 1.36 cm3 g−1, which is the highest among HOF materials to date (Table 1).
| HOFs | N2 uptake [cm3 g−1] | SABET [m2 g−1] | Pore volume [cm3 g−1] |
|---|---|---|---|
| a Calculated by the CO2 uptake at 195 K. | |||
| TTBI28 | 754 | 2796 | 1.02 |
| PFC-124 | 613 | 2122 | 0.95 |
| HOF-TCPB24 | 535 | 2066 | 0.83 |
| HOF-5a9 | — | 1101a | 0.44 |
| PFC-229 | 415 | 1014 | 0.64 |
| CBPHAT-1a25 | 362 | 1288 | 0.56 |
| HOF-1917 | 287 | 685 | 0.45 |
| tcpb30 | 266 | 1095 | 0.42 |
| IISERP-HOF131 | 405 | 1025a | 0.26a |
| SOF-7a32 | — | 900 | 0.23 |
| HOF-14 | 881 | 2573 | 1.36 |
Considering the relatively strong hydrogen bonding interactions between carboxylic groups and the presence of strong face to face π–π stacking interactions in HOF-14, we tried to explore its chemical stability. Firstly, the HOF-14 samples were treated in water, concentrated HCl aqueous solution, and NaOH aqueous solution (pH = 10) at room temperature, as well as in boiling water. Upon soaking in these solutions for 24 h, HOF-14 exhibited maintained crystallinity and an unchanged structure as confirmed by PXRD measurements (Fig. 2a). Furthermore, the N2 sorption isotherms for the samples after being immersed in water, boiling water, HCl aqueous solution (pH = 1), and NaOH aqueous solution (pH = 10) for 24 h were measured and almost the same uptakes were found (Fig. 2b). In order to further confirm the excellent chemical stability of HOF-14, the morphologies of the samples before and after acid/base aqueous solution treatment were characterized by scanning electron microscopy (SEM). As shown in Fig. S3, ESI,† after being treated in HCl (pH = 1) or NaOH (pH = 10) aqueous solutions for 24 h, the surface of the sample still remained smooth and no obvious flaw was spotted. All the characterization mentioned above together confirmed the excellent chemical stability of HOF-14, rarely observed in HOF materials.
In addition to the chemical stability, we further explored the thermal stability of HOF-14. As shown in Fig. S2, ESI,† thermo gravimetric analysis (TGA) shows that the activated HOF-14 is stable up to 673 K in an air atmosphere. Then the samples of HOF-14 were placed in ovens with different temperatures. It was found that even when exposed to a 573 K oven for 2 h, the crystallinity of HOF-14 still remained (Fig. 2c). Furthermore, we explored the changes in the N2 uptake of HOF-14 at 77 K after being activated at high temperature and under high vacuum. As shown in Fig. 2d, initially, to fully remove the guest molecules in the channel of HOF-14, the sample was activated under high vacuum at 373 K for 10 h. The saturated N2 uptake was 881 cm3 g−1. Further activating the sample at 473 K for 2 h led to a decrease of the N2 uptake amount (from 881 to 625 cm3 g−1), indicating that the partial framework of the samples collapsed. Meanwhile, this also showed that the crystallinity of about 71% of the sample was retained, indicating the high thermal stability of HOF-14, rarely seen in HOFs. To recover the framework of HOF-14, the samples after being treated at high temperatrue or in strong base aqueous solutions were dissolved in a small amout of DMSO by heating, and then a large amount of THF was introduced to obtain a microcrystalline sample. The saturated N2 uptake of this sample was 858 cm3 g−1, which is almost the same as that of the crystal sample (881 cm3 g−1, Fig. S4, ESI†).
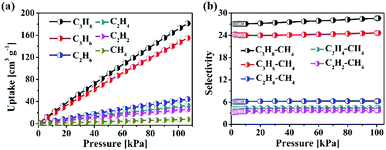
Recently, natural gas purification has become an important research field in industry. The removal of C3 hydrocarbons from CH4 can reduce the dew point of natural gas and prevent condensation during transportation. In addition, the separation of CH4 over C2 hydrocarbons is also important. Acetylene is principally obtained from the cracking of natural gas. The separation of C2H2 from CH4 is necessary to meet the requirements of Grade A C2H2 for organic synthesis. Besides, the oxidative coupling of CH4 to C2H4 or C2H6 ultimately needs to separate CH4 from them because of the incomplete conversion of CH4.33 We thus explored the separation of light hydrocarbons using HOF-14 under ambient conditions. Single-component gas sorption isotherms were recorded up to 100 kPa at 298 and 273 K, respectively (Fig. 3a and Fig. S5, ESI†). As can be seen from these isotherm data, HOF-14 shows the maximum C3H8, C3H6, C2H6, C2H4, and C2H2 uptake of 181.4, 154.9, 44.2, 32.5, and 25.6 cm3 g−1, respectively, at 298 K and 100 kPa (Fig. 3a). In contrast, the CH4 adsorption amount is only 7.8 cm3 g−1 under the same conditions, which is much lower than those of C3 and C2 hydrocarbons. Such a difference between the adsorption amounts of higher hydrocarbons and that of CH4 indicates that HOF-14 may enable highly selective separation of CH4 from these C3 and C2 hydrocarbons. To analyse the gas separation abilities of HOF-14, ideal adsorbed solution theory (IAST) was used to predict the selectivity of binary mixtures of C3 and C2 hydrocarbons with CH4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) at 298 K (Fig. 3b). As anticipated, the selectivities in C3H8/CH4, C3H6/CH4, C2H6/CH4, C2H4/CH4, and C2H2/CH4 mixtures were estimated to be 28.6, 24.6, 6.3, 4.4 and 3.7 at 298 K and 100 kPa, respectively (Fig. 3b). In addition, the isosteric heats of adsorption of C3 and C2 hydrocarbons are larger than that of CH4 (Fig. S6, ESI†), which indicates stronger interactions between the C3 and C2 hydrocarbons and the pore surfaces. Consequently, HOF-14 can highly selectively adsorb the light hydrocarbons under ambient conditions, thus separating them.
50) at 298 K (Fig. 3b). As anticipated, the selectivities in C3H8/CH4, C3H6/CH4, C2H6/CH4, C2H4/CH4, and C2H2/CH4 mixtures were estimated to be 28.6, 24.6, 6.3, 4.4 and 3.7 at 298 K and 100 kPa, respectively (Fig. 3b). In addition, the isosteric heats of adsorption of C3 and C2 hydrocarbons are larger than that of CH4 (Fig. S6, ESI†), which indicates stronger interactions between the C3 and C2 hydrocarbons and the pore surfaces. Consequently, HOF-14 can highly selectively adsorb the light hydrocarbons under ambient conditions, thus separating them.
In summary, a novel chemically and thermally stable mesoporous HOF, HOF-14, was constructed. HOF-14 has a high surface area and a large pore volume and has potential applications for separation and purification of light hydrocarbons.
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (21971038, 21975044, 21805039, 21673039, and 573042), the Fujian Science and Technology Department (2019H6012 and 2018J07001), the China Postdoctoral Science Foundation (Grant No. 2018M642556), and the Welch Foundation (AX-1730). Work at the Molecular Foundry and Advanced Light Source was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC.
- A. I. Cooper, Angew. Chem., Int. Ed., 2012, 51, 7892–7894 CrossRef CAS.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, eaal1585 CrossRef.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef.
- H. Cui, Y. Ye, H. Arman, Z. Li, A. Alsalme, R.-B. Lin and B. Chen, Cryst. Growth Des., 2019, 19, 5829–5835 CrossRef CAS.
- J. Lu and R. Cao, Angew. Chem., Int. Ed., 2016, 55, 9474–9480 CrossRef.
- Y. He, S. Xiang and B. Chen, J. Am. Chem. Soc., 2011, 133, 14570–14573 CrossRef CAS.
- P. Li, Y. He, H. D. Arman, R. Krishna, H. Wang, L. Weng and B. Chen, Chem. Commun., 2014, 50, 13081–13084 RSC.
- H. Wang, B. Li, H. Wu, T. L. Hu, Z. Yao, W. Zhou, S. Xiang and B. Chen, J. Am. Chem. Soc., 2015, 137, 9963–9970 CrossRef CAS.
- H. Wang, H. Wu, J. Kan, G. Chang, Z. Yao, B. Li, W. Zhou, S. Xiang, J. Cong-Gui Zhao and B. Chen, J. Mater. Chem. A, 2017, 5, 8292–8296 RSC.
- W. Yang, B. Li, H. Wang, O. Alduhaish, K. Alfooty, M. A. Zayed, P. Li, H. D. Arman and B. Chen, Cryst. Growth Des., 2015, 15, 2000–2004 CrossRef CAS.
- D. W. Kang, M. Kang, H. Kim, J. H. Choe, D. W. Kim, J. R. Park, W. R. Lee, D. Moon and C. S. Hong, Angew. Chem., Int. Ed., 2019, 58, 16152–16155 CrossRef CAS.
- I. Hisaki, Y. Suzuki, E. Gomez, Q. Ji, N. Tohnai, T. Nakamura and A. Douhal, J. Am. Chem. Soc., 2019, 141, 2111–2121 CrossRef CAS.
- W. Liang, F. Carraro, M. B. Solomon, S. G. Bell, H. Amenitsch, C. J. Sumby, N. G. White, P. Falcaro and C. J. Doonan, J. Am. Chem. Soc., 2019, 141, 14298–14305 CrossRef CAS.
- T. H. Chen, I. Popov, W. Kaveevivitchai, Y. C. Chuang, Y. S. Chen, O. Daugulis, A. J. Jacobson and O. S. Miljanic, Nat. Commun., 2014, 5, 5131 CrossRef CAS PubMed.
- A. Karmakar, R. Illathvalappil, B. Anothumakkool, A. Sen, P. Samanta, A. V. Desai, S. Kurungot and S. K. Ghosh, Angew. Chem., Int. Ed., 2016, 55, 10667–10671 CrossRef CAS.
- B. Han, H. Wang, C. Wang, H. Wu, W. Zhou, B. Chen and J. Jiang, J. Am. Chem. Soc., 2019, 141, 8737–8740 CrossRef CAS.
- W. Gong, D. Chu, H. Jiang, X. Chen, Y. Cui and Y. Liu, Nat. Commun., 2019, 10, 600 CrossRef.
- Q. Yin, P. Zhao, R. J. Sa, G. C. Chen, J. Lu, T. F. Liu and R. Cao, Angew. Chem., Int. Ed., 2018, 57, 7691–7696 CrossRef CAS.
- P. Li, Y. He, J. Guang, L. Weng, J. C. Zhao, S. Xiang and B. Chen, J. Am. Chem. Soc., 2014, 136, 547–549 CrossRef CAS.
- P. Li, Y. He, Y. Zhao, L. Weng, H. Wang, R. Krishna, H. Wu, W. Zhou, M. O'Keeffe, Y. Han and B. Chen, Angew. Chem., Int. Ed., 2015, 54, 574–577 CAS.
- H. Wang, Z. Bao, H. Wu, R. B. Lin, W. Zhou, T. L. Hu, B. Li, J. C. Zhao and B. Chen, Chem. Commun., 2017, 53, 11150–11153 RSC.
- W. Yang, F. Yang, T.-L. Hu, S. C. King, H. Wang, H. Wu, W. Zhou, J.-R. Li, H. D. Arman and B. Chen, Cryst. Growth Des., 2016, 16, 5831–5835 CrossRef CAS.
- F. Hu, C. Liu, M. Wu, J. Pang, F. Jiang, D. Yuan and M. Hong, Angew. Chem., Int. Ed., 2017, 56, 2101–2104 CrossRef CAS.
- I. Hisaki, Y. Suzuki, E. Gomez, B. Cohen, N. Tohnai and A. Douhal, Angew. Chem., Int. Ed., 2018, 57, 12650–12655 CrossRef CAS.
- Y. Jin, Y. Hu and W. Zhang, Nat. Rev. Chem., 2017, 1, 0056 CrossRef.
- During the prepartion of this manuscript, we noticed that the same structure named HOF-102 has been reported in a preprinting work by Farha Omar and co-workers. However, they did not obtain the single crystal structure of HOF-102, they constructed its structure by using Material Studio software. K. Ma, P. Li, J. Xin, Y. Chen, Z. Chen, S. Goswami, X. Liu, S. Kato, H. Chen, X. Zhang, J. Bai, M. Wasson, R. Maldonado, R. Snurr and O. Farha, ChemRxiv, 2019 DOI:10.26434/chemrxiv.9729494.v1.
- M. Mastalerz and I. M. Oppel, Angew. Chem., Int. Ed., 2012, 51, 5252–5255 CrossRef CAS.
- Q. Yin, Y. L. Li, L. Li, J. Lu, T. F. Liu and R. Cao, ACS Appl. Mater. Interfaces, 2019, 11, 17823–17827 CrossRef CAS.
- C. A. Zentner, H. W. Lai, J. T. Greenfield, R. A. Wiscons, M. Zeller, C. F. Campana, O. Talu, S. A. FitzGerald and J. L. Rowsell, Chem. Commun., 2015, 51, 11642–11645 RSC.
- S. Nandi, D. Chakraborty and R. Vaidhyanathan, Chem. Commun., 2016, 52, 7249–7252 RSC.
- J. Lu, C. Perez-Krap, M. Suyetin, N. H. Alsmail, Y. Yan, S. Yang, W. Lewis, E. Bichoutskaia, C. C. Tang, A. J. Blake, R. Cao and M. Schroder, J. Am. Chem. Soc., 2014, 136, 12828–12831 CrossRef CAS.
- Z. Bao, G. Chang, H. Xing, R. Krishna, Q. Ren and B. Chen, Energy Environ. Sci., 2016, 9, 3612–3641 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1955714. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc07802a |
| This journal is © The Royal Society of Chemistry 2020 |