A hierarchical hollow-on-hollow NiCoP electrocatalyst for efficient hydrogen evolution reaction†
Juntao
Zhang
ab,
Lipeng
Zhang
ab,
Xingdong
Wang
ab,
Wei
Zhu
 *ab and
Zhongbin
Zhuang
*ab and
Zhongbin
Zhuang
 *abc
*abc
aState Key Lab of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, China
bBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: zhuwei@mail.buct.edu.cn; zhuangzb@mail.buct.edu.cn
cBeijing Key Laboratory of Energy Environmental Catalysis, Beijing University of Chemical Technology, Beijing 100029, China
First published on 25th November 2019
Abstract
A hierarchical hollow NiCoP nanopolyhedron composed of assembled hollow nanoparticles was prepared by a facile synthetic route. It showed superior activity and durability for the hydrogen evolution reaction. The unique hierarchical hollow-on-hollow structure was demonstrated to be the origin of the promoted performance.
Hydrogen energy shows excellent potential to substitute fossil fuels owing to its high energy density and eco-friendly features.1,2 After considering the secondary pollution and carbon emission issues, water electrolysis has been proven to be the chosen one for the production of clean hydrogen.3,4 Since the high price and low abundance of noble metal cathodic catalysts for the hydrogen evolution reaction (HER) stunt the large-scale application of water electrolysis, considerable efforts have been made to look for noble-metal-free HER electrocatalysts to reduce the cost.5–9 3d transition metal phosphides are widely accepted as one of the most promising HER catalysts because of the relatively high HER performance.5,10,11 However, the HER activity of metal phosphides is still more than two orders of magnitude lower than that of Pt/C, and they are also prone to dissolution in long-term operation. Hence, it is essential to improve the HER activity and durability via structural engineering strategies by increasing the density and robustness of accessible active sites.12
By virtue of the high surface-to-volume ratios, abundant exposed active sites, sufficient reaction zone, and short mass transport path, high-performance hollow nanostructures have gained a lot of attention for various applications, particularly for energy-related electrocatalysis.13–15 Furthermore, hollow structures can also serve as perfect hosts to accommodate other unique structural features (e.g., nanowires, nanosheets, etc.) and evolve into hierarchical hollow nanomaterials.16–18 The hierarchical hollow nanomaterials integrate the edges of multiple structures, achieving enhanced performance or multifunctionality. Various efficient hierarchical hollow nanocatalysts, e.g., nanocages consisting of thin Ni-Co layered double hydroxide (LDH) nanosheets,19 and nano-polyhedrons composed of CoPx NPs,20 have been synthesized. Construction of hierarchical hollow-on-hollow nanomaterials can significantly facilitate mass transport and charge transfer of catalytic processes by assembling hollow building blocks into crosslinked hollow shells with single-particle thickness, promoting the surface utilization of hollow NPs as well as the possible interparticle cooperativity.21,22 Meanwhile, the self-supported feature strengthens the robustness of tiny building units, resulting in high durability. Hence, the design of hierarchical hollow-on-hollow metal phosphide nanostructures is a valid structural engineering method for the HER performance promotion of noble metal-free catalysts. Herein, we demonstrated a multi-step strategy for the synthesis of hierarchical hollow-on-hollow NiCoP nanocatalysts (HiHo-NiCoP) for efficient HER, which were hollow polyhedrons composed of assembled hollow NiCoP NPs.
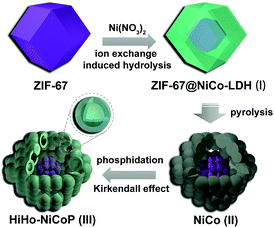
As illustrated in Scheme 1, the synthesis of HiHo-NiCoP was started with the typical preparation of ZIF-67 NPs by stirring the mixed solution of Co(NO3)2 and 2-methylimidazole (HMIM) at room temperature. Fig. S1 (ESI†) shows the TEM image and XRD pattern of the as-obtained ZIF-67 nanopolyhedrons of several hundred nanometres in size. Then, the ZIF-67 nanopolyhedrons were added into an ethanol solution of Ni(NO3)2 and stirred at room temperature for 2 h. The transmission electron microscopy (TEM) and the X-ray diffraction (XRD) pattern of the product in Fig. S2 (ESI†) suggested the formation of yolk–shell structures with layered double hydroxide shells (denoted as NiCo-LDH, I in Scheme 1).23,24 Then, the yolk–shell ZIF-67@NiCo-LDH nanopolyhedrons were pyrolysis at 900 °C in N2 atmosphere to form carbon-supported NiCo NPs (denoted as NiCo, II in Scheme 1, Fig. S3 in ESI†). Finally, the HiHo-NiCoP (III in Scheme 1) was obtained via the phosphidation of NiCo by using NaH2PO2 as the phosphorous source.
Fig. 1a shows the HiHo-NiCoP polyhedrons with a diameter of ca. 400 nm. The outer shells were composed by assembled hollow NPs with a diameter of 10–70 nm, and the inner voids accommodated porous particles originating from the pyrolysis and phosphidation of the ZIF-67 cores. As illustrated in Fig. 1b, a carbon shell was observed on the surface of the hollow phosphide NPs. It probably resulted from the carbonization of the organic ligand during the pyrolysis. Fig. S4 (ESI†) shows the X-ray dispersive energy spectrum (EDS) of the HiHo-NiCoP. It confirmed the co-existence of Co, Ni, and P with a Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 31
P ratio of 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36. The XRD pattern (Fig. 1c) demonstrated the complete conversion from metals to phosphides in the phosphidation step. It matched well with the standard diffraction patterns of Ni2P (JCPDS No. 65-9706) and CoP (JCPDS No. 65-1474), indicating that the HiHo-NiCoP was composed of Ni2P and CoP. The high angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image (Fig. 1d) confirmed the hierarchical hollow-on-hollow structure of the HiHo-NiCoP NPs. The corresponding EDS elemental mapping profiles in Fig. 1d demonstrated that Ni, Co, and P dispersed uniformly in the as-obtained HiHo-NiCoP, indicating the incorporation of Ni into the lattice of ZIF-67 during the ion-exchange process.
36. The XRD pattern (Fig. 1c) demonstrated the complete conversion from metals to phosphides in the phosphidation step. It matched well with the standard diffraction patterns of Ni2P (JCPDS No. 65-9706) and CoP (JCPDS No. 65-1474), indicating that the HiHo-NiCoP was composed of Ni2P and CoP. The high angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image (Fig. 1d) confirmed the hierarchical hollow-on-hollow structure of the HiHo-NiCoP NPs. The corresponding EDS elemental mapping profiles in Fig. 1d demonstrated that Ni, Co, and P dispersed uniformly in the as-obtained HiHo-NiCoP, indicating the incorporation of Ni into the lattice of ZIF-67 during the ion-exchange process.
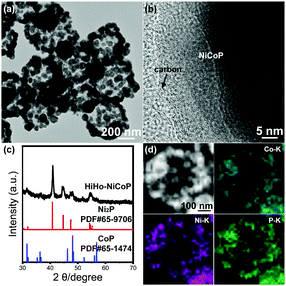 | ||
| Fig. 1 (a) TEM image, (b) HRTEM image, (c) XRD pattern, (d) HAADF-STEM image, and the corresponding elemental mapping profiles of the HiHo-NiCoP. | ||
Fig. 2 shows the TEM images of the ZIF-67@NiCo-LDH at different reaction times of the ion-exchange-induced hydrolysis, and the corresponding products after the pyrolysis and phosphidation. By comparing the contours of the products at each stage (I, II, and III) in Fig. 2, the formation of yolk–shell ZIF-67@NiCo-LDH determined the primary hollow structure of the HiHo-NiCoP. As illustrated in Fig. 2a–d, the size of the core structure was tunable by controlling the ion exchange step. Hollow shell structures with large cores inside generated at 1 h (Fig. 2b). When the reaction time was 2 h, the shell wall grew thicker with a small polyhedral core. When the reaction time was 4 h, the ZIF-67 core was consumed, leaving only the hollow shell, which was made up of tiny NiCo-LDH nanosheets (Fig. 2d). Hence, an ion-exchange-induced hydrolysis mechanism probably resulted in the primary hollow structure, as denoted by the following equations.25
| Ion exchange (core): Co(MIM)2 + Ni2+ → Ni(MIM)2 + Co2+ | (1) |
| Dissolution of ZIF-67 (core): Co(MIM)2 ⇌ Co2+ + 2(MIM)− | (2) |
| Hydrolysis of deprotonated HMIM (MIM−) (shell): (MIM)− + H2O ⇌ HMIM + OH− | (3) |
| Hydrolysis into NiCo-LDH (shell): Co2+ + Ni2+ + xOH− + (4 − x)L− → CoNi(OH)xL(4−x) | (4) |
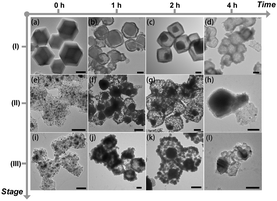 | ||
| Fig. 2 TEM images of the ZIF-67@NiCo-LDH intermediates reacted for (a) 0 h, (b) 1 h, (c) 2 h, and (d) 4 h. TEM images of (e–h) the corresponding pyrolysis products and (i–l) the subsequent phosphidation products. The x-axis represents the reaction time of the ion exchange step, and the y-axis represents the products for the different reaction stages in Fig. 1, i.e., (I) for the ion exchange hydrolysis, (II) for the pyrolysis, (III) for the phosphidation. All the scale bars represented 200 nm. | ||
Ostwald ripening was the probable driving force for mass transport from small cores to large shells.26 At the pyrolysis step, the pristine ZIF-67@NiCo-LDH converted into cross-linked carbon-supported NiCo NPs and maintained its original hollow contour. The HMIM ligand decomposed into nitrogen-doped carbon, and also reduced the Ni2+ and Co2+ into metallic NPs. Since the primary hollow structures were NiCo-LDH shells, the remaining ZIF-67 cores in the yolk–shell spheres served as the reducing agent as well as the carbon source. Consequently, these results indicated that the hollow ZIF-67@NiCo-LDH intermediates played a vital role in the formation of the primary hollow structure of the HiHo-NiCoP, and the reaction time of 2 h is the optimal condition for the formation of ZIF-67@NiCo-LDH.
For the secondary hollow structures, the pyrolysis of the yolk–shell ZIF-67@NiCo-LDH prior to the phosphidation step was proven to be the critical process. We directly conducted the phosphidation treatment on ZIF-67@NiCo-LDH without pyrolysis, and nanocages composed of solid phosphide NPs were finally obtained, as shown in Fig. S5 (ESI†) (denoted as H1-NiCoP). The pyrolysis step made the ZIF-67@NiCo-LDH convert into hollow spheres with metallic NiCo NPs embedded in the shell walls. Only after the pyrolysis procedure, the solid NiCo NPs can transform into hollow NiCoP NPs with the help of the Kirkendall effect during the successive phosphidation step, leading to the formation of secondary hollow structures.27,28
X-ray photoelectron spectroscopy (XPS) was conducted to investigate the surface composition and chemical environment of the HiHo-NiCoP. For the high-resolution Co 2p core-level spectrum (Fig. S6b in ESI†), the two peaks located at 778.6 and 793.7 eV were assigned to Co 2p3/2 and 2p1/2 in cobalt phosphide, respectively.29,30 Similar results were found for the Ni 2p core-level spectrum (Fig. S6c in ESI†). For the high-resolution P 2p core-level spectrum (Fig. S6d in ESI†), the peak at 130.0 eV was assigned to the metal phosphides, and the peak at 134.2 eV was assigned to the oxidized species due to the exposure to air.
The HER performance evaluations of the as-synthesized HiHo-NiCoP catalysts were conducted in H2-saturated 0.5 M H2SO4 aqueous electrolyte by using a standard three-electrode system. The catalyst loadings on the glassy carbon electrodes were 0.41 mg cm−2. To investigate the merits of the hierarchical hollow-on-hollow structure, we also performed the HER activity evaluations on the reference samples, i.e., one solid NiCoP NPs and two hollow NiCoP NPs. We synthesized solid NiCoP NPs by the phosphidation of Ni-doped ZIF-67 (synthetic methods in ESI†). The TEM image and XRD pattern in Fig. S7 (ESI†) confirmed the dendritic morphology and bimetallic phosphide phase of the NiCoP NPs. Two hollow NiCoP reference samples were employed to mimic the primary and secondary hollow structure features of the HiHo-NiCoP respectively (synthetic methods in ESI†): the H1-NiCoP was a simple shell structure assembled by NiCoP nanosheets and possessed the primary hollow structure feature (Fig. S5 in ESI†), and both the synthetic method and structure were similar to those of the reported NiCoP hollow quasi-polyhedra;31 the H2-NiCoP, carbon-supported hollow NiCoP NP, was prepared by the pyrolysis and phosphidation of NiCo-ZIF-67, and it showed the secondary hollow feature of the HiHo-NiCoP (Fig. S8 in ESI†).
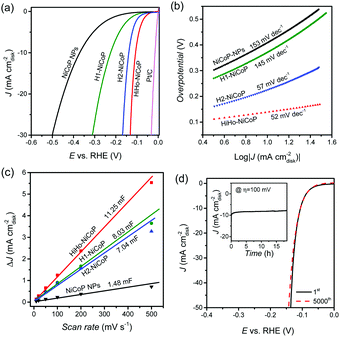
Fig. 3a shows the iR-compensated polarization curves of the aforementioned NiCoP samples at a sweep rate of 5 mV s−1. We chose the overpotential value at the HER current density of 10 mA cm−2 (denoted as η10) as the criterion to evaluate the HER activity. Among all the samples, the solid NiCoP NPs showed the worst HER activity, and required an overpotential of 360 mV to achieve the HER current density of 10 mA cm−2 (η10 = 360 mV). The η10 values for H1-NiCoP and H2-NiCoP were 230 mV and 140 mV, respectively, exhibiting higher HER activity than the solid counterpart. It demonstrated the promotion effect of the primary and secondary hollow structures for the HER. As expected, the HiHo-NiCoP showed the highest HER activity with the η10 value of 103 mV at a low loading of 0.41 mg cm−2, which was comparable to the best reported NiCoP catalysts (Table S1 in ESI†). It was demonstrated that both the primary and secondary hollow structures contributed to the overall HER activity of the HiHo-NiCoP catalyst. Furthermore, as shown in Fig. S9 (ESI†), the HiHo-NiCoP also exhibited much higher activity than the monometallic phosphide NPs, i.e., Ni2P and Co2P (Fig. S10 ESI†), suggesting the probable synergistic effect. As shown in Fig. 3b, the Tafel slope values followed the sequence: HiHo-NiCoP (52 mV dec−1) < H2-NiCoP (57 mV dec−1) < H1-NiCoP (145 mV dec−1) < NiCoP NPs (153 mV dec−1). It was indicated that the HiHo-NiCoP showed the most effective electrocatalytic kinetics towards the HER among all the NiCoP samples. Hence, the activity evaluation results suggested that the hierarchical hollow-on-hollow NiCoP integrated the merits of the primary and secondary hollow structures, and thus displayed the enhanced catalytic activity of the HER.
Typically, the catalysts with a hollow structure possess large surface area and facilitate the mass transport of the reactions. Then, the electrochemically active surface areas of the catalysts were estimated by the double-layer capacitance values. Fig. S11 (ESI†) shows the CV curves of the catalysts in the non-faradaic range with different sweep rates, and Fig. 3c shows the corresponding double-layer capacitance plots. The two simple-hollow NiCoP references showed larger double-layer capacitance than the solid counterpart; the HiHo-NiCoP had the largest capacitance value, demonstrating the highest electrochemical active surface areas. Therefore, it was deduced that the structure effect of the HiHo-NiCoP for the HER was originated from the abundant accessible active sites and the mass-transfer-improved micro-environment created by the high surface area.
The durability was another significant parameter for the HER electrocatalysts. We performed the accelerating degradation test (ADT) on the HiHo-NiCoP sample by cycling the catalysts between −0.2 and 0 V with a scan rate of 100 mV s−1. Fig. 3d shows the polarization curves of the HiHo-NiCoP before and after the 5000 cycle CV scan. The η10 value remained unchanged in the ADT, and the overpotential at the current density of 50 mA cm−2 merely increased from 136 mV to 142 mV. The microstructure of the HiHo-NiCoP was almost retained after the long-term ADT measurement (Fig. S12, ESI†). As shown in Fig. S13, a loss of η10 value by 28 mV and 40 mV took place after 1000 cycle ADT experiments for H1-NiCoP and H2-NiCoP, respectively. We also conducted the chronoamperometric measurement (inset of Fig. 3d) at the overpotential of 100 mV. No significant loss of the cathodic current was observed during 18 h continuous operation. As compared with the reported NiCoP catalysts in Table S1 (ESI†), the superior long-term stability of the HiHo-NiCoP for the HER has been demonstrated. The self-supported hierarchical hollow framework was deduced as the probable reason for the high structure robustness,32 while the bimetallic phosphide was proven to be favourable for the surface stabilization in our previous work.27 Hence, the unique structure and bimetallic composition probably prevented the HiHo-NiCoP catalyst from agglomeration and oxidative passivation, leading to high durability in the long-term HER operation.
In summary, we took advantage of Ostwald ripening and the Kirkendall effect and prepared a hierarchical hollow-on-hollow NiCoP catalyst by using ZIF-67 as the precursors. The HiHo-NiCoP were hollow polyhedrons composed of assembled hollow nanospheres, and their HER activities surpassed those of the solid and simple hollow NiCoP counterparts. Abundant accessible active sites and improved mass transport brought by the hierarchical hollow-on-hollow structure were deduced as the reason for the superior activity. Moreover, excellent long-term HER durability was demonstrated in the ADT and chronoamperometric measurements. The high robustness of the self-supported structure probably protected the reactive sites from degeneration.
This work was financially supported by the National Natural Science Foundation of China (No. 21671014) and the Fundamental Research Funds for the Central Universities (buctrc201823).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Chow, R. J. Kopp and P. R. Portney, Science, 2003, 302, 1528–1531 CrossRef.
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS.
- J. D. Holladay, J. Hu, D. L. King and Y. Wang, Catal. Today, 2009, 139, 244–260 CrossRef CAS.
- S. Q. Lu and Z. B. Zhuang, Sci. China Mater., 2016, 59, 217–238 CrossRef CAS.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, J. Am. Chem. Soc., 2013, 135, 9267–9270 CrossRef CAS.
- X. Liang, B. Zheng, L. Chen, J. Zhang, Z. Zhuang and B. H. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 23222–23229 CrossRef CAS.
- K. Xu, P. Chen, X. Li, Y. Tong, H. Ding, X. Wu, W. Chu, Z. Peng, C. Wu and Y. Xie, J. Am. Chem. Soc., 2015, 137, 4119–4125 CrossRef CAS.
- D. Voiry, R. Fullon, J. Yang, C. C. E. S. C. De, R. Kappera, I. Bozkurt, D. Kaplan, M. J. Lagos, P. E. Batson and G. Gupta, Nat. Mater., 2018, 15, 1003 CrossRef.
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- M. Sun, H. J. Liu, J. H. Qu and J. H. Li, Adv. Energy Mater., 2016, 6, 1600087 CrossRef.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. B. Chorkendorff, J. K. Norskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef.
- Y. M. Chen, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 5990–5993 CrossRef CAS.
- G. Prieto, H. Tuysuz, N. Duyckaerts, J. Knossalla, G. H. Wang and F. Schuth, Chem. Rev., 2016, 116, 14056–14119 CrossRef CAS.
- W. Zhu, Z. Chen, Y. Pan, R. Dai, Y. Wu, Z. Zhuang, D. Wang, Q. Peng, C. Chen and Y. Li, Adv. Mater., 2019, 31, 1800426 CrossRef.
- H. B. Yao, H. Y. Fang, X. H. Wang and S. H. Yu, Chem. Soc. Rev., 2011, 40, 3764–3785 RSC.
- M. Fang, G. F. Dong, R. J. Wei and J. C. Ho, Adv. Energy Mater., 2017, 7, 1700559 CrossRef.
- J. Y. Wang, J. W. Wan and D. Wang, Acc. Chem. Res., 2019, 52, 2169–2178 CrossRef CAS.
- C. C. Sun, J. Yang, X. H. Rui, W. N. Zhang, Q. Y. Yan, P. Chen, F. W. Huo, W. Huang and X. C. Dong, J. Mater. Chem. A, 2015, 3, 8483–8488 RSC.
- B. You, N. Jiang, M. L. Sheng, S. Gul, J. Yano and Y. J. Sun, Chem. Mater., 2015, 27, 7636–7642 CrossRef CAS.
- T. Li, B. Xue, B. Wang, G. Guo, D. Han, Y. Yan and A. Dong, J. Am. Chem. Soc., 2017, 139, 12133–12136 CrossRef CAS.
- Y. M. Chen, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 5990–5993 CrossRef CAS.
- Z. Jiang, Z. P. Li, Z. H. Qin, H. Y. Sun, X. L. Jiao and D. R. Chen, Nanoscale, 2013, 5, 11770–11775 RSC.
- T. Wang, S. L. Zhang, X. B. Yan, M. Q. Lyu, L. Z. Wang, J. Bell and H. X. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 15510–15524 CrossRef CAS.
- M. Kim, J. F. Cahill, H. H. Fei, K. A. Prather and S. M. Cohen, J. Am. Chem. Soc., 2012, 134, 18082–18088 CrossRef CAS.
- X. W. Lou, Y. Wang, C. L. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325–2329 CrossRef CAS.
- H. M. Li, Y. Wang, W. Zhu and Z. B. Zhuang, Int. J. Hydrogen Energy, 2019, 44, 22806–22815 CrossRef CAS.
- R. K. Chiang and R. T. Chiang, Inorg. Chem., 2007, 46, 369–371 CrossRef CAS.
- A. P. Grosvenor, S. D. Wik, R. G. Cavell and A. Mar, Inorg. Chem., 2005, 44, 8988–8998 CrossRef CAS.
- I. I. Abu and K. J. Smith, J. Catal., 2006, 241, 356–366 CrossRef CAS.
- Y. Li, J. Liu, C. Chen, X. Zhang and J. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 5982–5991 CrossRef CAS.
- X. Ren, D. Wu, R. X. Ge, X. Sun, H. M. Ma, T. Yan, Y. Zhang, B. Du, Q. Wei and L. Chen, Nano Res., 2018, 11, 2024–2033 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc08256e |
| This journal is © The Royal Society of Chemistry 2020 |