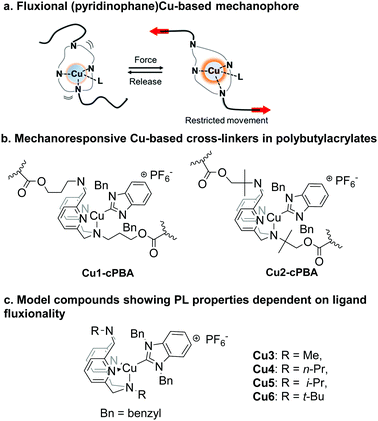
Highly sensitive mechano-controlled luminescence in polymer films modified by dynamic CuI-based cross-linkers†
Ayumu
Karimata‡
 a,
Pradnya H.
Patil‡
a,
Pradnya H.
Patil‡
 a,
Eugene
Khaskin
a,
Eugene
Khaskin
 a,
Sébastien
Lapointe
a,
Sébastien
Lapointe
 a,
Robert R.
Fayzullin
a,
Robert R.
Fayzullin
 b,
Pavlos
Stampoulis
c and
Julia R.
Khusnutdinova
b,
Pavlos
Stampoulis
c and
Julia R.
Khusnutdinova
 *a
*a
aCoordination Chemistry and Catalysis Unit, Okinawa Institute of Science and Technology Graduate University, 1919-1 Tancha, Onna-son, Okinawa, 904-0495, Japan. E-mail: juliak@oist.jp
bArbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center, Russian Academy of Sciences, 8 Arbuzov Street, Kazan, 420088, Russia
cJEOL RESONANCE Inc., Musashino, Akishima, Tokyo 196-8558, Japan
First published on 18th November 2019
Abstract
Dynamic CuI-based mechanophores used as cross-linkers in polybutylacrylates enable highly sensitive detection of mechanical stress even at small strain (<50%) and stress (<0.1 MPa) values via reversible changes in luminescence intensity. Such sensitivity is superior to previously reported systems based on classical organic mechanophores and it allows for direct visualization of mechanical stress by imaging methods.
Mechanoresponsive materials are a class of “smart” materials that demonstrate specific property changes induced by mechanical stimuli, for example, showing self-reporting or self-healing properties. Given the widespread use of synthetic polymers for engineering applications, the design of mechanoresponsive polymers capable of self-reporting mechanical stress is of high importance not only for preventing the materials’ failure, but also for the better understanding of polymer response to mechanical force. An interesting approach to the design of diverse mechanosensitive materials includes the use of mechanophores covalently incorporated into polymer samples, which can change their spectroscopic properties in response to mechanical force.1 The majority of commonly used mechanophores are based on organic molecules such as spiropyran,2 1,2-dioxetane,3 diaryldibenzofuranone,4 and others,5 in which scission of a weak covalent chemical bond is caused by mechanical force. Intrinsically, the requirement for such covalent bond scission, and its associated structural reorganization, often leads to an irreversible response, or a slow recovery of the mechanophore's original state. Such drawbacks hinder the development of practical mechanical stress probes that can directly visualize subtle stress changes repeatably in real-time. Although some progress has been made in developing molecular mechanophores that do not require covalent bond scission,6 including our previous report,7 these systems typically require significant strain (>100%, e.g. stretching to more than two-fold of the original length during a tensile test) and stress (several MPa) to observe a response. In general, the development of a mechanosensitive system with fast, reversible and sensitive response remains a tremendous opportunity, as well as a challenge.
Our group has recently reported a CuI iodide complex with a macrocyclic pyridinophane ligand covalently incorporated into a linear polyurethane chain that shows gradual photoluminescence intensity changes in response to tensile stress (Scheme 1a).7 However, that initial system suffered from fast degradation under air, low photoluminescence quantum yield (PLQY), and it also required stretching to several times of the sample's initial length (strain >100%) in order to observe the response, meaning an overall low sensitivity and an inefficient transmission of mechanical force.
In the current work we report new, photoluminescent (NHC)CuI complexes covalently incorporated into polybutylacrylate as a cross-linker. As a result, cross-linked polybutylacrylate (cPBA) samples Cu1-cPBA and Cu2-cPBA (Scheme 1b) demonstrate a highly sensitive response to mechanical stress even at small strain (<50%) and stress (<0.1 MPa) values. Such sensitivity is unprecedented when compared to many currently known stress-responsive polymers containing organic-based mechanophores. This system enables direct visualization of mechanical stress via imaging methods. In addition, we achieved good air-stability as the samples showed only minor degradation after several days under air.
We propose that the emission intensity increases in response to mechanical force, due to restricting the mobility of the cross-linker which contains the fluxional, Cu-based mechanophore. Thus, these systems represent a new type of mechanophore in which the mechanism of response is not caused by the cleavage, or the formation of a covalent bond, letting us achieve a fast and reversible response.6 Our mechanistic proposal is supported by the study of model compounds Cu3–6 (Scheme 1c), which display good correlation between complex fluxionality and their non-radiative decay rate, as well as their PLQY.
Before attempting to make cross-linkers and incorporating them into a polymer, we optimized the synthesis of model complexes Cu3–6 containing the RN4 ligand, with its steric hindrance modified by the R substituent (R = Me, n-Pr, i-Pr, t-Bu). The complexes were synthesized by reacting the corresponding macrocyclic ligands RN4 with (BnNHC)CuCl (1,3-dibenzylbenzimidazoyl-2-ylidene)copper(I) chloride, followed by the counterion exchange with KPF6. These complexes were isolated and characterized by NMR, IR, UV-vis spectroscopies, single-crystal X-ray diffraction (Fig. S72, ESI†), and elemental analysis.8 Their dynamic behavior in solution was studied in detail by NMR (vide infra)8 and was found to be similar to previously described (RN4)CuII complexes.9 Photophysical properties of Cu3–6 are summarized in Table 1.
| Complex | R | λ max [nm] | PLQY | τ [μs] | k r 10−4 [s−1] | k nr 10−4 [s−1] |
|---|---|---|---|---|---|---|
| a Excitation at 380 nm. b Emission maximum. c Emission lifetime. d Radiative decay rate constant. e Non-radiative decay rate constant. | ||||||
| Cu3 | Me | 567 | 0.06 | 8.20 | 0.732 | 11.5 |
| Cu4 | n Pr | 561 | 0.19 | 7.59 | 2.50 | 10.7 |
| Cu5 | iPr | 551 | 0.30 | 9.98 | 3.00 | 7.01 |
| Cu6 | t Bu | 549 | 0.72 | 18.0 | 4.00 | 1.56 |
We then set out to prepare cross-linked polybutylacrylate (cPBA) samples using the bis(acrylate) functionalized pyridinophane ligand as a cross-linker. PBA was selected for this study due to its widespread use in industry, its suitable mechanical properties such as elasticity, and the possibility to further tune a wide range of properties by a rich choice of monomers. Cross-linked sample Cu1-cPBA was prepared by photo-initiated radical polymerization using butyl acrylate and bis(acrylate)-functionalized ligand L1 (1 mol%), followed by incorporation of (BnNHC)CuI by dipping the film into a solution of (BnNHC)CuCl precursor (Scheme S5, ESI†). Cu2-cPBA was prepared by the analogous procedure using acrylate-functionalized ligand L2 (Scheme S6, ESI†).8 PLQYs of Cu1-cPBA and Cu2-cPBA were determined to be 0.075 and 0.27, respectively.
The mechanical properties of Cu1-cPBA and Cu2-cPBA were investigated using a tensile testing machine under an argon atmosphere. The representative stress–strain (S–S) curves are given in Fig. S31 (ESI†) showing good sample elongation (323% for Cu1-cPBA, 476% for Cu2-cPBA).
To investigate the mechanoresponsive properties of the new cross-linked Cu1-cPBA film, it was stretched uniaxially and at the same time the luminescence spectrum during stretching was measured by monitoring the film's central area. As the Cu1-cPBA film was being stretched, its luminescence intensity increased (Fig. 1a). The plot of integrated luminescence intensity vs. tensile strain (Fig. 1b) shows that luminescence intensity gradually increased even when the strain was less than 50% and the tensile stress was less than 0.1 MPa. Such sensitivity is much higher than that found in the majority of previously reported organic mechanophore systems, which typically require stress of several MPa to reliably observe a response.2–6
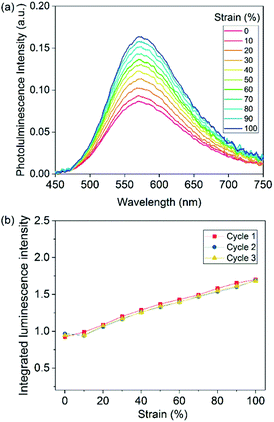 | ||
| Fig. 1 (a) Emission spectra of Cu1-cPBA during stretching. (b) Plot of integrated photoluminescence intensity vs. strain of Cu1-cPBA. | ||
The film was stretched and released three times from 0 to 100% of strain, showing only a slight photoluminescence (PL) intensity decrease when compared to the original value measured during the first cycle. The luminescence enhancement was observed in a reproducible and reversible manner over more than 30 cycles of applied stress (Fig. 2). Furthermore, an additional intensity enhancement in response to tensile elongation was also observed up to the film's breaking point (Fig. S21, ESI†).8 Up to a 200% strain value, the maximum wavelength and the shape of the emission spectrum remains unchanged, indicating that no significant structural changes occur upon stretching.
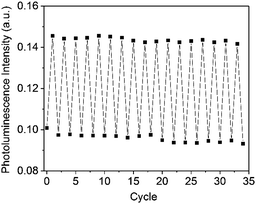 | ||
| Fig. 2 Photoluminescence intensity of Cu1-cPBA during repeated stretching (strain varies from 0 to 100%). | ||
A similar mechanoresponse which showed a measurable emission intensity increase at low strain and stress values was also observed for Cu2-cPBA (Fig. S23, ESI†).8
A sensitive response and good PLQY suggest that it should be possible to directly visualize applied mechanical stress via optical imaging. Accordingly, we monitored the luminescence intensity change in the Cu1-cPBA and Cu2-cPBA samples by a CCD camera during repeated cycles of stretching and release under UV-light irradiation in the 0–200% elongation range. To our delight, luminescence intensity smoothly and steadily increased in response sample stretching (Fig. 3 and Fig. S33, ESI†). This response was quick and reversible, and upon releasing the sample to its original length, PL intensity decreased accordingly. A movie showing real-time changes in the emission brightness in response to stress is supplied in the ESI.†
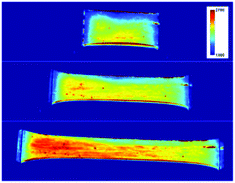 | ||
| Fig. 3 Imaging analysis of Cu1-cPBA during stretching: images of a film during stretching at 0% (top), 100% (middle), 200% (bottom) strain. | ||
We performed a control experiment to investigate whether the emission intensity changes are caused by mechanical force that is directly transmitted to the cross-linker, or if it's influenced by a constrained environment. A reference sample of cross-linked cPBA was prepared using hexamethylene diacrylate as a cross-linker mechanically mixed with 1 wt% of model compounds Cu4 and Cu6. The control samples with the complexes mechanically incorporated into hexamethylene diacrylate-cross linked polybutylacrylate showed insignificant changes in spectroscopically detected emission intensity in response to tensile stress. The imaging analysis also confirmed that there are no noticeable emission intensity changes in response to stretching in the control samples with mechanically incorporated complexes Cu4 and Cu6 (Fig. S34–S35, ESI†). Thus, covalent incorporation of the mechanophore as a cross-linker is required to clearly observe sensitive mechanoresponsive behavior.4e
To investigate their air-stability, films of Cu1-cPBA and Cu2-cPBA were exposed to air for several days and the PLQYs were recorded (see Fig. S29, ESI†).8 Both polymers showed good air-stability with only slight decrease of PLQY (3–4%) after 4 days under air, presumably due to oxidation to trace CuII with oxygen. Thus, while not ideal, the strategy of introducing a carbene moiety makes the copper mechanophores suitable for practical applications.
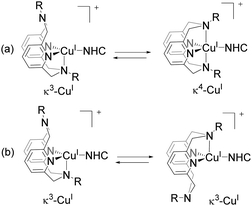
To shed light on the mechanoresponse mechanism, we analyzed the dynamic behavior and photophysical properties of model complexes Cu3–6. All these complexes show isomerization in solution that involves conformational fluxionality of the macrocyclic ligand RN4 (Scheme 2). Variable temperature (VT) NMR studies in CD2Cl2 solution show that as the steric hindrance of the RN4 ligand increases, fluxionality of the complexes decreases leading to slower isomerization. Interestingly, PLQY in CH2Cl2 solution increases from Cu3 to Cu6, correlating with the increase of steric hindrance and an inversely proportional decrease in fluxionality, accompanied by a blue shift of the emission peak (Table 1). Notably, this trend shows very good correlation with the gradual decrease of the non-radiative decay rate constant knr from Cu3 to Cu6, showing that suppression of non-radiative decay is likely the main reason for PLQY's increase.
Based on these observations and by analogy with other photoluminescent CuI complexes reported in the literature, we propose that the increase of PLQY in more rigid model systems is due to the suppression of Jahn–Teller distortions in the excited state in the presence of sterically hindered ligands. A similar effect was reported for substituted phenanthroline CuI complexes, where ligand sterics were varied.10 By analogy, we propose that applied mechanical force restricts cross-linker mobility, making it responsible for the observed increase of emission intensity upon elongation.
Additionally, formation of an exciplex by coordination of a pendant N-donor of the RN4 ligand to form a five-coordinate species could be another mechanism for photoluminescence quenching.10a,11 Indeed, NMR studies show that only the κ3-isomer is present in solutions of bulky complexes Cu5 and Cu6, while both κ4- and κ3-isomers were observed to be present in equilibrium for Cu3 and Cu4 (Scheme 2). Less favorable pendant amine arm binding in bulkier complexes is also consistent with cyclic voltammetry studies.8
Notably, the rate of isomerization of complex Cu6 in solution was found to be ca. 16 s−1 and it is expected to be even slower in a polymer matrix, making it several orders of magnitude slower than non-radiative and radiative decay processes (Table 1).8 Based on this, we attribute the observed luminescence response to changes in the dynamics of the excited state, rather than to isomerization suppression that was proposed for an analogous system.12
In summary, we report a highly sensitive mechanophore based on the (RN4)Cu(NHC)+ complex, which was incorporated as a cross-linker in polybutylacrylate. This system shows a highly sensitive response to mechanical force, allowing for the detection of mechanical stress changes even at small strain and stress values, and it enables the direct visualization of mechanical stress. The covalent incorporation of the Cu-based mechanophore as a cross-linker is necessary to observe of a mechanoresponse. Such high sensitivity is superior to many currently reported system based on classical mechanophores such as spiropyrans, diaryldibenzofuranone and others.2–5 The mechanism of mechanoresponse is different from commonly used organic-based mechanophores as it does not involve covalent bond cleavage/formation, but is based on restricting the mobility of the fluxional Cu complex and thus suppressing non-radiative decay as confirmed by our studies of the model systems. Considering the high sensitivity and good air stability of these systems, they may find use in real-time visualization and in situ sensing of mechanical stress via coating or incorporation into common construction materials in aerospace or civil engineering. Generally, the conformationally flexible macrocyclic ligands resemble allosteric enzyme mimic systems13 showing stimuli-responsive structural changes, and we plan to further utilize these ligands to study mechanocontrolled reactivity and properties of coordination compounds.
This work was supported by JSPS KAKENHI Grant Number JP18K05247. We thank Dr Takuya Miyazawa, Dr Kieran Deasy (MEMS, OIST), Dr Michael Roy (IAS, OIST), Engineering support section, and Izuru Karimata (Kobe University) for technical support and for helpful discussions.
Conflicts of interest
There are no conflicts to declare.References
- (a) J. Li, C. Nagamani and J. S. Moore, Acc. Chem. Res., 2015, 48, 2181–2190 CrossRef CAS; (b) C. Calvino, L. Neumann, C. Weder and S. Schrettl, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 640–652 CrossRef CAS.
- (a) D. A. Davis, A. Hamilton, J. Yang, L. D. Cremar, D. Van Gough, S. L. Potisek, M. T. Ong, P. V. Braun, T. J. Martinez, S. R. White, J. S. Moore and N. R. Sottos, Nature, 2009, 459, 68–72 CrossRef CAS; (b) C. K. Lee, B. A. Beiermann, M. N. Silberstein, J. Wang, J. S. Moore, N. R. Sottos and P. V. Braun, Macromolecules, 2013, 46, 3746–3752 CrossRef CAS; (c) Y. Lin, M. H. Barbee, C. C. Chang and S. L. Craig, J. Am. Chem. Soc., 2018, 140, 15969–15975 CrossRef CAS.
- (a) Y. Chen and R. P. Sijbesma, Macromolecules, 2014, 47, 3797–3805 CrossRef CAS; (b) Y. Chen, A. J. Spiering, S. Karthikeyan, G. W. Peters, E. W. Meijer and R. P. Sijbesma, Nat. Chem., 2012, 4, 559–562 CrossRef CAS; (c) J. M. Clough, A. Balan, T. L. van Daal and R. P. Sijbesma, Angew. Chem., Int. Ed., 2016, 55, 1445–1449 CrossRef CAS PubMed; (d) E. Ducrot, Y. Chen, M. Bulters, R. P. Sijbesma and C. Creton, Science, 2014, 344, 186–189 CrossRef CAS.
- (a) K. Imato, A. Irie, T. Kosuge, T. Ohishi, M. Nishihara, A. Takahara and H. Otsuka, Angew. Chem., Int. Ed., 2015, 54, 6168–6172 CrossRef CAS; (b) K. Imato, T. Kanehara, S. Nojima, T. Ohishi, Y. Higaki, A. Takahara and H. Otsuka, Chem. Commun., 2016, 52, 10482–10485 RSC; (c) K. Imato, T. Kanehara, T. Ohishi, M. Nishihara, H. Yajima, M. Ito, A. Takahara and H. Otsuka, ACS Macro Lett., 2015, 4, 1307–1311 CrossRef CAS; (d) T. Kosuge, X. Zhu, V. M. Lau, D. Aoki, T. J. Martinez, J. S. Moore and H. Otsuka, J. Am. Chem. Soc., 2019, 141, 1898–1902 CrossRef CAS; (e) H. Oka, K. Imato, T. Sato, T. Ohishi, R. Goseki and H. Otsuka, ACS Macro Lett., 2016, 5, 1124–1127 CrossRef CAS.
- (a) R. Göstl and R. P. Sijbesma, Chem. Sci., 2016, 7, 370–375 RSC; (b) M. J. Robb, T. A. Kim, A. J. Halmes, S. R. White, N. R. Sottos and J. S. Moore, J. Am. Chem. Soc., 2016, 138, 12328–12331 CrossRef CAS; (c) T. Wang, N. Zhang, J. Dai, Z. Li, W. Bai and R. Bai, ACS Appl. Mater. Interfaces, 2017, 9, 11874–11881 CrossRef CAS.
- (a) H. Yabu, Y. Saito, S. Saito, S. Yamagichi and S. Nobusue, US Pat. 20190031820A1, 2019 Search PubMed; (b) Y. Sagara, M. Karman, E. Verde-Sesto, K. Matsuo, Y. Kim, N. Tamaoki and C. Weder, J. Am. Chem. Soc., 2018, 140, 1584–1587 CrossRef CAS.
- G. A. Filonenko and J. R. Khusnutdinova, Adv. Mater., 2017, 29, 1700563 CrossRef.
- See ESI† for details.
- P. H. Patil, G. A. Filonenko, S. Lapointe, R. R. Fayzullin and J. R. Khusnutdinova, Inorg. Chem., 2018, 57, 10009–10027 CrossRef CAS.
- (a) D. R. McMillin, J. R. Kirchhoff and K. V. Goodwin, Coord. Chem. Rev., 1985, 64, 83–92 CrossRef CAS; (b) C. T. Cunningham, K. L. H. Cunningham, J. F. Michalec and D. R. McMillin, Inorg. Chem., 1999, 38, 4388–4392 CrossRef CAS; (c) D. Felder, J.-F. Nierengarten, F. Barigelletti, B. Ventura and N. Armaroli, J. Am. Chem. Soc., 2001, 123, 6291–6299 CrossRef CAS; (d) A. Lavie-Cambot, M. Cantuel, Y. Leydet, G. Jonusauskas, D. M. Bassani and N. D. McClenaghan, Coord. Chem. Rev., 2008, 252, 2572–2584 CrossRef CAS; (e) O. Green, B. A. Gandhi and J. N. Burstyn, Inorg. Chem., 2009, 48, 5704–5714 CrossRef CAS; (f) M. W. Mara, K. A. Fransted and L. X. Chen, Coord. Chem. Rev., 2015, 282–283, 2–18 CrossRef CAS.
- (a) E. M. Stacy and D. R. McMillin, Inorg. Chem., 1990, 29, 393–396 CrossRef CAS; (b) L. X. Chen, G. B. Shaw, I. Novozhilova, T. Liu, G. Jennings, K. Attenkofer, G. J. Meyer and P. Coppens, J. Am. Chem. Soc., 2003, 125, 7022–7034 CrossRef CAS.
- (a) G. A. Filonenko, J. A. M. Lugger, C. Liu, E. P. A. van Heeswijk, M. Hendrix, M. Weber, C. Muller, E. J. M. Hensen, R. P. Sijbesma and E. A. Pidko, Angew. Chem., Int. Ed., 2018, 57, 16385–16390 CrossRef CAS; (b) G. A. Filonenko, D. Sun, M. Weber, C. Muller and E. A. Pidko, J. Am. Chem. Soc., 2019, 141, 9687–9692 CrossRef CAS.
- (a) H. J. Yoon and C. A. Mirkin, J. Am. Chem. Soc., 2008, 130, 11590–11591 CrossRef CAS; (b) H. J. Yoon, J. Kuwabara, J.-H. Kim and C. A. Mirkin, Science, 2010, 330, 66–69 CrossRef CAS PubMed; (c) H. J. Yoon, J. Heo and C. A. Mirkin, J. Am. Chem. Soc., 2007, 129, 14182–14183 CrossRef CAS PubMed; (d) M. Raynal, P. Ballester, A. Vidal-Ferran and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2014, 43, 1734–1787 RSC; (e) J. Kuwabara, H. J. Yoon, C. A. Mirkin, A. G. Di Pasquale and A. L. Rheingold, Chem. Commun., 2009, 4557–4559 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization. CCDC 1903712 (Cu4), 1903713 (Cu6), 1937851 (Cu3), and 1937852 (Cu5). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc08354e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |