Anthropogenic gadolinium in lakes and rivers near metrocities in Korea†
Intae
Kim
 *a,
Suk Hyun
Kim
a and
Guebuem
Kim
b
*a,
Suk Hyun
Kim
a and
Guebuem
Kim
b
aMarine Environmental Research Center, Korea Institute of Ocean Science and Technology, Busan 49111, South Korea. E-mail: ikim@kiost.ac.kr
bSchool of Earth and Environmental Sciences/RIO, Seoul National University, Seoul 08826, South Korea
First published on 5th November 2019
Abstract
We measured dissolved rare earth elements (REEs) in the water samples from Shihwa Lake (SL), which was assumed to be highly polluted, and in the downstream portion of the Han River (HR), which runs through Seoul, Korea. Among the investigated REEs, only gadolinium (Gd) was found to be significantly enhanced after REE concentrations were shale-normalized (SN). The calculated Gd anomaly (Gd/Gd* = 3 × GdSN/(SmSN + 2 × TbSN)) was about 1.5 ± 0.1 (n = 16) in SL and 1.6 ± 0.3 in the HR (n = 26) water relative to other types of natural water such as groundwater, seawater, and river water in uncontaminated areas (Gd/Gd* ∼ 1.2, n > 400). These significant Gd anomalies seem to be due to the inputs of anthropogenic Gd (Gdanth), especially from the use of Gd-based contrast agents for magnetic resonance imaging (MRI) tests from a number of hospitals and medical institutes surrounding our study areas. The amount of Gdanth was estimated to be 190 ± 80 g and 680 ± 360 kg Gd in SL and the HR (watersheds in our study area), respectively. The Gdanth flux to the Yellow Sea from the HR is estimated to be 530 ± 330 g Gd d−1. These results suggest that quantitative evaluation of anthropogenic REEs in natural waters near big cities is needed, because considerable amounts of REEs are now used by modern high-tech industries.
Environmental significanceThis study presents that gadolinium (Gd), one of the rare earth elements (REEs), is noticeably enhanced in the lake and river water near big cities where over 10 million people live. This enhanced Gd, unlike the other 14 REEs, seems to be due to the use of Gd-based contrast agents for MRI tests from a number of hospitals and medical institutes surrounding big cities. This study, therefore, implies that future impact of this artificially derived Gd and some other REEs should be significant in natural waters in the polluted area. Overall, these results suggest that quantitative evaluation of man-made REEs for associated human risk assessments is needed, because considerable amounts of REEs are now used by modern high-tech industries. |
1. Introduction
Rare earth elements (REEs) include Sc and Y, and all lanthanide series of elements (possessing atomic numbers ranging from 57 to 71). In general, all REEs except for cerium (Ce4+) and europium (Eu2+) behave similarly in aquatic environments because they are found in trivalent oxidation in seawater.1–3 Recently, REEs have been recognized as essential resources of a number of state-of-the-art industries, including the manufacture of high-tech devices (e.g., memory devices, batteries, cell phones, liquid crystal displays (LCDs), catalytic converters, magnets, and electric car engines),4–6 and they play critical roles in the economy and some diplomacy matters. Thus, over the past few decades, the demands and resultant uses of these goods have caused an increase in anthropogenic REE discharges to the environment.Among REEs, gadolinium (Gd) has been used to trace the origins of anthropogenic substances in water because it is used in specific medical purposes. For example, the major sources of anthropogenic Gd in aquatic systems are water-soluble Gd-based contrast agents, such as Gd-diethylenetriaminepenta-acetic acid (DTPA). Gd-DTPA and other Gd-complexes (Gd-DOTA, Gd-DTPA-DMA, Gd-BOPTA, and etc.) have been used in magnetic resonance imaging (MRI) over the last three decades because of the paramagnetic properties of Gd(III) ions.7–9 Because of the high stability of these Gd complexes, they readily pass through the human body, resulting in positive Gd anomalies in surrounding aquatic systems.10,11 Even though Gd is used in the form of a complex with strong organic chelators (e.g., polyaminocarboxylic acid chelating agents) for the safety of MRI examinees, the safety of the product for biological toxicity at environmental concentrations has not yet been demonstrated.
According to a recent report on medical devices by the Korea Health Industry Development Institute (KHIDI),12 the number of MRI facilities in South Korea is 26.3 per million population. This number of MRI facilities in South Korea, which is the fifth largest number in OECD member countries is much higher than the average number (15.9) in Organization for Economic Co-operation and Development (OECD) statistics in 2017. Considering that the number of MRI tests per 1000 population in South Korea was 31.2 (paid only),12 more than 1.5 million MRI tests were conducted, until now. However, the impacts of the anthropogenic Gd input from MRI tests on environments have been rarely studied in South Korea. Thus, in this study, we hypothesized that natural water systems near large cities in South Korea have been significantly influenced by anthropogenic Gd. We chose Shihwa Lake (SL) and the Han River (HR) as study sites representative of lakes surrounded by heavily industrialized areas and urban rivers, respectively. More than 45% of the total population of South Korea lives near these sites (>10 million in Seoul and >13 million in other parts of Gyeonggi Province). Here, we present the REE distributions and attempt to assess the impact of Gd in these regions.
2. Materials and methods
2.1. Study area
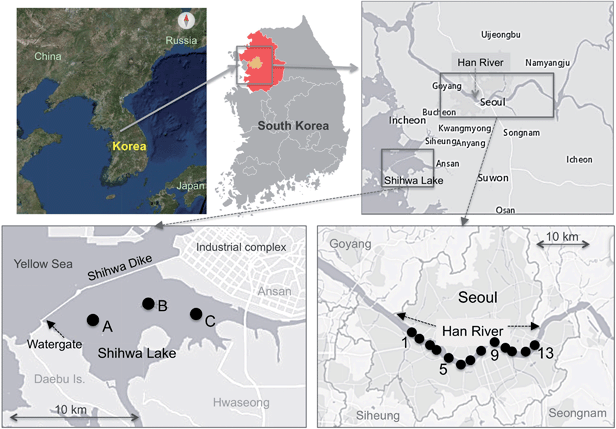
SL is an enclosed saltwater lake with an area of approximately 61 km2 located on the western coast of South Korea (Fig. 1). SL was artificially constructed by entrapment of water from the Yellow Sea (YS) in the early 1990s to supply fresh water to farming and industrial areas. A significant amount of chemical substances, including pollutants from nearby industrial complexes, is introduced into SL through six major streams.13 As a result, the government decided to open the eight water gates of SL to allow the water to mix with Yellow Sea water to improve the water quality, during which time SL became saline.14 In summer, SL becomes strongly stratified because of an increase in fresh water in response to precipitation. During this season, the water column of the lake is separated into two layers, a brackish surface layer with a salinity of 6–20 and a saline hypoxic bottom layer with a salinity of 17–27.15The HR is the largest river in South Korea, with a length of ∼490 km and a watershed area of ∼26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2. During summer (June–August), the average discharge rate in the HR ranges from 480 to 880 m3 s−1 (average 640 ± 140) (Water Resource Management Information System, http://water.nier.go.kr/, Observation at Seoul station, 2018). Among the watershed regions of the HR, more than 30% are residential and business areas including Seoul and other large cities in Gyeonggi Province. Additionally, more than 50% of the total residents in Seoul live near the HR watersheds, where more than 160 MRI instruments are currently in operation.16 The mean annual precipitation is 1400 mm per year, about 70% of which occurs between June and September (Water Resource Management Information System, http://water.nier.go.kr/).
000 km2. During summer (June–August), the average discharge rate in the HR ranges from 480 to 880 m3 s−1 (average 640 ± 140) (Water Resource Management Information System, http://water.nier.go.kr/, Observation at Seoul station, 2018). Among the watershed regions of the HR, more than 30% are residential and business areas including Seoul and other large cities in Gyeonggi Province. Additionally, more than 50% of the total residents in Seoul live near the HR watersheds, where more than 160 MRI instruments are currently in operation.16 The mean annual precipitation is 1400 mm per year, about 70% of which occurs between June and September (Water Resource Management Information System, http://water.nier.go.kr/).
2.2. Sampling
Water samples were collected from three stations in SL in July of 2011 (Fig. 1) onboard a private fishing boat: the outer part of the lake near the water gate (Station A; total depth: 7.7 m), the middle part of the lake where the depth is greatest (Station B; total depth: 13 m), and the inner part of the lake, where the surface stream inflow occurs (Station C; total depth: 6 m). A non-metal submersible pump was used for vertical water sampling (Global Water, USA) (5, 6, and 4 depths at Stations A, B, and C, respectively). Samples for the analyses of REEs were collected in polyethylene bottles (1 L), which had been pre-cleaned by soaking in 6 M HNO3 and then rinsing with de-ionized water.For the HR, 26 water samples were collected from 13 stations (just below the Han River Bridges from western to eastern Seoul) in June 2012 (Fig. 1) onboard a water taxi used for both public transport and sightseeing. Surface river water samples were taken using a handle sampler (1 L polyethylene dipper with a 4 m handle length) to avoid contamination from the ship. The dippers were also soaked and pre-cleaned with double-distilled 6 M ultrapure HNO3 for at least 24 h before sampling.
All of the sample bottles were double-bagged with clean plastic zip-lock bags in the field and then transferred to the laboratory. All samples were filtered through a membrane filter (47 mm, 0.45 μm pore size, mixed cellulose ester, Millipore) into a new acid cleaned bottle and acidified to pH < 2 using 6 M ultrapure grade HNO3 (65%, Thermo Fisher Scientific, Waltham, MA, USA) within 12 h of sampling.17 The sample bottles were then stored with clean, plastic zip-lock bags in a clean laboratory (class 100) until analyses.
2.3. Analytical procedure
The REE concentrations in all water samples were measured using a method developed by Kim et al.18 Briefly, the aqueous samples (500 mL) were adjusted to pH 5.8–6.0 using CH3COOH (99.7%, Junsei, Japan) and NH3 (28%, Junsei, Japan), after which they were passed through a Chelex 100 resin (1.2 g, wet weight) column at a flow rate of less than 1.2 mL min−1. Next, 8 mL of 1 M ammonium acetate solution (Sigma Aldrich, USA) and 5 mL of de-ionized water were used to wash out the interfering elements. The REEs absorbed on the resin were then eluted with 1.5 mL of 2 M HNO3. All procedures for the extraction of REEs from seawater were conducted on a laminar flow clean bench (class-100). The quantitative extraction efficiencies of the REEs were confirmed using Tm as a spike.18 To check the validity of this method, we analyzed common seawater reference samples (CASS-4 and NASS-5, National Research Council of Canada).REEs (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Er, Tm, Yb, Lu, and Y) and Ba were measured by inductively coupled plasma mass spectrometry (ICP-MS) (Model: X-II, Thermo Scientific Inc., UK). The procedural blank values for La, Ce, Nd, Tb, Tm, and Lu were 0.62, 1.92, 0.19, 0.07, 0.11, and 0.09 ng L−1, respectively, whereas the values for the other REEs were not detected. Our total blank value for Nd was about 1–2% of the lowest concentrations in seawater (8–12 ng L−1). The instrumental detection limits of La and Gd were 0.55 and 0.42, respectively, whereas those of the other elements were <0.20 ng L−1. The BaO/Eu and CeO/Ce ratios were found to be lower than 2% and 1%, respectively, when 1 ppb of REE standard was measured before the sample measurement.17
The extraction efficiencies monitored using the Tm spike were >95%, and the analytical precisions of the REEs were lower than 2%, 3%, and 5% of the relative standard deviations (RSD) for light REEs (LREEs), middle REEs (MREEs), and heavy REEs (HREEs), respectively. Although the certified values of REEs are not available for certified reference materials (CRMs) used in this study, CASS-4 and NASS-5, we compared our results for the same CRMs with those reported by Zhu et al.19 Our findings were in good agreement with those of Zhu et al.19 for all REEs, with uncertainties (relative standard deviation for 5 repeated measurements) of <10% for Ce, Nd, Gd, and Tb and <5% for the other REEs. Previously, Hennebrüder et al.20 tested the effect of Gd-DTPA on Chelex-100 extraction (similar method to our study), and they reported that the recoveries (%) of spiked Gd could decline from 71–78% under 0.2 μg L−1 of Gd-DTPA spiked. Here, we noted that our Gd values in this study could be underestimated up to (max.) 20–30%.
3. Results and discussion
3.1. REE concentrations
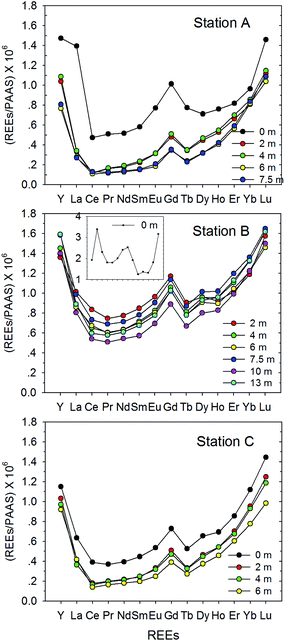
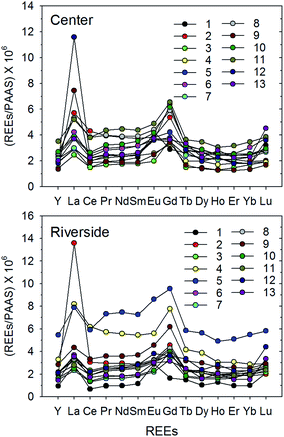
The concentrations of dissolved Nd (as a representative of REEs) ranged from 3.4 to 46.7 ng L−1 (mean 12.8 ± 10.8 ng L−1) and from 25.2 to 193 ng L−1 (mean 75.0 ± 34.6 ng L−1) in SL and the HR, respectively (Tables 1 and 2). Because the 14 REEs are coherent, their geochemical behaviors in the environments are the same. Therefore, here we only present the results for Nd, which is a LREE that is commonly used as a representative of REEs for convenience (the results for the other REEs are presented in Tables 1 and 2). The highest concentrations were shown in the surface of SL (Station B) and Stations 3 and 5 (both are riversides) in the HR, near Seogang and Wonhyo Bridges, respectively (Fig. 2 and 3). In SL, the concentrations of dissolved REEs decreased with depth, which was likely because of removal owing to increasing salinity (mixing between newly introduced high-REE freshwater and saline water) and the strong stratification during summer (Table 1). These trends were also observed in vertical distributions of DO, pH, water temperature, and some trace metals.21 In the HR, the concentrations of the surface REE varied by about 1–3-fold, which was assumed to be a result of different local input sources and spatial variability of suspended solids.| Station | Depth (m) | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Yb | Lu | Gd/Gd* | Gdanth |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0 | 32.4 | 41.8 | 30.3 | 3.62 | 13.5 | 2.62 | 0.68 | 3.85 | 0.49 | 2.49 | 0.61 | 1.89 | 2.13 | 0.47 | 1.4 | 1.15 |
| A | 2 | 22.9 | 10.0 | 7.4 | 1.16 | 4.67 | 1.01 | 0.27 | 1.82 | 0.22 | 1.56 | 0.43 | 1.52 | 1.78 | 0.35 | 1.6 | 0.66 |
| A | 4 | 23.9 | 10.2 | 7.7 | 1.20 | 4.99 | 1.07 | 0.27 | 1.93 | 0.22 | 1.64 | 0.45 | 1.61 | 1.89 | 0.37 | 1.6 | 0.74 |
| A | 6 | 16.9 | 8.3 | 7.0 | 0.85 | 3.36 | 0.68 | 0.17 | 1.32 | 0.16 | 1.12 | 0.33 | 1.30 | 1.80 | 0.33 | 1.7 | 0.52 |
| A | 7.5 | 17.8 | 8.2 | 8.5 | 0.90 | 3.63 | 0.71 | 0.18 | 1.35 | 0.14 | 1.12 | 0.35 | 1.37 | 1.85 | 0.35 | 1.7 | 0.57 |
| B | 0 | 42.4 | 101.2 | 146.9 | 12.8 | 46.7 | 8.98 | 2.11 | 9.58 | 1.22 | 4.36 | 1.09 | 3.03 | 3.98 | 1.02 | 1.3 | 2.20 |
| B | 2 | 29.9 | 30.4 | 53.5 | 5.31 | 20.2 | 3.82 | 0.85 | 4.45 | 0.57 | 3.36 | 0.74 | 2.34 | 2.61 | 0.51 | 1.3 | 1.09 |
| B | 4 | 31.9 | 26.5 | 41.7 | 4.30 | 16.5 | 3.23 | 0.73 | 4.03 | 0.52 | 3.35 | 0.76 | 2.54 | 2.92 | 0.53 | 1.4 | 1.05 |
| B | 6 | 30.7 | 25.8 | 43.1 | 4.27 | 16.5 | 3.17 | 0.71 | 3.89 | 0.51 | 3.19 | 0.73 | 2.41 | 2.68 | 0.47 | 1.4 | 1.01 |
| B | 8 | 34.9 | 29.6 | 46.9 | 4.90 | 18.5 | 3.52 | 0.79 | 4.33 | 0.56 | 3.56 | 0.81 | 2.76 | 2.99 | 0.53 | 1.4 | 1.12 |
| B | 10 | 30.8 | 24.2 | 34.6 | 3.62 | 14.1 | 2.59 | 0.61 | 3.38 | 0.43 | 2.81 | 0.66 | 2.29 | 2.70 | 0.47 | 1.4 | 0.96 |
| B | 13 | 35.0 | 26.7 | 38.3 | 4.10 | 15.9 | 3.05 | 0.68 | 3.90 | 0.49 | 3.25 | 0.78 | 2.59 | 2.92 | 0.53 | 1.4 | 1.07 |
| C | 0 | 25.3 | 19.1 | 24.9 | 2.62 | 10.3 | 2.02 | 0.47 | 2.77 | 0.33 | 2.29 | 0.56 | 1.97 | 2.46 | 0.46 | 1.5 | 0.87 |
| C | 2 | 22.7 | 12.4 | 11.4 | 1.42 | 5.68 | 1.10 | 0.29 | 1.93 | 0.21 | 1.63 | 0.43 | 1.61 | 2.09 | 0.40 | 1.7 | 0.79 |
| C | 4 | 21.4 | 10.9 | 10.7 | 1.39 | 5.52 | 1.13 | 0.27 | 1.79 | 0.21 | 1.56 | 0.43 | 1.56 | 2.04 | 0.39 | 1.6 | 0.66 |
| C | 6 | 20.2 | 12.6 | 8.9 | 1.17 | 4.70 | 0.89 | 0.21 | 1.49 | 0.17 | 1.32 | 0.36 | 1.39 | 1.71 | 0.32 | 1.6 | 0.55 |
| Station | Location | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Yb | Lu | Gd/Gd* | Gdanth |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Center | 42.6 | 88.0 | 96.6 | 13.7 | 52.8 | 10.1 | 3.75 | 11.0 | 1.67 | 7.36 | 1.80 | 4.38 | 3.94 | 5.9 | 1.2 | 1.56 |
| 2 | Center | 50.8 | 171.4 | 275.3 | 27.4 | 99.1 | 16.7 | 3.39 | 20.4 | 1.83 | 9.33 | 1.68 | 4.85 | 4.31 | 3.7 | 1.7 | 8.42 |
| 3 | Center | 31.4 | 74.0 | 96.3 | 11.8 | 45.6 | 8.0 | 1.75 | 13.92 | 0.95 | 5.14 | 1.04 | 3.38 | 3.79 | 3.5 | 2.3 | 7.90 |
| 4 | Center | 41.3 | 87.5 | 130.7 | 15.6 | 60.9 | 10.8 | 2.28 | 12.6 | 1.29 | 6.89 | 1.37 | 4.18 | 4.05 | 3.5 | 1.5 | 4.42 |
| 5 | Center | 54.9 | 113.1 | 163.3 | 20.6 | 78.8 | 14.0 | 3.36 | 16.0 | 1.81 | 9.17 | 1.95 | 5.62 | 5.35 | 5.3 | 1.4 | 4.88 |
| 6 | Center | 54.4 | 127.1 | 150.6 | 19.8 | 75.7 | 14.1 | 3.21 | 24.3 | 1.95 | 9.26 | 2.09 | 5.87 | 6.70 | 6.9 | 2.1 | 12.5 |
| 7 | Center | 47.6 | 89.6 | 126.2 | 16.6 | 64.5 | 11.7 | 3.22 | 13.2 | 1.56 | 7.98 | 1.67 | 4.89 | 5.07 | 4.9 | 1.4 | 3.76 |
| 8 | Center | 56.8 | 159.6 | 245.1 | 27.7 | 100.7 | 17.5 | 3.89 | 22.4 | 2.07 | 10.0 | 1.98 | 5.54 | 5.59 | 5.1 | 1.7 | 9.30 |
| 9 | Center | 29.8 | 223.5 | 120.4 | 13.4 | 48.5 | 8.44 | 2.19 | 13.0 | 1.06 | 4.91 | 1.04 | 2.93 | 2.96 | 3.1 | 2.0 | 6.37 |
| 10 | Center | 59.3 | 116.7 | 170.2 | 22.5 | 85.1 | 15.7 | 3.66 | 23.4 | 2.02 | 10.1 | 2.00 | 5.81 | 5.55 | 5.4 | 1.9 | 11.0 |
| 11 | Center | 77.2 | 156.2 | 244.5 | 30.3 | 115.8 | 19.5 | 4.30 | 24.9 | 2.34 | 12.2 | 2.44 | 7.29 | 7.63 | 7.1 | 1.7 | 10.1 |
| 12 | Center | 39.7 | 347.4 | 142.8 | 17.1 | 60.8 | 11.1 | 3.18 | 14.4 | 1.84 | 6.92 | 1.83 | 4.12 | 4.03 | 6.6 | 1.4 | 3.95 |
| 13 | Center | 44.6 | 111.3 | 144.3 | 17.4 | 63.5 | 11.8 | 3.30 | 13.9 | 2.15 | 8.00 | 2.26 | 5.17 | 5.07 | 8.3 | 1.2 | 1.98 |
| 1 | Riverside | 21.0 | 83.7 | 43.7 | 6.6 | 25.2 | 5.1 | 2.54 | 6.26 | 0.95 | 3.59 | 1.01 | 2.23 | 2.18 | 3.8 | 1.2 | 1.02 |
| 2 | Riverside | 43.2 | 407.6 | 194.5 | 20.6 | 76.4 | 12.9 | 2.87 | 17.3 | 1.48 | 7.51 | 1.40 | 4.28 | 4.13 | 3.6 | 1.8 | 7.72 |
| 3 | Riverside | 47.3 | 96.9 | 148.7 | 18.4 | 71.2 | 12.6 | 2.72 | 15.7 | 1.49 | 8.13 | 1.57 | 4.75 | 4.50 | 4.0 | 1.7 | 6.20 |
| 4 | Riverside | 72.7 | 246.0 | 396.3 | 39.7 | 144.1 | 24.3 | 4.94 | 29.6 | 2.67 | 13.6 | 2.44 | 7.09 | 6.33 | 5.3 | 1.7 | 12.1 |
| 5 | Riverside | 120.0 | 237.2 | 379.2 | 50.8 | 193.2 | 32.4 | 7.58 | 36.3 | 3.73 | 19.7 | 3.92 | 11.7 | 12.1 | 10.7 | 1.5 | 12.3 |
| 6 | Riverside | 43.4 | 110.1 | 138.7 | 17.4 | 67.2 | 12.3 | 2.80 | 13.9 | 1.54 | 7.30 | 1.55 | 4.55 | 4.77 | 4.8 | 1.4 | 4.29 |
| 7 | Riverside | 32.9 | 69.5 | 85.0 | 11.1 | 42.3 | 8.2 | 1.93 | 12.6 | 1.11 | 5.51 | 1.24 | 3.71 | 4.98 | 4.4 | 1.9 | 5.85 |
| 8 | Riverside | 47.9 | 99.5 | 120.8 | 16.6 | 64.8 | 11.6 | 2.81 | 16.3 | 1.54 | 7.75 | 1.68 | 4.92 | 5.19 | 5.0 | 1.7 | 6.89 |
| 9 | Riverside | 62.7 | 130.5 | 213.3 | 24.8 | 92.5 | 16.4 | 4.01 | 23.5 | 2.03 | 10.5 | 2.09 | 5.96 | 5.59 | 5.3 | 1.8 | 10.8 |
| 10 | Riverside | 36.2 | 74.9 | 87.7 | 12.3 | 47.2 | 8.6 | 2.05 | 15.1 | 1.18 | 5.90 | 1.34 | 3.97 | 4.41 | 4.7 | 2.1 | 8.02 |
| 11 | Riverside | 44.6 | 83.9 | 123.2 | 16.0 | 61.6 | 11.2 | 2.68 | 14.3 | 1.45 | 7.43 | 1.50 | 4.33 | 4.26 | 4.3 | 1.6 | 5.36 |
| 12 | Riverside | 46.9 | 105.6 | 141.3 | 17.6 | 64.9 | 12.5 | 3.36 | 13.7 | 2.15 | 8.35 | 2.28 | 5.29 | 5.12 | 8.1 | 1.1 | 1.67 |
| 13 | Riverside | 32.2 | 106.5 | 110.0 | 13.2 | 47.1 | 8.8 | 2.64 | 11.9 | 1.59 | 5.64 | 1.62 | 3.50 | 3.36 | 6.1 | 1.4 | 3.13 |
3.2. Shale-normalized REE patterns
Generally, average-shale normalized REE patterns have been investigated to identify the REE fractionation relative to their pristine source (i.e., continents) and thus allow easy comparison with other REE data.22 In this study, we used Post-Archean average Australian Shale (PAAS) values, which are commonly used in REE studies, for normalization.23,24In SL, the shale-normalized REE (REESN) showed HREE-enrichment patterns similar to those found in typical seawater,2 with the YbSN/NdSN (as a representative of HREESN/LREESN, that is commonly used) ratio in the range of 1.5–6.0 (average 3.4 ± 1.0, n = 15) (Fig. 2). This YbSN/NdSN ratio was the lowest (1.0–1.8) on the surface and increased with depth to the highest level of 2.2–6.8 at the bottom for all 3 stations, indicating that surface water was more influenced by REE inputs from land origin (showing relatively flat REESN patterns) superimposed on strong stratification in SL. This YbSN/NdSN ratio in SL is similar to that of open ocean water (3–5 in the Indian Ocean),25 which is very similar to the results observed for the Korean Marginal Sea, East/Japan Sea (EJS) (mean ∼ 4, n > 20).17 A large amount of suspended solids or terrestrial particles may be responsible for the substantially enhanced HREESN/LREESN in SL since the LREEs were efficiently removed relative to HREEs under the same sample conditions.26
The REESN patterns in the HR were found to be completely different from those in SL. The REESN patterns in the HR showed a “flat” type REE pattern, with an YbSN/NdSN ratio of approximately ∼1 (mean = 0.83 ± 0.17, n = 26) (Fig. 3). This type of REESN pattern indicates a more continental source (rock or soil-like feature) because of the widespread distribution of sedimentary rocks in the drainage area and chemical weathering.27 Here, we also observed a significantly enhanced Gd anomaly (p < 0.00) for all 26 samples, indicating the result of significant inputs of artificially-derived Gd. The slope between Gd and its neighbor elements, Eu and Tb, morphologically, is also very similar to that of SL samples (we will show this quantitatively in the Results and Discussion section). Lanthanum (La) is also remarkably elevated in few samples (riverside of Station 2, and center of Stations 9 and 12) from the HR (Fig. 3). A previous study has reported that in addition to Gd anthropogenic La anomalies may be found in aquatic systems. Kulaksız and Bau28 reported for the first time the strong enrichment of anthropogenic La due to effluent from a production plant for fluid catalytic cracking catalysts in the Rhine River water. The significantly higher La and Gd values observed in our study region evidently show that this area is a region of considerable anthropogenic sources of REEs.
3.3. Gd enrichments in Shihwa Lake and the Han River
We consistently observed positive Gd anomalies in all samples collected from SL and the HR (Fig. 2 and 3). These anomalies were primarily because of the large inputs of refractory Gd-based contrast agents, such as Gd-DTPA, which are mainly used for conducting magnetic resonance image (MRI) tests, as previously reported by several studies.7–11,29 Because of the extremely high stability of Gd-(DTPA)−2, Gd complexes are not removed well by typical wastewater treatment plants (WWTPs) and instead pass into coastal surface waters.29The common method for estimation of the additional (i.e., anthropogenic sources) Gd, known as the Gd anomaly, is calculation of the relative ratio of GdSN over the mathematically estimated GdSN, which is known as Gd*. In general, the Gd anomaly (GdSN/GdSN*) is calculated by linear or geometric extrapolations between GdSN and neighbor REESN, such as SmSN, EuSN and TbSN (hereafter, Gd/Gd*). In this study, the Gd anomaly Gd/Gd* was calculated using the following equation:
| Gd/Gd* = 3 × GdSN/(SmSN + 2 × TbSN) | (1) |
Because of the exceptional redox chemistry of Eu (usually Eu2+ in seawater),2 we used SmSN instead of EuSN, although the atomic number differs from Gd by two. The calculated Gd/Gd* ranged from 1.30 to 1.72 (average of 1.48 ± 0.14, n = 15) and 1.20–2.31 (average of 1.63 ± 0.31, n = 26) in SL and the HR, respectively. The highest Gd/Gd* was shown at depths of 7.5 m (near bottom) (Gd/Gd* ∼ 1.73) for Station A in SL and Station 11 (Gd/Gd* ∼ 2.31) in the HR.
In SL, contrary to the Gd (and REE) concentration, the Gd/Gd* increased with increasing depth (Fig. 4). The higher Gd anomaly in deep water in SL was probably because the stratified deep water was staying much longer than the newly introduced fresh surface water. These findings indicate that the deeper water received more contaminants than shallower water from both new fresh water in the upper portion and sediment in the lower portion at the same time. In a previous study (with the same sampling in this study) a large benthic flux of trace metals was reported in SL.21
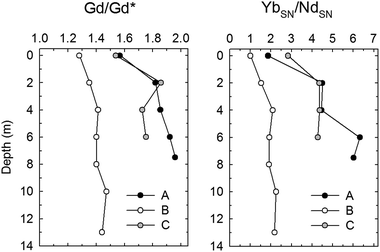 | ||
| Fig. 4 The vertical profiles of Gd anomaly (Gd/Gd*) and the ratio of shale-normalized HREE/NREE (Yb/Nd) in Shihwa Lake water. | ||
In the HR, the Gd/Gd* (average of 1.63 ± 0.31, n = 26) values were notably higher than those in SL (average of 1.48 ± 0.14, n = 15) and varied more (1.20–2.31) than those in SL (1.30–1.72), probably because of higher levels of anthropogenic contaminant inputs as a result of the much longer watershed area surrounding large cities in contrast to the enclosed environment of SL. Overall, the mean Gd anomaly (Gd/Gd*) in SL (1.48 ± 0.14, n = 15) and HR (1.63 ± 0.31, n = 26) samples was noticeably larger than that in other natural aquatic systems such as groundwater, coastal water, stream water (rural areas are supposed to have less anthropogenic pollution) and the marginal sea (East/Japan Sea) around South Korea (n > 400) (Table 3).30 These Gd anomalies in SL and the HR are very similar to those recently reported in Lake Erie (Gd/Gd* of 1.58) and Niagara River (Gd/Gd* of 1.63) although there are differences in sampling years,29 but slightly lower than those recently reported in San Francisco Bay water (average Gd/Gd* of 2.3 ± 0.7).31
| Shihwa Lake (this study) | Han River (this study) | Coastal groundwater | Coastal seawater | East/Japan Sea | Stream | |
|---|---|---|---|---|---|---|
| a Data from Kim and Kim.17,30 | ||||||
| Gd anomaly (Gd/Gd*) | 1.48 ± 0.14 | 1.63 ± 0.31 | 1.27 ± 0.18 | 1.23 ± 0.15 | 1.21 ± 0.08 | 1.20 ± 0.20 |
| Sample types | Brackish lake water (N = 15) | River water (N = 20) | Brackish/fresh-groundwater (N = 69a) | South sea coastal water (N = 121a) | Offshore seawater (N = 96a) | Stream/river (N = 22a) |
3.4. Impact of anthropogenic Gd on Shihwa Lake and the Han River
In this study, we attempted to determine the amount of anthropogenic Gd in SL and the HR based on the significant positive Gd anomaly (Gd/Gd* > 1). First, we calculated the anthropogenic components (Gdanth) in the water samples using the below equation as previously suggested:31| Gdanth = Gdmeasured − Gd* | (2) |
The Gdanth levels ranged from 0.55 to 2.20 ng L−1 (mean 0.94 ± 0.39 ng L−1, n = 15) and 1.56 to 12.6 ng L−1 (mean 6.61 ± 3.46 ng L−1, n = 26) in SL and the HR, respectively. Considering the entire volume of SL (61 km2 with 3.2 m average depths), the mean inventory of Gdanth was estimated to be 190 ± 80 g Gdanth during our sampling date. For the HR, the watershed area of our sampling region was approximately 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 km2 (7.5 m of average depths); therefore, the inventory of Gdanth was estimated to be 680 ± 360 kg Gdanth during our sampling period. In addition, considering the discharge rate in the HR during our sampling period (950 ± 120 m3 s−1 from June to August 2012) (Water Resource Management Information System, http://water.nier.go.kr/), the mean flux of Gdanth to the coastal ocean in the HR downstream/estuary was estimated to be 530 ± 330 g Gdanth d−1 in summer. This Gdanth flux is one order of magnitude higher than that in the Allegheny (83 g Gdanth d−1) and Monongahela Rivers (33 g Gdanth d−1) in the United States and similar to that of (330 g Gdanth d−1) the Ohio River.29
780 km2 (7.5 m of average depths); therefore, the inventory of Gdanth was estimated to be 680 ± 360 kg Gdanth during our sampling period. In addition, considering the discharge rate in the HR during our sampling period (950 ± 120 m3 s−1 from June to August 2012) (Water Resource Management Information System, http://water.nier.go.kr/), the mean flux of Gdanth to the coastal ocean in the HR downstream/estuary was estimated to be 530 ± 330 g Gdanth d−1 in summer. This Gdanth flux is one order of magnitude higher than that in the Allegheny (83 g Gdanth d−1) and Monongahela Rivers (33 g Gdanth d−1) in the United States and similar to that of (330 g Gdanth d−1) the Ohio River.29
Recently, Hatje et al.31 reported that the Gd/Gd* in the lower south region of the SFB increased steadily annually, from Gd/Gd* ∼1.6 in the mid-1990s to Gd/Gd* ∼2.4 in the mid-2000s and recently to 2.9 (in 2013). These findings demonstrate that Gd levels can steadily increase in aquatic environments. Even though the absolute amount of Gdanth seems less important in SL, the continuous Gdanth input together with the release of Gdanth accumulated in the bottom sediments to the water column is likely to have a significant impact in the future.
4. Conclusion
We observed a noticeable anomaly of Gd in both lake and river water near cities with >13 million people in Korea. Because its use in medical facilities is essential, it is assumed that the supply of these anthropogenic sources of Gd to the surrounding natural water will be steadily increased. Anthropogenic Gd complexes are very stable; therefore, they can remain in any environment for a long time. As a result, they eventually enter the ocean, where they can be transferred in natural marine products consumed by humans between trophic levels. Moreover, other anthropogenic rare earth elements (REEs) are also being produced increasingly, particularly by high-tech industries in the production of batteries, lighting/LCD systems, catalytic converters and aircraft engines, as well as in oil refining, and it is expected that their input to environments will gradually increase. Therefore, more extensive investigations of Gd and other anthropogenic REEs, such as source identification using isotope or element ratios and quantification of anthropogenic REEs using speciation analysis of Gd and other REEs, together with assessment of the risk they pose to human health should be conducted in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank all our colleagues who helped with sampling onboard. All datasets used in this study are available upon request from the corresponding author (E-mail: ikim@kiost.ac.kr). This research was supported by the KIOST project titled “Biogeochemical cycling and marine environmental change studies” (PE99712).References
- E. D. Goldberg, M. Koide, R. A. Schmitt and R. H. Smith, Rare-Earth distributions in the marine environment, J. Geophys. Res., 1963, 68(14), 4209–4217 CrossRef CAS.
- H. Elderfield and M. J. Greaves, The rare earth elements in seawater, Nature, 1982, 296(5854), 214 CrossRef CAS.
- Rare Earth Element Geochemistry, ed. P. Henderson, Elsevier, 2013, vol. 2 Search PubMed.
- Y. Kanazawa and M. Kamitani, Rare earth minerals and resources in the world, J. Alloys Compd., 2006, 408, 1339–1343 CrossRef.
- C. H. E. N. Zhanheng, Global rare earth resources and scenarios of future rare earth industry, J. Rare Earths, 2011, 29(1), 1–6 CrossRef.
- S. Massari and M. Ruberti, Rare earth elements as critical raw materials: focus on international markets and future strategies, Resour. Policy, 2013, 38(1), 36–43 CrossRef.
- D. H. Carr, J. Brown, G. M. Bydder, R. E. Steiner, H. J. Weinmann, U. Speck, A. S. Hall and I. R. Young, Gadolinium-DTPA as a contrast agent in MRI: initial clinical experience in 20 patients, Am. J. Roentgenol., 1984, 143(2), 215–224 CrossRef CAS PubMed.
- J. P. Stack, R. T. Ramsden, N. M. Antoun, R. H. Lye, T. Isherwood and J. P. J. Jenkins, Magnetic resonance imaging of acoustic neuromas: the role of gadolinium-DTPA, Br. J. Radiol., 1988, 61(729), 800–805 CrossRef CAS PubMed.
- P. Caravan, Strategies for increasing the sensitivity of gadolinium based MRI contrast agents, Chem. Soc. Rev., 2006, 35(6), 512–523 RSC.
- S. Kulaksız and M. Bau, Contrasting behaviour of anthropogenic gadolinium and natural rare earth elements in estuaries and the gadolinium input into the North Sea, Earth Planet. Sci. Lett., 2007, 260(1–2), 361–371 CrossRef.
- S. Kulaksız and M. Bau, Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities, Appl. Geochem., 2011, 26(11), 1877–1885 CrossRef.
- KHIDI, Brief of Health Industry, Market Analysis of Medical Device (In Korean), Korea Health Industry Development Institute, 2018, vol. 60, http://medicaldevice.khidi.or.kr Search PubMed.
- Y. C. Park, J. K. Park, M. W. Han, S. K. Son, M. K. Kim and S. H. Huh, Biogeochemical study of dissolved organic and inorganic compounds under oxic/anoxic environment in Lake Shihwa, The Sea, 1997, 2(2), 53–68 Search PubMed.
- J. K. Park, E. S. Kim, S. R. Cho, K. T. Kim and Y. C. Park, Annual variation of water qualities in the Shihwa Lake, Ocean Polar Res., 2003, 25(4), 459–468 CrossRef CAS.
- J. K. Choi, E. H. Lee, J. H. Noh and S. H. Huh, The study on the phytoplankton bloom and primary productivity in lake Shihwa and adjacent coastal areas, The Sea, 1997, 2(2), 78–86 Search PubMed.
- J. Mainali and H. Chang, Landscape and anthropogenic factors affecting spatial patterns of water quality trends in a large river basin, South Korea, J. Hydrol., 2018, 564, 26–40 CrossRef CAS.
- I. Kim and G. Kim, Submarine groundwater discharge as a main source of rare earth elements in coastal waters, Mar. Chem., 2014, 160, 11–17 CrossRef CAS.
- I. Kim, S. Kim and G. Kim, Analytical artifacts associated with the chelating resin extraction of dissolved rare earth elements in natural water samples, Aquat. Geochem., 2010, 16(4), 611–620 CrossRef CAS.
- Y. Zhu, A. Itoh, E. Fujimori, T. Umemura and H. Haraguchi, Determination of rare earth elements in seawater by ICP-MS after preconcentration with a chelating resin-packed minicolumn, J. Alloys Compd., 2006, 408, 985–988 CrossRef.
- K. Hennebrüder, R. Wennrich, J. Mattusch, H. J. Stärk and W. Engewald, Determination of gadolinium in river water by SPE preconcentration and ICP-MS, Talanta, 2004, 63(2), 309–316 CrossRef PubMed.
- T. H. Kim and G. Kim, Estimating benthic fluxes of trace elements to hypoxic coastal waters using 210Po, Estuarine, Coastal Shelf Sci., 2014, 151, 324–330 CrossRef CAS.
- S. R. Taylor and S. M. McLennan, The Continental Crust: Its Composition and Evolution, 1985 Search PubMed.
- D. S. Alibo and Y. Nozaki, Rare earth elements in seawater: particle association, shale-normalization, and Ce oxidation, Geochim. Cosmochim. Acta, 1999, 63(3–4), 363–372 CrossRef CAS.
- S. R. Taylor and S. M. McLennan, The significance of the rare earths in geochemistry and cosmochemistry, Handbook on the physics and chemistry of rare earths, 1988, vol. 11, pp. 485–578 Search PubMed.
- Y. Nozaki and D. S. Alibo, Importance of vertical geochemical processes in controlling the oceanic profiles of dissolved rare earth elements in the northeastern Indian Ocean, Earth Planet. Sci. Lett., 2003, 205(3–4), 155–172 CrossRef CAS.
- I. Kim and G. Kim, Role of colloids in the discharge of trace elements and rare earth elements from coastal groundwater to the ocean, Mar. Chem., 2015, 176, 126–132 CrossRef CAS.
- J. S. Ryu, K. S. Lee, S. G. Lee, D. Lee and H. W. Chang, Seasonal and spatial variations of rare earth elements in rainwaters, river waters and total suspended particles in air in South Korea, J. Alloys Compd., 2007, 437(1–2), 344–350 CrossRef CAS.
- S. Kulaksız and M. Bau, Rare earth elements in the Rhine River, Germany: first case of anthropogenic lanthanum as a dissolved microcontaminant in the hydrosphere, Environ. Int., 2011, 37(5), 973–979 CrossRef PubMed.
- M. Bau, A. Knappe and P. Dulski, Anthropogenic gadolinium as a micropollutant in river waters in Pennsylvania and in Lake Erie, northeastern United States, Chem. Erde, 2006, 66(2), 143–152 CrossRef CAS.
- I. Kim and G. Kim, Large fluxes of rare earth elements through submarine groundwater discharge (SGD) from a volcanic island, Jeju, Korea, Mar. Chem., 2011, 127(1–4), 12–19 CrossRef CAS.
- V. Hatje, K. W. Bruland and A. R. Flegal, Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco Bay over a 20 year record, Environ. Sci. Technol., 2016, 50(8), 4159–4168 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00304e |
| This journal is © The Royal Society of Chemistry 2020 |