Using low-cost sensors to monitor indoor, outdoor, and personal ozone concentrations in Beijing, China
Meichen
Liu
 *a,
Karoline K.
Barkjohn
*a,
Karoline K.
Barkjohn
 a,
Christina
Norris
a,
James J.
Schauer
b,
Junfeng
Zhang
c,
Yinping
Zhang
d,
Min
Hu
ef and
Michael
Bergin
*a
a,
Christina
Norris
a,
James J.
Schauer
b,
Junfeng
Zhang
c,
Yinping
Zhang
d,
Min
Hu
ef and
Michael
Bergin
*a
aDuke University, Civil and Environmental Engineering, Durham, NC, USA. E-mail: meichen.liu@duke.edu; mike.bergin@duke.edu
bUniversity of Wisconsin at Madison, Civil and Environmental Engineering, Madison, Wisconsin, USA
cDuke University, Nicholas School of the Environment, Durham, NC, USA
dTsinghua University, School of Architecture, Beijing, China
eState Key Joint Laboratory of Environmental Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China
fBeijing Innovation Center for Engineering Sciences and Advanced Technology, Peking University, Beijing, 100871, China
First published on 7th November 2019
Abstract
High concentrations of ground-level ozone (O3) have been measured outdoors across China but there are limited measurements of O3 in microenvironments, including in homes, and for personal exposure. This highlights the need for cheaper methods to accurately make these measurements and to better capture fine-scale spatial variability in O3 across cities. With this in mind, we conducted a pilot study at six homes in Beijing, China, over 12 days to evaluate the use of portable, low-cost, time-resolved monitors for measuring O3 indoors and outdoors. We also assessed personal exposure for one adult in each home for two 48 hour periods using backpack-mounted monitors. Prior to and following sampling we collocated all monitors with a reference analyzer; we used data from these colocations to generate linear calibrations which we applied to all monitor data. Calibration slopes did not change significantly over the study although some intercepts differed. The average limit of detection (LOD) was 7.0 ppb, average root mean square error was 16.7 ppb, mean absolute error was 13.3 ppb and normalized root mean square error was 33%. Performance varied substantially between sensors, underscoring the importance of monitor-specific calibrations and determinations of measurement error. Outdoor concentrations varied spatially, with home-specific peak hourly averages of 32–165 ppb; indoor concentrations ranged from below the LOD to 15 ppb. Hour-averaged personal exposure was generally higher than O3 indoors, and at times exceeded ambient O3 indicating contributions to personal exposure from ambient sources of O3 away from the home. This work illustrates the feasibility of using these monitors to characterize distributions of O3 spatially and temporally when differences in concentrations are large, and outlines considerations for using these monitors to measure personal exposure.
Environmental significanceGround-level ozone (O3), which affects both human and environmental health, has become one of the main harmful air pollutants in China. In spite of this, limited data exists about personal exposure to O3 and about concentrations of O3 in microenvironments across a region. In this study, we illustrate the feasibility of using low-cost sensors to address these knowledge gaps and describe considerations when calibrating the sensors to improve data accuracy. We highlight the large gradients in concentrations and exposures to O3 that can be measured in a relatively polluted urban area (here, Beijing) using these small, portable, real-time sensors and show that if homes are well-sealed, the concentrations of O3 indoors are often quite low. |
1. Introduction
Ground-level ozone (O3) pollution is a global concern, and O3 has become one of the most problematic air pollutants in China with average daily concentrations reaching as high as 242 μg m−3 (124 ppb) in Beijing.1 The Chinese National air quality standards are 100 μg m−3 (51 ppb) for Class 1 O3 concentrations and 160 μg m−3 (82 ppb) for Class 2 O3 concentrations, measured as daily 8 hour maximum averages.2 Class 1 standards apply to areas in need of special protection, i.e., national parks, while Class 2 standards apply to urban and industrial environments.1 Many other cities in China including Shanghai, Nanjing, Xi 'an, Jinan, and Zhengzhou also have high concentrations of ground-level O3, with concentrations highest from July to September.3 Factors such as high temperature, strong sunlight, and emissions of precursor pollutants (i.e. NOx and specific volatile organic compounds) can contribute to elevated concentrations of O3 throughout the year with values typically highest in summer due to the increased presence of sunlight (UV irradiation). Monitoring ground-level O3 is an important first step in understanding its spatial and regional patterns and concentrations, which are necessary in assessing environmental and health effects.Current approaches to measuring O3 concentrations are limited, in part due to the instrumentation available.4 Conventional air pollution monitoring systems typically employ instruments with relatively complicated methods of measurement and use additional tools or equipment including temperature and relative humidity controllers, and air filters or sensors for particulate matter and gases. They also have built-in calibrators, aiming to guarantee the accuracy and performance of the instruments. Consequently, these instruments tend to be large, expensive, and work best with a constant supply of stable power.5 These factors limit their use to environments with a reliable power supply and where theft or vandalism of expensive equipment are not a major concern and make it challenging to use them in smaller spaces including homes or to transport instruments readily between locations. These limitations further hinder the development of systematic networks of time-resolved monitoring instruments, resulting in the inefficiency of the monitoring of strong gradients in pollutant concentrations both spatially and temporally.4 Understanding the impacts of air pollutants on human health and the environment can be challenging without these data. Small, low-cost (e.g., costing a few hundred dollars) sensors which can collect measurements integrated over short intervals of time (e.g., minutes or hours) provide a means to fill these gaps in the data.5 While the potential for such devices is well understood, low-cost O3 sensors have not been used as widely as might be expected due, in part, to the challenges of obtaining precise and accurate measurements of specific gaseous pollutants using low-cost sensors, especially over a long period of time, as compared to using conventional monitoring instruments.5,6 Detailed published measures of performance of low-cost gas sensors are relatively sparse and each low-cost sensor must be thoroughly evaluated to understand its limitations and to determine the best methods of calibration to maximize data quality.7–10 Moreover, networks of time-resolved monitoring instruments or systems are not being widely applied in China.
This study was a pilot preceding an extensive field campaign in Shanghai; as such, an overarching goal of the research was to evaluate the performance of a particular type of low-cost O3 monitor and to determine how well the methods of deployment and calibration work in these highly polluted environments in urban China where there are large temporal–spatial and indoor/outdoor variabilities in concentrations of O3. The specific aims of the pilot study were to (a) test portable, low-cost, time-resolved monitors for measuring concentrations of O3 indoors and outdoors at homes in Beijing, (b) to measure personal exposure to O3 and (c) to evaluate the performance of these monitors for these purposes.
2. Methods and equipment
2.1 Study location and participant recruitment
This study was conducted in Beijing, China (40° N, 116° E, 43 m asl.). Beijing is the world's third most populous city proper, with a population of over 17 million and an area of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 km2 (38% flatlands and 62% mountain areas). Beijing is surrounded by high mountains to the north, southwest, and west.11 This geography makes Beijing vulnerable to air pollution because easterly or southerly winds can transport large amounts of pollution to Beijing where it accumulates.11 From 2010–2014, the average concentration of O3-8 h in urban Beijing increased at a rate of 1.8 μg m−3 (∼0.9 ppb) per year, with concentrations in 2010 of approximately 80 μg m−3 (∼40 ppb).12 The combined effects of urbanization and pollution caused by combustion of fossil fuels has resulted in Beijing becoming one of the most polluted cities in the world with several intense air pollution events occurring each year.13
800 km2 (38% flatlands and 62% mountain areas). Beijing is surrounded by high mountains to the north, southwest, and west.11 This geography makes Beijing vulnerable to air pollution because easterly or southerly winds can transport large amounts of pollution to Beijing where it accumulates.11 From 2010–2014, the average concentration of O3-8 h in urban Beijing increased at a rate of 1.8 μg m−3 (∼0.9 ppb) per year, with concentrations in 2010 of approximately 80 μg m−3 (∼40 ppb).12 The combined effects of urbanization and pollution caused by combustion of fossil fuels has resulted in Beijing becoming one of the most polluted cities in the world with several intense air pollution events occurring each year.13
We included six homes from across Beijing in this pilot study (Fig. 1). Homes were selected through collaborators at Tsinghua University. As participants were selected from university contacts, we do not expect them to reflect the general population; however, as homes were located across Beijing, we expect them to illustrate a range of pollutant concentrations. Home 1 was located in a busy commercial area at the third-ring road in Beijing. Homes 2 and 3 were on the Tsinghua University campus in northwestern Beijing. Home 4 was in western Beijing, and homes 5 and 6 were in east Beijing. These latter three homes were in residential apartments and neighborhoods. During personal sampling, participants engaged in their usual routine, with some time spent at work, some time spent at home, and some time spent commuting. Participants who lived on campus also worked on campus.
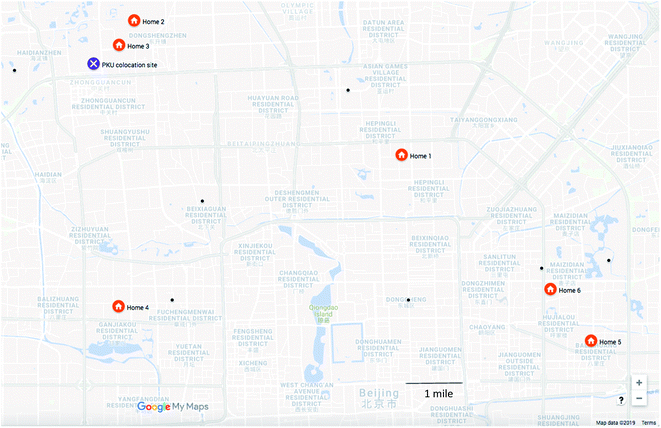 | ||
| Fig. 1 Sampling locations in Beijing, with regulatory monitoring stations indicated with a black dot. | ||
Prior to sampling in the homes and on individuals, all monitors were collocated with a stationary reference analyzer (Thermo Electron Corporation UV Photometric O3 Analyzer, Model 49i) at a base station at the College of Computer Science, Peking University (PKU) in order to ensure monitors were working properly and to derive calibrations for the monitors. The reference analyzer logged minute averages of O3 in addition to temperature and relative humidity. All instruments were located on the roof of the building – five floors above ground level – and the site had relatively unobstructed airflow.
2.2 Description of low-cost monitors
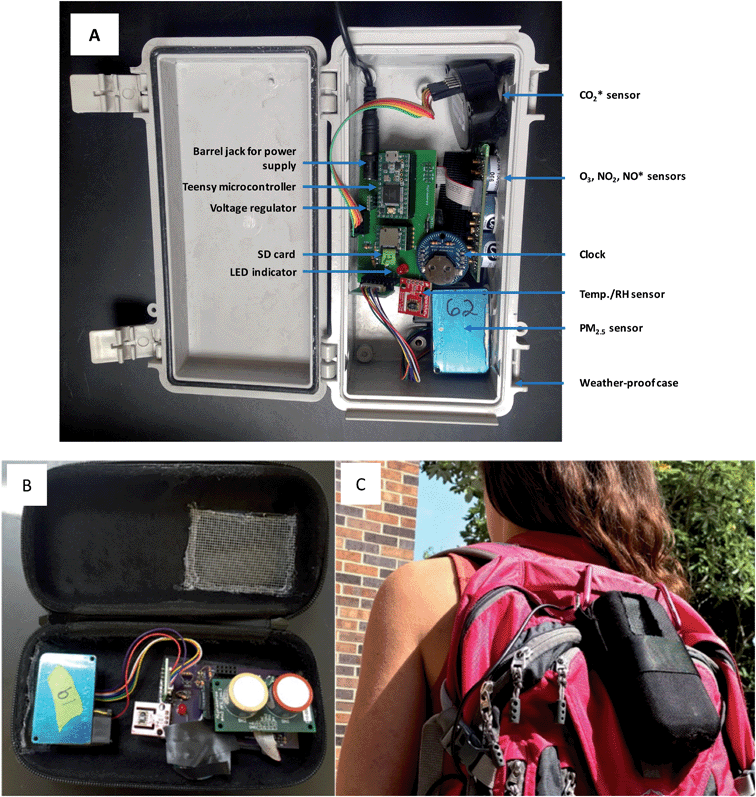
We used indoor/outdoor and personal exposure monitors to measure time-resolved concentrations of pollutants (Fig. 2A). Monitoring packages, which cost approximately 350 USD, included 2-way (i.e., comprising oxidant and NO2 sensors) or 3-way (i.e., comprising oxidant, NO2 and NO sensors) Alphasense 4-Electrode gas sensors (OX-A4 and NO2-A4 series), a Plantower PMS3003 particulate matter (PM2.5) sensor, in some cases a Cozir CO2 sensor, an SD card, LED indicators, a Sensirion SHT15 temperature and relative humidity (RH) sensor, a clock, a voltage regulator, a barrel jack for wall power supply, and a Teensy microcontroller. The Alphasense gas sensors were connected to Alphasense analogue front end (AFE) support circuits and these in turn, along with all other components, were connected to a printed circuit board (PCB) designed specifically for these monitors. Diodes, resistors and transformers on the PCB were used to convert the AC power supplied via wall power to DC power required by the sensors. Sensors were located flush with the edge of the monitoring box so that they were directly exposed to ambient air – this minimizes the possibility of O3 reacting with the inside surfaces of the monitor before it reaches the sensor. When using these monitors, minute averages of gas phase pollutant concentrations are recorded on an SD card connected to the Teensy microcontroller, and data can subsequently be downloaded by inserting the SD card into a computer. An LED indicates the status of the monitor (off, logging data, not logging data, error). The barrel jack is used to connect the monitor to a portable battery or to wall power. The indoor/outdoor and personal monitors all contain the same components, although the external packaging differs and thus the components are arranged in a slightly different configuration in the packages. Fig. 2B and C show a personal monitor which can be attached to a backpack to evaluate time-resolved personal exposure to air pollutants. Although additional pollutants were measured using these monitors, in this paper we focus only on the O3 data; PM2.5 data are discussed in a separate publication.14Alphasense gas sensors operate in the amperometric mode, generating a current that is linearly proportional to the fractional volume of a specific gas. The sensors comprise four electrodes – a working electrode, a reference electrode, a counter electrode, and an auxiliary electrode. The working electrode is designed to optimize the oxidation or reduction of the gas to be measured, while the other three electrodes are used to balance the chemical reaction of the working electrode and provide a stable environment such as equivalent current and stable potential. The sensor's analog output ranges from 0–3 volts and is converted to ppb based on sensor-specific factors – total working electrode zero (mV), total auxiliary electrode zero (mV), and sensitivity to O3 and NO2 (mV ppb−1) – provided by Alphasense. In these monitors, the conversion from sensor signal to oxidant and NO2 concentration (ppb) is done using these factors via Arduino code uploaded to the Teensy microcontroller. As is the case for many electrochemical sensors, these sensors experience cross-sensitivity to multiple pollutants. The Alphasense platform thus uses an oxidant sensor – responsive to NO2 and O3 – and an NO2-specific sensor in tandem to measure concentrations of O3; the NO2 sensor filters out O3 using a manganese dioxide filter to convert O3 to O2, and the concentration of O3 can be determined by subtracting the response of the NO2 sensor from the response of the oxidant sensor. Previous work has shown these sensors detect O3, NO2, and NO with high sensitivity, stability, and reliability for long-term repeatability,6,15 although one study found that concentrations of O3 tend to be underestimated in mixtures of NO2 and O3.16 While O3 concentrations are logged to the SD card, these monitor-derived concentrations should still be calibrated through colocation and comparison with a reference analyzer, as detailed in Section 2.3.1.
2.3 Study design and timeline
A limit of detection (LOD) was calculated for each monitor using data from periods when monitors were colocated at PKU and the reference analyzer recorded O3 concentrations less than or equal to 3 ppb. The standard deviation (σ) of the readings of the monitors during these periods of low O3 concentration was calculated for each monitor separately, and monitor-specific LODs were calculated as 3σ.17 Although we might expect concentrations of O3 in the homes to be low due to rapid reactions with surfaces, they are likely not consistently zero; we believe that excluding values below the LOD altogether would bias the data. As such, LODs were applied to hour-averaged data collected during home and personal exposure monitoring and values below the LOD were replaced with 3/4 the LOD.
2.4 Data processing
Minute averages of data (temperature, relative humidity, concentrations of O3 and NO2) were logged during this study. Most studies using these Alphasense O3 sensors have employed these sensors to collect stationary ambient measurements or in well-controlled chamber studies, and to our knowledge we are one of the first to use these sensors to measure personal exposure. In a very recent study, these sensors were deployed in both a stationary and mobile configuration with minute-averages of data analyzed. In the aforementioned study, agreement with reference analyzer data was worse with the mobile configuration illustrating some of the challenges of collecting mobile data.19 In the current study, raw, one-minute data from the low-cost monitors and the reference analyzer were hour-averaged as one-minute data for both personal exposure (i.e. mobile measurements) and indoor/outdoor (static measurements) were relatively noisy and time-activity data were collected by hour. The decision to hour-average was made to balance the need for higher time-resolution with the need to ensure data robustness.These Alphasense electrochemical sensors require time to stabilize upon startup, and outliers representing erroneous values occur frequently at the very beginning of sampling. To account for this warm-up period, we removed the first two hours of data after monitor start up. In some instances, the sensor became dislodged from its connection to the rest of the monitor resulting in very high and variable values (e.g., values several hundred ppb higher than those measured from all other sensors). These erroneous values were easily identifiable when data from numerous monitors were plotted simultaneously over time, and such data were removed prior to further analysis. Lastly, from the remaining data we removed hour-averaged data collected during the colocation periods that were greater than the third quartile (Q3) plus 1.5 times the interquartile range (IQR) or less than the first quartile (Q1) minus 1.5 × IQR of each single set of data (reference analyzer or monitor). This process preceded the determination of calibration constants for the monitors.
3. Results
3.1 Field evaluation of O3 monitors
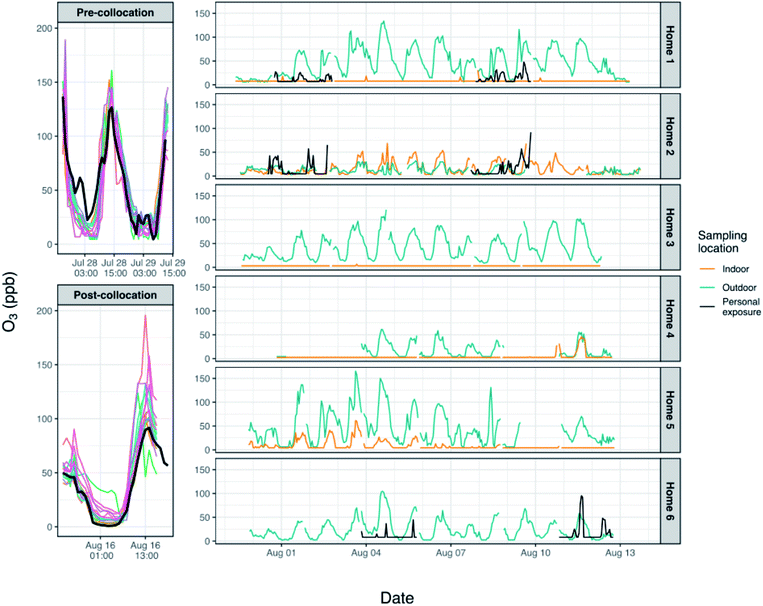
After removal of outliers and erroneous data and after integrating by hour, unique calibration constants (slope, intercept) were derived for each monitor (Table 1) using linear regression of the monitor concentrations on data from the reference analyzer during periods when all instruments were collocated at PKU. During calibrations such as these it is important to collect data over a wide range of concentrations and especially over the range of concentrations and environmental conditions one might expect to see over the course of the study. During the ∼3 days of colocation, O3 measured by the reference analyzer ranged from <1 ppb to 142 ppb. This is comparable to the values measured during home monitoring, which ranged from 2–165 ppb; four data points fell outside of the range of concentrations measured during colocation. Conditions were slightly warmer and with higher humidity (t-test, p < 0.05), on average, during the pre-colocation (mean: 28 °C, 75%) than during the post-colocation (mean: 26 °C, 66%) although it was generally warm and humid during both periods (pre, range: 22–36 °C, 50–100%; post, range: 21–33 °C, 48–90%). There was substantial overlap between environmental conditions measured during the colocations and those encountered indoors and outdoors at homes during home monitoring (hour averages across homes, indoor: 28–38 °C and 36–72% RH; outdoor: 25–46 °C and 21–86% RH), and we would not expect the slightly higher temperatures and lower humidity encountered infrequently during home sampling to have a large impact on our results.
| Monitor use | Pre-colocation | Post-colocation | Combined colocation data (pre + post) | MAE (ppb) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept (ppb) | Slope | R 2 | Intercept (ppb) | Slope | R 2 | Intercept (ppb) | Slope | R 2 | RMSE (ppb) | NRMSE (%) | ||
| a Sensor was not working properly. Note: data from home 6, indoor, were excluded due to sensor malfunction. | ||||||||||||
| Base station #1 | −21 | 0.6 | 0.66 | −16 | 1.0 | 0.87 | −14 | 0.6 | 0.57 | 30.8 | 61 | 21.7 |
| Base station #2 | 21 | 1.4 | 0.83 | 24 | 1.9 | 0.94 | 28 | 1.4 | 0.83 | 16.8 | 33 | 14.0 |
| Home 1 – indoor | 0 | 1.3 | 0.65 | 18 | 1.5 | 0.76 | 14 | 1.2 | 0.64 | 26.9 | 53 | 20.6 |
| Home 1 – outdoor | 35 | 1.0 | 0.81 | 33 | 1.0 | 0.96 | 34 | 1.0 | 0.86 | 15.1 | 30 | 11.8 |
| Home 2 – indoor | −12 | 1.4 | 0.87 | 0 | 1.4 | 0.98 | −4 | 1.3 | 0.88 | 13.8 | 27 | 11.2 |
| Home 2 – outdoor | −16 | 1.4 | 0.84 | −7 | 1.3 | 0.97 | −11 | 1.3 | 0.87 | 14.1 | 28 | 11.2 |
| Home 3 – indoor | 17 | 1.3 | 0.83 | 26 | 1.2 | 0.91 | 22 | 1.2 | 0.85 | 15.3 | 30 | 12.1 |
| Home 3 – outdoor | −13 | 1.3 | 0.88 | −13 | 1.3 | 0.88 | 14.3 | 28 | 12.4 | |||
| Home 4 – indoor | 11 | 1.2 | 0.97 | 11 | 1.2 | 0.97 | 3.6 | 7 | 2.2 | |||
| Home 4 – outdoor | −3 | 1.6 | 0.97 | −3 | 1.6 | 0.97 | 4.8 | 9 | 3.7 | |||
| Home 5 – indoor | −9 | 1.5 | 0.90 | 13 | 1.4 | 0.98 | 4 | 1.4 | 0.89 | 13.1 | 26 | 10.7 |
| Home 5 – outdoor | 9 | 0.8 | 0.80 | 3 | 1.1 | 0.92 | 8 | 0.8 | 0.82 | 17.2 | 34 | 13.5 |
| Home 6 – outdoor | 2 | 1.4 | 0.89 | 12 | 1.4 | 0.97 | 7 | 1.4 | 0.91 | 11.6 | 23 | 9.1 |
| Personal #1 | 0 | 0.9 | 0.85 | 23 | 0.9 | 0.37 | 14 | 0.8 | 0.69 | 24.9 | 49 | 21.4 |
| Personal #2 | −23 | 0.6 | 0.83 | 1 | 0.2 | 0.95 | −12 | 0.4 | 0.76 | 21.2 | 42 | 18.0 |
| Personal #3 | −7 | 0.6 | 0.82 | 9 | 0.6 | 0.83 | 3 | 0.6 | 0.72 | 23.5 | 46 | 19.3 |
| Average | −1 | 1.1 | 0.82 | 10 | 1.2 | 0.89 | 6 | 1.1 | 0.82 | 16.7 | 33 | 13.3 |
| Standard deviation | 17 | 0.4 | 0.08 | 14 | 0.4 | 0.16 | 15 | 0.4 | 0.11 | 7.4 | 14 | 5.8 |
During pre-colocation, the intercepts ranged from −23 to 35 ppb (mean ± SD: −1 ± 17 ppb), and the slopes ranged from 0.6 to 1.5 (mean ± SD: 1.1 ± 0.4) (Table 1). During post-colocation, the intercepts ranged from −16 to 33 ppb (mean ± SD: 10 ± 14 ppb), and the slopes ranged from 0.2 to 1.9 (mean ± SD: 1.2 ± 0.4). Coefficients of determination (R2) for monitor vs. reference analyzer data were 0.56–0.90 during pre-colocation and 0.36–0.98 during post-colocation. A paired t-test was used to compare, by monitor, the intercepts and the slopes between the pre- and post-colocations; differences in the slopes were not statistically significant (p > 0.05) although differences in the intercepts were. Some monitors had colocation data from only one of the two periods due to issues of poor sensor connection or for logistical reasons. As such, final calibration constants were derived after aggregating pre- and post-colocation data for each monitor and conducting linear regression between these data and data from the reference analyzer. Although the slopes of the individual monitors did not change appreciably over the several weeks of the study, over longer periods of time in these highly polluted environments this may not hold true, especially if environmental conditions change during this time due to a change in season. Furthermore, previous work by others has indicated that over multiple months of use these Alphasense electrochemical sensors may experience drift, although the nature of the drift may not be uniform across sensors thus indicating the need for careful sensor-by-sensor recalibration over time.20
The parameters derived from regression were used to calibrate all data collected by the monitors during colocation, during home sampling, and for personal exposure. Monitor errors were calculated as the difference between the calibrated sensor values and data from the reference analyzer during the colocation periods (Table 1). After applying the calibration, root-mean-square deviations (RMSE) were calculated to measure the differences between the predicted values and observed values. The average RMSE was 16.7 ppb, and the mean absolute error (MAE) was 13.3 ppb. This RMSE is comparable to what was found in China in June by others using the same Alphasense O3 sensors (14.7 ppb).19 Others have previously found slightly lower RMSE and MAE values for Alphasense O3 sensors using larger data sets and including more parameters for calibration, although some of these differences may relate to the conditions experienced in these environments which may not reflect the conditions typical in urban China. For example, in Cross' examination of these sensors in Boston, USA, a prolonged colocation (4.5 months) led to the collection of more than 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 data points (25% used for model training), for an RMSE of 9.71 ppb and a MAE of 7.34 ppb, although the examination was of an older model of the O3 sensor (OX-B421).21 Employing a machine learning approach, Zimmerman et al. obtained a MAE of 3.36 ± 0.41 ppb in Pittsburgh, USA.22 On average, our R2 values were consistent with those measured previously (mean: 0.82),7,22 although some individual sensors in our study had low R2 values (i.e., 5 sensors <0.8). Taking into account the average concentration during the calibration periods (51 ppb) the normalized root mean squared error (NRMSE) in our study was 33%, with sensor-specific values ranging from 7 to 61%. If we exclude sensors that had an R2 < 0.7 during either of the two calibration periods (n = 3), our metrics improve to a mean R2 of 0.86, RMSE of 14.2 ppb, NRMSE of 28% and MAE of 11.5 ppb. Substantial variability in the values for these metrics across sensors highlights the importance of conducting sensor-specific evaluations prior to use in the field as performance is quite variable even when sensors are brand new; such evaluations may allow researchers to identify and remove from further use any sensors that are under-performing.21
000 data points (25% used for model training), for an RMSE of 9.71 ppb and a MAE of 7.34 ppb, although the examination was of an older model of the O3 sensor (OX-B421).21 Employing a machine learning approach, Zimmerman et al. obtained a MAE of 3.36 ± 0.41 ppb in Pittsburgh, USA.22 On average, our R2 values were consistent with those measured previously (mean: 0.82),7,22 although some individual sensors in our study had low R2 values (i.e., 5 sensors <0.8). Taking into account the average concentration during the calibration periods (51 ppb) the normalized root mean squared error (NRMSE) in our study was 33%, with sensor-specific values ranging from 7 to 61%. If we exclude sensors that had an R2 < 0.7 during either of the two calibration periods (n = 3), our metrics improve to a mean R2 of 0.86, RMSE of 14.2 ppb, NRMSE of 28% and MAE of 11.5 ppb. Substantial variability in the values for these metrics across sensors highlights the importance of conducting sensor-specific evaluations prior to use in the field as performance is quite variable even when sensors are brand new; such evaluations may allow researchers to identify and remove from further use any sensors that are under-performing.21
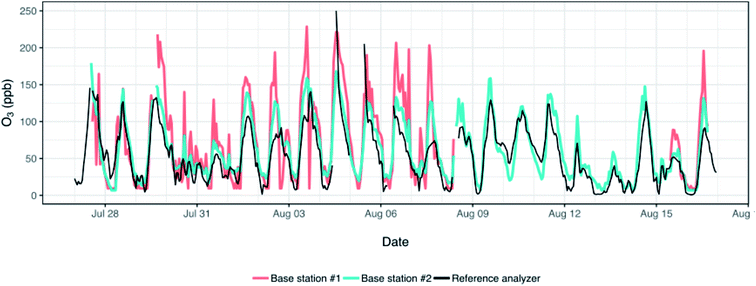
As we left two monitors (base station #1 and #2 in Table 1) alongside the reference analyzer for the duration of the study (n = 21 days) we were able to evaluate calibrations derived from a prolonged colocation against the 3 day colocation used for calibration in this study. This longer colocation captured a broader range of concentrations of O3 (up to ∼250 ppb) than was seen during our 3 day colocation. The RMSE for the two monitors based on the prolonged colocation/calibration were 37.3 and 17.9 ppb – slightly higher than the 30.8 and 16.8 ppb obtained for these same monitors when evaluation was restricted to the 3 days of colocation. Similarly, R2 for the prolonged colocation was 0.58 (3 day: 0.57) and 0.80 (3 day: 0.83). This is comparable to work done by others with these same Alphasense O3 sensors in which they found an R2 of 0.65 during a 5 day colocation period used for sensor calibration.19 In sum, in this particular instance conducting a substantially longer colocation does not appear to improve data quality. Our 3 days of colocation already capture a broad range of concentrations and environmental conditions and although the longer colocation included higher concentrations of O3, few measurements at homes (n = 4) exceeded the concentrations measured during the 3 days of colocation anyways.
As is evident in Fig. 4, the monitors seem to generally track the O3 data measured by the reference analyzer and to pick up the diurnal trends in concentrations; the monitors correlate well with each other (R2: 0.82). However, there are numerous periods during which the monitors substantially overestimate or underestimate the reference analyzer; one monitor experienced spikes and drops in concentrations (see Fig. 4, ∼Aug 3) that were either attenuated in the data measured by the other monitor and the reference, or which did not match up at all with these data. In previous work with Alphasense oxidant sensors, concentrations of O3 were underestimated by up to 187% when there was a mix of NO2 and O3,16 and overestimated by 20–40% when concentrations were at their peak.23 The differences we observed between the reference and our sensors may thus reflect, in part, diurnal changes in the concentrations of NOx,. The marked differences in the concentrations measured by the reference analyzer and the sensors point to the need for a greater investigation of calibration methods and environmental conditions or cross-sensitivities to other pollutants that may be biasing these monitor-based measurements in this environment. This also highlights the need for careful comparison of measurements collected by different sensors following collocation as differences between measurements at different locations may fall within the error of the two sensors used to make those measurements.
3.2 Outdoor, indoor, and personal ozone concentrations
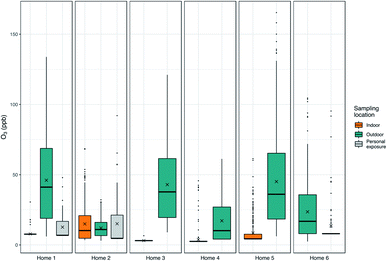
Significant spatial variation can exist in O3 concentrations across small (i.e., indoor vs. outdoor) and larger (i.e., between sampling sites) distances.30 Where the homes were located likely impacts the concentrations of O3 – for example, home 1 was in an area that was in close proximity to traffic, near several large commercial centers and companies at the third-ring road. Home 5 was also next to a busy road, and there was a construction site behind the apartment where the monitor was located. Previous work in Beijing found lower O3 concentrations at traffic sites versus other urban sites, although the exact definition of a “traffic site” (e.g. proximity to the roadway) was not clear from the paper.31 Although home 1 is in a neighborhood close to a major roadway, the home itself is set back in the neighborhood. In spite of a relative proximity to main roads, it is possible that homes 1 and 5 are also close to sources of volatile organic compounds (VOCs) leading to their higher concentrations of O3. For example, at home 1, nearby restaurants and an automobile service centre may be emitting VOCs and for home 5, emissions may be coming from a nearby cleaner. As we did not methodically identify these types of nearby pollutant sources during the field campaign, we are unable to determine what these sources are with certainty. Home 4 may have experienced lower ozone concentrations due to nearby sources of NOx and fewer sources of VOCs. Although both on the Tsinghua campus, there were large and significant (p < 0.05, paired Wilcoxon sign rank test) differences in the concentrations of O3 measured at home 2 (median: 11.0 ppb, range: 3.0–32.2 ppb; sensor RMSE: 14.1 ppb, MAE: 11.2 ppb) and home 3 (median: 37.8 ppb, range: 9.0–121.1 ppb; sensor RMSE: 14.3 ppb, MAE: 12.4 ppb). These homes were located on different parts of the campus which are likely influenced by differences in NOx emissions that influence O3 concentrations. The mean difference in concentrations between these two homes was 27.3 ppb (median 19.5 ppb), with differences ranging from −7.0 to 118.1 ppb or 44.9–190.3% (relative percent difference); we believe these to be real differences in concentrations between the two homes although the exact magnitude of the difference may be slightly more or less than indicated due to the error associated with these measurements.
There are numerous additional ambient monitors in Beijing, and we compared values from our low-cost ambient monitors to data from the nearest monitors. Our monitors showed similar trends but substantially lower values than those from the nearest reference monitors. As the distance between the closest regulatory monitor and home monitors ranged from ∼0.6–3.5 km we might expect to see differences between the concentrations in these environments. However, the systematically lower values measured by the monitors as compared to the reference data may be due to a short-coming of the technology itself; previous work with Alphasense sensors for measuring O3 has shown that when sensors are exposed to a mixture of O3 and NO2 – as is common when collecting ambient measurements due to traffic-related pollution – O3 concentrations are often underestimated.16 In Beijing, additional sources of NOx in the summer may include fossil fuel combustion for manufacturing, construction, and for the generation of electricity.32
No homes had central heat or ventilation units, although some homes did have an air conditioner and all participants indicated the air conditioner was used some or most of the time. Additional information about the homes has been previously published.14 In addition, high summer temperatures led to some participants opening windows to reduce temperatures indoors. Compliance was initially determined by examining the simultaneous indoor and outdoor pollutant concentrations at the home. It was readily apparent in the data when indoor concentrations closely mirrored ambient concentrations in terms of trends in concentrations but, more importantly, also in the magnitude of the concentrations. While indoor concentrations of O3 tend to be quite low with the doors and windows closed, the concentrations are higher when the doors and windows are open providing a relatively constant source of O3. These indicators of non-compliance in the data were further supported by visits to home 2 during which we noted that the patio door and/or windows between outdoors and the bedroom were open when we arrived. While homes 2 and 5 experienced many hour-averaged values above 10 ppb, most concentrations of O3 indoors at other homes were quite low (<10 ppb), and for a number of homes (homes 1, 3 and 4) indoor concentrations fell almost exclusively below the LODs. The higher concentrations indoors at home 5 may indicate that the doors and/or windows were opened periodically in this home, although not as often as home 2, leading to greater concentrations of O3 indoors than were seen in most other homes; it is also possible that this home was less well-sealed than the other homes sampled.
Others have previously measured the response times of Alphasense O3 sensors to various concentrations of O3 and found them to be two minutes or less.35 This lagged response may lead to some error as participants move between environments with drastically different concentrations of ozone (i.e., moving between indoor and outdoor environments), although for this study data were ultimately hour-averaged and this small lag is thus less of a concern. In addition, if environments differ drastically in terms of humidity and sensors are exposed to a change in RH of 20% min−1 or more they can experience a shock response and may exhibit an artificial spike in output signal; it typically take up to 40 minutes to recover from such a shock.23 In contrast, gradual changes in RH appear not to appreciably impact sensor output.23 During personal exposure monitoring, <0.03% of minute readings were associated with a change in RH > 20% min−1 and the mean change in RH was 0.004% min−1; in the current dataset, we think it unlikely that the shock response mentioned is biasing our results.
Personal exposure data from homes 1 and 6 were lower, on average, than outdoor concentrations (Fig. 5), and these differences were significant (p < 0.05, paired Wilcoxon signed rank test). Home 2 had the highest personal exposure even though this home had the lowest outdoor concentrations, and differences between personal exposure and concentrations measured indoors and outdoors were not significant. The differences noted for homes 1 and 6 may relate to the specific activities that the individual was doing during personal monitoring – the outdoor O3 monitor reflects the concentrations at the individual's home, but the individual may have been exposed to higher or lower ozone concentrations during their commute, while at work, in other rooms in the home without air purification, or while they were doing other activities outside. In addition, personal care and other products and materials used in the home are known sources of volatile organic compounds, which are ozone precursors. It is possible that the personal monitoring captured some generation of ozone due to these products. During personal monitoring we asked participants to complete a brief time-activity form indicating whether they were at home, at work/school, or outdoors each hour. The participant in home 1 spent, on average, 13.25 hours in their bedroom, 4.25 hours in other rooms at home, 6 hours at the office, and 0.5 hours outside per day. The participant at home 5 spent 19 hours in the bedroom, 4 hours in other rooms at home and 1 hour outside, and the participant at home 6 spent 14 hours in the bedroom, no time in other rooms at home, 8 hours at the office, and 2 hours outside. It is likely that even within these environments there were gradients in O3, and the collection of more detailed time-activity data would facilitate the interpretation of the contributions of different microenvironments to overall exposure. Furthermore, the personal exposure data readily highlight times when the participant was outdoors and away from the home, where concentrations of O3 were higher than those measured indoors or directly outside the home (see Fig. 3 – home 6). Data like these – that are not consistently reflected in data from stationary monitors – help us to better understand the minutiae of personal exposure and which environments may be contributing more to exposure.
4. Strengths, limitations and further research
As a small pilot study, this work has numerous limitations. First and foremost, the sample size (6 homes) and duration of the study (∼2 weeks in homes) allowed us to gain a preliminary understanding of the concentrations of O3 in Beijing but limit any broad conclusions about O3 in these types of highly polluted urban environments in China. Second, due to variation in the performance of the sensors even when they are brand new, differences in calibrated measurements cannot be taken at face value. Measurements must be carefully examined in the context of the performance of the sensor(s) being used to make the measurements.A strength of this work is that it highlights the potential of these monitors, once calibrated and when evaluating measurements in the context of sensor performance, for capturing spatial and temporal variability in O3 concentrations; sensors captured substantial differences in ambient concentrations between homes located in different parts of the city, daily variability in ambient O3 concentrations, and stark contrasts between indoor and outdoor concentrations. This study also illustrates a potential alternative use for these sensors – to monitor compliance in a study such as this in which it is important to keep doors and windows closed (for example, when evaluating the maximum benefit of a filtration intervention). In our data, high concentrations of O3 indoors were a good indicator of a poorly sealed home or one in which windows and doors were routinely opened.
As these are low-cost devices and therefore less accurate out-of-the-box than reference analyzers, it is critical to determine an appropriate method of calibration to maximize data quality. Although the inclusion of temperature and relative humidity did not greatly improve data accuracy in this context, it may do so under different conditions (e.g., in Shanghai in seasons other than summer and for a longer duration); each research study should consider similar evaluations of parameters for inclusion during calibrations. Others have recently examined the use of random forest models to calibrate data from Alphasense O3 sensors and have seen large improvements in accuracy with these models over linear regression models.22 However, in the aforementioned work the random forest model approach was applied to a much larger (∼6 months of data, using 4 weeks of data for model training) and more complex (containing numerous pollutants, temperature and RH data) dataset; although such an approach could lead to improvements in data accuracy with our monitors, these techniques were not applied to the much smaller dataset used in the current study.
The variation in calibration parameters observed in this study illustrates the need to generate calibration constants for each individual sensor. For this study, a minimum of 2 days of colocation with the reference instrument was required – one day prior to deployment in homes and one day following – to examine potential drift of the sensors. Furthermore, due to the daily pattern in O3 concentrations (i.e., with concentrations highest during daylight hours and lowest at night), conducting colocations over a period of 24 hours minimum ensured we captured a broad range of concentrations; this would not have been achieved had we collocated for a much shorter period. This approach also allowed us to capture times with the low O3 concentrations needed to generate appropriate calibration constants for in-home measurements as concentrations of O3 indoors can be, and were in this study, quite low. Environmental conditions and pollutant concentrations were generally stable during the field campaign and colocations beyond the 3 day colocations used for the majority of monitors in this study led to little improvement in data accuracy. Nonetheless, a prolonged colocation may lead to greater gains under different conditions, if, for example, a few days does not capture the full suite of environmental conditions possible during the study; prolonged colocations should not be ruled out in future research. In short studies such as this the potential benefits of long colocations must also be weighed with restrictions on study resources and time limitations.
Lastly, although we integrated data over an hour, personal exposure monitoring may benefit from integrating data over a shorter period of time, especially if individuals are moving frequently between environments with different conditions. However, this must be balanced by losses in data quality that may occur with increased granularity. In recent work by others using these sensors the R2 between sensor data and reference data while both were collocated but in motion was determined to be 0.56 for minute-averaged data; this highlights some of the challenges to data quality that arise when sensors are in motion and data are assessed over fine time-scales.19 In the current study, it is possible that different averaging periods would have led to different LODs for the sensors, although this was not investigated. This pilot highlighted some of the shortcomings of the design of the monitors, thereby allowing us to modify them to be more robust for the subsequent study in Shanghai; while the components used in these monitors seem to work well, there is a need to improve upon how sensors and other monitoring package components are affixed inside the package housing and to the in-house, custom-designed printed circuit board in order to prevent data loss.
5. Conclusions
Our overall objectives in this study were to evaluate low-cost, time-resolved O3 monitors in an urban area with relatively poor air quality (i.e. Beijing) for monitoring indoor and outdoor concentrations of O3 and for use in personal exposure monitoring. Most monitors performed well with high R2 between monitors and a reference analyzer with values ranging from 0.66–0.90 during the pre-colocation period (mean = 0.82) and 0.37–0.98 during the post-colocation period (mean = 0.89). After applying calibrations to the monitors, the mean RMSE was 16.7 ppb, the MAE was 13.3 ppb and the NRMSE was 33%, although performance varied substantially between sensors. Monitor-specific LODs ranged from 3.3 to 12.6 ppb, highlighting the need to determine LOD values and quantify performance on a sensor-by-sensor basis. A great deal of variability was seen for outdoor concentrations at the homes across Beijing while the bulk of indoor data (77%) fell below the detection limit of the monitors. Personal exposure reflected, at times, the low concentrations indoors, and at other times contributions that far exceeded the concentrations measured in and near the home. Overall, the monitors show promise for providing a low-cost option for accurately measuring concentrations of O3 outdoors, where pollutant concentrations tend to be relatively high (i.e., greater than the monitor LODs). However, measurements must be evaluated in the context of the sensor-specific parameters of performance as there is large variation in these parameters. Regardless of differences in performance it is clear that these sensors can be used to identify temporal changes in concentrations of O3. This study also illustrates some, although perhaps limited, utility for these sensors in environments with lower concentrations of O3 (e.g., indoors); utility will depend on just how low the concentrations are in these environments. Lastly, in an urban area with relatively high levels of pollution, our results indicate that these sensors may be useful for capturing large differences in O3 concentrations experienced by individuals as they move between environments. Further iterations of the monitoring package require more robust attachment of the sensor itself within the monitor to prevent erroneous data or data loss.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The project team thanks Underwriters Laboratory (UL) Inc. for support and funding. This study was also supported in part by grants from the National Natural Science Foundation of China (51420105010). Thanks to Amway (China) Co., Limited, for lending the air purifiers for use in this study; however, the company had no involvement in study design, implementation, or data interpretation. Thanks to Yusheng Wu from Peking University for their support including access to and data from their rooftop monitoring lab. Thank you also to Linchen He of Duke University, and Wei Jingya of Tsinghua University for their assistance with logistics and data collection in the field, and to the participants in this study for allowing us access to their homes.References
- Chinese National Environmental Monitoring Center, July 2016 City Air Quality Status Report, Chinese National Environmental Monitoring Center, 2016 July.
- China, Ambient Air Quality Standards: GB 3095-2012, 2012.
- K. He, H. Huo and Q. Zhang, Urban Air Pollution in China: Current Status, Characteristics, and Progress, Annu. Rev. Energy Environ., 2002, 27(1), 397–431 CrossRef.
- D. E. Williams, G. S. Henshaw, M. Bart, G. Laing, J. Wagner and S. Naisbitt, et al., Validation of low-cost ozone measurement instruments suitable for use in an air-quality monitoring network, Meas. Sci. Technol., 2013, 24(6), 065803 CrossRef.
- W. Y. Yi, K. M. Lo, T. Mak, K. S. Leung, Y. Leung and M. L. Meng, A Survey of Wireless Sensor Network Based Air Pollution Monitoring Systems, Sensors, 2015, 15(12), 31392–31427 CrossRef PubMed.
- A. C. Lewis, J. D. Lee, P. M. Edwards, M. D. Shaw, M. J. Evans and S. J. Moller, et al., Evaluating the performance of low cost chemical sensors for air pollution research, Faraday Discuss., 2016, 189, 85–103 RSC.
- L. Spinelle, M. Gerboles, M. G. Villani, M. Aleixandre and F. Bonavitacola, Field calibration of a cluster of low-cost available sensors for air quality monitoring. Part A: Ozone and nitrogen dioxide, Sens. Actuators, B, 2015, 215, 249–257 CrossRef CAS.
- R. M. Duvall, R. W. Long, M. R. Beaver, K. G. Kronmiller, M. L. Wheeler and J. J. Szykman, Performance Evaluation and Community Application of Low-Cost Sensors for Ozone and Nitrogen Dioxide, Sensors, 2016, 16(10), 1698 CrossRef PubMed.
- K. Sadighi, E. Coffey, A. Polidori, B. Feenstra, Q. Lv and D. K. Henze, et al., Intra-urban spatial variability of surface ozone in Riverside, CA: viability and validation of low-cost sensors, Atmos. Meas. Tech., 2018, 11(3), 1777–1792 CrossRef CAS.
- E. Hall, S. Kaushik, R. Vanderpool, R. Duvall, M. Beaver and R. Long, et al., Integrating Sensor Monitoring Technology into the Current Air Pollution Regulatory Support Paradigm: Practical Considerations, Am. J. Environ. Eng., 2014, 4(6), 147–154 Search PubMed.
- L. Li, Y. Wang, Q. Zhang, J. Li, X. Yang and J. Jin, Wheat straw burning and its associated impacts on Beijing air quality, Sci. China, Ser. D: Earth Sci., 2008, 51(3), 403–414 CrossRef CAS.
- J. Lang, Y. Zhang, Y. Zhou, S. Cheng, D. Chen and X. Guo, et al., Trends of PM2.5 and Chemical Composition in Beijing, 2000–2015, Aerosol Air Qual. Res., 2017, 17(2), 412–425 CrossRef CAS.
- Z. Wang, Y. Li, T. Chen, D. Zhang, L. Li, B. Liu, et al., Science–Policy Interplay: Improvement of Air Quality from 2008 to 2014 in Beijing and the Scientific Approach to Achieve APEC Blue, 2016 Search PubMed.
- K. K. Barkjohn, M. H. Bergin, C. Norris, J. J. Schauer, Y. Zhang and M. Black, et al., Using Low-cost sensors to Quantify the Effects of Air Filtration on Indoor and Personal Exposure Relevant PM2.5 Concentrations in Beijing, China, Aerosol Air Qual. Res., 2018 DOI:10.4209/aaqr.2018.11.0394.
- M. I. Mead, O. A. M. Popoola, G. B. Stewart, P. Landshoff, M. Calleja and M. Hayes, et al., The use of electrochemical sensors for monitoring urban air quality in low-cost, high-density networks, Atmos. Environ., 2013, 70, 186–203 CrossRef CAS.
- C. Zuidema, N. Afshar-Mohajer, M. Tatum, G. Thomas, T. Peters and K. Koehler, Efficacy of Paired Electrochemical Sensors for Measuring Ozone Concentrations, J. Occup. Environ. Hyg., 2018, 1–12 Search PubMed.
- L. Spinelle, M. Aleixandre and M. Gerboles, Protocol of evaluation and calibration of low-cost gas sensors for the monitoring of air pollution, Report No.: Report EUR26112EN, European Commission, 2013 Search PubMed.
- Y. Zhan, K. Johnson, C. Norris, M. M. Shafer, M. H. Bergin and Y. Zhang, et al., The influence of air cleaners on indoor particulate matter components and oxidative potential in residential households in Beijing, Sci. Total Environ., 2018, 626, 507–518 CrossRef CAS.
- L. Chatzidiakou, A. Krause, O. A. M. Popoola, A. Di Antonio, M. Kellaway and Y. Han, et al., Characterising low-cost sensors in highly portable platforms to quantify personal exposure in diverse environments, Atmos. Meas. Tech., 2019, 2019, 1–19 Search PubMed.
- A. Ripoll, M. Viana, M. Padrosa, X. Querol, A. Minutolo and K. M. Hou, et al., Testing the performance of sensors for ozone pollution monitoring in a citizen science approach, Sci. Total Environ., 2019, 651, 1166–1179 CrossRef CAS.
- E. S. Cross, L. R. Williams, D. K. Lewis, G. R. Magoon, T. B. Onasch and M. L. Kaminsky, et al., Use of electrochemical sensors for measurement of air pollution: correcting interference response and validating measurements, Atmos. Meas. Tech., 2017, 10(9), 3575–3588 CrossRef CAS.
- N. Zimmerman, A. A. Presto, S. P. N. Kumar, J. Gu, A. Hauryliuk and E. S. Robinson, et al., A machine learning calibration model using random forests to improve sensor performance for lower-cost air quality monitoring, Atmos. Meas. Tech., 2018, 11(1), 291–313 CrossRef.
- X. Pang, M. D. Shaw, A. C. Lewis, L. J. Carpenter and T. Batchellier, Electrochemical ozone sensors: A miniaturised alternative for ozone measurements in laboratory experiments and air-quality monitoring, Sens. Actuators, B, 2017, 240, 829–837 CrossRef CAS.
- N. Castell, F. R. Dauge, P. Schneider, M. Vogt, U. Lerner and B. Fishbain, et al., Can commercial low-cost sensor platforms contribute to air quality monitoring and exposure estimates?, Environ. Int., 2017, 99, 293–302 CrossRef CAS PubMed.
- A. C. Rai, P. Kumar, F. Pilla, A. N. Skouloudis, S. Di Sabatino and C. Ratti, et al., End-user perspective of low-cost sensors for outdoor air pollution monitoring, Sci. Total Environ., 2017, 607–608, 691–705 CrossRef CAS PubMed.
- Alphasense Ltd, Alphasense Application Note AAN 106 – Humidity Extremes: Drying Out and Water Absorption, 2013, ch. 12 Search PubMed.
- Alphasense Ltd, OX-A431 Oxidising Gas Sensor: Ozone + Nitrogen Dioxide 4-Electrode, 2016 Search PubMed.
- China Meteorological Administration, The Public Weather Service Center of CMA, 2019, http://www.cma.gov.cn/.
- China Environmental Monitoring Center, 2019, http://113.108.142.147:20035/emcpublish/.
- T.-Y. Lin, L.-H. Young and C.-S. Wang, Spatial variations of ground level ozone concentrations in areas of different scales, Atmos. Environ., 2001, 5799–5807 CrossRef CAS.
- H. Zhao, Y. Zheng, T. Li, L. Wei and Q. Guan, Temporal and Spatial Variation in, and Population Exposure to, Summertime Ground-Level Ozone in Beijing, Int. J. Environ. Res. Public Health, 2018, 15(4), 628 CrossRef.
- M. Wang, T. Zhu, J. Zheng, R. Y. Zhang, S. Q. Zhang and X. X. Xie, et al., Use of a mobile laboratory to evaluate changes in on-road air pollutants during the Beijing 2008 Summer Olympics, Atmos. Chem. Phys., 2009, 9(21), 8247–8263 CrossRef CAS.
- C. J. Weschler, Ozone in Indoor Environments: Concentration and Chemistry, Indoor Air, 2000, 10(4), 269–288 CrossRef CAS.
- S. A. Abdul-Wahab, C. S. Bakheit and S. M. Al-Alawi, Principal component and multiple regression analysis in modelling of ground-level ozone and factors affecting its concentrations, Environ. Model. Softw., 2005, 20(10), 1263–1271 CrossRef.
- L. Spinelle, M. Gerboles and M. Aleixandre, Performance Evaluation of Amperometric Sensors for the Monitoring of O3 and NO2 in Ambient Air at ppb Level, Procedia Eng., 2015, 120, 480–483 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |