Open Access Article
Open Access ArticlePotential emerging chemical risks in the food chain associated with substances registered under REACH†
J.
Oltmanns
 *a,
O.
Licht
*a,
O.
Licht
 b,
M.-L.
Bohlen‡
a,
M.
Schwarz
a,
S. E.
Escher
b,
V.
Silano
c,
M.
MacLeod
b,
M.-L.
Bohlen‡
a,
M.
Schwarz
a,
S. E.
Escher
b,
V.
Silano
c,
M.
MacLeod
 c,
H. P. J. M.
Noteborn
c,
G. E. N.
Kass
c,
H. P. J. M.
Noteborn
c,
G. E. N.
Kass
 d and
C.
Merten
*d
d and
C.
Merten
*d
aForschungs- und Beratungsinstitut Gefahrstoffe GmbH (FoBiG), Klarastraße 63, 79106 Freiburg, Germany. E-mail: jan.oltmanns@fobig.de; markus.schwarz@fobig.de
bFraunhofer Institute for Toxicology and Experimental Medicine ITEM, Nikolai-Fuchs-Strasse 1, 30625 Hannover, Germany. E-mail: oliver.licht@item.fraunhofer.de; sylvia.escher@item.fraunhofer.de
cEuropean Food Safety Authority, Standing Working Group on Emerging Risks, via Carlo Magno 1/a, 43126 Parma, Italy. E-mail: vittorio.silano@alice.it; Matthew.MacLeod@aces.su.se; Hub.Noteborn@xs4all.nl
dEuropean Food Safety Authority, Scientific Committee and Emerging Risks Unit, via Carlo Magno 1/a, 43126 Parma, Italy. E-mail: georges.kass@efsa.europa.eu; caroline.merten@efsa.europa.eu
First published on 2nd December 2019
Abstract
A screening procedure for the identification of potential emerging chemical risks in the food and feed chain developed in a previous EFSA-sponsored pilot study was applied to 15021 substances registered under the REACH Regulation at the time of evaluation. Eligible substances were selected from this dataset by excluding (a) intermediates handled under strictly controlled conditions, (b) substances lacking crucial input data and (c) compounds considered to be outside the applicability domain of the models used. Selection of eligible substances resulted in a considerable reduction to 2336 substances. These substances were assessed and scored for environmental release (tonnage and use information from REACH registration dossiers), biodegradation (predictions from BIOWIN models 3, 5 and 6 evaluated in a battery approach), bioaccumulation in food/feed (ACC-HUMANsteady modelling) and chronic human health hazards (classification according to the CLP Regulation for carcinogenicity, mutagenicity, reproductive toxicity and repeated dose toxicity as well as IARC classification for carcinogenicity). Prioritisation based on the scores assigned and additional data curation steps identified 212 substances that are considered potential emerging risks in the food chain. Overall, 53% of these substances were prioritised due to chronic hazards identified in REACH registrations dossiers only (i.e. hazards not identified in classifications from other sources). Bioaccumulation in food and feed predicted on the basis of ACC-HUMANsteady modelling identified many substances that are not considered bioaccumulative in aquatic or terrestrial organisms based on screening criteria of the relevant ECHA guidance documents. Furthermore, 52% of the priority substances have not yet been assessed for their presence in food/feed by EU regulatory agencies. This finding and illustrative examples suggest that the screening procedure identified substances that have the potential to be emerging chemical risks in the food chain. Future research should investigate whether they actually represent emerging chemical risks as defined in EFSA's mandate.
Environmental significanceA substantial amount of information on chemicals is collected under the European Union REACH Regulation. This study applied a scoring system to all chemicals registered under the REACH Regulation with the goal of identifying emerging chemical risks to food and feed. The scoring system evaluated (i) environmental release based on maximum aggregated tonnages and environmental release categories; (ii) biodegradation in the environment; (iii) bioaccumulation in food and (iv) chronic human health hazards. 212 ‘potential emerging chemical risks’ were identified, most of which have not yet been evaluated by regulatory agencies in the EU for their presence in food. The data generated in this screening study are made available to interested stakeholders to facilitate further evaluations. |
Introduction
The European Food Safety Authority (EFSA) is responsible for the risk assessment of all aspects of food safety as established by Regulation (EC) no. 178/2002. According to Article 34 of this regulation, EFSA has the responsibility to establish procedures to systematically search for and evaluate information with a view to identify emerging risks in the field of food safety. An ‘emerging risk’ is understood to be a risk resulting from (a) a newly identified hazard to which significant exposure may occur or (b) an unexpected new or increased significant exposure or susceptibility to a known hazard.1 Recognising that the available information is often insufficient to conclude whether a risk actually exists, EFSA subsequently introduced the term ‘emerging issue’ to describe cases in which ‘the information collected is preliminary and too limited to be able to assess whether it is (or it could develop) into an emerging risk’.2Within the framework of its responsibility to identify emerging risks, EFSA has pursued options to use data generated under the European chemicals legislation (Regulation (EC) no. 1907/2006 on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)) in order to identify emerging chemical risks in the food chain. An EFSA-sponsored pilot study developed a novel scoring system that was tested on 100 substances registered under REACH3,4 in order to gain experience for a possible application to all chemicals registered under REACH. The methodology developed and applied in the pilot study assessed data in four blocks:
Block A (environmental releases): assessed on the basis of tonnage and use information from REACH registration dossiers.
Block B (biodegradation): assessed on the basis of experimental data from REACH registration dossiers.
Block C (bioaccumulation in food/feed): assessed on the basis of modelling using ACC-HUMANsteady.5,6
Chronic toxicity blocks: assessed on the basis of experimental data on repeated dose toxicity, genotoxicity, and reproductive and developmental toxicity from REACH registration dossiers.
The methodology was applied successfully in the pilot study.3,4 However, experience gained in the pilot study demonstrated that extraction, curation and evaluation of experimental data on biodegradation and chronic toxicity was not suitable for semi-(automated) procedures for all chemicals registered under REACH. Therefore, the pilot study also tested an assessment of biodegradation based on predicted biodegradation data evaluated in a battery approach and found a good agreement of predicted biodegradation with experimental biodegradation data. The use of predicted biodegradation data was therefore recommended for screening large databases of chemicals. For chronic toxicity, the pilot study recommended using the classification of substances according to the CLP Regulation (Regulation (EC) no. 1272/2008) instead of experimental toxicity data.3
This paper presents the results of the application of the refined methodology to a large set of substances registered under REACH in a second EFSA-sponsored study. While the methodology and detailed results have been published by EFSA in an external scientific report,7 this paper presents key findings and conclusions, with a focus on the substance selection, evaluation and prioritisation results in the overall workflow, and reality checks carried out by additional analyses and evaluations.
Materials and methods
The materials and methods for the multi-step procedure are described in detail elsewhere.3,4,7 Briefly, eligible substances were first selected from among all substances registered under the REACH Regulation. These eligible substances were then evaluated with respect to environmental releases (block A), biodegradation (blocks B), bioaccumulation in food/feed (block C) and chronic toxicity, which involved scoring in each of the four blocks. Based on the scoring results, substances were prioritised and the prioritisation results assessed as described below.Data sources and evaluation
The overall procedure was geared towards (semi-)automated data extraction and evaluation procedures that allowed processing of a large number of chemicals. The QSAR Toolbox (version 3.4)8 was used at various stages, e.g. to retrieve SMILES notations, to profile substances according to their structure, to predict the degree of ionisation, to predict biodegradation and as a source of physico-chemical input data for ACC-HUMANsteady modelling. Since CAS numbers had to be used for batch processing in the QSAR Toolbox and different SMILES notations (of variable quality) may be assigned to a given CAS number within the QSAR Toolbox, procedures were implemented to ensure that the SMILES notation is of a high quality and actually represents the substance to be assessed. These procedures are described in detail elsewhere.7The next step involved exclusion of substances lacking a CAS number, since a CAS number is required for several evaluation steps. The CAS numbers of the remaining substances were loaded into the QSAR Toolbox to retrieve SMILES notations. Substances without SMILES notations were also excluded, since it is a pre-requisite for subsequent substance selection and evaluation steps (e.g. the prediction of biodegradation; see below). The SMILES notation was subsequently used to exclude substances that are considered to be outside the applicability domain of the models used to predict biodegradation and bioaccumulation in food/feed (see below). To this end, several profilers in the QSAR Toolbox were used to exclude metals, metalloids, organometallic substances, inorganic and ionisable substances. Ionisable substances were defined as substances predicted in the QSAR Toolbox to be ionised by more than 90% at pH 7.4. Furthermore, this step aimed at excluding UVCB substances (‘substance of unknown or variable composition, complex reaction products or biological materials’ under the REACH Regulation) under the assumption that single SMILES notations for UVCB mixtures would not provide a reliable basis for modelling. All the evaluation and curation steps in the substance selection process are described in detail elsewhere.7
Environmental releases (block A). Environmental releases were assessed on the basis of data submitted under the REACH Regulation: (a) the maximum of the total tonnage band, resulting in a Tonnage Score and (b) the potential releases as indicated by the Environmental Release Category (ERC), resulting in an ERC Score as described previously.3 If tonnage or ERC information was not available, the maximum score of 5 was assigned if the registration applied to a group of companies, which was the vast majority of cases. In the few cases in which the substance was registered by an individual company, one half of the maximum tonnage score (i.e. 2.5) was assigned for missing tonnage information. Score A is defined as the sum of the Tonnage Score (5 possible scores, maximum of 5) and the ERC Score (12 possible scores, maximum of 5) with a total maximum Score A of 10 (see Table 1).
| Block | Possible scores | Prioritisation criteria |
|---|---|---|
| a Since the minimum Tonnage Score is 1 and the minimum ERC score is 0.0025, the minimum Score A is 1.0025. | ||
| Block A: environmental release | 1.0025a – 10 (60 possible scores) | Score A > 5 OR Score B > 5 |
| Block B: biodegradation | 1, 6, 8, 10 | |
| Block C: bioaccumulation in food/feed | 1, 3, 6, 10 | AND |
| Score C > 5 | ||
| Toxicity block | 1, 10 | AND |
| Toxicity Score = 10 | ||
Biodegradation (block B). Biodegradation was evaluated in a battery approach based on biodegradation predicted by BIOWIN models 3, 5 and 6 in the QSAR Toolbox, resulting in a Score B of 1, 6, 8 or 10 (see Table 1). Higher scores indicate a lower degree of biodegradability. Full details of this approach and comparisons with experimental biodegradation data from REACH registration dossiers are described elsewhere.3,7
Bioaccumulation in food feed (block C). Bioaccumulation was assessed on the basis of ACC-HUMANsteady5,6 modelling as described previously.3,7 Required input data, such as physico-chemical properties and biotransformation half-lives, were generated using the QSAR Toolbox. Assuming equal emissions of all substances, Score C is based on the concentration of a substance predicted in each of the 12 evaluated food/feed items relative to the quartiles of the distribution of the concentrations in each food/feed item for all 2336 substances. For example, if for a given substance the modelled concentration in apples was higher than the 75th percentile of all 2336 modelled concentrations in apples, the substance was assigned a score of 10 for the food item apple. At the other extreme, a concentration below the 25th percentile of all concentrations led to a score of 1, with scores of 3 and 6 in between those extremes (Table 1). The maximum score assigned in any food/feed item was taken as the final Score C.
Chronic toxicity (toxicity blocks). The assessment of chronic toxicity was based on classification information as recommended in the pilot study.3 The classification for the following four endpoints representing chronic human health hazards was assessed on the basis of classification information provided under the CLP Regulation (Regulation (EC) no. 1272/2008 on the classification, labelling and packaging of substances and mixtures) in ECHA's Classification & Labelling Inventory database:10 (a) carcinogenicity, (b) mutagenicity, (c) reproductive toxicity and (d) repeated dose toxicity (specific target-organ toxicity after repeated exposure (STOT RE) in REACH terminology).
Throughout this paper, the term ‘classification’ refers to classifications for these four endpoints (called ‘relevant endpoints’ hereafter).
For carcinogenic effects, classifications by the International Agency for Research on Cancer (IARC)11 were considered in addition to those reported in ECHA's Classification & Labelling Inventory database. Classification information was evaluated in a hierarchical order: (1) harmonised classification agreed upon by EU Member States (HARMON hereafter), (2) IARC classifications (IARC), (3) classifications from joint and individual submissions of REACH registration dossiers (REACH) and (4) other classifications in ECHA's Classification & Labelling Inventory database (OTHER). Since it was evident that other classifications include unreliable classifications, an extensive procedure was applied to assess the reliability of such classifications as described in more detail by Oltmanns et al.7 A reliable classification for any of the four endpoints resulted in a Toxicity Score of 10 irrespective of the level of evidence. For example, suspected carcinogens, mutagens or reproductive toxicants (Category 2) were assigned the same Toxicity Score of 10 as those for which the evidence is generally considered conclusive (Category 1A or 1B). Similarly, all substances in IARC groups 1, 2A and 2B for carcinogenicity as well as substances classified for repeated dose toxicity (STOT RE 1 or 2) were assigned a Toxicity Score of 10 (Table 1). For some of the evaluations presented below, substances classified for carcinogenicity, mutagenicity or reproductive toxicity (CMR properties) were differentiated from those classified for repeated dose toxicity only.
Prioritisation of evaluated substances
An evaluation of different approaches to identify priority substances found that applying heuristic rules for scores in each block represents the best option. Weighting scenarios using predefined algorithms that put different weight on the scores in individual blocks were also evaluated. The algorithms provide quantitative rankings of all substances, and are discussed in detail elsewhere.7Table 1 illustrates the possible scores in each block and also describes the heuristic rules applied in the prioritisation. The heuristic rules are designed to prioritise substances that combine relatively high toxicity with high potential for exposure of the food chain. Thus substances with a score of 10 in the toxicity block are prioritised since chronic human health hazards are most relevant as potential emerging risks. Either high environmental releases (Score A > 5) or little potential for biodegradation (Score B > 5) combined with high potential for bioaccumulation in food or feed (Score C > 5) was considered sufficient for prioritisation due to high potential exposure of the food chain.
Evaluation of the prioritisation
The priority substances identified by the screening approach described above are likely to include (a) substances that have already been assessed in detail for their presence in food/feed, (b) substances that have received some attention under EU chemicals legislation and (c) substances that have not been assessed in more detail. Therefore, we analysed whether substances have already been assessed by regulatory agencies for their presence in food/feed.We retrieved all substances included in EFSA OpenFoodTox database12 as well as substances included in seven lists related to EU chemicals legislation (Table 2) and the priority substances identified in this study were checked against the substances included in these eight lists. More information on the background and purpose of the corresponding databases and listings is available from the sources provided.
| Listing | Abbreviation |
|---|---|
| a Sources (all accessed in May 2018): https://www.efsa.europa.eu/en/data/chemical-hazards-data. b Sources (all accessed in May 2018): https://echa.europa.eu/candidate-list-table. c Sources (all accessed in May 2018): https://echa.europa.eu/authorisation-list. d Sources (all accessed in May 2018): https://echa.europa.eu/substances-restricted-under-reach. e Sources (all accessed in May 2018): https://echa.europa.eu/information-on-chemicals/evaluation/community-rolling-action-plan/corap-table. f Sources (all accessed in May 2018): https://echa.europa.eu/de/pact. g Sources (all accessed in May 2018): https://echa.europa.eu/information-on-chemicals/biocidal-active-substances. h Sources (all accessed in May 2018): https://echa.europa.eu/information-on-chemicals/information-from-existing-substances-regulation. | |
| EFSA OpenFoodTox databasea | EFSA |
| Candidate list of substances of very high concern (SVHC) for authorisationb | CL |
| Authorisation list (Annex XIV of the REACH Regulation)c | AL |
| Restriction list (Annex XVII of the REACH Regulation)d | RL |
| CoRAP list (community rolling action plan for substance evaluation)e | CoRAP |
| PACT list (the public activities coordination tool)f | PACT |
| Biocides list (substances assessed under EU legislation as biocidal active substances)g | Biocides |
| EU RAR (substances for which a risk assessment report was prepared under the former EU chemicals legislation)h | RAR |
It was not feasible to check why substances included in these eight lists were not prioritised by our approach. All eight lists combined contain almost 6000 substances and the respective documentation would need to be checked in order to identify reasons for the listing. It is also not meaningful to perform such checks, since in almost all cases the reason for listing is not expected to be related to risks resulting from human exposure via the food chain. For example, inclusion in the Candidate list, Authorisation list and Restriction list is usually only hazard-based. While inclusion in the CoRAP may consider exposure-related concerns, these are based on general information (high tonnage, wide dispersive uses or assumed environmental exposure) rather than a consideration of pathway-specific information.
Illustrative examples
The overall evaluation applied (semi-)automated procedures that allowed screening of a large number of substances. The identified priority substances can be considered potential emerging chemical risks or ‘emerging issues’ in EFSA's terminology.2 Additional data are required to establish whether these substances actually represent ‘emerging chemical risks’.1 Oltmanns et al.7 evaluated such additional data for ten of the priority substances identified. This evaluation forms the basis of the four illustrative examples discussed here.Presentation of results
As discussed in the Introduction, EFSA has developed a terminology that differentiates ‘emerging risks’ and ‘emerging issues’. In the context of this study, the term ‘priority substances’ is used for the stages of evaluation and prioritisation, while the term ‘potential emerging chemical risks’ is used in the context of conclusions reached. This term may be understood as synonymous with ‘emerging issues’,2 but is more specifically related to chemicals and risk. Illustrative examples emphasise the problems associated with concluding whether a potential emerging chemical risk actually represents an emerging chemical risk. Apart from the evaluation and prioritisation results, this study also makes public the data generated in this study (see ESI†) for other applications by all stakeholders.Results
Substance selection for evaluation of the four blocks
The requirement that substances be registered with a full registration excluded almost 9000 substances from the prioritisation (Table 3). About half of these substances are excluded because they are used as intermediates under strictly controlled conditions. Tonnage information is not publicly available for such ‘intermediate registrations’. According to our methodology, in nearly all cases missing tonnage information would have led to a maximum default Tonnage Score (see Materials and methods). Our choice to exclude intermediates therefore invokes the assumption that this use pattern results in low environmental releases. With respect to NONS, the corresponding substances cannot be assumed to be associated with low environmental releases. Since information on the tonnage and on the use pattern are not available for these substances, default worst case scores for block A indicating high environmental releases would have resulted in prioritisation of all 4511 substances with ‘NONS registrations’. Prioritisation of such a high number of substances due to lack of data was considered not meaningful. Additional analyses showed that only 25% of all NONS registrations have a CAS number assigned and a SMILES notation could only be retrieved in the QSAR Toolbox for 43% of the NONS with a CAS number. Furthermore, limited analyses indicated that most of these substances are manufactured at comparatively low tonnages.7| Substances selection step | Number of substances remaining in selection | Number of substances excluded |
|---|---|---|
a The sum of substances excluded (4456 + 4511 = 8967) is higher than the difference between the total number and those registered with a full registration (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021–6843 = 8178) since substances with an intermediate registration or a NONS registration may also have a full registration; see Oltmanns et al.7 for details. 021–6843 = 8178) since substances with an intermediate registration or a NONS registration may also have a full registration; see Oltmanns et al.7 for details.
|
||
| All registered substances | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021 021 |
|
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Selection by registration type | ||
| Intermediate registrations | 4456 | |
| NONS registrations | 4511 | |
| Full registrations | 6843 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Selection by required input data | ||
| CAS number availability | 5380 | 1463 |
| SMILES notation availability | 4330 | 1050 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Selection by applicability domain and curation | ||
| Applicability domain considerations | 2374 | 1956 |
| Final data curation steps | 2336 | 38 |
The data in Table 3 illustrate that 1463 substances with a full registration were excluded due to a lacking CAS number. While most of these substances have an EC number (an identification number assigned under European Union chemicals legislation), batch processing in the QSAR Toolbox requires a CAS number thus necessitating their exclusion. Additional analyses showed that the fraction of substances with comparatively high tonnages (Tonnage Score > 2) is lower among the excluded substances (8.1%) than among the substances with a CAS number (18%). Most of these high volume substances lacking a CAS number represent UVCBs or inorganic substances and the same applies to most of the substances lacking a SMILES notation.7
Finally, applicability domain considerations excluded 1956 substances and most of these substances (66%) were excluded because they were predicted to be ionised by more than 90% at environmentally relevant pH values.7
Overall, 2336 substances (16% of those entering the substance selection) were selected and subsequently evaluated with respect to the four blocks.
Evaluation of the four blocks and prioritisation of evaluated substances
All 2336 substances were assessed in the four blocks and scored according to the approach described in Materials and methods. The resulting scores in each of the four blocks as well as additional data and evaluations are documented in detail elsewhere.7 This paper focusses on the prioritisation approach using the criteria shown in Table 1. Based on these criteria, it is meaningful to present the overall results differentiated by the Toxicity Score and Score C (Bioaccumulation), since scores > 5 in these two blocks are a requirement for prioritisation. Fig. 1 shows the distribution of substances based on these prioritisation criteria. For these plots, scores for the individual blocks have been translated into more meaningful descriptions (see Fig. 1 for details).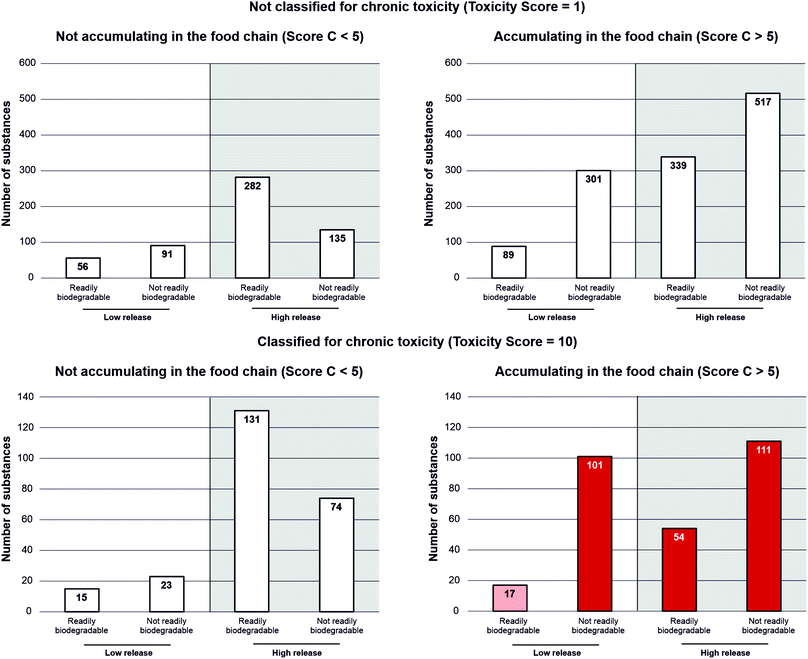 | ||
| Fig. 1 Distribution of scores for substances assigned a Toxicity Score of 1 (top two plots, N = 1810) and a Toxicity Score of 10 (bottom two plots, N = 526). Low release: Score A < 5, high release: Score A > 5; readily biodegradable: Score B < 5, not readily biodegradable: Score B > 5; dark red columns identify substances meeting the prioritisation criteria shown in Table 1, the light red column relates to substances meeting the prioritisation criteria for block C and toxicity only, while white columns identify all other substances. Note: a score of exactly 5 was not assigned in any of the blocks. | ||
Eighty-five percent (N = 1810) of the 2336 substances were assigned a Toxicity Score of 1 and most of these (N = 1610) were not classified for relevant endpoints in any notification in the CLP Inventory. A relevant toxicity classification was available in the remaining 200 cases, but was considered to be of very low reliability and therefore not assigned a Toxicity Score of 10. Out of these 1810 substances, 517 substances have high scores (scores > 5) in all other blocks (Fig. 1, top right). Therefore, these substances are candidates for future screening for relevant toxicity endpoints.
The majority of the 526 substances assigned a Toxicity Score of 10 were classified by harmonised classifications (N = 281; 53%), IARC classifications (N = 24; 4.6%) or classifications from REACH registration dossiers (N = 209; 40%). The remaining 12 substances (2.3%) have other classifications.7
Among the 526 substances assigned a Toxicity Score of 10, 266 substances met the prioritisation criteria shown in Table 1 (dark red columns in Fig. 1, bottom right), while 17 substances only met the criteria for Score C and the Toxicity Score, but had a score < 5 in both block A and block B (light red column in Fig. 1, bottom right). Due to the uncertainties associated with block A, these substances were manually evaluated with respect to possible environmental releases. As discussed in more detail in Oltmanns et al.,7 Score A was underestimated in the semi-automated scoring procedure for one of these 17 substances (hydroquinone, CAS no. 123-31-9) that was therefore added to the list of prioritised substances.
Overall, 267 out of 2336 substances were therefore initially prioritised. Most of these substances (212/267; 79%) were predicted not to be readily biodegradable (see Fig. 1). Further analyses of these 267 substances revealed that – despite the effort made to exclude UVCB substances during substance selection – 50 of these 267 substances are UVCB substances (almost exclusively petroleum products). These analyses also showed that the toxicity classification may have been impacted by impurities in five cases. Exclusion of these 55 substances resulted in 212/2336 substances (9.1%) that are considered priority substances for further evaluation (i.e. ‘potential emerging risks’ as discussed below).
In this final selection, 171/212 substances (81%) had a Score B > 5, a fraction that is almost identical to the one obtained for the 267 substances initially selected (79%; see above). In fact, 155/212 substances (73%) are predicted not to be biodegradable at all (Score B = 10). This finding is important since Score A is associated with an uncertainty due to the possibility that the tonnage (leading to the Tonnage Score) and the use (leading to the ERC Score) may not be related. For example, 99% of the tonnage may be used in applications with much lower (or even negligible) environmental releases than indicated by the ERC Score. The finding that most of the priority substances are predicted to show little or no biodegradation makes them potential candidates for further evaluation even if releases to the environment are comparatively small.
Evaluation of the prioritisation
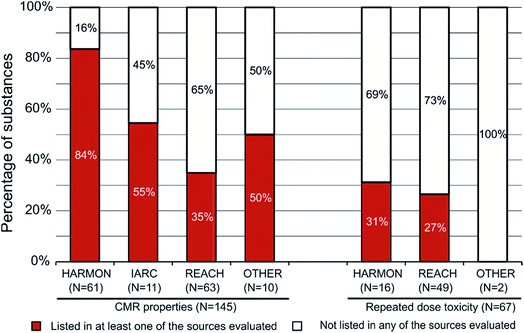
Of the 212 priority substances, 145 substances were assigned a Toxicity Score of 10 due to classifications for CMR properties (31 of these 145 substances were also classified for repeated dose toxicity). The remaining 67 substances were assigned a Toxicity Score of 10 on the basis of classifications for repeated dose toxicity only (i.e. without any concurrent classification for CMR properties). Most of these 212 substances had a harmonised classification, IARC classification for carcinogenicity or a classification from REACH registration dossiers. Only 12 substances were classified in other classifications (Fig. 2).We subsequently analysed whether there is a difference between the priority substances not listed in any source and those listed in at least one of the eight sources (see Table 2 for explanations) depending on (a) the endpoints (CMR properties versus repeated dose toxicity) and (b) the source of the classification. Most of the 212 priority substances (110 out of 212, 52%) were not listed in any of the eight sources evaluated. They are therefore unlikely to have been assessed in the EU. This finding illustrates that the screening approach identified substances that have not received much attention by EU regulatory agencies in the past. However, it should be noted that (a) 40 substances (19%) were included in EFSA's OpenFoodTox database and may therefore have received at least some attention in relation to their presence in food/feed (7 of these are also included in the Candidate List of Substances of Very High Concern (SVHC) for authorisation); (b) 27 substances (13%) were included in the Candidate list (including the 7 substances also included in EFSA's OpenFoodTox database) and may therefore be subject to inclusion in the Authorisation list with the ultimate aim of substitution; however, only one third (9/27) of the substances in the Candidate list was also on the Authorisation list (Annex XIV of the REACH Regulation) at the time of evaluation, and (c) 42 substances (20%) were listed in sources other than EFSA's OpenFoodTox database and the Candidate list.
The prioritisation approach successfully identified substances that (i) have relevant hazardous properties, (ii) are predicted to occur in the environment, (iii) are predicted to be potentially relevant in food/feed and (iv) do not appear to have been assessed for this exposure pathway in the past. This is likely to be the case for the 110 substances not listed in any of the sources evaluated, but may also apply to substances listed in some of these sources (see below for examples).
Fig. 2 shows in more detail the differentiation by endpoint, the source of the classification and whether a substance is listed in any of the eight sources evaluated or not. For example, 61 of the 212 substances have a harmonised classification for CMR properties and 51 of these (84%) are listed in at least one of the sources evaluated. In contrast, only 31% of the 16 substances with a harmonised classification for repeated dose toxicity (but not for CMR properties) are listed in at least one of these sources. This suggests that CMR properties more likely result in a listing than a classification for repeated dose toxicity. However, the source of the classification is also important. The data in Fig. 2 suggest that a harmonised classification for CMR properties more likely results in a listing (84% of the 61 substances) than a classification for CMR properties in REACH registration dossiers (35% of the 63 substances). The same pattern is not evident for substances classified for repeated dose toxicity (but not CMR properties), for which the fraction of substances listed is similar (31% for harmonised classifications and 27% for classifications from REACH registration dossiers).
Collectively, these data suggest that prioritisation on many lists is based on harmonised classifications for CMR properties, while classifications for CMR properties in other sources or classifications for repeated dose toxicity in any source less often lead to prioritisation in other schemes. This observation is in line with the prioritisation approaches for many of the REACH-related lists. Thus, no substances are currently included in the Candidate list under the REACH Regulation based on a classification for repeated dose toxicity alone. In fact, there were only nine entries in the Candidate list for which repeated dose toxicity was a reason for inclusion: all of these refer to cadmium compounds for which carcinogenic effects were also given as a reason for inclusion.13 The findings for IARC and other classifications shown in Fig. 2 should not be given too much weight due to the small number of substances in each group.
The data in Fig. 2 also demonstrate that 112 of the 212 priority substances (53%) were identified as hazardous due to a classification in REACH registration dossiers: 63 substances classified for CMR properties and 49 substances classified for repeated dose toxicity. The hierarchical evaluation of classification information (see Materials and methods) implies that these 112 substances did not have a harmonised or IARC classification for the relevant endpoints. This study therefore identified more than half of the priority substances by making use of classification information from REACH registration dossiers. Fig. 2 illustrates that the majority of these 112 substances has previously not been selected for further evaluation: 65% of the 63 substances classified for CMR properties and 73% of the 49 substances classified for repeated dose toxicity in REACH registration dossiers (in total: N = 77/112, 69%).
| No. | Name | CAS no. | Listed in | Tonnagec |
|---|---|---|---|---|
| a See Table 2 for the meaning of the abbreviations used. b Only substances with a reliable Toxicity Score were included in this analysis (one high ranking substance excluded). c Upper end of the REACH registration total tonnage band (in t/a) in original evaluation in February 2017/when checked again in November 2018. d One or several additional intermediate registrations. e One or several additional full registrations. f Not contained in EFSA's OpenFoodTox database (as identified by name and CAS number), but in fact evaluated by EFSA.14 | ||||
| Assessed by EFSA (N = 7) | ||||
| 1 | Bisphenol A (BPA) | 80-05-7 | EFSA, CL, RL, PL, CoRAP, RAR | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 2 | Hexabromocyclododecane (HBCDD) | 25637-99-4 | EFSAf, CL, AL, RAR | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/10 000/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 3 | Tetrabromobisphenol A (TBBPA) | 79-94-7 | EFSA, PL, CoRAP, RAR | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 4 | Melamine | 108-78-1 | EFSA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000d 000d |
| 5 | 4-Nitroaniline | 100-01-6 | EFSA | 10d |
| 6 | Retinol acetate | 127-47-9 | EFSA | 10 |
| 7 | 2,2-(1,4-Phenylene)bis-((4H-3,1-benzoxazin-4-one)) | 18600-59-4 | EFSA | 100 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Not yet assessed by EFSA but on other lists (N = 7) | ||||
| 8 | 4,4′-Methylenediphenyl diisocyanate | 101-68-8 | CoRAP, PL, RL | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000d 000d |
| 9 | Triphenyl phosphite | 101-02-0 | CoRAP, PL | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000d 000d |
| 10 | 6,6′-Di-tert-butyl-2,2′-methylenedi-p-cresol | 119-47-1 | CoRAP | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 11 | A mixture of triphenylthiophosphate and tertiary butylated phenyl derivatives | 192268-65-8 | CoRAP | 1000 |
| 12 | 2,4-Dihydroxybenzophenone | 131-56-6 | PL | 10/1000 |
| 13 | Piperonyl butoxide | 51-03-6 | CoRAP, biocides | TDC/10e |
| 14 | 1,2-Bis(2-methoxyethoxy)ethane | 112-49-2 | CL | 100 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Not listed in any of the sources evaluated (N = 6) | ||||
| 15 | Phenol, isopropylated, phosphate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
68937-41-7 | Not listed | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 16 | N,N′-Di-sec-butyl-p-phenylenediamine | 101-96-2 | Not listed | 1000 |
| 17 | 2-Benzyl-2-dimethylamino-4′-morpholinobutyrophenone | 119313-12-1 | Not listed | 1000e |
| 18 | 2,5,8,11,14-Pentaoxapentadecane | 143-24-8 | Not listed | 1000 |
| 19 | 4-Aminophenol | 123-30-8 | Not listed | 100d |
| 20 | 2,3-Bis((2-mercaptoethyl)thio)-1-propanethiol | 131538-00-6 | Not listed | 10 |
Among these 20 high-ranking substances there are seven substances that have already been assessed by EFSA: bisphenol A (BPA),15 hexabromocyclododecane (HBCDD),14 tetrabromobisphenol A (TBBPA)16 and melamine17 were assessed in detail by EFSA and all except melamine were also found in several other lists. At least for HBCDD and TBBPA, the occurrence in food/feed is largely considered to be the result of releases of these substances to the environment and subsequent accumulation in the food chain.14,16 This finding confirms the general validity of the screening procedure applied in this study. Two of these substances are included in the Candidate list and one of them (HBCDD) is also included in the Authorisation list. In fact, the only authorisations granted for this substance have expired in August 2017 so that all uses (except manufacture and use as an intermediate, which are exempted from authorisation under the REACH Regulation) are prohibited in the EU. The decrease in the tonnage between February 2017 and November 2018 (see Table 4) may reflect this fact. The assessment of the other three substances by EFSA was more limited in scope.18–20 For example, 4-nitroaniline was only evaluated as an impurity in a specific feed supplement for chicken.18 Retinol acetate (vitamin A), which was classified in the REACH registration dossier as a reproductive toxicant (Repr. Cat. 1B), was only assessed for its use as a feed supplement.19 These examples illustrate that an assessment by EFSA does not necessarily include all pathways of exposure, whereas this study identified substances that may enter the food chain due to releases to the environment, i.e. by a pathway not yet addressed by EFSA.
Of the remaining 13 substances not assessed by EFSA, seven are included in at least one list. Further analyses of the documents related to these listings showed that the assessments were primarily hazard-based and none of the evaluations involved an appraisal of human exposure via the food chain. For example, the substance evaluation under the CoRAP listing for 6,6′-di-tert-butyl-2,2′-methylenedi-p-cresol (DBMC) did not include any exposure considerations. This substance is also discussed in the illustrative examples below. Finally, six of the 20 high-ranking substances are not included in any of the lists evaluated.
Overall, these data suggest that the prioritisation approach identified a few substances that were already assessed in detail for their presence in food, thus demonstrating the validity of the procedure. However, most priority substances were (a) not yet assessed for their presence in food by EU regulatory agencies or (b) were assessed in food/feed only due to specific uses but not in relation to possible entry into the food chain from environmental releases. This observation indicates that the approach also successfully identified potential emerging chemical risks in relation to exposure via the food chain.
Illustrative examples
Ten of these 212 priority substances (or potential emerging chemical risks) identified in this study were evaluated in more depth by Oltmanns et al.7 with the aim to establish whether they actually represent emerging chemical risks. These evaluations confirmed the screening assessment for all ten substances in relation to block A, block B and the toxicity block. Confirmation of the results for block C (bioaccumulation in food/feed), however, is more difficult and in many cases impossible, since few substances are monitored in food/feed. At the same time, information on the occurrence in food/feed is crucial in establishing whether a potential emerging chemical risk may actually represent an emerging chemical risk. Therefore, the occurrence of these substances in other media (e.g. surface water) was also evaluated based on the understanding that the presence of a substance in environmental media is a pre-requisite for uptake into food/feed (e.g. via irrigation water). While occurrence in environmental media in itself does not indicate a high bioaccumulation potential, this surrogate approach was considered helpful in the evaluation. Note that any occurrence in environmental media also confirms the assessment of blocks A and B. The following four examples illustrate general issues observed in the evaluation of occurrence data that have an impact on the decision whether a substance may be considered an emerging chemical risk.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (melamine cyanurate) and 1
000 (melamine cyanurate) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per annum (melamine)). Considering these volumes, there is a surprising paucity of information on the presence of melamine in the environment. In the German Federal State of North Rhine-Westphalia, melamine was detected in all four rivers monitored (including the Rhine river) and in the Dutch part of the Rhine catchment area, melamine concentrations regularly exceeded 1 μg L−1.21,22
000 tonnes per annum (melamine)). Considering these volumes, there is a surprising paucity of information on the presence of melamine in the environment. In the German Federal State of North Rhine-Westphalia, melamine was detected in all four rivers monitored (including the Rhine river) and in the Dutch part of the Rhine catchment area, melamine concentrations regularly exceeded 1 μg L−1.21,22
Overall, this example highlights the importance of considering different pathways by which a substance may enter food and feed and illustrates the lack of robust information on the occurrence in environmental compartments even for high volume substances.
TDCIPP was analysed in a Swedish market basket survey involving 53 composite food samples from 12 food categories. The substance was detected in several food items and the highest mean concentrations were found in fats/oils, beverages, sugar/sweets, cereals and vegetables.35 The number of samples per food category was very small (N = 2–5). TDCIPP was also detected in 165 food samples from 14 food categories sampled in Belgium. The highest mean concentrations were found in cheese, baby food, potatoes and fats/oils, the latter category showing the highest concentrations due to inclusion of fish oil supplements.36 Again, the number of samples was small (N = 4–17 per category). TDCIPP was also analysed in duplicate diets of a Norwegian cohort (N = 61) collected over a 24 h period. In contrast to the findings in Belgium and Sweden, all samples were below the LoD.37 In Sweden, TDCIPP concentrations in marine and freshwater fish were consistently below the LoD, except for freshwater fish sampled close to sources (e.g. STPs) that showed TDCIPP concentrations of 36–140 ng g−1 lipid.38 No bioaccumulation was observed in benthic and pelagic food webs of the Western Scheldt estuary in the Netherlands.39 Finally, the uptake of TDCIPP in plants (strawberry, lettuce) was shown experimentally.40,41 Overall, these data generally support the assessment of block C in this study. The fact that TDCIPP concentrations were below the limit of detection in all samples of the Norwegian study is somewhat surprising, but may be related to the methodology. In this study, one sample consisted of all food and drink consumed over the past 24 h, thus potentially diluting high concentrations in specific foods.
In human biomonitoring studies, the relevant TDCIPP metabolite was frequently found in urine samples in concentrations above the LoD in studies in Sweden,42 Norway43 and Belgium.44 The fraction of samples above the LoD appeared to be higher in Sweden and Norway (52–91%) than in Belgium (25%). These data demonstrate existing human exposure, but are unable to identify the sources of exposure. For organophosphorus flame retardants in general, exposure by other pathways (e.g. inhalation and ingestion of house dust) is generally believed to be higher than intake from food.38,43 However, the sample sizes of the available studies on the occurrence in food are too small to allow a final conclusion with respect to the relevance of this pathway for TDCIPP.
Overall, this example illustrates that our screening assessment correctly predicted the occurrence in environmental media including food. The relevance of dietary exposure in comparison with other pathways of exposure needs to be assessed in more detail and a more robust data basis is also required to establish whether TDCIPP represents an emerging chemical risk.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1). The maximum concentrations in raw water abstracted from the Ruhr river for drinking water preparation was 13.4 μg L−1.47 The data reflect a specific incident (total sulfolane amount emitted estimated to be 3–4 tonnes) and may not represent typical situations. However, it must be noted that the source was only identified by non-target screening analyses and most likely would have gone unnoticed under normal monitoring arrangements.
000 μg L−1). The maximum concentrations in raw water abstracted from the Ruhr river for drinking water preparation was 13.4 μg L−1.47 The data reflect a specific incident (total sulfolane amount emitted estimated to be 3–4 tonnes) and may not represent typical situations. However, it must be noted that the source was only identified by non-target screening analyses and most likely would have gone unnoticed under normal monitoring arrangements.
Sulfolane was shown to occur in a variety of crops cultivated in an area affected by contaminated groundwater in North Pole (Alaska). The highest sulfolane concentrations were observed in green beet leaf, leaf lettuces, currant, tomato and zucchini fruit (up to 198 μg kg−1).48 Experimental studies have demonstrated rapid uptake and translocation into the shoots of soybean and tomato plants,49 uptake from irrigation water and translocation into leaves and fruit of apple trees50 as well as uptake by wetland vegetation.51,52 Taken together, sulfolane appears to be taken up into crops, but it remains unclear whether environmental contamination is limited to cases of contamination close to sites of use or is more widespread. Furthermore, the data is too limited to conclude whether the substance represents an emerging chemical risk in the food chain.
Discussion and conclusions
Substance selection for evaluation of the four blocks
Only 2336 substances out of 15021 substances registered under the REACH Regulation as of January 2017 (16%) were able to be evaluated in this study. Three main reasons for the high rate of exclusion of substances were: (a) assumed very low environmental releases: this applies to intermediates handled under strictly controlled conditions where tonnage data is also not publicly available; (b) missing data: first, NONS are regarded as registered under the REACH Regulation, but no tonnage or use information is publicly available; second, substances without CAS number and/or SMILES notation: their removal was required since both a CAS number and a SMILES notation is required in the approach used in this study; and (c) substances potentially outside the applicability domain of the models used. This includes e.g. ionisable substances and metals.Exclusion of 84% of all substances entering the substance selection appears non-desirable at first sight. However, the approach applied in this study – as in any screening procedure – has to strike a balance between targeting as many substances as possible, while at the same time preventing the generation of an excessive number of high scoring substances due to the application of conservative defaults for missing data (i.e. tonnage and/or use information).
For the 2513 substances lacking a CAS number and/or a SMILES notation (see Table 3 and Fig. 3), exclusion is necessary since both parameters are required in the approach used in this study. Substances lacking a CAS number could not be loaded for batch processing into the QSAR Toolbox. In this context, it would be helpful if the QSAR Toolbox would enable loading lists of EC numbers, which are available for 90% of the substances lacking a CAS number. However, many of the substances lacking a CAS number or a SMILES notation are produced at low tonnages and those with higher tonnages are often UVCB substances that present other problems for risk assessment.
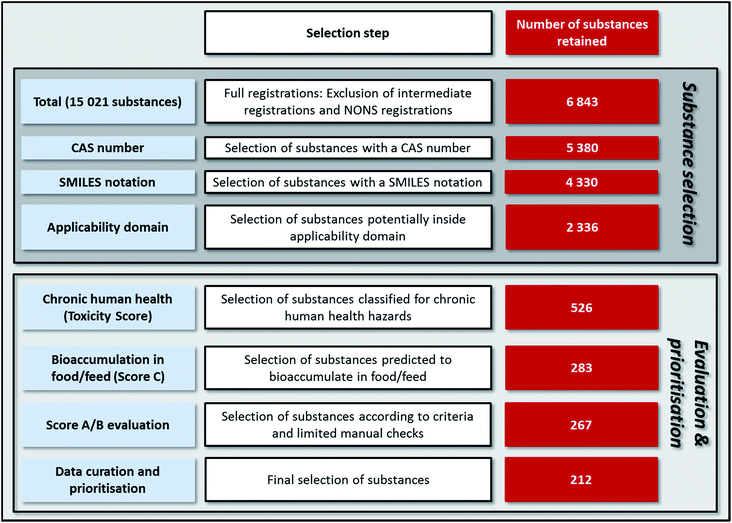 | ||
| Fig. 3 Summary of the workflow of substance selection as well as evaluation and prioritisation applied in this study with the number of substances retained after each step. | ||
Among the 4330 substances with a full registration, a CAS number and a SMILES notation, almost one half (see Table 3 and Fig. 3) were potentially outside the applicability domain of the models used. In contrast to the reasons for exclusion discussed above, this step involves a scientific rationale rather than technical or data availability issues.
Overall, while the large fraction of substances excluded may appear less than satisfactory, the approach ensures that the selected substances (a) can be assessed on the basis of actual data rather than default worst case assumptions for missing data, (b) represent discrete chemicals that are (c) potentially inside the applicability domain of the models used for the prediction of biodegradation and bioaccumulation in food/feed. The approach therefore does not aim to screen all substances but rather to have greater confidence in the assessment performed on the selected substances.
Evaluation of the four blocks and prioritisation of evaluated substances
Out of a total of 2336 substances registered under REACH and assessed in this study, 212 priority substances (9.1%) were identified that (a) have high potential to occur in food/feed due to releases to the environment and (b) possess a chronic human health hazard. Therefore, they are potential emerging risks or emerging issues according to EFSA's mandate. Most of these 212 substances have not yet been assessed by regulatory agencies in the EU in relation to their presence in food resulting from environmental releases.The evaluation applied in this study followed approaches applied by others with respect to block A (environmental releases). Thus, a combination of the tonnage and the use pattern is also applied in prioritisation for various regulatory instruments under the REACH Regulation, such as substance evaluation (CoRAP listing),60 as well as scientific evaluations, as performed most recently e.g. by Schulze et al.61 However, all these approaches suffer from the fact that use-specific tonnages are not publicly available and that a large fraction of the tonnage may in fact be used in applications without any significant releases to the environment. The missing link between tonnage and use is also a major uncertainty of the present study. This limitation notwithstanding, the illustrative examples discussed above indicate that the prioritised substances have been detected in environmental media at least in some locations, a finding that also applies to most of the remaining substances evaluated in more depth.7 This observation supports the general approach for blocks A and B in this study. However, the apparent lack of robust data on the concentrations in environmental media even for most of these high volume substances (see e.g. melamine and DBMC above) is another main finding of this study.
Battery approaches for the evaluation of predicted biodegradation data (block B) are also commonly employed in an attempt to increase the performance of biodegradability predictions.62–64 We applied a new battery combining BIOWIN models 3, 5 and 6, an approach that correctly predicted or overpredicted the persistence in 93% of the cases when compared against experimental biodegradation data from REACH registration dossiers.7 This study did not consider abiotic degradation processes, such as hydrolysis and phototransformation, due to the fact that abiotic degradation reflects primary rather than ultimate degradation and the degradation products would require additional evaluations. As a consequence, abiotic processes alone should not be used to assess the persistence of a substance.65 In practical terms, consideration of all possible degradation products would face the problem of identifying such degradation products. While tools are available to simulate hydrolysis, several degradation products are predicted for each substance. In addition, the number and/or identity of hydrolysis products may change depending on the pH value and the relative importance of the predicted degradation products is typically unknown. The assessment for block B, block C and chronic toxicity would therefore need to be performed for all these compounds without knowing whether a predicted degradation product actually occurs in significant fractions under environmental conditions. Overall, consideration of all degradation products would thus increase the uncertainty of the results obtained and is not considered meaningful in the context of a screening approach. While abiotic degradation could thus not be assessed in this study, it is clear that such processes should be considered prior to any time-consuming in-depth evaluation to avoid focussing on potentially irrelevant substances.
The assessment of bioaccumulation in food/feed (block C) is an uncertain element of this study, since the results cannot be checked against other data in a consistent way. In this context, the high ranks found in this study for some substances known to be present in food/feed (see Table 4) suggest that the modelling approach was successful. Furthermore, the experimental and survey data for some of the illustrative examples also support the assessment for block C. However, robust information on the presence in food/feed is unlikely to be available for the majority of the 212 priority substances, preventing an independent evaluation of our assessment for block C. This study used ACC-HUMANsteady modelling to assess the potential for bioaccumulation in the food chain. This model takes into consideration a variety of possible pathways (e.g. differentiation of uptake in above ground and below ground crops) and allows consideration of additional parameters such as biotransformation within organisms. It may therefore be superior to simple screening approaches. For example, 168 of the 212 priority substances have a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow ≤ 4.5 and thus do not meet the screening criterion for bioaccumulation in aquatic organisms according to the relevant ECHA Guidance.65 When compared with the screening criteria for bioaccumulation in terrestrial organisms in the same ECHA Guidance (log
Kow ≤ 4.5 and thus do not meet the screening criterion for bioaccumulation in aquatic organisms according to the relevant ECHA Guidance.65 When compared with the screening criteria for bioaccumulation in terrestrial organisms in the same ECHA Guidance (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow > 2 AND log
Kow > 2 AND log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koa > 5), 97 of the 212 priority substances do not meet these criteria. These comparisons illustrate that the approach applied in this study identified substances that would escape any prioritisation based on simple screening criteria.
Koa > 5), 97 of the 212 priority substances do not meet these criteria. These comparisons illustrate that the approach applied in this study identified substances that would escape any prioritisation based on simple screening criteria.
It must be noted that exposure of humans via the environment (including exposure through the food chain) was likely assessed in Chemical Safety Reports (CSRs) under the REACH Regulation for some of the 212 priority substances. However, CSRs are not publicly available and the corresponding results could therefore not be used for a comparison with the results of this study. Furthermore, EUSES software is generally used to model human exposure via the food chain presented in CSRs. While the need for several updates in EUSES affecting the assessment of human exposure via the food chain has been identified,66 they have not yet been implemented. A comparison of ACC-HUMANsteady and EUSES has shown the latter to be less up-to-date than ACC-HUMANsteady software.67
In relation to the toxicity assessment, our approach not only used harmonised classifications for relevant endpoints, but also classifications by IARC, from REACH registration dossiers and other classifications from the Classification and Labelling Inventory database.10 This led to the identification of considerably more priority substances (N = 212) than would have been identified based on harmonised classifications alone (N = 77; see Fig. 2). Most of the additional 135 substances were identified based on classifications in REACH registration dossiers (N = 112; see Fig. 2). Since listings for various regulatory instruments are often based on harmonised classifications, our approach therefore identified many substances not yet addressed by these instruments. It is also interesting to note that some of the substances identified on the basis of classifications from ‘non-harmonised’ sources do in fact have a harmonised classification, but not for any of the four endpoints relevant in the context of this study. Among the ‘top 20’ substances, seven substances were assigned a Toxicity Score of 10 on the basis of a harmonised classification for relevant endpoints. The remaining 13 substances were assigned this score due to a ‘non-harmonised’ classification for relevant endpoints, with the majority (N = 8) coming from REACH registration dossiers. Of these 13 substances, nine substances did not have a harmonised classification for any endpoint, while four substances had a harmonised classification, but this did not cover any of the four relevant endpoints. For example, triphenyl phosphite has a harmonised classification for skin and eye irritation only, while the substance is also classified for repeated dose toxicity in the REACH registration dossier.9 Similarly, TBBPA has a harmonised classification for aquatic toxicity, but not for CMR properties. However, the substance was recently classified by IARC as being probably carcinogenic to humans68 and the REACH registration dossier includes a classification as a suspected carcinogen.9
The findings of this study therefore show that REACH registration dossiers (a) contain classifications for substances that have no harmonised classification or (b) contain classifications for additional endpoints for substances that do have a harmonised classification. This finding is in agreement with observations in a previous study on a different dataset.69 This study did not differentiate the level of evidence for toxicity (see Materials and methods). For example, suspected and confirmed CMR substances were all assigned a Toxicity Score of 10. It may thus be assumed that we primarily identified suspected CMR substances based on ‘non-harmonised’ classifications. However, additional analyses showed that this is not the case.7
Overall, Fig. 3 illustrates that application of the toxicity criterion substantially reduces the number of substances from 2336 to 526 (23%). Consideration of the bioaccumulation criterion almost halves the number of substances to 283. In contrast, additional consideration of the criteria for environmental release and/or biodegradation has almost no effect. In fact, the final data curation step (i.e. predominantly the exclusion of UVCB substances) has a higher impact on the substances prioritised than the results for blocks A and B.
Evaluation of the prioritisation, illustrative examples and recommendations
This procedure created a list of 212 priority substances. In a few cases, the prioritised substances are known to occur in food/feed. While the example of melamine shows that the presence of a substance in food/feed may result from several sources, the occurrence due to releases to the environment is well-established in some of the cases (e.g. HBCDD and TBBPA). This demonstrates the general validity of the screening procedure applied in this study. Most of these 212 priority substances, however, have not been previously assessed for their presence in food. In fact, the majority of the 212 priority substances has not yet been assessed by EFSA or included in any list related to EU chemicals legislation. The screening procedure was therefore also successful in identifying ‘emerging chemicals’. This was primarily achieved by (a) the application of a more sophisticated approach to assess bioaccumulation in the food chain than using simple screening criteria, (b) making full use of classification information on the toxic hazards of substances by considering classifications in REACH registration dossiers and (c) combining these two approaches. For example, 112 substances were prioritised due to a classification for relevant endpoints in REACH registration dossiers and 84 of these do not fulfil the screening criterion for bioaccumulation in aquatic organisms according to the ECHA Guidance.65All of the 212 priority substances can be considered ‘potential emerging risks’ (or ‘emerging issues’ in EFSA's terminology1,2). In principle, those not yet assessed in detail by EFSA should be evaluated in-depth to conclude whether they qualify as ‘emerging chemical risks’ or not. While such in-depth evaluations increase our knowledge on the occurrence of these substances in food/feed, the four illustrative examples presented in this paper and the more detailed evaluation in Oltmanns et al.7 suggest that existing information on the occurrence in food/feed as well as in environmental compartments is generally too limited to allow such a conclusion. In some respects, this observation is related to the inherent limitation of the term ‘emerging risk’: robust and representative data are required to conclude on the existence of a risk, but substances meeting this requirement at some stage cease to be ‘emerging chemicals’.
Given these limitations of literature-based in-depth evaluations, it may also be useful to develop and apply analytical methods to monitor these substances in food/feed. This may be limited to subsets of the 212 priority substances (e.g. those not listed in any of the eight sources, but assigned the maximum Score C of 10 and produced at high tonnages) or take the form of suspect screening analyses on all 212 priority substances. The examples discussed in this paper show that some of the priority substances identified may enter the food chain from food contact materials. In order to identify an occurrence in food as a result of environmental releases, monitoring of unprocessed and unpackaged food may be most meaningful. Even if such analyses do not produce robust and reliable data that would allow concluding on the presence of ‘emerging chemical risk’, the results from such analyses could confirm or refute the results of the screening assessment and thus help prioritising substances for more representative monitoring programmes. In order to facilitate such activities, the data generated in this study, including the scores in all food items, are made available in the ESI.† These data may also be useful for interested stakeholders in several other applications. Oltmanns et al.7 discuss a variety of examples for such applications and the use of the data generated is greatly encouraged.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The work described in this paper was sponsored by the European Food Safety Authority under contract OC/EFSA/SCER/2016/01-CT1. The authors would like to thank Emma Undeman (Department of Environmental Science and Analytical Chemistry, Stockholm University, Stockholm, Sweden) for her help with the ACC-HUMANsteady software.References
- EFSA, European Food Safety Authority, Definition and Description of “Emerging Risks” within the EFSA's Mandate, (adopted by the scientific committee on 10 July 2007), EFSA/SC/415 final, http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/escoemriskdefinition.pdf, 2007 Search PubMed.
- EFSA, European Food Safety Authority, Towards a methodological framework for emerging risk identification, Technical report supporting publications 2012:EN-243, https://www.efsa.europa.eu/en/supporting/pub/en-243, 2012 Search PubMed.
- A. Bitsch, M. L. Bohlen, S. Escher, O. Licht, J. Oltmanns, K. Schneider and A. Wibbertmann, Final report: testing a procedure for the identification of emerging chemical risks in the food chain, External scientific report, OC/EFSA/SCER/2014/03, EFSA supporting publication 2016:EN-1050, https://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/1050e.pdf, 2016 Search PubMed.
- J. Oltmanns, O. Licht, A. Bitsch, M. L. Bohlen, S. E. Escher, V. Silano, M. MacLeod, R. Serafimova, G. E. N. Kass and C. Merten, Development of a novel scoring system for identifying emerging chemical risks in the food chain, Environ. Sci.: Processes Impacts, 2018, 20, 340–353 RSC.
- G. Czub, E. Arbaban, E. Undeman and M. McLachlan, Model description for ACC-HUMANsteady 1.0, Department of Applied Environmental Science, Stockholm University, 2011 Search PubMed.
- G. Czub and M. S. McLachlan, A food chain model to predict the levels of lipophilic organic contaminants in humans, Environ. Toxicol. Chem., 2004, 23, 2356–2366 CrossRef CAS PubMed.
- J. Oltmanns, M. L. Bohlen, S. Escher, M. Schwarz and O. Licht, Final report: Applying a tested procedure for the identification of potential emerging chemical risks in the food chain to the substances registered under REACH – REACH 2, EFSA supporting publication 2019:EN-1597. http://www.efsa.europa.eu/en/publications, 2019 Search PubMed.
- OECD, Organisation for Economic Co-Operation and Development, The OECD QSAR Toolbox for Grouping Chemicals into Categories. Version 3.4, https://www.qsartoolbox.org/home, 2016 Search PubMed.
- ECHA Dissemination, Information on Chemicals - Registered Substances, European Chemicals Agency, online: http://echa.europa.eu/web/guest/information-on-chemicals/registered-substances, disclaimer: http://echa.europa.eu/web/guest/legal-notice, 2019 Search PubMed.
- ECHA C&L Inventory, Information on Chemicals – Classification & Labelling Inventory, European Chemicals Agency, online: http://echa.europa.eu/information-on-chemicals/cl-inventory, disclaimer: http://echa.europa.eu/web/guest/legal-notice, 2018 Search PubMed.
- IARC, International Agency for Research on Cancer, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, WHO, World Health Organization, Lyon, France, http://monographs.iarc.fr, 2018 Search PubMed.
- J. L. Dorne, J. Richardson, G. Kass, N. Georgiadis, M. Monguidi, L. Pasinato, S. Cappe, H. Verhagen and T. Robinson, Editorial: OpenFoodTox: EFSA's open source toxicological database on chemical hazards in food and feed, EFSA J., 2017, 15(1), e15011 Search PubMed.
- ECHA, European Chemicals Agency, Candidate List of substances of very high concern for Authorisation (published in accordance with Article 59(10) of the REACH Regulation), https://echa.europa.eu/candidate-list-table, assessed Dececmber 2018, 2018 Search PubMed.
- EFSA, European Food Safety Authority, Scientific Opinion on Hexabromocyclododecanes (HBCDDs) in Food. EFSA Panel on Contaminants in the Food Chain (CONTAM), EFSA J., 2011, 9, 2296 Search PubMed.
- EFSA, European Food Safety Authority, Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Panel on Contaminants in the Food Chain (CONTAM), EFSA J., 2015, 13, 3978 CrossRef.
- EFSA, European Food Safety Authority, Scientific Opinion on Tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA Panel on Contaminants in the Food Chain (CONTAM), EFSA J., 2011, 9, 2477 CrossRef.
- EFSA, European Food Safety Authority, Scientific Opinion on Melamine in Food and Feed. EFSA Panel on Contaminants in the Food Chain (CONTAM) and EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF), EFSA J., 2010, 8, 1573 CrossRef.
- EFSA, European Food Safety Authority, Scientific Opinion on the safety and efficacy of Koffogran (nicarbazin) as a feed additive for chickens for fattening. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), EFSA J., 2010, 8, 1551 Search PubMed.
- EFSA, European Food Safety Authority, Scientific Opinion on the safety and efficacy of vitamin A (retinyl acetate, retinyl palmitate and retinyl propionate) as a feed additive for all animal species and categories. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), EFSA J., 2013, 11, 3037 CrossRef.
- EFSA, European Food Safety Authority, Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 18th list of substances for food contact materials. EFSA Panel on food additives, flavourings, processing aids and materials in contact with food (AFC), EFSA J., 2008, 6, 628 CrossRef.
- RIWA-Rijn, Annual reports 2015–2017 in German, https://www.riwa-rijn.org/de/riwa-rijnpublikationen/, accessed August 2018, 2017 Search PubMed.
- LANUV, Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Non Target New #3, online: https://www.lanuv.nrw.de/fileadmin/lanuv/analytik/non_target/Melamin.pdf, accessed 2 November 2018, 2016 Search PubMed.
- A. Marklund, B. Andersson and P. Haglund, Organophosphorus flame retardants and plasticizers in Swedish sewage treatment plants, Environ. Sci. Technol., 2005, 39, 7423–7429 CrossRef CAS PubMed.
- T. Reemtsma, J. B. Quintana, R. Rodil, M. García-López and I. Rodríguez, Organophosphorus flame retardants and plasticizers in water and air I. Occurrence and fate, TrAC, Trends Anal. Chem., 2008, 27, 727–737 CrossRef CAS.
- R. Rodil, J. B. Quintana, E. Concha-Graña, P. López-Mahía, S. Muniategui-Lorenzo and D. Prada-Rodríguez, Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain), Chemosphere, 2012, 86, 1040–1049 CrossRef CAS PubMed.
- I. Van der Veen and J. de Boer, Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis, Chemosphere, 2012, 88, 1119–1153 CrossRef CAS.
- K. M. Blum, P. L. Andersson, L. Ahrens, K. Wiberg and P. Haglund, Persistence, mobility and bioavailability of emerging organic contaminants discharged from sewage treatment plants, Sci. Total Environ., 2018, 612, 1532–1542 CrossRef CAS.
- K. M. Blum, P. Haglund, Q. Gao, L. Ahrens, M. Gros, K. Wiberg and P. L. Andersson, Mass fluxes per capita of organic contaminants from on-site sewage treatment facilities, Chemosphere, 2018, 201, 864–873 CrossRef CAS.
- U. E. Bollmann, A. Möller, Z. Xie, R. Ebinghaus and J. W. Einax, Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters, Water Res., 2012, 46, 531–538 CrossRef CAS PubMed.
- K. Quednow and W. Püttmann, Organophosphates and synthetic musk fragrances in freshwater streams in Hessen/Germany, Clean: Soil, Air, Water, 2008, 36, 70–77 CAS.
- H. Wolschke, R. Sühring, Z. Xie and R. Ebinghaus, Organophosphorus flame retardants and plasticizers in the aquatic environment: A case study of the Elbe River, Germany, Environ. Pollut., 2015, 206, 488–493 CrossRef CAS PubMed.
- JRC, Joint Research Centre, EMBLAS II – Joint Black Sea Survey 2017. JRC Chemical Contaminant Measurements. Sampling, analytical methodologies and results of ultra-trace organic contaminants monitoring, JRC Technical report, European Commission Joint Research Centre, Directorate D – Sustainable Resources, Unit D.02 Water and Marine Resources, Ispra (VA), Italy, 2018 Search PubMed.
- J. Regnery and W. Püttmann, Organophosphorus flame retardants and plasticizers in rain and snow from middle Germany, Clean: Soil, Air, Water, 2009, 37, 334–342 CAS.
- A. Marklund, B. Andersson and P. Haglund, Traffic as a source of organophosphorus flame retardants and plasticizers in snow, Environ. Sci. Technol., 2005, 39, 3555–3562 CrossRef CAS.
- G. Poma, A. Glynn, G. Malarvannan, A. Covaci and P. O. Darnerud, Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations, Food Chem. Toxicol., 2017, 100, 1–7 CrossRef CAS.
- G. Poma, C. Sales, B. Bruyland, C. Christia, S. Goscinny, J. Van Loco and A. Covaci, Occurrence of Organophosphorus Flame Retardants and Plasticizers (PFRs) in Belgian Foodstuffs and Estimation of the Dietary Exposure of the Adult Population, Environ. Sci. Technol., 2018, 52, 2331–2338 CrossRef CAS.
- F. Xu, J.-H. Tay, A. Covaci, J. A. Padilla-Sanchez, E. Papadopoulou, L. S. Haug, H. Neels, U. Sellström and C. A. de Wit, Assessment of dietary exposure to organohalogen contaminants, legacy and emerging flame retardants in a Norwegian cohort, Environ. Int., 2017, 102, 236–243 CrossRef CAS.
- A. M. Sundkvist, U. Olofsson and P. Haglund, Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk, J. Environ. Monit., 2010, 12, 943–951 RSC.
- S. H. Brandsma, P. E. Leonards, H. A. Leslie and J. de Boer, Tracing organophosphorus and brominated flame retardants and plasticizers in an estuarine food web, Sci. Total Environ., 2015, 505, 22–31 CrossRef CAS PubMed.
- K. C. Hyland, A. C. Blaine, E. R. Dickenson and C. P. Higgins, Accumulation of contaminants of emerging concern in food crops-part 1: Edible strawberries and lettuce grown in reclaimed water, Environ. Toxicol. Chem., 2015, 34, 2213–2221 CrossRef CAS PubMed.
- K. C. Hyland, A. C. Blaine and C. P. Higgins, Accumulation of contaminants of emerging concern in food crops-part 2: Plant distribution, Environ. Toxicol. Chem., 2015, 34, 2222–2230 CrossRef CAS PubMed.
- E. Norén, E. Larsson, M. Littorin, M. Maxe, B. A. Jönsson and C. H. Lindh, Biomonitoring of organophosphorus flame retardants in a Swedish population–Results from four investigations between years 2000–2013, 2017 Search PubMed.
- E. Cequier, A. K. Sakhi, R. M. Marce, G. Becher and C. Thomsen, Human exposure pathways to organophosphate triesters – A biomonitoring study of mother-child pairs, Environ. Int., 2015, 75, 159–165 CrossRef CAS PubMed.
- A.-M. Saillenfait, S. Ndaw, A. Robert and J.-P. Sabaté, Recent biomonitoring reports on phosphate ester flame retardants: a short review, Archives of toxicology, 2018, pp. 1–30 Search PubMed.
- CCME, Canadian Council of Ministers of the Environment, Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health – Sulfolane Factsheet, 2006, http://ceqg-rcqe.ccme.ca/en/index.html.
- CCME, Canadian Council of Ministers of the Environment, Canadian Environmental Quality Guidelines for Sulfolane: Water and Soil. Scientific Supporting Document, 2006, http://https://www.ccme.ca/files/Resources/supporting_scientific_documents/sulfolane_ssd_soil_water_1.1_e.pdf.
- LANUV, Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Jahresbericht 2009, online: https://www.lanuv.nrw.de/fileadmin/lanuvpubl/2_jahresberichte/JB_2009.pdf, accessed September 2018, 2010 Search PubMed.
- Alaska DHSS, Alaska Department of Health and Social Services, Division of Public Health, Section of Epidemiology, Environmental Public Health Program, Health Consultation – Sulfolane Plume in Groundwater: Evaluation of Community Concerns about Sulfolane in Private Water Wells, http://dec.alaska.gov/spar/csp/sites/north-pole-refinery/documents.aspx, accessed August 2018, 2012 Search PubMed.
- E. M. Dettenmaier, W. J. Doucette and B. Bugbee, Chemical hydrophobicity and uptake by plant roots, Environ. Sci. Technol., 2009, 43, 324–329 CrossRef CAS.
- B. K. Chard, W. J. Doucette, J. K. Chard, B. Bugbee and K. Gorder, Trichloroethylene uptake by apple and peach trees and transfer to fruit, Environ. Sci. Technol., 2006, 40, 4788–4793 CrossRef CAS.
- W. Doucette, J. Chard, B. Moore, W. Staudt and J. Headley, Uptake of sulfolane and diisopropanolamine (DIPA) by cattails (Typha latifolia), Microchem. J., 2005, 81, 41–49 CrossRef CAS.
- J. V. Headley, K. M. Peru and L. C. Dickson, Gas chromatographic-mass spectrometric determination of sulfolane in wetland vegetation exposed to sour gas-contaminated groundwater, J. Chromatogr. A, 1999, 859, 69–75 CrossRef CAS.
- R. Liu, T. Ruan, S. Song, Y. Lin and G. Jiang, Determination of synthetic phenolic antioxidants and relative metabolites in sewage treatment plant and recipient river by high performance liquid chromatography–electrospray tandem mass spectrometry, J. Chromatogr. A, 2015, 1381, 13–21 CrossRef CAS PubMed.
- N. Mali, S. Cerar, A. Koroša and P. Auersperger, Passive sampling as a tool for identifying micro-organic compounds in groundwater, Sci. Total Environ., 2017, 593, 722–734 CrossRef.
- K. Bouma, F. M. Nab and R. C. Schothorst, Migration of N-nitrosamines, N-nitrosatable substances and 2-mercaptobenzthiazol from baby bottle teats and soothers: a Dutch retail survey, Food Addit. Contam., 2003, 20, 853–858 CrossRef CAS.
- K. Bouma and R. C. Schothorst, Identification of extractable substances from rubber nettings used to package meat products, Food Addit. Contam., 2003, 20, 300–307 CrossRef CAS.
- M. S. Dopico-García, J. M. López-Vilariñó and M. V. González-Rodríguez, Antioxidant content of and migration from commercial polyethylene, polypropylene, and polyvinyl chloride packages, J. Agric. Food Chem., 2007, 55, 3225–3231 CrossRef.
- M. A. Lago and L. K. Ackerman, Identification of print-related contaminants in food packaging, Food Addit. Contam., Part A, 2016, 33, 518–529 CrossRef CAS.
- J. H. Petersen, P. Togeskov, J. Hallas, M. B. Olsen, B. Jørgensen and M. Jakobsen, Evaluation of retail fresh meat packagings covered with stretch films of plasticized PVC and non-PVC alternatives, Packag. Technol. Sci., 2004, 17, 53–66 CrossRef CAS.
- ECHA, European Chemicals Agency, Background document to the decision of the Executive Director of ECHA, ED/32/2011: Selection criteria to prioritise substances for Substance Evaluation (2011 CoRAP selection criteria), 2011, http://echa.europa.eu/documents/10162/13628/background_doc_criteria_ed_32_2011_en.pdf.
- S. Schulze, D. Sättler, M. Neumann, H. P. H. Arp, T. Reemtsma and U. Berger, Using REACH registration data to rank the environmental emission potential of persistent and mobile organic chemicals, Sci. Total Environ., 2018, 625, 1122–1128 CrossRef CAS.
- R. Boethling, Comparison of ready biodegradation estimation methods for fragrance materials, Sci. Total Environ., 2014, 497–498, 60–67 CrossRef CAS.
- R. S. Boethling, D. G. Lynch, J. S. Jaworska, J. L. Tunkel, G. C. Thom and S. Webb, Using BIOWIN™, Bayes, and batteries to predict ready biodegradability, Environ. Toxicol. Chem., 2004, 23, 911–920 CrossRef CAS PubMed.
- R. Posthumus, T. P. Traas, W. J. Peijnenburg and E. M. Hulzebos, External validation of EPIWIN biodegradation models, SAR QSAR Environ. Res., 2005, 16, 135–148 CrossRef CAS.
- ECHA, European Chemicals Agency, Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.11: PBT/vPvB Assessment. Version 3.0, June 2017, http://echa.europa.eu/support/guidance, 2017 Search PubMed.
- D. van de Meent, J. Quik and T. Traas, Identification and preliminary analysis of update needs for EUSES, Contract no. ECHA/2014/253, RIVM, National Institute of Public Health and the Environment, Bilthoven, The Netherlands, https://echa.europa.eu/documents/10162/13630/echa_2014_253_euses_report_en.pdf, 2014 Search PubMed.
- E. Undeman and M. S. McLachlan, Assessing model uncertainty of bioaccumulation models by combining chemical space visualization with a process-based diagnostic approach, Environ. Sci. Technol., 2011, 45, 8429–8436 CrossRef CAS.
- IARC, International Agency for Research on Cancer, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 115. Some Industrial Chemicals, WHO, World Health Organization, Lyon, France, https://monographs.iarc.fr/wp-content/uploads/2018/06/mono115.pdf, 2018 Search PubMed.
- J. Oltmanns, D. Bunke, W. Jenseit and C. Heidorn, The impact of REACH on classification for human health hazards, Regul. Toxicol. Pharmacol., 2014, 70, 474–481 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Apart from the list of 212 priority substances, this study also generated a substantial amount of data for the 2336 substances that were evaluated in the screening procedure. See DOI: 10.1039/c9em00369j |
| ‡ Present address: KIST Europe, Campus E 7.1, 66123 Saarbrücken, Germany; E-mail: E-mail: mlbohlen@kist-europe.de |
| § Technically, these extractions from the ECHA website involve registrations rather than substances, since there may be several registrations for any given substance. However, the numbers of unique substances were derived in addition to the number of registrations and are presented here for the sake of simplicity. |
| This journal is © The Royal Society of Chemistry 2020 |