Energy-generating potential of anaerobically enhanced primary treatment of domestic wastewater using multiple-compartment bioreactors†
Andrew
Pfluger
 *abc,
Rebecca
Erickson
ab,
Gary
Vanzin
ab,
Martha
Hahn
ab,
Jennie
Callahan
ab,
Junko
Munakata-Marr
*abc,
Rebecca
Erickson
ab,
Gary
Vanzin
ab,
Martha
Hahn
ab,
Jennie
Callahan
ab,
Junko
Munakata-Marr
 ab and
Linda
Figueroa
ab
ab and
Linda
Figueroa
ab
aColorado School of Mines, Department of Civil & Environmental Engineering, Golden, CO 80401, USA. E-mail: apfluger@mines.edu
bEngineering Research Center for Reinventing the Nation's Urban Water Infrastructure (ReNUWIt), USA
cUnited States Military Academy, Department of Geography & Environmental Engineering, West Point, NY, USA
First published on 5th November 2019
Abstract
Wastewater reclamation facilities have the potential to be net energy producers if anaerobic bioreactors coupled with energy-generating technologies, such as combined heat and power (CHP), are employed. To characterize the energy-generating potential of multiple-compartment anaerobic bioreactors used for enhanced primary treatment of domestic wastewater, organic removal and observed methane (CH4) generation from two pilot-scale anaerobic baffled bioreactors operating for more than 2400 days over a range of wastewater temperatures (11 to 24 °C) were characterized. Aggregated data from both bioreactor systems were subjected to uncertainty analysis and modeling to increase confidence in results and to determine the energy-generating potential from five different CHP technologies. Results suggest that multiple-compartment anaerobic reactors converted 76% of the chemical oxygen demand (COD) removed to methane-rich biogas (effective energy content of 2.0 kW h kg−1 COD removed). Observed CH4 production was most accurately modeled using total COD measurements, not biodegradable COD estimates. The use of the aerobic biochemical oxygen demand (BOD) assay underestimated the amount of anaerobically biodegradable COD. Modeled scenarios suggest that energy generated from several CHP technologies with heat recovery (i.e., effective electrical energy) can provide power equivalent to the amount used by many conventional activated sludge systems. A modeled future scenario where dissolved methane (dCH4) is recovered for energy generation also suggests that dCH4 capture provides additional energy generation and is needed to reduce greenhouse gas emissions. Based on COD, mass balances indicate that using multiple-compartment anaerobic reactors for anaerobically enhanced primary treatment increases the portion of COD in the influent wastewater going to electrical energy from ∼8.5% to 21%. Results from this study suggest that replacing conventional primary treatment with anaerobic bioreactors can enhance energy-generating potential at water resource recovery facilities.
Water impactAnaerobic primary treatment of domestic wastewater using baffled bioreactors converted 76% of the COD removed to methane-rich biogas. Uncertainty modeling suggests that anaerobic primary treatment coupled with combined heat and power technologies can produce electrical energy equivalent to the amount used by conventional activated sludge and can be a path forward for energy-positive wastewater treatment. |
1. Introduction
Medium-strength domestic wastewater (tCOD = 430 mg L−1 and NH4+-N = 40 mg L−1) has a maximum potential chemical energy of 1.80 kW h m−3. Most of the chemical energy potential is found in the organics, i.e., the chemical oxygen demand (COD) (1.49 kW h m−3). If harnessed, the maximum energy potential of wastewater is three to six times the energy required for wastewater treatment using conventional technologies (e.g., conventional activated sludge).1,2 In practice, however, the transport and treatment of domestic wastewater is very energy-intensive, accounting for approximately 3% of the U.S. electrical energy supply,3 a proportion similar to that in other countries.4 Conventional activated sludge (CAS), the most commonly employed wastewater treatment approach, is energy intensive requiring 0.3 to 0.6 kW h per m3 wastewater treated.5 Electricity use can account for as much as 40% of a WWTF's operating budget, with approximately 25% of electricity use coming from aeration alone.6,7Potential energy-generating alternatives to the energy-intensive aerobic wastewater treatment paradigm center on anaerobic bioreactors. Anaerobic bioreactor technologies generate methane-rich biogas from the degradation of organics such as fats, carbohydrates, and proteins commonly found in domestic wastewater via hydrolysis, acidogenesis, acetogenesis, and methanogenesis. While anaerobic digestion of wastewater sludge (i.e., primary sludge and waste activated sludge) is a common method for sludge treatment,8 mainstream anaerobic treatment of domestic wastewater is the focus of several current research efforts. Bioreactor systems such as the anaerobic membrane bioreactor (AnMBR) have demonstrated the ability to achieve discharge standards for wastewater organics and suspended solids set by the U.S. EPA (30 mg L−1 for 5 day biochemical oxygen demand (BOD5) and total suspended solids (TSS)); however, AnMBRs currently use more energy than can be recovered from the methane (CH4) they generate.9 Anaerobic sludge blanket processes, such as the upflow anaerobic sludge blanket (UASB) or the anaerobic baffled reactor (ABR), if located within the hydraulic gradient of the facility, can require no energy input but currently fail to meet wastewater discharge standards,10,11 suggesting that such technologies may be best employed as biologically enhanced primary wastewater treatment. To date, few studies have characterized the methane-generating potential of ABRs treating dilute domestic wastewater under low temperatures.12 Gopala-Krishna et al. (2008) reported that a bench-scale ABR (10-liter reactor volume) treating synthetic wastewater at temperatures ranging from 20 to 32 °C generated 0.18 to 0.23 L CH4 per g COD removed (41 to 55% conversion of COD to CH4) under various HRTs (6–20 hours).13 Shoener et al. (2014) used results from Gopala-Krishna et al. (2008), as well as results from three other bench-scale ABRs treating swine wastewater (further details in section 3.1), to model potential energy recovery from ABRs. Shoener et al. (2014) found that the ABR had greater energy recovery efficiency relative to several other anaerobic technologies, including UASBs, AnMBRs, microbial fuel cells, anaerobic fluidized bed reactors, and anaerobic sequencing batch reactors.14 While these results are promising, further modeling with data from pilot-scale bioreactors treating raw domestic wastewater under low wastewater temperatures over long timescales (i.e., taking seasonal variations in wastewater temperature into account) is required to more fully understand the energy-generating potential of ABRs employed for biologically enhanced primary treatment of domestic wastewater.
The objective of this study was therefore to examine the generation of CH4 and the energy-generating potential of two pilot-scale multiple-compartment anaerobic sludge blanket bioreactors operated over long timescales (cumulatively >2400 days) under cooler temperatures (11–24 °C) and variable organic loading. Observed CH4 generation was compared to the theoretical maximum generation of CH4 from COD removal in the reactor systems. To increase confidence in the measured values for future full-scale anaerobic primary wastewater treatment applications, uncertainty modeling of COD removal, methane generation, and potential energy generation using several combined heat and power (CHP) technologies was employed using Oracle Crystal Ball and Monte Carlo simulation.
2. Materials & methods
2.1. Anaerobic reactor configurations
Schematics for the two multiple-compartment anaerobic bioreactors examined in this study are shown in Fig. S1.† ABR 1 (Fig. S1.a†) was a four-compartment ABR that consisted of four equal-sized rectangular compartments (0.46 m wide/long and 1.22 m tall). The hydraulic volume of ABR 1 was held constant at 869 liters; however, the hydraulic retention time (HRT) was modified from 12 hours to 24 hours after 1357 days of operation to evaluate the impact on substrate removal and CH4 generation. ABR 1 was characterized for 1740 total days during this study. The second bioreactor, henceforth called ABR 2 (Fig. S1.b†), was operated as a three-compartment ABR with equal-sized cylindrical compartments (0.15 m radius and 3.66 m tall) for 390 days prior to the addition of a fourth cylindrical compartment (0.15 m radius and 1.22 m tall) that contained media for biofilm growth (i.e., an anaerobic fixed film reactor (AFFR)). The ABR-AFFR (i.e., ABR 2) was characterized for a total of 720 days during this study. The total hydraulic volume of the first three compartments of ABR 2 was 720 liters, which increased to 810 liters when the AFFR was added. Correspondingly, the HRT for the ABR portion of ABR 2 was 24 hours, which increased to 27 hours when the AFFR was added.Both reactors had the same hydraulic flow pattern based on a baffled design.15,16 In each reactor, raw, unheated influent wastewater was treated as it flowed sequentially through a series of four spatially separated reactor compartments. Each reactor compartment contained a downcomer pipe that routed influent wastewater from the feed tank or the previous compartment to the bottom of the compartment beneath the sludge blanket. Wastewater then flowed upward through the sludge blanket into a clarified zone. Wastewater exited each reactor compartment through an effluent pipe located at the top of each compartment, but below the water surface. For ABR 2, each compartment contained a gas–liquid–solid separator that was located above the sludge bed, but below the water surface. The separators were installed after 118 days of operation in ABR 2 as biogas-induced lifting of the sludge bed was observed. Gas–liquid–solid separators were not required in ABR 1. For the AFFR in ABR 2, media for biofilm growth was held in the upper portion of the reactor compartment by the gas–liquid–solid separator. Further reactor description is provided in ESI† Section S1.a. Influent and effluent wastewater characteristics for each ABR, as each was operated in a different location, are summarized in Table S1.† Influent wastewater characteristics for Plum Creek Wastewater Reclamation Authority, the location of ABR 1, are further described in Hahn and Figueroa (2015).17 Influent wastewater characteristics for Mines Park, the location of ABR 2, are further described in Vuono et al. (2013) and Pfluger et al. (2018).18,19 Fluid flow through each ABR was powered by a peristaltic pump; however, a pump would not be required for anaerobic primary treatment using ABRs if the bioreactors were placed within the hydraulic gradient of the facility. Therefore, energy use by ABRs for modeling was assumed negligible and was not included in the analysis.
2.2. Data collection and analyses
Measurements collected from both ABRs included temperature, pH, TSS, volatile suspended solids (VSS), total COD (tCOD), dissolved COD (dCOD), particulate COD (pCOD), BOD5, alkalinity, biogas production, biogas composition (CH4 and CO2), and dissolved CH4 (dCH4). Continuously monitored parameters included temperature and pH for both reactor systems. Grab samples were collected weekly from the influent and effluent of each reactor compartment for TSS, VSS, tCOD, dCOD, pCOD, BOD5, and alkalinity. Grab samples were collected periodically (at least twice monthly) for biogas production, biogas composition, and dCH4. In total, biogas production and dCH4 were simultaneously taken on 82 occasions and were used for analysis. COD measurements used for calculation of theoretical CH4 production were taken on 450 occasions. Table S2† provides further detail concerning the operational conditions under which each sample was taken, to include wastewater temperature, which varied from approximately 11 to 24 °C.Analyses for TSS, VSS, tCOD, dCOD, pCOD, BOD5, and alkalinity were conducted according to Standard Methods.20 Specific methods used are listed in Section S1.b.† For ABR 1, pH values were collected with Broadley James pH ProcessProbes and temperature was monitored and logged with submersible HOBO Temp Pro V2 temperature loggers. For ABR 2, pH was measured with Cole-Parmer pH electrodes (100 Ohm Pt RTD, EW-27003-23). Temperature was measured with LabJack EI-1034 probes. Biogas flowrate in ABR 1 was measured using Cole Parmer 0 to 500 SSCM gas flow meters. Biogas flowrate in ABR 2 was measured using an Agilent Digital Flow Meter (Optiflow 520). For ABR 1, methods for biogas composition and dCH4 sampling during the first 900 days of operation are described in Hahn & Figueroa (2015).17 Biogas composition was measured using a Shimadzu GC-17A or a Shimadzu GC-8A with TCD detectors and a HayeSep Q 80/100 column with UHP helium carrier gas at 30 mL min−1. For measurements taken after day 900 of ABR 1's operation and for all ABR 2 measurements, biogas composition was determined on a Hewlett Packard 6890 with Agilent 5973 Mass Selective Detector GC-MS with an Agilent 113-3133 GS-Carbonplot capillary column at max temperature of 360 °C, flowrate of 1.2 mL min−1, and helium carrier gas. Section S1.c† provides additional information regarding GC measurements. For ABR 2, dCH4 was analyzed using equilibrium partitioning from the dissolved phase to the gas phase according to the method described in Pfluger et al. (2011) with minor modification (described in Section S1.d†).21 Results were consistent between dCH4 sampling procedures (Table S3†). Methane production from ABR 2 is further described in Pfluger et al. (2018).19 Sludge retention time (SRT) was estimated using the approach described in Hahn & Figueroa (2015), which accounts for the total mass of volatile solids in the reactor (determined from sludge VSS (g L−1) and sludge volume (L)), the mass removal rate of effluent VSS (g d−1), periodic scum removal from the top of each reactor compartment (g d−1), and sludge removed from episodic events such as biological sampling (g d−1).18 Methods for determining COD mass balances for the ABR systems based on influent COD, effluent COD, and biogas produced are found in Erickson (2018).22 All “±” values presented in this study represent one standard deviation. All 10th and 90th percentile values from uncertainty analyses are shown with brackets, i.e., “[10th percentile value, 90th percentile value]”. Confidence intervals (95%) are only graphically depicted in Fig. 1.
2.3. Energy-related calculations
The biodegradable fraction of COD (bCOD) is degraded in anaerobic systems to create CO2 and CH4. A theoretical maximum volume of CH4 for any given quantity of bCOD removed can be calculated using the relationship 0.35 m3 CH4 per kg of BODultimate (i.e., bCOD) removed at STP, which is derived using the ideal gas law and stoichiometry. This relationship is modified at temperatures and pressures other than STP. Theoretical CH4 production for reactors in this study occurred at a lower atmospheric pressure (0.83 atm) and under variable air temperatures (ABR 1 ranged from 9.9 to 25.8 °C; ABR 2 ranged from 8.9 to 28.9 °C). Using the ideal gas law, the calculated range of theoretical CH4 production in this study therefore varied from 0.43 to 0.47 m3 CH4 per kg BODultimate removed.BOD5 measurements were used to estimate bCOD in this study. To estimate bCOD removal, measurements of tCOD and BOD5 were first used to determine the tCOD-to-BOD5 ratio for each reactor system. The bCOD for each tCOD measurement was then calculated using experimentally derived tCOD-to-BOD5 and BOD5-to-bCOD relationships (values provided in Table 1). Using this approach, theoretical CH4 production was calculated from the estimated biodegradable fraction of all tCOD measurements (eqn (1a)). For comparison, theoretical CH4 production was also calculated directly from tCOD measurements (eqn (1b)).
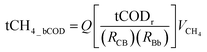 | (1a) |
| tCH4_tCOD = (Q)(tCODr)(VCH4) | (1b) |
tCH4_tCOD = total theoretical CH4 production (m3 CH4 per d) from total COD
Q = wastewater flowrate (m3 d−1)
tCODr = total COD removed by ABR system (kg tCOD m−3 wastewater)
R CB = ratio of tCOD to BOD5
R Bb = ratio of BOD5 to bCOD
V CH4 = theoretical volume of CH4 produced per kg tCOD removed (m3 kg−1) adjusted for temperature & pressure.
Note: values used in uncertainty analyses for RCB and RBb are found in Table 1.
| (a) Organic removal and theoretical methane generation values | ||||||
|---|---|---|---|---|---|---|
| Uncertainty parameter | Units | Distribution | Temperature | Baseline value | Low value | High value |
| g tCOD removed per d | g tCOD d−1 | Triangular | Warm | 393.5 | 107.4 | 679.6 |
| Cold | 329.2 | 36.2 | 622.2 | |||
| % COD removal | % | Triangular | Warm | 57 | 47 | 66 |
| Cold | 51 | 42 | 61 | |||
| g tCOD removed per m3 WW | g tCOD m−3 | Triangular | Warm | 419.8 | 97.5 | 742.2 |
| Cold | 355.9 | 29.7 | 682.0 | |||
| tCOD-to-BOD5 ratio | g tCOD g−1 BOD5 | Triangular | Warm | 2.73 | 2.19 | 3.27 |
| Cold | 2.47 | 2.02 | 2.92 | |||
| BOD rate constant (k) | d−1 | Triangular | Warm/cold | 0.20 | 0.16 | 0.24 |
| BOD5-to-bCOD ratio | g BOD5 g−1 bCOD | Triangular | Warm/cold | 0.63 | 0.54 | 0.70 |
| L CH4 per g bCOD removed | L CH4 per g bCOD | Triangular | Warm | 0.46 | 0.45 | 0.47 |
| Cold | 0.45 | 0.44 | 0.46 | |||
| Organic to CH4 conversion | Efficiency (%) | Uniform | Warm/cold | 81 | 81 | 95 |
| Air temperature | K | Triangular | Warm | 295.4 | 292.6 | 298.2 |
| Cold | 288.4 | 285.2 | 291.7 | |||
| Dissolved CH4 recovery future scenario | % | Uniform | Warm/cold | 0 | 0 | 100 |
| (b) Methane generation values from Crystal Ball predictor | ||||||
|---|---|---|---|---|---|---|
| Uncertainty parameter | Units | Distribution | Temperature | Baseline value | Low value | High value |
| Gas CH4 per m3 WW | L m−3 WW | Triangular | Warm | 85.6 | 66.0 | 105.3 |
| Cold | 72.0 | 42.6 | 101.3 | |||
| Dissolved CH4 per m3 WW | L m−3 WW | Triangular | Warm | 38.5 | 26.9 | 50.1 |
| Cold | 31.8 | 15.7 | 47.9 | |||
| Total CH4 per m3 WW | L m−3 WW | Triangular | Warm | 124.5 | 95.9 | 153.0 |
| Cold | 103.8 | 59.4 | 148.2 | |||
| Energy from CH4 gas | kW h m−3 WW | Triangular | Warm | 0.64 | 0.49 | 0.78 |
| Cold | 0.53 | 0.32 | 0.75 | |||
| Energy potential (gas & dissolved) | kW h m−3 WW | Triangular | Warm | 0.93 | 0.71 | 1.15 |
| Cold | 0.77 | 0.44 | 1.09 | |||
McCarty et al. (2011) state that approximately 20% of biodegradable energy potential may be lost in the wastewater treatment process.5 Specifically, around 8% of energy potential is lost in the conversion of wastewater organics (e.g., carbohydrates, fats, and proteins) to methane. A further 7% of energy potential is lost during anaerobic cell synthesis, while another 5% may be lost in the inefficiency of wastewater treatment itself. Such losses should be accounted for when determining the theoretical CH4 production of an anaerobic system. In this study, decreases in energy-generating potential due to such losses are accounted for in uncertainty analyses by including an energy potential loss adjustment factor in some modeled scenarios. Specifically, the modeled CH4 production was multiplied by a factor of 0.8 to simulate 20% loss in energy potential.
The energy content of CH4 was calculated using the factor 0.222 kW h mol−1 CH4.14,23 Electrical energy conversion efficiency is dependent on the CHP technology used and ranges from as low as 5% recovery for some steam engines to as high as 63% recovery for some fuel cells.24 Recovery of additional electrical energy from the conversion of heat, i.e., the effective electrical efficiency, can increase energy recovery efficiency to as high as 80% for reciprocating engines and fuel cells.24 The range of electrical energy conversion efficiency for each CHP technology used in the uncertainty analysis is provided in Table S4.a.†
2.4. Uncertainty analyses
To address uncertainty in pilot-scale ABR data, measurements from both ABR reactors were subjected to uncertainty analysis in Oracle Crystal Ball (release 11.1.2.4.850) using Monte Carlo analysis (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 simulations). To increase confidence in measurements for organic removal and CH4 generation, performance data from both reactor systems were normalized to m3 wastewater treated (e.g., g COD removed per m3 wastewater treated), aggregated, and analyzed over 60 forecast periods in Oracle's Crystal Ball Predictor. The probable low-end, baseline, and high-end values were then incorporated into the uncertainty analysis. Uncertainty parameters for organic removal, theoretical CH4 generation, and observed CH4 generation are listed in Table 1. Uncertainty parameters for energy recovery from CHP and energy use in other wastewater treatment processes for comparison (e.g., CAS) are listed in Table S4.b.† As both organic removal and CH4 generation were impacted by temperature, uncertainty parameters were subset into a cold weather condition (15 ± 3 °C) and a warm weather condition (21 ± 3 °C) prior to Monte Carlo simulations. Last, as dCH4 recovery for energy generation is not currently feasible above bench-scale,25 a current scenario (i.e., 0% recovery) and future scenario (0 to 100% recovery, uniform distribution) were constructed. For all data, a triangular probability distribution was assumed when low-end, baseline, and high-end values were available. When only two data points were available, i.e., a low-end and high-end value, a uniform probability distribution was assumed.
000 simulations). To increase confidence in measurements for organic removal and CH4 generation, performance data from both reactor systems were normalized to m3 wastewater treated (e.g., g COD removed per m3 wastewater treated), aggregated, and analyzed over 60 forecast periods in Oracle's Crystal Ball Predictor. The probable low-end, baseline, and high-end values were then incorporated into the uncertainty analysis. Uncertainty parameters for organic removal, theoretical CH4 generation, and observed CH4 generation are listed in Table 1. Uncertainty parameters for energy recovery from CHP and energy use in other wastewater treatment processes for comparison (e.g., CAS) are listed in Table S4.b.† As both organic removal and CH4 generation were impacted by temperature, uncertainty parameters were subset into a cold weather condition (15 ± 3 °C) and a warm weather condition (21 ± 3 °C) prior to Monte Carlo simulations. Last, as dCH4 recovery for energy generation is not currently feasible above bench-scale,25 a current scenario (i.e., 0% recovery) and future scenario (0 to 100% recovery, uniform distribution) were constructed. For all data, a triangular probability distribution was assumed when low-end, baseline, and high-end values were available. When only two data points were available, i.e., a low-end and high-end value, a uniform probability distribution was assumed.
3. Results & discussion
3.1. Comparison of observed organic removal and methane generation to other sludge blanket bioreactor studies
Table 2 summarizes a comparison of ABR operating conditions (HRT, temperature, water volume, length of study), tCOD removal (%), and CH4 generation (L) per g tCOD removed. Table 3 provides a comparison between observed and theoretical CH4 generation, as well as projected energy generation (kW h m−3 wastewater treated), and energy recovery efficiency (kW h kg−1 tCOD removed). COD removal varied from 43% to 72% in ABRs 1 and 2 depending on the operating condition; however, observed total (gaseous and dissolved) CH4 production (L) per g tCOD removed was less variable, with mean values ranging from 0.31 to 0.40 L CH4 per g tCOD removed.| Reactor | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reactor | Substrate | Configuration | HRT (h) | Temperature (°C) | Volume (L) | Study length (d) | tCOD removal (%) | L CH4 per g tCODa |
| ABR 1b | Raw DWW | 4-Compart. | 12 | 12–23 | 869 | 1357 | 43 ± 13 | 0.31 ± 0.19 |
| ABR 1 | Raw DWW | 4-Compart. | 24 | 12–23 | 869 | 383 | 72 ± 8 | 0.34 ± 0.09 |
| ABR 2c | Raw DWW | 3-Compart. | 24 | 11–24 | 720 | 390 | 43 ± 20 | 0.31 ± 0.29 |
| ABR 2c | Raw DWW | 4-Compart. | 27 | 11–24 | 810 | 330 | 54 ± 15 | 0.40 ± 0.24 |
| Comparison to studies examined in Shoener et al. (2014)14 | ||||||||
| Study | Substrate | Configuration | HRT | Temperature (°C) | Volume (L) | Study length (d) | tCOD removal (%) | L CH4 per g tCOD |
|---|---|---|---|---|---|---|---|---|
| Studies: (1) Yang & Moengangongo (1987);33 (2) & (3) Boopathy & Sievers (1991);34 (4) Gopala Krishna et al. (2008)13 & Gopala Krishna et al. (2009).35a Liters of CH4 produced includes both gaseous and dissolved CH4b COD removal and methane generation for ABR 1 with a 12 hour HRT is further described in Hahn & Figueroa (2015).18c COD removal and methane generation for ABR 2 is further described in Pfluger et al. (2018).20d Study reported that the reactor was housed in a chamber held at a constant temperature of 30 °C, but that the influent wastewater was between 20 and 32 °C.e CH4 production per g COD removed varied with HRT. | ||||||||
| 1 | Swine WW supernatant | Horizontal baffled (6 total) | 2.5 d | 30 ± 1 | 20 | ∼180 | 75 | 0.17 |
| 2 | Whole swine wastewater | 2 chamber | 15 d | 35 | 15 | ∼300 | 69 | 0.04 |
| 3 | Whole swine wastewater | 3 chamber | 15 d | 35 | 15 | ∼300 | 62 | 0.04 |
| 4 | Low strength synthetic WW | Hanging baffles (45°) | 6–20 h | 30d | 10 | 592 | >90 | 0.18–0.23e |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 simulations). 10th and 90th percentile values are depicted in brackets. Maximum calculated values are compared to values accounting for 20% loss in chemical energy potential as suggested by McCarty et al. (2011)5
000 simulations). 10th and 90th percentile values are depicted in brackets. Maximum calculated values are compared to values accounting for 20% loss in chemical energy potential as suggested by McCarty et al. (2011)5
| (a) Theoretical total methane and energy production calculated from total COD removal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reactor | HRT (h) | L CH4 per d (no loss) | L CH4 per d (20% loss) | L CH4 per m3 WW treated (no loss) | L CH4 per m3 WW treated (20% loss) | kW h m−3 WW treated (no loss) | kW h m−3 WW treated (20% loss) | ||
| ABR 1 | 12 | 120 [111, 130] | 96 [89, 104] | 69 [64, 75] | 55 [51, 60] | 1.36 [1.26, 1.47] | 1.19 [1.14, 1.22] | ||
| ABR 1 | 24 | 174 [151, 188] | 138 [129, 150] | 200 [186, 217] | 160 [149, 173] | 1.94 [1.81, 2.10] | 1.66 [1.55, 1.79] | ||
| ABR 2 | 24 | 107 [97, 119] | 86 [78, 95] | 150 [135, 166] | 119 [108, 132] | 1.20 [1.09, 1.34] | 0.96 [0.87, 1.07] | ||
| ABR 2 | 27 | 105 [95, 116] | 84 [76, 93] | 132 [120, 146] | 107 [97, 118] | 1.29 [1.41, 1.43] | 1.03 [0.94, 1.14] | ||
| (b) Theoretical total methane and energy production calculated from estimated biodegradable COD removal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reactor | HRT (h) | L CH4 per d (no loss) | L CH4 per d (20% loss) | L CH4 per m3 WW treated (no loss) | L CH4 per m3 WW treated (20% loss) | kW h m−3 WW treated (no loss) | kW h m−3 WW treated (20% loss) | ||
| ABR 1 | 12 | 70 [61, 80] | 56 [49, 64] | 40 [35, 46] | 32 [28, 37] | 0.79 [0.69, 0.90] | 0.69 [0.65, 0.72] | ||
| ABR 1 | 24 | 101 [88, 115] | 80 [71, 92] | 116 [102, 133] | 93 [82, 106] | 1.13 [1.00, 1.29] | 0.91 [0.80, 1.04] | ||
| ABR 2 | 24 | 62 [52, 74] | 50 [42, 59] | 87 [72, 103] | 69 [58, 82] | 0.70 [0.59, 0.84] | 0.56 [0.47, 0.67] | ||
| ABR 2 | 27 | 61 [51, 73] | 49 [41, 58] | 77 [65, 91] | 62 [52, 73] | 0.75 [0.63, 0.89] | 0.60 [0.51, 0.71] | ||
| (c) Observed methane production (total and gas), projected energy generation, and projected energy recovery efficiency, over the course of each study period (mean values ± one standard deviation). Total CH4 is defined as the sum of gaseous and dissolved CH4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reactor | HRT | L CH4 per d (total) | L CH4 per m3 WW treated (total) | kW h m−3 WW treated (total) | L CH4 per d (gas) | L CH4 per m3 WW treated (gas) | kW h m−3 WW treated (gas) | kW h kg−1 tCOD removed | Energy recovery efficiency (%) (tCOD removal) |
| ABR 1 | 12 h | 164 ± 39 | 95 ± 23 | 0.7 ± 0.2 | 118 ± 28 | 68 ± 16 | 0.5 ± 0.1 | 2.4 ± 1.4 | 67 ± 22 |
| ABR 1 | 24 h | 151 ± 28 | 175 ± 32 | 1.3 ± 0.2 | 100 ± 18 | 116 ± 20 | 0.9 ± 0.2 | 1.5 ± 0.4 | 76 ± 20 |
| ABR 2 | 24 h | 76 ± 34 | 106 ± 47 | 0.8 ± 0.4 | 54 ± 24 | 75 ± 34 | 0.6 ± 0.3 | 1.4 ± 1.3 | 68 ± 18 |
| ABR 2 | 27 h | 83 ± 23 | 115 ± 33 | 0.9 ± 0.3 | 59 ± 18 | 82 ± 24 | 0.6 ± 0.2 | 2.5 ± 1.2 | 87 ± 20 |
| All reactors | N/A | 109 ± 52 | 115 ± 47 | 0.9 ± 0.4 | 76 ± 36 | 80 ± 31 | 0.6 ± 0.2 | 2.0 ± 1.2 | 76 ± 20 |
These results are near the theoretical limit of ∼0.45 L CH4 per g tCOD removed (adjusted from STP for temperature and pressure), or 67 to 87% conversion of tCOD to CH4. These results are higher than reported values for UASB and UASB variants treating ≥1 m3 of raw domestic wastewater under temperatures ≤20 °C, which have been reported to range from 0.03 to 0.25 L CH4 per g tCOD removed, representing ∼9 to 71% conversion (at STP).26–32 Results from this study are also higher than the bench-scale ABR studies examined in Shoener et al. (2014),14 which reported a range of 0.04 to 0.23 L CH4 per g tCOD removed at temperatures of 30 to 35 °C (Table 2).13,33–35 The increase in observed CH4 production per g tCOD removed is likely attributable to the long SRT observed in the pilot-scale ABRs. Estimated SRT was 45 ± 13 days for ABR 1 and 61 ± 42 days for ABR 2, which was approximately 60 to 90 times the HRT. Purposeful solids wasting was not required in either ABR, which decoupled SRT from HRT and allowed for increased removal of particulate organic matter and settled solids via degradation in the sludge bed over time. Analysis of the sludge in ABR 1 reactor compartments indicated that ≈1% (unpublished) of COD removed by the ABR was retained in the sludge blanket, suggesting degradation by hydrolysis and eventual conversion to methane over time. This long-term degradation likely supported the generation of additional CH4 relative to the bench-scale ABRs Shoener et al. (2014)14 examined in their review (listed in Table 2), which contained substantially lower sludge bed volumes and may not have experienced this phenomenon.
The theoretical energy potential in typical domestic wastewater has been estimated using bomb calorimeters to range from 4.1 kW h kg−1 COD to 4.9 kW h kg−1 COD.2,36 However, Heidrich et al. (2011) apparently included the energy value of ammonia in the measured energy value normalized to COD, thereby overestimating the energy content from COD.2 Energy content of COD alone has been recorded as 3.86 kW h kg−1 COD based on the higher heat value, and 3.47 kW h kg−1 COD based on the lower (or net) heat value.1,23 Given uncertainty in domestic wastewater energy content estimates, this study conservatively estimated energy content based on the lower (or net) heat value (i.e., 3.47 kW h kg−1 COD). The mean potential effective energy production, measured in kW h kg−1 COD removed, between reactors and under varying operational conditions was 2.0 ± 1.2, which equates to 76 ± 20% energy recovery efficiency (compared to theoretical energy potential from COD removal adjusted from STP, ∼0.45 L CH4 per g tCOD removed) (Table 3). Despite some variation between reactors, no statistically significant difference was observed. The observed energy recovery efficiency from COD degradation in this study (76 ± 20%) and the mean potential effective energy production (2.0 ± 1.2 kW h kg−1 COD removed) exceed the values determined by Shoener et al. (2014) (1.1 to 2.0 kW h kg−1 COD removed with 29 to 53% energy recovery efficiency) likely due to differences in ABR operating conditions.14 Shoener et al. (2014) examined bench-scale reactors (10–20 liters) operated under wastewater temperatures (30–35 °C) higher than those commonly observed at wastewater treatment facilities, using wastewaters that are not representative of raw domestic wastewater (i.e., high-strength swine wastewater or low-strength synthetic wastewater) (Table 2).13,14,33–35
3.2. Comparison of theoretical and observed methane generation
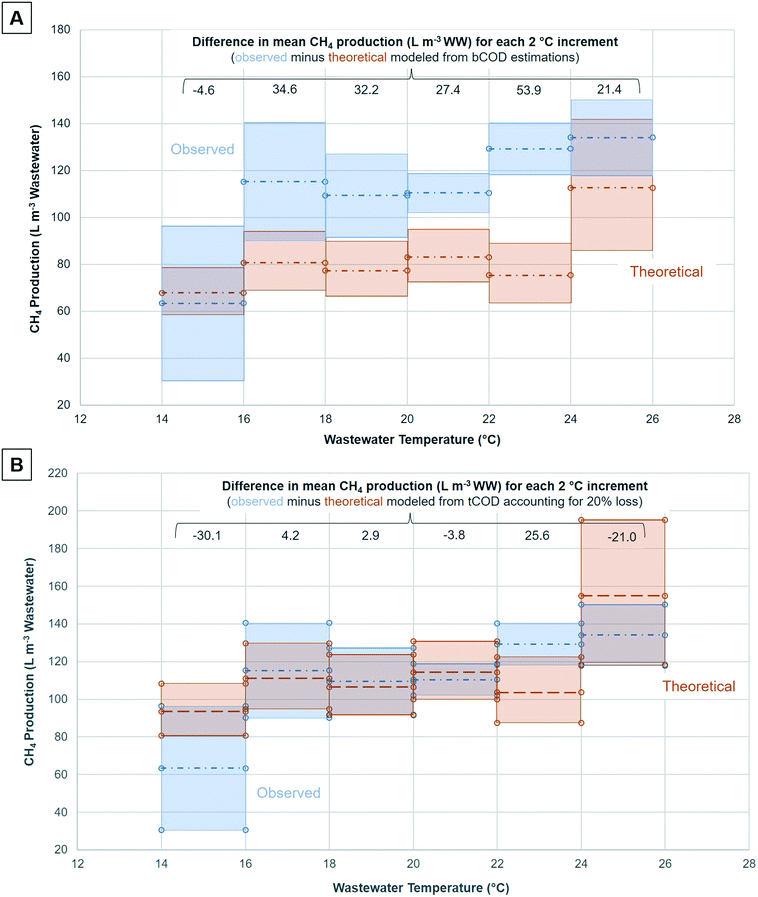
Four scenarios based on organic removal were modeled for comparison to observed CH4 generation: (1) tCOD removal; (2) tCOD removal with 20% loss of biodegradable energy potential; (3) bCOD estimated from BOD5 measurements; (4) bCOD estimated from BOD5 measurements with 20% loss of biodegradable energy potential. Both examined ABRs treated differing volumes of wastewater, therefore, CH4 production was normalized to m3 of wastewater treated for comparison. This study used BOD5 to estimate bCOD rather than biochemical methane potential (BMP tests). Zhang et al. (2013 and 2018) used BMP tests (15 °C incubation) to predict maximum potential CH4 generation for an upflow anaerobic sludge blanket (UASB) with digester bioreactor system receiving influent domestic wastewater.37,38 BMP and observed CH4 production results from Zhang et al. (2013 and 2018) suggest that the BMP test underestimates bCOD more than BOD5 (discussed further in Section S.1e†).37,38 Modeled theoretical CH4 and energy production for each scenario is shown in Table 3. Specifically, Table 3a provides results for scenarios involving modeled CH4 production from tCOD (scenarios 1 and 2) and Table 3b provides results for scenarios involving CH4 production from estimated bCOD (scenarios 3 and 4). Additionally, values for both observed total and gaseous CH4 production (L CH4 per d and L CH4 per m WW treated) are displayed separately in Table 3c. dCH4 is accounted for in total CH4 production but is not separately listed in Table 3 (instead found in Table S3†).Fig. 1 depicts results for scenarios that most closely modeled observed CH4 production: tCOD removal with 20% loss of biodegradable energy potential (scenario 2) and bCOD estimated from BOD5 measurements (scenario 3). Scenario 1 significantly overestimated observed CH4 production, while scenario 4 significantly underestimated observed CH4 production. Scenario 1 (tCOD removal without loss of energy potential) was expected to estimate methane generation beyond observed CH4 because it represents an absolute maximum CH4 production. As shown in Fig. 1A, the model based on estimated bCOD (scenario 3) underestimated CH4 production relative to observed CH4 measurements. In aerobic conditions bCOD can be accurately estimated from either tCOD and/or BOD5 based on well-studied relationships; however, for anaerobic sludge blanket bioreactors, a higher fraction of the wastewater organic matter is anaerobically degraded through hydrolysis of particulate COD and settled solids in the sludge blanket and endogenous decay of cells. To predict CH4 production in an ABR or similar sludge blanket system, 80% of tCOD removed is supported by our analysis in scenario 2. Fig. 1A depicts the difference between mean values of observed CH4 production and modeled CH4 production from estimated bCOD using 95% confidence intervals (for observed production) and modeled 10th and 90th percentile values from the uncertainty analysis (for modeled production) for each 2 °C change in wastewater temperature (14 to 26 °C). A comparison of mean values of observed CH4 and modeled CH4 production for each 2 °C temperature interval suggests that observed CH4 production exceeded modeled CH4 production from estimated bCOD by at least 21.4 L CH4 per m3 of wastewater treated for temperatures >16 °C. The difference in means (observed minus theoretical CH4 production) for each temperature interval (L CH4 per m3 of wastewater treated) is depicted along the x-axis in Fig. 1A. The range of differences was 21–54 L CH4 per m3 at wastewater temperatures over 16 °C, which equates to a 25 to 42% increase of observed CH4 relative to theoretical modeled production from estimated bCOD removal. These results suggest that bCOD estimates based on BOD5 should not be used to estimate CH4 production.
As shown in Fig. 1B, CH4 production modeled by tCOD with 20% loss of biodegradable energy potential (scenario 2) is a more accurate predictor of observed CH4 production for temperatures between 16 and 24 °C. The differences in means (observed minus theoretical) are very similar for 16–18 °C (difference = 4.2 L CH4 per m3 wastewater), 18–20 °C (difference = 2.9), and 20–22 °C (difference = −3.8). For the 22–24 °C temperature range, observed was greater than theoretical by a larger amount (25.6 L CH4 per m3 wastewater). For the coldest (14–16 °C) and warmest (24–26 °C) temperature ranges, modeled CH4 generation was larger. As shown in Table S2,† the number of observed CH4 measurements was lower in the coldest and warmest temperature ranges examined, suggesting the model could be refined with additional measurements. Total COD measurements overestimate biochemical oxygen demand for aerobic systems due to the oxidation of all organic matter rather than aerobically biodegradable organics. The oxidation of all the organic matter by the tCOD test mimics anaerobic biodegradation at long SRT, which includes anaerobic hydrolysis and endogenous decay in the sludge blanket. The inclusion of an energy potential loss factor (20%) improves the model by accounting for the presence of recalcitrant carbon and carbon sequestered in biomass. These factors make scenario 2 a better predictor of organic material removal by anaerobic sludge bed processes, and therefore CH4 generation, relative to BOD5 or bCOD estimations (i.e., scenarios 3 and 4).
CH4 generation from anaerobic degradation of physically retained organic solids (i.e., hydrolysis and endogenous decay) in the sludge blanket has also been observed to increase the observed ratio of CH4 produced per mass of tCOD removed in baffled anaerobic bioreactors.17,19,39 An examination of ABR 1 before and after an increase in HRT also shows this phenomenon. When the wastewater flowrate to ABR 1 was reduced from 1738 L d−1 to 869 L d−1 after 1357 days of operation, the influent organic loading was reduced by approximately half; however, ABR 1 produced almost the same volume of CH4, only decreasing from 164 ± 39 L CH4 per d to 151 ± 28 L CH4 per d. This result suggests that degradation of retained organic solids was a significant contributor to CH4 production; despite the decrease in organic loading from the influent wastewater by approximately one-half, observed CH4 production only decreased by ∼8%.
A model of CH4 production for anaerobic sludge blanket processes, therefore, must include a factor accounting for anaerobic activity such as hydrolysis and endogenous decay within the sludge blanket. A model based on tCOD removal, however, is a better predictor as the tCOD measurement oxidized material beyond readily biodegradable organics. Refinement to this model over time is required, especially for lower wastewater temperatures where microbial activity is suppressed and degradation in the sludge blanket may be reduced.
3.3. Modeled energy generation from combined heat and power technologies
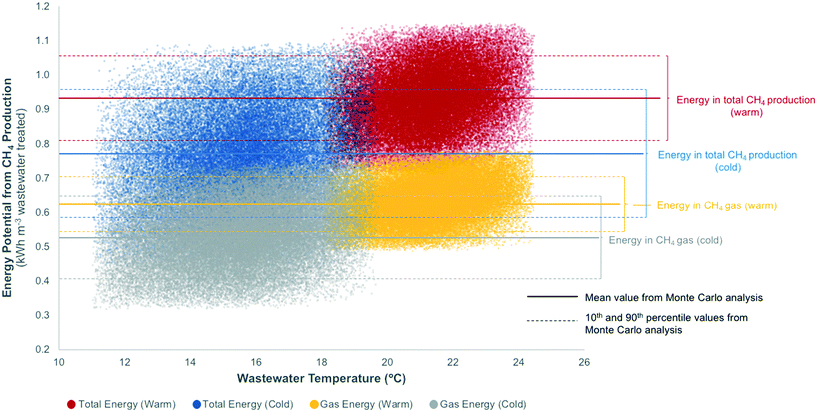
Fig. 2 depicts results of uncertainty modeling (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Monte Carlo simulations) for energy potential from observed CH4 production (kW h m−3 wastewater treated) in ABRs over a range of wastewater temperatures. Results are subset into four categories: energy potential from gaseous CH4 production under warm and cold wastewater temperatures, and energy potential from total CH4 production (i.e., gaseous and dCH4) under warm and cold wastewater temperatures. Energy recovery from total CH4 production represents a future scenario as full-scale dCH4 recovery schemes are not currently viable. Future dCH4 recovery, therefore, was modeled using a uniform distribution ranging from 0 to 100% recovery (Table 1). All modeled values (total of 200
000 Monte Carlo simulations) for energy potential from observed CH4 production (kW h m−3 wastewater treated) in ABRs over a range of wastewater temperatures. Results are subset into four categories: energy potential from gaseous CH4 production under warm and cold wastewater temperatures, and energy potential from total CH4 production (i.e., gaseous and dCH4) under warm and cold wastewater temperatures. Energy recovery from total CH4 production represents a future scenario as full-scale dCH4 recovery schemes are not currently viable. Future dCH4 recovery, therefore, was modeled using a uniform distribution ranging from 0 to 100% recovery (Table 1). All modeled values (total of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) are displayed in Fig. 2, which shows the variation in potential energy production at any given wastewater temperature. Modeled potential energy from gaseous CH4 increased from a minimum value of 0.32 kW h m−3 (32 occurrences, temperature range = 11.1 to 16.7 °C) to a maximum value of 0.78 kW h m−3 (65 occurrences, temperature range = 19.6 to 24.4 °C). Similarly, the modeled total potential energy increases from a minimum value of 0.44 kW h m−3 (3 occurrences, temperature range = 12.1 to 14.6 °C) to a maximum value of 1.15 kW h m−3 (14 occurrences, temperature range = 20.0 to 23.9 °C). These results suggest that seasonal variations in wastewater temperature will impact potential energy production; however, the extent of the variation in modeled potential energy under varying temperatures will likely decrease as future studies provide more data for modeling.
000) are displayed in Fig. 2, which shows the variation in potential energy production at any given wastewater temperature. Modeled potential energy from gaseous CH4 increased from a minimum value of 0.32 kW h m−3 (32 occurrences, temperature range = 11.1 to 16.7 °C) to a maximum value of 0.78 kW h m−3 (65 occurrences, temperature range = 19.6 to 24.4 °C). Similarly, the modeled total potential energy increases from a minimum value of 0.44 kW h m−3 (3 occurrences, temperature range = 12.1 to 14.6 °C) to a maximum value of 1.15 kW h m−3 (14 occurrences, temperature range = 20.0 to 23.9 °C). These results suggest that seasonal variations in wastewater temperature will impact potential energy production; however, the extent of the variation in modeled potential energy under varying temperatures will likely decrease as future studies provide more data for modeling.
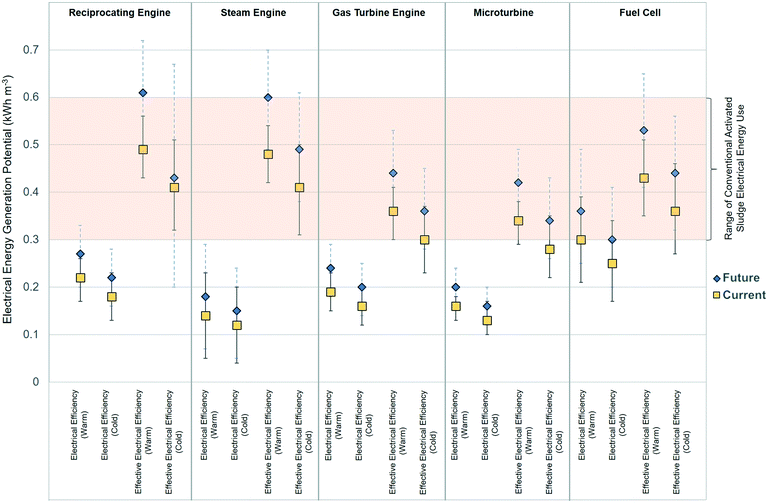
Modeled energy potential from ABR-generated CH4 represents maximum available energy. CHP technologies, however, are not 100% efficient and losses are observed in the electricity generation process. The U.S. EPA's Combined Heat and Power Partnership Catalog of CHP Technologies (U.S. EPA, 2017), which describes the state-of-the-art concerning commonly used CHP technologies, provides a range of recovery efficiencies, including electrical energy efficiency and effective electrical efficiency (which accounts for additional electrical energy recovered from produced heat).24 This study uses uncertainty analysis to examine the electrical energy efficiency and effective electrical energy efficiencies for five CHP technologies: reciprocating engine, steam turbine, gas turbine, microturbine, and fuel cells. The range of electrical energy efficiency and effective electrical energy efficiencies used for uncertainty analysis for each CHP technology are listed in Table S4.a.†Fig. 3 depicts electrical energy generation potential from each CHP technology from the uncertainty analysis for warm and cold wastewater temperatures (21 ± 3 °C and 15 ± 3 °C, respectively). Both the modeled current scenario (no dCH4 recovery) and future scenarios where dCH4 is recovered for energy generation are shown. Fig. 3 also compares CHP electrical energy generation potential to the typical range of CAS energy use (i.e., 0.3–0.6 kW h m−3 wastewater treated). As shown, the fuel cell has the highest current electrical energy recovery from modeled ABR gaseous CH4 production. Under both warm and cold temperatures, electrical energy generated from the fuel cell approaches the lower range of CAS energy use. Considering effective electrical efficiency, however, reciprocating and steam engines have the highest potential electrical energy generation and can generate enough electrical energy to power many CAS scenarios. Considering a future scenario where dCH4 is recovered, the reciprocating engine and the steam engine may produce enough effective electrical energy to power even the most energy intensive CAS scenario.
The choice of which CHP technology to implement usually depends on factors beyond electrical or heat energy generating capability. Costs, wastewater flowrate, biogas treatment requirements, physical space, and maintenance requirements are additional considerations for water resource recovery facilities (WRRFs).24,40 Microturbines, for example, provide relatively low electrical energy recovery, but may be more applicable for WRRFs treating lower wastewater flowrates.41 Reciprocating engines are the most widely installed CHP technology in the U.S. today and are located at 51.9% of CHP sites. The gas turbine, however, generates more electrical capacity (53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 MW or 64%), despite being at only 15.8% of CHP locations.24 Fuel cells, which have the highest electrical efficiency (up to 63%), are still an emerging technology and are currently employed at only 2.9% of CHP locations and account for approximately 0.1% of CHP capacity in the U.S.24
320 MW or 64%), despite being at only 15.8% of CHP locations.24 Fuel cells, which have the highest electrical efficiency (up to 63%), are still an emerging technology and are currently employed at only 2.9% of CHP locations and account for approximately 0.1% of CHP capacity in the U.S.24
3.4. Implications for integration of anaerobic primary treatment using ABRs into WRRFs
The current wastewater treatment paradigm centers on aerobic degradation of organics using technologies such as CAS.1 In the near term, multiple-compartment anaerobic reactor systems for mainstream treatment of domestic wastewater can replace conventional primary treatment technologies, such as primary clarification. Conventional primary treatment typically removes 25–35% of BOD and 50–65% of TSS,42 which is less than ABRs, which remove 50–70% of organics (Table 2) and 70–80% of TSS.17,19,39 The additional removal of organics and suspended solids from anaerobic primary treatment using ABRs will reduce organic and suspended solids loading for downstream activated sludge treatment, which will reduce required energy use for aeration. While aeration for CAS and associated energy requirements can vary between WRRFs, energy consumption in a typical CAS process with medium strength wastewater (i.e., 430 mg COD L−1) can be estimated as 1.0 kW h electrical input per kg COD removed.43 Using this approximation and typical values for organic removal in conventional primary treatment (25–35%) and observed organic removal in ABRs examined in this study (Table 1), results from uncertainty analysis suggest a decrease in CAS energy use of approximately 30% when ABRs are used as primary treatment. More specifically, modeled energy use in CAS (i.e., from uncertainty analysis; Table S4†) after conventional primary treatment was 0.47 ± 0.11 kW h m−3 wastewater treated, while energy use in CAS after anaerobic primary using ABRs was 0.29 ± 0.07 kW h m−3 under warm wastewater temperatures and 0.33 ± 0.08 kW h m−3 under cold wastewater temperatures. This result suggests that anaerobic primary treatment using ABRs would not only generate CH4 for electrical energy production but would substantially decrease electrical energy requirements for CAS.Given no apparent requirement to waste ABR sludge, the requirement to digest and stabilize sludge normally removed by conventional primary treatment would be eliminated. The reduced organic loading to CAS would also likely result in a reduced volume of waste activated sludge produced. Follow-on sludge digestion and stabilization requirements in a WRRFs employing anaerobic primary treatment could, therefore, be substantially reduced. Reduction in sludge processing requirements would likely further result in a reduced facility physical footprint and additional reduction in energy use from sludge processing equipment, such as sludge dewatering and thickening. While many processes may be reduced in size due to the use of ABRs, the ABRs themselves may require more physical space than commonly used primary sedimentation basins, which typically have lower hydraulic retention times (i.e., 1.5 to 2.5 hours) and corresponding lower volumes.1 While additional analysis outside the scope of this study is required to quantify footprint modifications, Fig. 4 provides a comparative COD mass balance between a typical WWRF with conventional primary treatment and CAS and a WWRF with anaerobic primary treatment using ABRs with CAS. As shown in Fig. 4a, approximately 35% of COD from the influent wastewater goes to CHP post anaerobic digestion, of which approximately 8.5% is recovered as electrical energy and 16% is converted to heat. An additional 25% of influent COD goes to follow-on solids management processes after anaerobic digestion. In comparison, approximately 62% of the influent COD goes to CHP when anaerobic primary treatment and anaerobic digestion of waste activated sludge are employed, of which approximately 15% is converted to electrical energy via CHP and 28.5% is converted to heat. Further, only 20% of the influent COD goes to anaerobic sludge digestion, suggesting that the digester capacity could be reduced by approximately one-third, and only 8% of COD goes to follow-on solids management processes after anaerobic digestion (Fig. 4b).
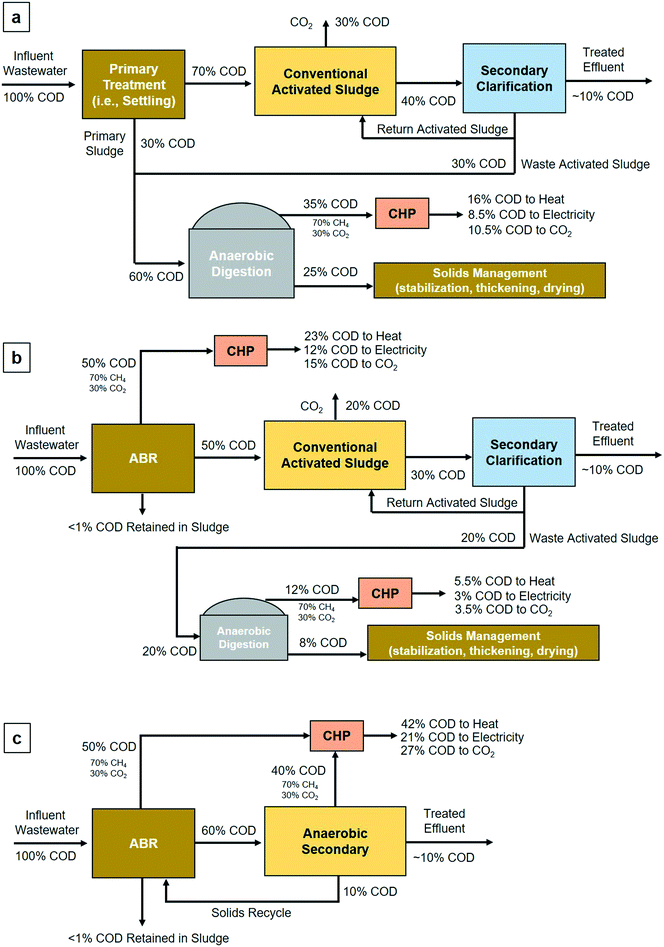 | ||
| Fig. 4 COD mass balances for: (a) conventional activated sludge with primary treatment (i.e., gravitational settling), anaerobic digestion, and CHP; (b) conventional activated sludge with anaerobic primary (i.e., ABR), anaerobic digestion of waste activated sludge, and CHP; (c) anaerobic primary with an ABR coupled to an anaerobic secondary treatment process with CHP. COD mass balance for configuration (a) was adapted from Tchobanoglous et al. (2003),1 WEF (2007),42 and Wan et al. (2016).43 COD mass balance for configurations (b) and (c) were adopted from observed COD removal in anaerobic primary, Tchobanoglous et al. (2003)1 and the performance of AnMBRs for anaerobic secondary (Smith et al. 2012).49 Configuration (b) does not require two separate CHP processes; however, two are displayed for visual simplicity. Dissolved CH4 was assumed to move from the ABR to secondary treatment processes (e.g., conventional activated sludge or anaerobic secondary). For configuration (c), dissolved CH4 was assumed removed via stripping and is included in the COD fraction transferred to CHP. | ||
3.5. Path forward for anaerobic primary treatment using ABRs
While anaerobic primary treatment using ABRs is a promising energy-generating technology, further research is required prior to widespread implementation of full-scale systems. First, pilot-or full-scale anaerobic demonstrations coupled with aerobic secondary treatment, i.e., conventional activated sludge, need to be constructed and anaerobically recalcitrant COD removal characterized to determine if discharge standards are achieved. More promising than ABRs coupled with CAS is a treatment configuration coupling ABRs with a subsequent low-complexity anaerobic secondary treatment technology, such as an AFFR; however, further research is needed before full-scale low-complexity demonstrations anaerobic technologies are viable. Fig. 4c provides a generic COD mass balance for an ABR coupled to anaerobic secondary treatment process. Here, approximately 90% of COD from the influent wastewater goes to CHP with 21% conversion to electrical energy and 42% conversion to heat. This paradigm more than doubles the COD converted to CH4 and the anticipated electrical energy production.Second, any paradigm centered on anaerobic treatment of wastewater for carbon removal and CH4 generation will require further treatment for the constituents of anaerobic effluents, which include ammonia, phosphorus, hydrogen sulfide, and dCH4.44 Aerobic secondary, e.g. CAS, with anoxic denitrification is a common method for removing nitrogen; however, this approach can be energy-intensive.8 Anoxic denitrification could, however, have the tangential benefit of using dCH4 as an electron acceptor for denitrification, thereby reducing CH4 volatilization to the atmosphere and reducing greenhouse gas emissions. Aerobic methanotrophic activity in an aerobic secondary process would also likely remove the majority of dCH4 prior to volatilization.9,45,46 A possible low-energy solution that simultaneously removes carbon and nitrogen is partial nitritation coupled with anammox; however, full-scale mainstream demonstrations to date are limited.47,48 Several recent studies discuss approaches to biological and mechanical removal of dCH4 from anaerobic effluents.17,25,49 Such approaches include biogenic capture with the downflow hanging sponge, membrane degasification, and dCH4 recovery for energy generation using microbial fuel cells;25,50–54 however, no approach has been demonstrated to be energetically or economically viable at full-scale and none are ready for mainstream wastewater treatment. Recovery of dCH4 is imperative as volatilization to the atmosphere represents both a loss of energy and substantial increase in greenhouse gas emissions.
Third, practical barriers to widespread implementation must be addressed. Several studies have identified barriers to the beneficial use of biogas from anaerobic digestion of primary and waste activated sludge, which may be applicable to implementation of ABRs for anaerobic primary treatment with CHP.40,55 Identified barriers were mainly economic in nature (e.g., capital costs, operations and maintenance costs, limited availability of grants or loans), but technical (e.g., concerns over biogas cleaning requirements), social (e.g., lack of community interest), and regulatory (e.g., permitting requirements) barriers were also identified.40,55 While barriers are likely to vary by location, thorough study of each barrier category (i.e., economic, technical, social, and regulatory) will be required prior industry acceptance.55
4. Conclusions
Observed CH4 generation from two pilot-scale ABRs operating for more than 2400 total days indicates that reactors produce between 0.31 and 0.40 L CH4 per g tCOD removed, which equates to potential effective energy production from gaseous CH4 of 2.0 ± 1.2 kW h kg−1 COD removed or 76 ± 20% energy recovery efficiency. Observed CH4 production was most closely modeled by using tCOD measurements to predict CH4 generation. Observed CH4 production was also higher than values reported for pilot-scale or larger UASBs and bench-scale ABRs in other studies, likely due to degradation of particulate COD and settled solids as well as endogenous decay in the sludge blanket over time. Scenario modeling using Monte Carlo simulations suggests that energy generated from ABR gaseous CH4via CHP with heat recovery is enough to power coupled CAS systems, but that capture of dCH4 is required to enhance energy generation. Results of this study suggest that use of ABRs as biologically enhanced primary treatment with solids digestion for CAS systems, or as part of future complete anaerobic systems, is a viable wastewater treatment paradigm. The replacement of conventional primary treatment with multiple-compartment anaerobic bioreactors would enhance onsite energy-generating potential and reduce solids production at water resource recovery facilities.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The study was supported by the U.S. National Science Foundation (NSF) under CBET-1512787 and by the U.S. NSF Engineering Research Center (ReNUWIt) (EEC-1028968). The authors thank Mike Veres, Tani Cath, Kate Spangler, Mengyuan Yu, and Brett Van Houghton for their technical assistance.References
- G. Tchobanoglous, F. Burton and H. Stensel, Wastewater Engineering Treatment and Reuse, McGraw Hill, 2003 Search PubMed.
- E. S. Heidrich, T. P. Curtis and J. Dolfing, Determination of the Internal Chemical Energy of Wastewater, Environ. Sci. Technol., 2011, 45, 827–832 CrossRef CAS.
- U.S. EPA Office of Water, Wastewater Management Fact Sheet, Energy Conservation, EPA 832-F-06-024, Washington, DC, 2006, 1–7 Search PubMed.
- T. P. Curtis, ed. R. Mitchell and J.-D. Gu, Low-energy wastewater treatment: Strategies and technologies, in Environmental Microbiology, John Wiley & Sons, Inc., NJ, 2010, pp. 301–318 Search PubMed.
- P. L. McCarty, J. Bae and J. Kim, Domestic wastewater treatment as a net energy producer - can this be achieved?, Environ. Sci. Technol., 2011, 45, 7100–7106 CrossRef CAS PubMed.
- U.S. EPA, Ensuring a Sustainable Future: An Energy Management Guidebook for Wastewater and Water Utilities, 2008 Search PubMed.
- U.S. EPA, Energy Efficiency in Water and Wastewater Facilities, 2013 Search PubMed.
- S. Tarallo, A. Shaw, P. Kohl and R. Eschborn, A Guide to Net-Zero Energy Solutions for Water Resource Recovery Facilities, Water Environment Research Foundation, 2015 Search PubMed.
- A. L. Smith, L. B. Stadler, L. Cao, N. G. Love, L. Raskin and J. Steven, Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: A critical review, Environ. Sci. Technol., 2014, 5972–5981 CrossRef CAS PubMed.
- L. Foresti, Anaerobic treatment of domestic sewage: established technologies and perspectives, Water Sci. Technol., 2002, 45, 181–186 CrossRef CAS PubMed.
- W. P. Barber and D. C. Stuckey, The use of the anaerobic baffled reactor (ABR) for wastewater treatment: A review, Water Res., 1999, 33, 1559–1578 CrossRef CAS.
- D. L. Sills, V. L. Wade and T. D. DiStefano, Comparative life cycle and technoeconomic assessment for energy recovery from dilute wastewater, Environ. Eng. Sci., 2016, 33(11), 861–872 CrossRef CAS.
- G. V. T. Gopala Krishna, P. Kumar and P. Kumar, Treatment of low strength complex wastewater using an anaerobic baffled reactor (ABR), Bioresour. Technol., 2008, 99, 8193–8200 CrossRef CAS.
- B. D. Shoener, I. M. Bradley, R. D. Cusick and J. S. Guest, Energy positive domestic wastewater treatment: the roles of anaerobic and phototrophic technologies, Environ. Sci.: Processes Impacts, 2014, 16, 1204–1222 RSC.
- P. Bachmann, A. Beard and V. McCarty, Performance characteristics of the anaerobic baffled reactor, Water Res., 1985, 19, 99–106 CrossRef.
- A. M. W. Grobicki and D. C. Stuckey, Performance of the anaerobic baffled reactor under steady state and shock loading conditions, Biotechnol. Bioeng., 1991, 37, 344–355 CrossRef CAS.
- M. J. Hahn and L. A. Figueroa, Pilot scale application of anaerobic baffled reactor for biologically enhanced primary treatment of domestic wastewater, Water Res., 2015, 87, 494–502 CrossRef CAS.
- D. Vuono, J. Henkel, J. Benecke, T. Cath, T. Reid, L. Johnson and J. E. Drewes, Flexible Hybrid Membrane Treatment Systems for Tailored Nutrient Management: A New Paradigm in Urban Wastewater Treatment, J. Membr. Sci., 2013, 446, 34–41 CrossRef CAS.
- A. Pfluger, G. Vanzin, J. Munakata-Marr and L. Figueroa, An anaerobic hybrid bioreactor for biologically enhanced primary treatment of domestic wastewater under low temperatures, Environ. Sci.: Water Res. Technol., 2018, 4(11), 1851–1866 RSC.
- American Public Health Association, American Water Works Association and Water Environment Federation, Standard Methods for the Examination of Water and Wastewater, Washington, D.C., 21st edn, 2005 Search PubMed.
- A. R. Pfluger, W.-M. Wu, A. J. Pieja, J. Wan, K. H. Rostkowski and C. S. Criddle, Selection of Type I and Type II methanotrophic proteobacteria in a fluidized bed reactor under non-sterile conditions, Bioresour. Technol., 2011, 102, 9919–9926, DOI:10.1016/j.biortech.2011.08.054.
- R. R. Erickson, Stoichiometry and kinetics of the microbial degradation of substrate in an anaerobic baffeld reactor, ProQuest Dissertations Publishing, 10744717, Coloradd School of Mines, 2018 Search PubMed.
- J. Kim, K. Kim, H. Ye, E. Lee, C. Shin, P. L. McCarty and J. Bae, Anaerobic fluidized bed membrane bioreactor for wastewater treatment, Environ. Sci. Technol., 2011, 45, 576–581 CrossRef CAS.
- U.S. EPA Combined Heat and Power Partnership, Catalog of CHP Technologies, Sept. 2017.
- S. Chen and A. L. Smith, Methane-driven microbial fuel cells recover energy and mitigate dissolved methane emissions from anaerobic effluents, Environ. Sci.: Water Res. Technol., 2018, 4, 67–79 RSC.
- J. A. Álvarez, I. Ruiz, M. Gómez, J. Presas and M. Soto, Start-up alternatives and performance of an UASB pilot plant treating diluted municipal wastewater at low temperature, Bioresour. Technol., 2006, 97, 1640–1649 CrossRef.
- J. A. Álvarez, E. Armstrong, M. Gómez and M. Soto, Anaerobic treatment of low-strength municipal wastewater by a two-stage pilot plant under psychrophillic conditions, Bioresour. Technol., 2008, 99, 7051–7062 CrossRef.
- J. A. Álvarez, C. A. Zapico, M. Gómez, J. Presas and M. Soto, Anaerobic hydrolysis of a municipal wastewater in a pilot scale digester, Water Sci. Technol., 2003, 47, 223–230 CrossRef.
- P. Barros, I. Ruiz and M. Soto, Performance of an anaerobic digester-constructed wetland system for a small community, Ecol. Eng., 2008, 33, 142–149 CrossRef.
- M. Halalsheh, Z. Sawajneh, M. Zu'bi, G. Zeeman, J. Lier, M. Fayyad and G. Lettinga, Treatment of a strong domestic sewage in a 96 m3 UASB reactor operated at ambient temperatures: two-stage versus single-stage reactor, Bioresour. Technol., 2005, 96, 577–585 CrossRef CAS.
- J. J. Bogte, A. M. Breure and J. G, van Andel and G. Lettinga, Anaerobic treatment of domestic wastewater in small scale UASB reactors, Water Sci. Technol., 1993, 27(9), 75–82 CrossRef CAS.
- S. M. M. Vieira and A. D. Garcia Jr, Sewage Treatment by UASB reactor. Operation results and recommendations for design and operation, Water Sci. Technol., 1992, 25(7), 143–157 CrossRef CAS.
- T. H. Yang, P.Y., Moengangongo, Operational stability of a horizontally baffled-anaerobic reactor for diluted swine wastewater in the tropics, Trans. ASAE, 1987, 30, 1105–1110 Search PubMed.
- D. M. Boopathy, R., Sievers, Performance of a modified anaerobic baffled reactor to treat swine waste, Trans. ASAE, 1991, 34, 2573–2578 Search PubMed.
- G. V. T. Gopala Krishna, P. Kumar and P. Kumar, Treatment of low-strength soluble wastewater using an anaerobic baffled reactor (ABR), J. Environ. Manage., 2009, 90, 166–176 CrossRef CAS.
- I. Shizas and D. M. Bagley, Experimental determination of energy content of unknown organics in municipal wastewater streams, J. Energy Eng., 2004, 130, 45–53 CrossRef.
- L. Zhang, T. L. Hendrickx, C. Kampman, H. Temmink and G. Zeeman, Co-digestion to support low temperature anaerobic pretreatment of municipal sewage in a UASB–digester, Bioresour. Technol., 2013, 148, 560–566 CrossRef CAS.
- L. Zhang, J. De Vrieze, T. L. Hendrickx, W. Wei, H. Temmink, H. Rijnaarts and G. Zeeman, Anaerobic treatment of raw domestic wastewater in a UASB-digester at 10 C and microbial community dynamics, Chem. Eng. J., 2018, 334, 2088–2097 CrossRef CAS.
- A. Pfluger, M. Hahn, A. Hering, J. Munakata-Marr and L. Figueroa, Statistical exposé of a multiple-compartment anaerobic reactor treating domestic wastewater, Water Environ. Res., 2018, 90, 530–542, DOI:10.2175/106143017X15131012153068.
- J. Willis, L. Stone, K. Durden, N. Beecher, C. Hemenway and R. Greenwood, Barriers to Biogas Use for Renewable Energy, Water Environment Research Foundation, London, 2012 Search PubMed.
- Electric Power Research Institute and Water Research Foundation, Electricity use and management in the municipal water supply and wastewater industries, Report 3002001433, Palo Alto, CA, November 2013.
- WEF, Design of Municipal Wastewater Treatment Plants, 2007.
- J. Wan, J. Gu, Q. Zhao and Y. Liu, COD capture: a feasible option towards energy self-sufficient domestic wastewater treatment, Sci. Rep., 2016, 6, 1–9 CrossRef.
- J. Delgado Vela, L. B. Stadler, K. J. Martin, L. Raskin, C. B. Bott and N. G. Love, Prospects for biological nitrogen removal from anaerobic effluents during mainstream wastewater treatment, Environ. Sci. Technol. Lett., 2015, 2, 234–244 CrossRef CAS.
- M. R. J. Daelman, E. M. van Voorthuizen, U. G. J. M. van Dongen, E. I. P. Volcke and M. C. M. van Loosdrecht, Methane emission during municipal wastewater treatment, Water Res., 2012, 46, 3657–3670 CrossRef CAS.
- M. Waki, T. Yasuda, H. Yokoyama, D. Hanajima, A. Ogino, K. Suzuki, T. Yamagishi, Y. Suwa and Y. Tanaka, Nitrogen removal by co-curring methane oxidation, denitrification, aerobic ammonia oxidation, and anammox, Appl. Microbiol. Biotechnol., 2009, 84, 977–985 CrossRef CAS.
- S. Lackner, E. M. Gilbert, S. E. Vlaeminck, A. Joss, H. Horn and M. C. M. van Loosdrecht, Full-scale partial nitritation/anammox experiences - An application survey, Water Res., 2014, 55, 292–303 CrossRef CAS PubMed.
- M. Laureni, P. Falås, O. Robin, A. Wick, D. G. Weissbrodt, J. L. Nielsen, T. A. Ternes, E. Morgenroth and A. Joss, Mainstream partial nitritation and anammox: long-term process stability and effluent quality at low temperatures, Water Res., 2016, 101, 628–639 CrossRef CAS.
- A. L. Smith, L. B. Stadler, N. G. Love, S. J. Skerlos and L. Raskin, Perspectives on anaerobic membrane bioreactor treatment of domestic wastewter: A critical review, Bioresour. Technol., 2012, 122, 149–159 CrossRef CAS.
- J. Cookney, A. Mcleod, V. Mathioudakis, P. Ncube, A. Soares, B. Jefferson and E. J. McAdam, Dissolved methane recovery from anaerobic effluents using hollow fibre membrane contactors, J. Membr. Sci., 2016, 502, 141–150 CrossRef CAS.
- J. Cookney, E. Cartmell, B. Jefferson and E. J. McAdam, Recovery of methane from anaerobic process effluent using poly-di-methyl-siloxane membrane contactors, Water Sci. Technol., 2012, 65, 604–610 CrossRef CAS.
- M. Hatamoto, T. Miyauchi, T. Kindaichi, N. Ozaki and A. Ohashi, Dissolved methane oxidation and competition for oxygen in down-flow hanging sponge reactor for post-treatment of anaerobic wastewater treatment, Bioresour. Technol., 2011, 102, 10299–10304 CrossRef CAS.
- M. Hatamoto, H. Yamamoto, T. Kindaichi, N. Ozaki and A. Ohashi, Biological oxidation of dissolved methane in effluents from anaerobic reactors using down-flow hanging sponge reactor, Water Res., 2010, 44, 1409–1418 CrossRef CAS.
- N. Matsuura, M. Hatamoto, H. Sumino, K. Syutsubo, T. Yamaguchi and A. Ohashi, Closed DHS system to prevent dissolved methane emissions as greenhouse gas in anaerobic treatment by its recovery and biological oxidation, Water Sci. Technol., 2010, 61, 2407–2415 CrossRef CAS.
- A. Pfluger, J. Coontz, V. Zhiteneva, T. Gulliver, L. Cherry, L. Cavanaugh and L. Figueroa, Anaerobic digestion and biogas beneficial use at municipal wastewater treatment facilities in Colorado: A case study examining barriers to widespread implementation, J. Cleaner Prod., 2019, 206, 97–107 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00526a |
| This journal is © The Royal Society of Chemistry 2020 |