Removing scale-forming cations from produced waters†
Karen
Shafer-Peltier
 a,
Colton
Kenner
b,
Eric
Albertson
c,
Ming
Chen
a,
Colton
Kenner
b,
Eric
Albertson
c,
Ming
Chen
 ab,
Stephen
Randtke
b and
Edward
Peltier
ab,
Stephen
Randtke
b and
Edward
Peltier
 *b
*b
aUniversity of Kansas, Tertiary Oil Recovery Program, 1530 W. 15th St., Lawrence, KS 66045, USA
bUniversity of Kansas, Department of Civil, Environmental, and Architectural Engineering, 1530 W. 15th St., Lawrence, KS 66045, USA. E-mail: epeltier@ku.edu; Fax: +1 785 864 5631; Tel: +1 785 864 2941
cUniversity of Kansas, Department of Chemical and Petroleum Engineering, 1530 W. 15th St., Lawrence, KS 66045, USA
First published on 12th November 2019
Abstract
The formation of precipitates (scales) during reinjection limits the reuse of oil and gas production water (produced water) for additional oil recovery. Selective removal strategies that target Ba and Sr, the primary scale-forming cations, would limit produced water treatment costs, reduce waste generation, and increase produced water reuse. A novel treatment technique for targeted Ba and Sr removal, complexation with polyelectrolyte polymers, is compared with chemical precipitation (sulfate addition and precipitative softening) for the removal of Ba and Sr from Kansas oil field brines. Four polymers were examined for cation removal, both with and without ultrafiltration: poly-vinyl sulfonate (PVS), poly(4-styrenesulfonate) (PSS), polyacrylic acid (PAA), and poly(4-styrenesulfonic acid-co-maleic acid) (PSSM). PSSM and PSS were effective for Ba and Sr removal from the lower salinity brine (TDS of 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1), but exhibited limited Sr removal in the absence of Ba in the high salinity brine (TDS of 92
000 mg L−1), but exhibited limited Sr removal in the absence of Ba in the high salinity brine (TDS of 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg l−1). Similar results were achieved in both brines using sulfate addition. PSSM used in conjunction with ultrafiltration removed >99% of initial Sr and Ba from the lower salinity brine, while removing only 65% and 78% of Mg and Ca, respectively. These results compare favorably to precipitative softening, which removed >90% of all divalent cations from the same brine but was less selective for Ba and Sr. PAA plus ultrafiltration removed 58% of Sr (and 68% of Ca) from the high-salinity brine at pH 9. While increased Sr removal can be achieved by polymer-assisted ultrafiltration, further development of this process, including methods for polymer recovery and regeneration, will be needed to improve its performance compared to precipitative softening.
000 mg l−1). Similar results were achieved in both brines using sulfate addition. PSSM used in conjunction with ultrafiltration removed >99% of initial Sr and Ba from the lower salinity brine, while removing only 65% and 78% of Mg and Ca, respectively. These results compare favorably to precipitative softening, which removed >90% of all divalent cations from the same brine but was less selective for Ba and Sr. PAA plus ultrafiltration removed 58% of Sr (and 68% of Ca) from the high-salinity brine at pH 9. While increased Sr removal can be achieved by polymer-assisted ultrafiltration, further development of this process, including methods for polymer recovery and regeneration, will be needed to improve its performance compared to precipitative softening.
Water impactThis work examines the removal of scale-forming cations from oil and gas wastewater (produced water) using polyelectrolyte-enhanced ultrafiltration, a novel technology. Polyelectrolytes were more selective than chemical precipitation for removing Ba and Sr, the most common scale-forming elements. Improved cation removal will reduce one of the major barriers to increased reuse of produced water as a substitute for fresh water. |
1. Introduction
Global oil and gas (O&G) production was approximately 98 million barrels (16 billion liters) per day in 2017.1 O&G production is very water-intensive, both in its requirements for freshwater and in the production of formation water (or produced water) extracted from subsurface reservoirs.2 Overall, more than 20 billion barrels (3.2 trillion liters) of produced water are generated in the US each year, with a national average of >9 barrels of water generated per barrel of oil produced.3 The volume of produced water can be more than 10 times the volume of oil produced over the life of an oil field, increasing as a field matures. Disposal of this produced water has the potential for significant environmental impacts.4–6 Underground injection control wells have been connected to increased seismic activity in the United States,7 while surface disposal can have secondary impacts on water and sediment quality8,9 At the same time, oil and gas production often requires large volumes of water. Increasing reuse of produced water for oil production could thus provide important economic and conservation benefits by reducing disposal volumes while also reducing industry demands for fresh water.10–13A major issue limiting water reuse is the formation of precipitates (scales) during re-injection. These scales can plug injection and production wells, and coat production tubing and surface equipment, increasing costs and even shutting down operations.12,14–16 The most common cause of scale formation in oil and gas production activities is supersaturation with respect to sulfate (and to a lesser extent carbonate) salts of Ca, Sr, and Ba.16–19 Scale precursors can originate from the injection water, from dissolution of formation materials as water flows through the formation, or both, as when sulfate-containing waters are injected into a reservoir containing barium. Changes in temperature, pressure, and acidity can also contribute to scale buildup. While carbonate and hydroxide scales can easily be dissolved in acid,17,19,20 BaSO4 (barite), SrSO4 (celestite), and (Ba,Sr)SO4 (co-precipitates), once formed, are very difficult to remove. Selective removal of Ba and Sr from produced waters could thus reduce treatment requirements and waste disposal costs while also reducing the potential for sulfate scale formation during reuse.
Precipitation processes that rely on the insolubility of sulfate and carbonate scales to pre-emptively remove divalent cations during a controlled treatment process are relatively simple and inexpensive to implement. In single cation systems (no competing ions, organic material, or other interferents) the solubility of relevant salts is as follows: MgSO4 ≫ CaSO4 > SrSO4 > MgCO3 > SrCO3 > CaCO3 > BaCO3 ≫ BaSO4.17,18 Mg(OH)2 is also formed at high pH and is highly insoluble under those conditions. Precipitative softening with either lime or caustic soda has been widely used in produced water treatment for control of water hardness.4,21–23 Few studies, however, have directly addressed Ba and Sr removal by precipitative softening processes. As shown in Table 1, there is a wide range in the effectiveness of these different procedures, particularly for Sr removal. This variation is likely related to the wide variation in additives and in final pH during the treatment process. In fresh waters, however, lime softening has been effective at Ba and Sr removal.24,25 Sodium sulfate (Na2SO4) addition is also an industry-accepted method for removing alkaline earth metals from water to prevent scale formation.17 Studies using acid-mine drainage as a sulfate source have achieved good removal of Ba from Marcellus shale produced water (Table 1). Sr is removed in these processes primarily through substitution into barite, raising questions about the effectiveness of this process for Sr removal in waters containing lower Ba concentrations.26,27
| Additive | Water | pHa | Ba | Sr | Other | Ref. |
|---|---|---|---|---|---|---|
| a Adjusted pH used for softening processes. | ||||||
| Lime + alum | Illinois Basin Oilfield PW | 7.3–7.6 | 21% | No removal | No removal for Ca or Mg | 33 |
| NaOH | Oilfield PW, Kern County CA | 9.3 | 98% | 97% | 91% removal of total hardness | 34 |
| NaOH + alum | Marcellus shale PW | 10 | 48% | 20% | 46% and 19% removal of Ca and Mg | 35 |
| Na2SO4 | Synthetic flowback water | NA | 55–100% | 4–37% | Removal increased with sulfate concentration | 36 |
| Acid mine drainage | Marcellus shale flowback fluid | NA | >99% | 70% | Sr co-precipitated with barite | 26 |
| PAA | Reno County, KS PW | NA | 73% | 66% | 4 sequential additions of PAA | 32 |
The use of polymers, both sulfonates and carboxylates, to bind metals, followed by removal through ultrafiltration has been proposed previously for the removal from aqueous solution of a long list of metal cations, including Ag, As, Cu, Co, Ni, Cd, Zn, Pb, Ca, Mg, Sr, Cr, and Al.28–30 In previous studies, members of this project team explored divalent and monovalent cation affinity for two polyelectrolytes, poly(4-styrenesulfonate) (PSS) and polyacrylic acid (PAA) in both low and high salinity brine solutions.31,32 Both polyelectrolytes have a strong preference for Ba2+ complexation over other common produced water divalent cations (Ca2+, Sr2+, Mg2+), while PSS also prefers Sr2+ to Ca2+ and Mg2+. PAA formed polyelectrolyte complex precipitates with all scale-forming compounds, but this precipitation was inhibited by high concentrations of monovalent cations. An initial experiment was conducted using PAA to remove Ba and Sr from a field-collected high-salinity produced water (TDS = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg l−1) containing 3.6 mg l−1 B and 1800 mg l−1 Sr. After 4 sequential addition and separation steps, this experiment achieved 73% and 67% removal of Ba and Sr, respectively, while also removing >60% of Ca2+ and Mg2+.32
000 mg l−1) containing 3.6 mg l−1 B and 1800 mg l−1 Sr. After 4 sequential addition and separation steps, this experiment achieved 73% and 67% removal of Ba and Sr, respectively, while also removing >60% of Ca2+ and Mg2+.32
The current study investigates the removal of divalent cations (Ba2+, Sr2+, Ca2+, and Mg2+) from produced water as a stand-alone treatment strategy to reduce scale-formation potential of the treated waters. The goal of this treatment strategy is to decrease scale-formation potential and thereby increase produced water reuse for further oil production with minimal additional treatment, such as reverse osmosis or nanofiltration, which are costly and not ideal for treating high salinity brines.20,37 Chemical precipitation using sulfate addition and precipitative softening was used to treat two different field-collected oil-field brines. The results were compared to cation removal through complexation with four polyelectrolytes (PSS, PAA, poly-vinyl sulfonate (PVS), and poly(4-styrenesulfonic acid-co-maleic acid) (PSSM)) commonly used as scale inhibitors in the oil industry. Removal of precipitated or complexed cations was accomplished by either settling/centrifugation or ultrafiltration.
2. Experimental
2.1. Produced water collection
Produced water samples were collected from two locations in Kansas, one in Douglas County (DC) and one in Reno County (RC). Both waters were collected at the pump, with pH, conductivity, and temperature measured immediately in the field. The waters were then filtered in the field using a 0.2 micron cellulose-acetate filter, stored on ice during transport, and later stored in a laboratory refrigerator at 4 °C. At no point during transport did the samples freeze. No other sample pretreatment occurred. The initial characteristics of the two produced waters are shown in Table 2.| Douglas County (DC) | Reno County (RC) | |
|---|---|---|
| Note: concentrations are reported to 2 significant figures to reflect an overall CV of 10%. | ||
| Temperature, °C | 18.6 | 34.5 |
| pH | 6.6 | 6.2 |
| Specific conductivity (μS cm−1) | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Total organic carbon, mg L−1 | 3.9 | 48 |
| Total dissolved solids, mg L−1 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Total alkalinity, mg l−1 as CaCO3 | 700 | 180 |
| Sodium, mg L−1 | 9000 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Calcium, mg L−1 | 600 | 5600 |
| Strontium, mg L−1 | 80 | 1800 |
| Magnesium, mg L−1 | 260 | 1600 |
| Barium, mg L−1 | 430 | 3.6 |
| Chloride, mg L−1 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Bromide, mg L−1 | 46 | 300 |
| Bicarbonate, mg L−1 | 850 | 220 |
| Sulfate, mg L−1 | 3.0 | 110 |
Despite being collected from wells geographically located within 200 miles of each other, the two brines are quite different. The DC brine has a TDS concentration of 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 with both barium and strontium present, whereas the RC brine has a TDS concentration of 92
000 mg L−1 with both barium and strontium present, whereas the RC brine has a TDS concentration of 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1, a Sr concentration of 1800 mg L−1, and very little Ba. The RC brine does, however, have a significant sulfate concentration (110 mg L−1) that the DC brine does not have, while the DC brine has a higher bicarbonate concentration. In general, produced waters can also contain dispersed oils, production chemicals such as polymers and surfactants, and other organic components.19,38–40 The TOC concentrations for these two brines, however, were relatively small, (3.9 mg L−1 in the DC brine and 48 mg L−1 in the RC brine), indicating little presence of organic constituents. This is probably due to the extent to which the selected fields have previously been waterflooded.
000 mg L−1, a Sr concentration of 1800 mg L−1, and very little Ba. The RC brine does, however, have a significant sulfate concentration (110 mg L−1) that the DC brine does not have, while the DC brine has a higher bicarbonate concentration. In general, produced waters can also contain dispersed oils, production chemicals such as polymers and surfactants, and other organic components.19,38–40 The TOC concentrations for these two brines, however, were relatively small, (3.9 mg L−1 in the DC brine and 48 mg L−1 in the RC brine), indicating little presence of organic constituents. This is probably due to the extent to which the selected fields have previously been waterflooded.
The DC brine was chosen for method optimization as it is representative of Kansas brines that contain both barium and strontium; however, four of the polymers were tested against the RC brine as well to evaluate performance in the absence of barium and corresponding presence of sulfate.
2.2. Produced water characterization
Alkaline earth metals were measured by inductively coupled plasma-optical emission spectrometry (ICP-OES) immediately upon receipt in the lab, and then re-measured each time a sample was treated to establish the appropriate control value for a particular experiment. A Perkin Elmer Optima 2000 ICP-OES was used to measure Na, Ca, Sr, Mg, and Ba in the initial brines. All samples were diluted 200-fold in 2% nitric acid (Fisher Scientific PN A509) in deionized water (DI, 18.2 MΩ cm type 1 water prepared using a Milli-Q Direct 8 system) prior to analysis to achieve a sample pH of less than 2 as required by EPA method 200.7.41 Additional dilutions with 2% nitric acid were made as necessary to bring sample concentrations within the instrument's range.Temperature, pH, and total dissolved solids (TDS) were measured in the field using an Accumet AP85 portable meter. Total alkalinity and anion concentrations were measured immediately upon return of the sample to the laboratory, for characterization purposes only. Alkalinity was measured using a standard titration method. Reported bicarbonate values were calculated using the pH and alkalinity results. All other anion measurements were made using ion chromatography (Dionex ICS-2000, Ion Pac AS18 analytical column). Total organic carbon was measured using a Teledyne TORCH TOC analyzer.
2.3. Sulfate addition
A 0.2 M Na2SO4 solution, prepared by dissolving sodium sulfate (Na2SO4, Fisher Scientific PN S429) in DI water (type 1), was added to the oil-field brine in increasing amounts to induce sulfate precipitation. DI water was also added as appropriate to maintain a constant final solution volume. Samples were mixed using a vortex mixer and then allowed to sit for 24 hours (unless otherwise noted). These solutions were then centrifuged to separate out the precipitate (Centrifugation was later found to have no impact on cation removal using this method). Mg, Ca, Sr, and Ba concentrations in the supernatant were measured by ICP after acidification and dilution.2.4. Precipitative softening
Precipitative softening18 was accomplished through additions of sodium hydroxide (NaOH 50% solution, Fisher Science PN SS254-1) and sodium carbonate (Na2CO3, Fisher Science PN S263). NaOH, rather than lime (Ca(OH)2), was used to adjust the pH to avoid adding additional calcium to the samples. As calcium was the most common divalent cation in both brines, the lack of additional calcium should not substantially alter the softening mechanism. Furthermore, nearly all of the calcium in the brines was non-carbonate hardness, so lime addition would initially have resulted in little or no removal of calcium and would in fact have simply increased the calcium concentration, in proportion to the lime dosage, once any carbonate alkalinity present had been consumed.The brine pH was first adjusted using the NaOH solution until a pH greater than 11 was achieved. Increasing quantities of a 0.2 M solution of Na2CO3 were then added to achieve final solution concentrations up to 0.1 M in the final solution. For higher dosages of Na2CO3, powdered Na2CO3 was added directly to the brine. Finally, additional DI water was added to maintain a constant solution volume constant. These solutions were vortexed and then allowed to settle for 24 hours.
Initially, samples were centrifuged to separate out the precipitates. As with the sulfate experiments, this step was subsequently determined to have no impact on final dissolved cation concentration. At 24 hours, settling was complete, and the treated supernatant solution could be easily collected from the top of the centrifuge tube. Mg, Ca, Sr, and Ba in the treated solutions were measured by ICP after acidification and dilution.
2.5. Polymer addition
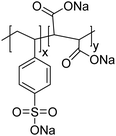
The polymers used in these experiments (Table 3) included one carboxylate-containing polymer, polyacrylic acid (PAA); two formulations of poly(4-styrenesulfonic acid-co-maleic acid) (PSSM), a co-block polymer containing sulfate and carboxylate moieties in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
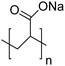
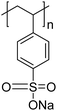
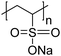
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios, respectively; and two sulfonate-containing polymers, poly-vinyl sulfonate (PVS) and poly(4-styrenesulfonate) (PSS). The average molecular weights were 100 kD for PAA, 20 kD for both of the PSSM formulations, 70 kD for PSS, and 4–6 kD for PVS.
1 ratios, respectively; and two sulfonate-containing polymers, poly-vinyl sulfonate (PVS) and poly(4-styrenesulfonate) (PSS). The average molecular weights were 100 kD for PAA, 20 kD for both of the PSSM formulations, 70 kD for PSS, and 4–6 kD for PVS.
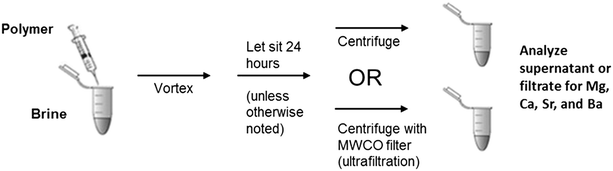
Cation removal using these polymers was tested in the manner depicted in Fig. 1. The polymer, diluted in DI water, was added directly to brine held in a tightly capped centrifuge tube. The sample was vortexed briefly and then allowed to sit for a specified period of time (24 hours unless stated otherwise).
After sitting, the sample was either centrifuged directly in the same tube or transferred to a 3 kD molecular weight cut-off (MWCO) combination filter/centrifuge tube (Vivaspin® 500, PN VS0192, 1.5 mL). Tubes were centrifuged between 15 and 90 minutes at 17.0 G for ultrafiltration. Although no significant differences in cation removal were observed between centrifuged and uncentrifuged samples when testing sulfate addition and precipitative softening, the centrifugation step was retained for polymer processing because no visible precipitate was formed. Once centrifugation was complete, the supernatant or filtered portion was removed from the tube, acidified, and diluted for ICP analysis.
In some cases (indicated where applicable), the pH of the polymer solution was adjusted using either sodium hydroxide (50% NaOH, Fisher Science PN SS254-1) or hydrochloric acid (6 N HCl, Ricca PN 375032) prior to addition to the brine. Polymer concentration was controlled through the addition of DI water to a constant volume. The pH values of the unadjusted polymer solutions (12.5% w/v) were as follows: 7.5 for both PSSM(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and PSSM(3
1) and PSSM(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 8.4 for PVS, 2.8 for PSS, and 1.7 for PAA.
1), 8.4 for PVS, 2.8 for PSS, and 1.7 for PAA.
2.6. Variance in the data
Each data point on a graph or in a table represents a single measurement (including repeated measurements on some samples, which were plotted as separate points). In cases where trends were being measured (e.g., addition of increasing concentrations of a chemical) single measurements were taken at each concentration to allow multiple concentrations to be tested. Where trends were less clear, more data points were collected (either at additional concentrations or at a specific concentration) and all data points were included in the figure.Control samples of the brine were analyzed with every experiment. To obtain an estimate of variance in the data measurement itself, the coefficients of variance 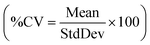 for two data sets (one with 6 samples, the other with 7) were calculated. The data in these sets were collected over a period of weeks and thus included run-to-run instrument variability as well as method variability. Both sets yielded %CVs of less than 10% (6% for one and 9% for the other). Based on the instrument variability and the run-to-run variability given by the coefficients of variance, differences between data points of less than 10% should not be considered significant. Differences greater than 10% may be considered significant within the context of the experiment. All reported error bars reflect this measurement variability.
for two data sets (one with 6 samples, the other with 7) were calculated. The data in these sets were collected over a period of weeks and thus included run-to-run instrument variability as well as method variability. Both sets yielded %CVs of less than 10% (6% for one and 9% for the other). Based on the instrument variability and the run-to-run variability given by the coefficients of variance, differences between data points of less than 10% should not be considered significant. Differences greater than 10% may be considered significant within the context of the experiment. All reported error bars reflect this measurement variability.
3. Results
3.1. Sulfate addition
Adding Na2SO4 to oil-field brine is an effective barium removal technique,42 and addition of as little as 0.020 moles of Na2SO4 per liter of brine achieved complete Ba removal from the DC brine after 48 hours (Fig. 2A). At this same dosage, only about 40% of the Sr was removed. As more Na2SO4 was added, Sr removal initially increased, but further additions had minimal effect, with removal plateauing at approximately 65%. No consistent removal of Ca or Mg was observed due to sulfate addition, and total divalent cation removal therefore did not increase significantly after this point (corresponding to the addition of approximately 0.045 moles Na2SO4 per L brine). Precipitation was clearly observable within the first five minutes, and further testing showed no changes in cation removal at different mixing times (5, 15 and 30 minutes) or standing times (2, 4, 24 and 48 hours) prior to separation.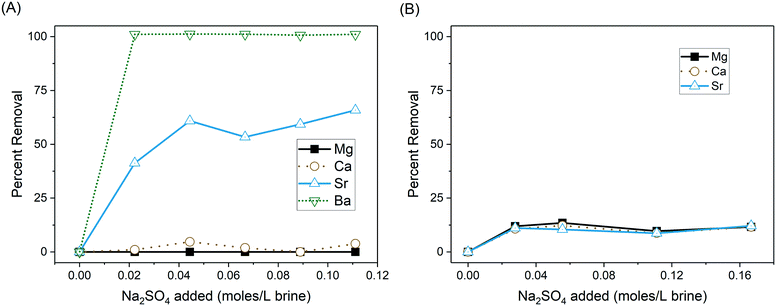 | ||
| Fig. 2 Removal of divalent cations through sodium sulfate addition to (A) DC brine and (B) RC brine. | ||
Strontium removal from the RC brine ranged from 8–12% with Na2SO4 addition, but there was no direct relationship between Sr removal and the amount of sulfate added (Fig. 2B). Ca and Mg were removed in similar proportion, and their removal was similarly unaffected by changes in sulfate addition. Equilibrium calculations using the geochemical speciation program PHREEQC43 indicate that the RC brine system was oversaturated with respect to celestite (SrSO4) formation (SI > 1) at all levels of Na2SO4 addition. However, the formation of strontium sulfate can be kinetically limited in produced waters.36 Additionally, the RC brine had a higher concentration of dissolved organic matter than the DC brine. Other studies have shown that organic acids, particularly carboxylic acids, can have an inhibitory effect on formation of sulfate precipitates.44
Overall, Sr removal from the DC brine accounts for ∼10–15% of total divalent cation removal on a molar basis. This ratio is consistent with previous reports of Sr removal through co-precipitation with barium sulfate,26,27,42,45 although the Sr substitution ratio here is near the low end of reported values. However, there is little evidence for direct precipitation of strontium sulfate in the absence of Ba. This is also consistent with previous reports that celestite formation is very slow when sulfate is added to produced waters.36 Ca and Mg sulfates are relatively soluble under most conditions in water and therefore sulfate addition has little effect on removal of these elements.
3.2. Precipitative softening
Adjustment of the brine to pH 11 using NaOH effectively removed all Mg from solution through formation of Mg(OH)2 (Fig. 3), while carbonate salts of other cations were formed with increasing dosages of Na2CO3 in the order Ca first, then Sr, and finally Ba. In the DC brine, NaOH addition (and its reaction with the brine alkalinity to form carbonate) removed 72%, 55% and 36% of the Ca, Ba and Sr ions. The RC brine required significantly more Na2CO3 to achieve complete removal (0.27 moles per L versus 0.029 moles per L for the DC brine) due to its much higher total divalent cation concentration. This approach to precipitative softening achieved significantly higher levels of Ba and Sr removal than previous softening processes that were targeted at general hardness removal.33,35 However, Ba and Sr removal can only be achieved after first removing calcium, which will require significant additional carbonate addition in many produced waters.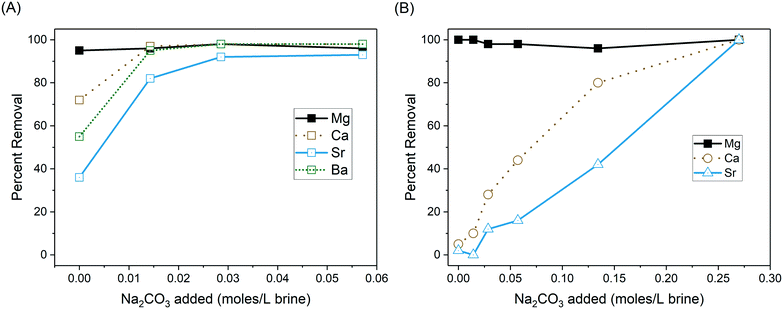 | ||
| Fig. 3 Removal of divalent cations through precipitative softening of (A) DC brine and (B) RC brine. | ||
3.3. Polymer-assisted removal
Results for Ba and Sr removal in the DC brine as a function of added polymer concentration are shown in Fig. 4. The PSS and PSSM polymers removed almost all of the barium and up to 60% of the strontium, although Sr removal required higher polymer doses. The PSSM co-block polymers and PSS performed much better than PAA and PVS for both Ba and Sr removal. PAA was able to achieve complete Ba removal at 3% polymer by weight (compared to 1% for the PSSM and PSS compounds), but no better than 40% Sr removal, while PVS showed little removal of either cation. No appreciable removal of calcium or magnesium was observed using any of the polymers (at dosages up to 10% by weight) in the absence of filtration. Thus, the PSSM and PSS polymers are selective for Ba and Sr removal under these conditions.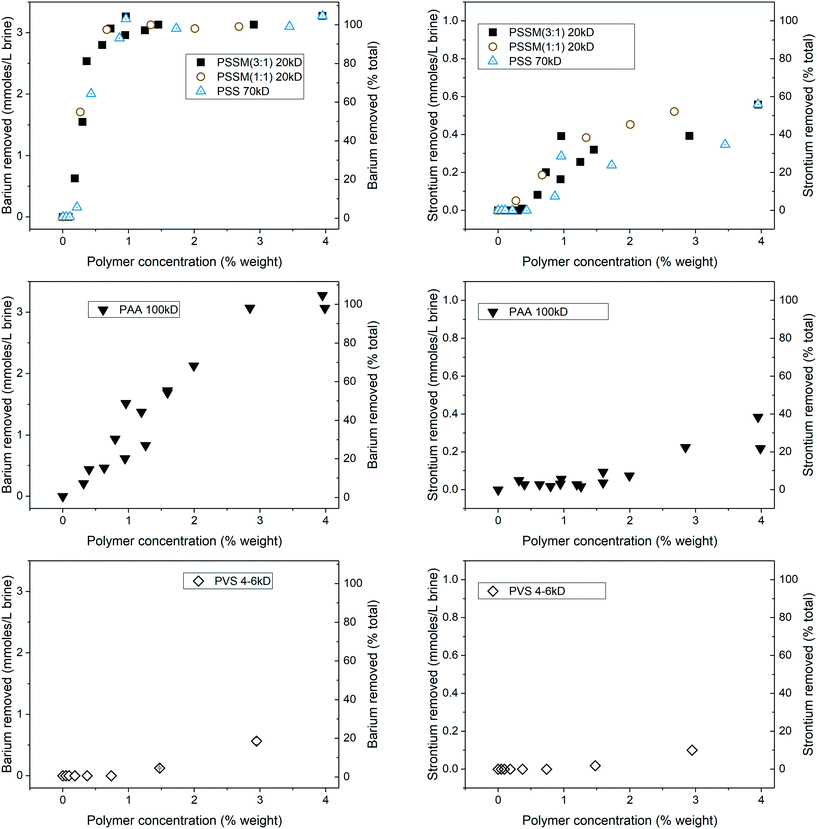 | ||
| Fig. 4 Ba and Sr removal from DC brine using different polymers. No appreciable magnesium or calcium removal was observed using these polymers at concentrations up to 10% by weight (not shown). | ||
Fig. 5 shows cation removal after ultrafiltration from both DC and RC brines with four of the five tested polymers (all except PVS). The use of a 3 kD MWCO filter substantially increased Sr removal for all four of these polymers. PVS, by contrast, did not show increased Sr removal with ultrafiltration, presumably because the PVS molecule has a similar median size (4–6 kD) to the MWCO. Without pH adjustment, PSS had the highest Sr removal percentage from the DC brine, with the PSSM and PAA compounds showing 60–75% removal. Sr removal was lower (as a percentage of initial concentration) from the RC brine, and there was less difference between polymers. For the DC brine, the use of ultrafiltration resulted in significant removal of both Ca and Mg, although at lower percentages than Sr or Ba. For the RC brine, all cations were removed at similar percentages in most cases. Increasing solution pH improved the effectiveness of PAA for cation removal, consistent with previously reported trends for PAA complexation with divalent cations.32 When the pH of the PAA solution was raised to 9 prior to adding it to the brine, all four divalent cations had >90% removal from the DC brine (Fig. 5A). Cation removal from the RC brine was incomplete, but substantially higher than for the other polymers or for the non-pH adjusted PAA at the same 4 wt% addition (Fig. 5B).
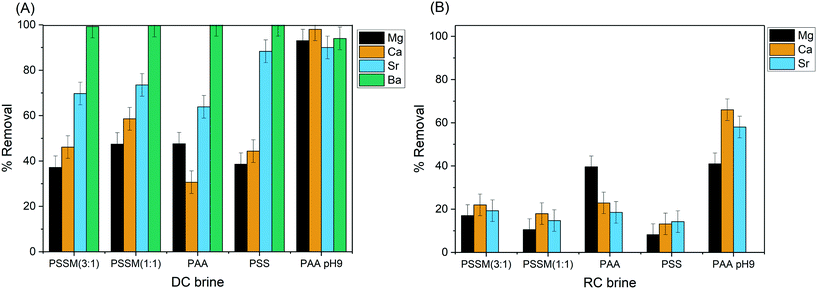 | ||
| Fig. 5 Removal of divalent cations using 4% polymer and ultrafiltration to treat (A) DC brine and (B) RC brine. | ||
Fig. 6 shows cation removal, both with and without ultrafiltration, from the DC brine using PAA (250 kD), PSS (70 kD), and PSSM(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions adjusted to a range of pH values between 2 and 9 prior to addition to the brine. PVS was not used in these additional studies due to poor performance in the initial tests. The impact of pH on cation removal by centrifugation only can be seen in Fig. 6. For the PSSM(3
1) solutions adjusted to a range of pH values between 2 and 9 prior to addition to the brine. PVS was not used in these additional studies due to poor performance in the initial tests. The impact of pH on cation removal by centrifugation only can be seen in Fig. 6. For the PSSM(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), removal of Mg, Ca, and Ba was independent of pH in the absence of filtration, with Ba completely removed under all conditions. Sr removal, however, was strongly affected by pH, with best performance between pH 3 and 5. Sr removal by PSS and PAA was not significantly impacted by pH.
1), removal of Mg, Ca, and Ba was independent of pH in the absence of filtration, with Ba completely removed under all conditions. Sr removal, however, was strongly affected by pH, with best performance between pH 3 and 5. Sr removal by PSS and PAA was not significantly impacted by pH.
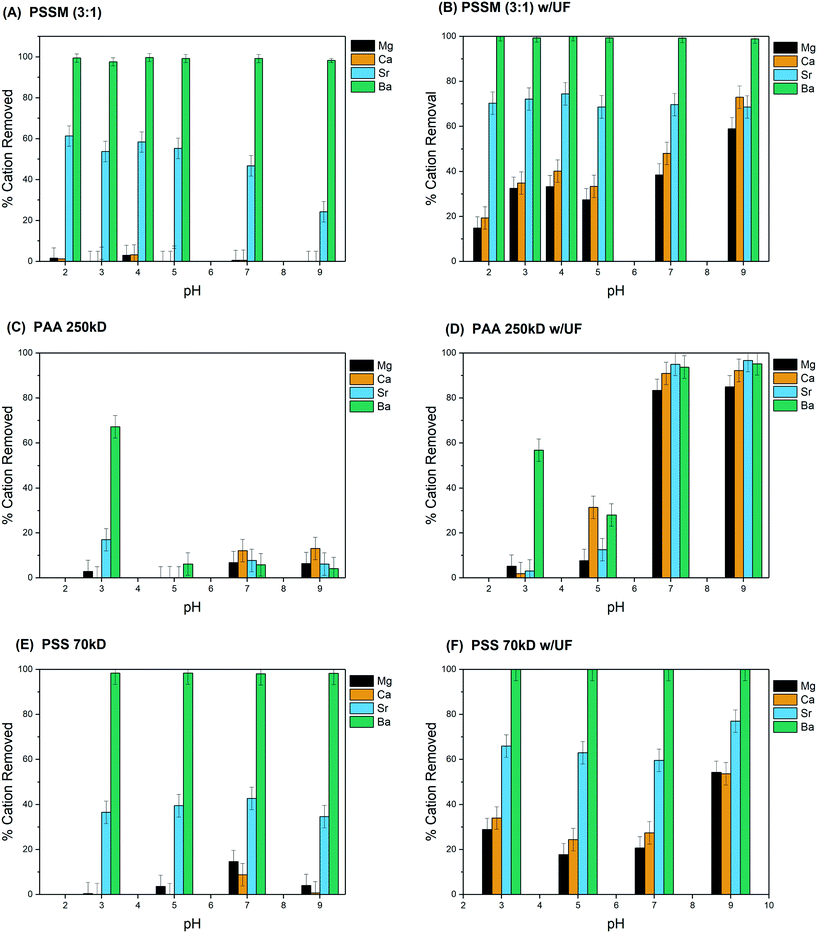 | ||
Fig. 6 pH dependence of divalent cation removal from DC brine using 4% PSSM(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), PAA 250 kD, and PSS 70 kD with and without ultrafiltration. 1), PAA 250 kD, and PSS 70 kD with and without ultrafiltration. | ||
Adding ultrafiltration to the removal process increased removal of Sr, Mg, and Ca with the PSSM(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) across the pH range (Fig. 6B). Ultrafiltration also increased Sr removal with PSS and PAA, although significant removal with PAA was only observed at pH ≥ 7 (Fig. 6D and F). Ca and Mg removal also increased above pH 7, decreasing the selectivity of the removal process. PSS, the only compound without a carboxylate functional group, showed the smallest increase in Ca and Mg removal.
1) across the pH range (Fig. 6B). Ultrafiltration also increased Sr removal with PSS and PAA, although significant removal with PAA was only observed at pH ≥ 7 (Fig. 6D and F). Ca and Mg removal also increased above pH 7, decreasing the selectivity of the removal process. PSS, the only compound without a carboxylate functional group, showed the smallest increase in Ca and Mg removal.
Fig. 7 compares the impact of pH adjustment on Sr removal using both the PSSM(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the PSSM(1
1) and the PSSM(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) polymers. From pH 2–5, more than 50% of the initial strontium can be removed by centrifugation alone, while ultrafiltration increases Sr removal by approximately 15%. As the pH increases above 5, Sr removal becomes less effective in the absence of ultrafiltration. This may indicate that the polymer aggregates less under neutral to basic conditions. Unlike the 3
1) polymers. From pH 2–5, more than 50% of the initial strontium can be removed by centrifugation alone, while ultrafiltration increases Sr removal by approximately 15%. As the pH increases above 5, Sr removal becomes less effective in the absence of ultrafiltration. This may indicate that the polymer aggregates less under neutral to basic conditions. Unlike the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 polymer, the 1
1 polymer, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 polymer shows greater Sr removal at high pH using the 3 kD MWCO filter. These results suggest that optimization of the PSSM polymer could result in higher levels of Sr removal than those reported in our initial tests. Additional parameters, such as polymer molecular weight and reaction timing, were also tested and found to have no effect on cation removal beyond the first few hours.
1 polymer shows greater Sr removal at high pH using the 3 kD MWCO filter. These results suggest that optimization of the PSSM polymer could result in higher levels of Sr removal than those reported in our initial tests. Additional parameters, such as polymer molecular weight and reaction timing, were also tested and found to have no effect on cation removal beyond the first few hours.
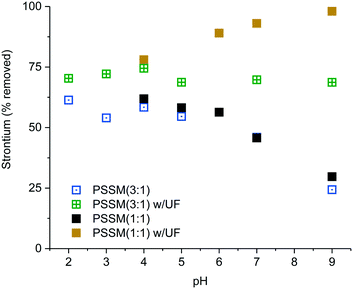 | ||
Fig. 7 pH dependence of Sr removal from DC brine using 4% PSSM(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 3 1 or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with and without ultrafiltration. 1) with and without ultrafiltration. | ||
4. Discussion
Table 4 shows the best results achieved for alkaline earth cation removal using the different methods from this study. These results show that the most effective treatment for Ba and Sr removal will likely vary substantially with the composition of the produced water. In waters with high concentrations of both Ba and Sr and moderate TDS, such as the DC brine, sulfate precipitation is an effective method of removing both elements. While complete Sr removal was not achieved, the sulfate precipitation process leaves Ca and Mg in solution, which limits the mass of solids generated. However, the RC brine results confirm, as others have reported, that sulfate precipitation cannot achieve Sr removal in produced waters where Ba is not already present. The same limitations would presumably apply in waters where Sr concentrations are much higher than Ba, since the major Sr removal pathway appears to be co-precipitation with barite.26,27 Under these conditions, sulfate precipitation will not be effective for controlling Sr concentrations.| % Removal Douglas County brine | % Removal Reno County brine | ||||||
|---|---|---|---|---|---|---|---|
| Mg | Ca | Sr | Ba | Mg | Ca | Sr | |
| a Sulfate addition of 0.11 moles SO4 per L brine. b Addition of 0.057 moles CO3 per L for DC brine and 0.27 moles CO3 per L for RC brine at pH 11. | |||||||
| Sulfate additiona | 0 | 4 | 66 | 100 | 12 | 11 | 11 |
| Precipitative softeningb | 96 | 98 | 93 | 98 | 100 | 100 | 100 |
4% PSSM(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) pH 8 w/UF 1) pH 8 w/UF |
65 | 78 | 100 | 100 | 11 | 18 | 15 |
| 4% PAA pH 9 w/UF | 93 | 98 | 90 | 94 | 41 | 66 | 58 |
Precipitative softening achieved nearly complete removal of both Ba and Sr from both brines, but at very high sodium carbonate dosages, particularly for the RC brine. As Sr removal occurred only after the formation of calcium carbonate, complete removal of Ca is also required. (Mg was also completely removed in these experiments, but that could be minimized by reducing the NaOH addition to maintain a lower pH). In produced waters containing high concentrations of both Ca and Sr, softening will therefore produce large amounts of precipitated sludge that will need to be disposed of as solid waste. This disposal will further add to the costs of this treatment process. Solids disposal could be a particular problem for produced waters containing Ra. While Ra was not included in this study, it is usually assumed to behave similarly to Ba due to their similar chemical properties. Thus, it is likely that Ra would co-precipitate with the other divalent cations when precipitative softening is carried out, which could result in the solid materials requiring disposal as hazardous waste.42
Without the use of ultrafiltration, maximum strontium removal from the DC brine using sulfonated polymers (PSS or PSSM) was similar to that achieved by sulfate precipitation. Polymer aggregation and settling (and therefore Sr removal) may be influenced by the presence of Ba, as previous results have shown co-removal of Sr with Ba precipitation when PSS is added to brine solutions.32 Addition of an ultrafiltration step increased removal of all cations, as this process captures additional cations that are complexed by PSS or PSSM but do not aggregate and settle from solution. Using ultrafiltration and pH adjustment, PSSM was able to achieve 100% removal of both Ba and Sr from the DC brine. This process also removed 65% and 78% of the initial Mg and Ca, respectively, substantially less than that obtained using precipitative softening. PSS was also able to remove more Sr (>80% under some conditions) than sulfate precipitation while limiting Ca and Mg removal to less than 60%. While PAA was also able to remove >90% of Ba and Sr from the DC brine, it demonstrated little selectivity for these ions over Ca and Mg. In the hypersaline RC brine, increased competition from Na molecules reduced the effectiveness of the sulfonate-based polymers (PSSM and PSS), resulting in less than 20% Sr removal. While PAA was able to remove 58% of Sr at pH 9, this removal was accompanied by a greater percentage removal of Ca. Thus, Sr control at very high salinities does not appear to be possible without substantial removal of other divalent cations as well.
These results show potential for development of polymer-based treatment processes for targeted removal of scale-forming compounds, particularly Sr, from produced waters, but additional method development and testing would be required to achieve this potential. Further optimization of polymer selectivity may be able to increase Sr removal over other divalent cations, particularly in moderately saline waters. If this objective could be achieved, it may provide an effective method for controlling Sr concentrations even in the absence of Ba. However, the high concentrations of polymer required would add to the expense of this approach. Treatment costs could be decreased substantially by regeneration and reuse of the added polymers for multiple treatment cycles. For both PSSM and PAA, complexation of divalent cations was sensitive to solution pH, which provides a possible means for polymer de-complexation and recovery. In a previous study,32 cation release from PAA in a synthetic cation solution was accomplished using HCl, and approximately three-quarters of the polymer recovered for reuse. Use of a larger molecular weight polymer could potentially increase this recovery, as even a 200 kDa (nominal) polymer can initially have a substantial fraction of material small enough to pass through a 10 kDa UF filter.31
In addition to decreasing chemical requirements, such a process could improve process waste management. Polymer regeneration and separation of the removed cation would result in a lower-volume waste brine containing Ba and Sr that could potentially be recovered for commercial purposes. Even if disposal is required, the reduction in brine volume (when coupled with recovery of the treated produced water for industrial reuse) would minimize the impact and cost of waste disposal. Further research into this this recovery process, as well as testing of ultrafiltration capabilities using cross-flow systems more commonly used in real treatment systems, will provide a more complete assessment of the viability of polymer-based treatment processes for produced waters.
5. Conclusions
A treatment method that removes Sr, Ba, and Ra, while leaving Mg and Ca behind, would result in reduced waste generation, reducing disposal costs. Sulfate addition achieves these goals when the produced water contains a significant excess of Ba over Sr. However, Sr removal by sulfate addition is not effective when Ba is not present, as in the sulfate-containing produced waters present in parts of the Central Plains and elsewhere. Precipitative softening (pH adjustment and addition of carbonate salts) will remove Sr even from hypersaline brines at >90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg l−1 TDS, but only concurrently with precipitation and removal of Ca as CaCO3. This requires high levels of chemical addition and generates significant waste solids. Increased Sr removal can be achieved by polymer-assisted ultrafiltration, but these processes are still not 100% effective, and become less selective as the overall TDS concentration increases. They will also likely cost significantly more than either precipitative softening or sulfate addition unless efficient methods are developed for polymer recovery and regeneration.
000 mg l−1 TDS, but only concurrently with precipitation and removal of Ca as CaCO3. This requires high levels of chemical addition and generates significant waste solids. Increased Sr removal can be achieved by polymer-assisted ultrafiltration, but these processes are still not 100% effective, and become less selective as the overall TDS concentration increases. They will also likely cost significantly more than either precipitative softening or sulfate addition unless efficient methods are developed for polymer recovery and regeneration.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr. Karla Leslie for sharing her expertise and helping to collect oil-field brine samples, Dr. Sheng-Xue Xie for performing preliminary experiments that helped to establish the feasibility of the studies presented herein, and Dr. Masoumeh Veisi for contributing to the characterization of the produced water samples collected. This work was supported by the National Science Foundation EPSCoR Track Research Infrastructure Improvement Program: Track-2 Focused EPSCoR Collaboration award (OIA-1632892) and by an internal Strategic Initiatives Grant from the University of Kansas.References
- USEIA, Short-Term Energy Outlook July 2018, U.S. Energy Information Administration, Washington, DC, 2018 Search PubMed.
- USEPA, Assessment of the Potential Impacts of Hydraulic Fracturing for Oil and Gas on Drinking Water Resources, Office of Research and Development, United States Environmental Protection Agency, Washington, DC, 2015 Search PubMed.
- J. A. Veil and C. E. Clark, Produced-water-volume estimates and management practices, SPE Prod. Oper., 2011, 26, 234–239, DOI:10.2118/125999-PA.
- S. Jimenez, M. M. Mico, M. Arnaldos, F. Medina and S. Contreras, State of the art of produced water treatment, Chemosphere, 2018, 192, 186–208, DOI:10.1016/j.chemosphere.2017.10.139.
- J. M. Estrada and R. Bhamidimarri, A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing, Fuel, 2016, 182, 292–303, DOI:10.1016/j.fuel.2016.05.051.
- T. L. S. Silva, S. Morales-Torres, S. Castro-Silva, J. L. Figueiredo and A. M. T. Silva, An overview on exploration and environmental impact of unconventional gas sources and treatment options for produced water, J. Environ. Manage., 2017, 200, 511–529, DOI:10.1016/j.jenvman.2017.06.002.
- W. L. Ellsworth, Injection-induced earthquakes, Science, 2013, 341, 1225942, DOI:10.1126/science.1225942.
- M. L. Hladik, M. J. Focazio and M. Engle, Discharges of produced waters from oil and gas extraction via wastewater treatment plants are sources of disinfection by-products to receiving streams, Sci. Total Environ., 2014, 466–467, 1085–1093, DOI:10.1016/j.scitotenv.2013.08.008.
- N. R. Warner, C. A. Christie, R. B. Jackson and A. Vengosh, Impacts of Shale Gas Wastewater Disposal on Water Quality in Western Pennsylvania, Environ. Sci. Technol., 2013, 47, 11849–11857, DOI:10.1021/es402165b.
- M. Abdou, A. Carnegie, S. Matthews, K. McCarthy, M. O'Keefe, B. Raghuramna, W. Wei and C. Xian, Finding Value in Formation Water, Oilfield Rev., 2011, 23, 24–35 Search PubMed.
- S. Monroe, D. McCracken, K. Dawson and S. Mouallem, Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, 2013, DOI:10.2118/166124-MS.
- D. L. Shaffer, L. H. A. Chavez, M. Ben-Sasson, S. R. V. Castrillon, N. Y. Yip and M. Elimelech, Desalination and Reuse of High-Salinity Shale Gas Produced Water: Drivers, Technologies, and Future Directions, Environ. Sci. Technol., 2013, 47, 9569–9583, DOI:10.1021/es401966e.
- D. A. Pierce, K. Bertrand and C. Certiu Vasiliu, Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Brisbane, Australia, 2010, DOI:10.2118/134137-MS.
- M. Crabtree, D. Eslinger, P. Fletcher, M. Miller, A. Johnson and G. King, Fighting scale--removal and prevention, Oilfield Rev., 1999, 30–45 CAS.
- K. B. Gregory, R. D. Vidic and D. A. Dzombak, Water management challenges associated with the production of shale gas by hydraulic fracturing, Elements, 2011, 7, 181–186, DOI:10.2113/gselements.7.3.181.
- J. Moghadasi, H. Muller-Steinhagen, M. Jamialahmade and A. Sharif, Model study on the kinetics of oil field formation damage due to salt precipitation from injection, J. Pet. Sci. Eng., 2004, 43, 201–217, DOI:10.1016/j.petrol.2004.02.014.
- W. W. Frenier and M. Ziauddin, Formation, Removal, and Inhibition of Inorganic Scale in the Oilfield Environment, Society of Petroleum Engineers, Richardson, TX, 2008 Search PubMed.
- S. J. Randtke, in Water Quality and Treatment: A Handbook on Drinking Water, ed. J. K. Edzwald, McGraw-Hill, Inc., New York, 6th edn, 2011, ch. 13 Search PubMed.
- M. A. Kelland, Production Chemicals for the Oil and Gas Industry, CRC Press, Boca Raton, FL, 2009, DOI:10.1201/b16648.
- J. M. Silva, H. Matis, W. L. Kostedt and V. Watkins, Produced water pretreatment for water recovery and salt production, RPSEA Report 08122-36, NETL, 2012 Search PubMed.
- G. F. Doran, F. H. Carini, D. A. Fruth, J. A. Drago and L. Y. C. Leong, Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, Texas, 1997, DOI:10.2118/38830-MS.
- C. F. Garbutt, Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, Texas, 1997, DOI:10.2118/38799-MS.
- F. R. Ahmadun, A. Pendashteh, L. C. Abdullah, D. R. A. Biak, S. S. Madaeni and Z. Z. Abidin, Review of technologies for oil and gas produced water treatment, J. Hazard. Mater., 2009, 170, 530–551, DOI:10.1016/j.jhazmat.2009.05.044.
- J. L. Parks and M. Edwards, Precipitative removal of As, Ba, B, Cr, Sr, and V using sodium carbonate, J. Environ. Eng., 2006, 132, 489–496, DOI:10.1061/(Asce)0733-9372(2006)132:5(489).
- A. J. O'Donnell, D. A. Lytle, S. Harmon, K. Vu, H. Chait and D. D. Dionysiou, Removal of strontium from drinking water by conventional treatment and lime softening in bench-scale studies, Water Res., 2016, 103, 319–333, DOI:10.1016/j.watres.2016.06.036.
- A. J. Kondash, N. R. Warner, O. Lahav and A. Vengosh, Radium and Barium Removal through Blending Hydraulic Fracturing Fluids with Acid Mine Drainage, Environ. Sci. Technol., 2014, 48, 1334–1342, DOI:10.1021/es403852h.
- C. He, T. Y. Zhang and R. D. Vidic, Co-treatment of abandoned mine drainage and Marcellus Shale flowback water for use in hydraulic fracturing, Water Res., 2016, 104, 425–431, DOI:10.1016/j.watres.2016.08.030.
- B. L. Rivas, E. D. Pereira, R. Cid and K. E. Geckeler, Polyelectrolyte-assisted removal of metal ions with ultrafiltration, J. Appl. Polym. Sci., 2005, 1091–1099, DOI:10.1002/app.21424.
- E. Hwang, K. Lee, K. Choo, S. Choi, S. Kim, C. Yoon and C. Lee, Effect of precipitation and complexation on nanofiltration of strontium-containing nuclear wastewater, Desalination, 2002, 289–294, DOI:10.1016/S0011-9164(02)00554-4.
- M. Chen, K. Shafer-Peltier, S. Randtke and E. Peltier, Modeling aresenic (V) removal from water by micellar enhacend ultrafiltration in the presence of competing anions, Chemosphere, 2018, 213, 285–294, DOI:10.1016/j.chemosphere.2018.09.046.
- M. Chen, K. Shafer-Peltier, S. Randtke and E. Peltier, Competitive association of cations with poly(sodium 4-styrenesulfonate) (PSS) and heavy metal removal from water by PSS-assisted ultrafiltration, Chem. Eng. J., 2018, 344, 155–164, DOI:10.1016/j.cej.2018.03.054.
- M. Chen, K. Shafer-Peltier, M. Veisi, S. Randtke and E. Peltier, Complexation and precipitation of scale-forming cations in oilfield produced water with polyelectrolytes, Sep. Purif. Technol., 2019, 222, 1–10, DOI:10.1016/j.seppur.2019.04.014.
- S. A. Dastgheib, C. Knutson, Y. Yang and H. H. Salih, Treatment of produced water from an oilfield and selected coal mines in the Illinois Basin, Int. J. Greenhouse Gas Control, 2016, 54, 513–523, DOI:10.1016/j.ijggc.2016.05.002.
- R. J. Jan and T. G. Reed, Jr., New Caustic Process for Softening Produced Water for Steam Generation, SPE Prod. Eng., 1992, 7, 199–202, DOI:10.2118/19759-PA.
- Z. Y. Zhang, X. W. Du, K. H. Carlson, C. A. Robbins and T. Z. Tong, Effective treatment of shale oil and gas produced water by membrane distillation coupled with precipitative softening and walnut shell filtration, Desalination, 2019, 454, 82–90, DOI:10.1016/j.desal.2018.12.011.
- C. He, M. Li, W. S. Liu, E. Barbot and R. D. Vidic, Kinetics and Equilibrium of Barium and Strontium Sulfate Formation in Marcellus Shale Flowback Water, J. Environ. Eng., 2014, 140 DOI:10.1061/(asce)ee.1943-7870.0000807.
- S. Mondal and S. R. Wickramasinghe, Produced water treatment by nanofiltration and reverse osmosis membranes, J. Membr. Sci., 2008, 322, 162–170, DOI:10.1016/j.memsci.2008.05.039.
- A. Fakhru'l-Razi, A. Pendashteh, A. Luqman Chuah, A. B. Dayang Radiah, S. S. Madaeni and A. Z. Zurina, Review of technologies for oil and gas produced water treatment, J. Hazard. Mater., 2009, 170, 530–551, DOI:10.1016/j.jhazmat.2009.05.044.
- K. L. Benko and J. E. Drewes, Produced water in the western United States: geographical distribution, occurrence, and composition, Environ. Eng. Sci., 2008, 25, 239–246, DOI:10.1089/ees.2007.0026.
- X. Wang, L. Goual and P. J. S. Colberg, Characterization and treatment of dissolved organic matter from oilfield produced waters, J. Hazard. Mater., 2012, 217, 164–170, DOI:10.1016/j.jhazmat.2012.03.006.
- USEPA, Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spetrometry, Revision 4.4, Cincinnati, OH, 1994 Search PubMed.
- T. Zhang, K. Gregory, R. W. Hammack and R. D. Vidic, Co-precipitation of radium with barium and strontium sulfate and its impact of the fate of radium during treatment of produced water from unconventional gas extraction, Environ. Sci. Technol., 2014, 48, 4596–4603, DOI:10.1021/es405168b.
- D. L. Parkhurst and C. A. J. Appelo, Description of input and examples for PHREEQC version 3: a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations, Report 6-A43, USGS, Reston, VA, 2013, DOI:10.3133/tm6A43.
- T. Rabizadeh, C. L. Peacock and L. G. Benning, Carboxylic acids: effective inhibitors for calcium sulfate precipitation?, Mineral. Mag., 2014, 78, 1465–1472, DOI:10.1180/minmag.2014.078.6.13.
- A. C. Todd and M. D. Yuan, Barium and Strontium sulfate solid-solution scale formation at elevated temperatures, SPE Prod. Eng., 1992, 7, 85–92, DOI:10.2118/19762-PA.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00643e |
| This journal is © The Royal Society of Chemistry 2020 |