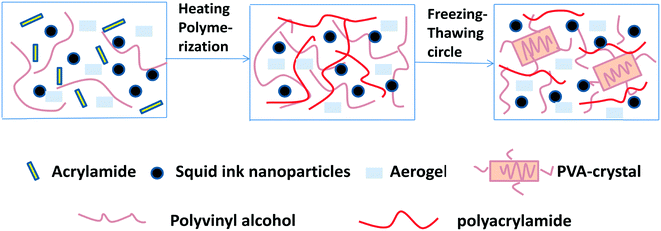
A novel floatable composite hydrogel for solar evaporation enhancement†
Liyuan
Zhao
a,
Jing
Tian
c,
Yangkaixi
Liu
a,
Longquan
Xu
b,
Yi
Wang
 c,
Xu
Fei
c,
Xu
Fei
 *ab and
Yao
Li
*ab and
Yao
Li
 *a
*a
aSchool of Light Industry and Chemical Engineering, Dalian Polytechnic University, 1# Qinggongyuan Road, Dalian 116034, China. E-mail: feixudlpu@163.com; liyaodlpu@163.com; Fax: +86 411 86322038; Tel: +86 411 86323691 201
bInstrumental Analysis Center, Dalian Polytechnic University, 1# Qinggongyuan Road, Dalian 116034, China
cSchool of Biological Engineering, Dalian Polytechnic University, Dalian 116034, China
First published on 15th November 2019
Abstract
Solar evaporation is an economically feasible method for desalination and distillation. Herein, we report a facile method to synthesize a floatable composite hydrogel with squid ink nanoparticles, silica aerogel, poly(vinyl alcohol) and acrylamide. Squid ink nanoparticles have good photothermal conversion efficiency. The silica aerogel can effectively reduce the density of the hydrogel and allow the composite hydrogel to float on the water surface. The good hydrophilicity of the hydrogel facilitates the transport of water molecules from the bottom to the top of the floatable composite hydrogel. In the evaporation process, these properties enable the floatable composite hydrogel to centrally heat the surface of the water, thus improving the water evaporation efficiency. The results of the evaporation experiments showed that the seawater evaporation rate of the floatable composite hydrogel was 4.73 times higher than that of the traditional process. Furthermore, the good mechanical strength and flexibility of the hydrogel provide a guarantee for its recovery and reuse in practical applications. This low-consumption, easy-to-manufacture and durable floatable composite hydrogel provides a new way for solar desalination.
Water impactFreshwater is one of the most important issues related to the future survival and development of human beings. Although the earth is rich in water resources, 97% of it is seawater that cannot be used directly. While wastewater reclamation partially relieves water shortages, only desalination technologies can broaden our supply to the ocean, which is the major source of water. In this work, inspired by the water transmission and evaporation mechanism of plants, we have synthesized a novel composite hydrogel with excellent solar evaporation properties. The floatable composite hydrogel achieved an evaporation rate of seawater more than 4.73 times that of the traditional process with the same light intensities and external environment. We believe that its excellent solar evaporation properties make it a promising candidate for establishing highly efficient desalination systems to produce freshwater. |
1. Introduction
Freshwater is one of the most important issues related to the future survival and development of human beings.1–3 Although the earth is rich in water resources, 97% of it is seawater that cannot be used directly.4–6 Therefore, it is necessary to find a suitable desalination method for human development. Solar energy is an abundant and clean energy source, which is a viable alternative to traditional energy.7–9 Solar energy can be used for seawater desalination,10,11 purification,12 sterilization,13 and distillation.14,15 In order to improve the photothermal conversion efficiency, some new types of light-to-heat nanomaterials such as copper chalcogenide nanocrystals,16–20 carbon-based nanomaterials,21–24 plasmonic absorbers,25–29 and conductive polymers30,31 have been developed in the past few years.Squid ink is the substance spurted by sea squids when they are in danger. From the perspective of micro-scale, squid ink is composed of numerous spherical nanoparticles with an average diameter of 100 nm. The inside of the spherical particles is the melanin core and the outside is the protein polysaccharide.32 The natural melanin nanoparticles extracted from squid ink show strong absorption capacity in the near-infrared region. The squid ink nanoparticles have been used to enhance the photothermal therapy of tumors.33
Recently, Jiang's group has developed a carbon-black-based superhydrophobic gauze for the solar evaporation enhancement at the air–water interface.34 The superhydrophobic gauze achieves an evaporation rate 2–3 times that of traditional evaporation. However, there is still much room for improvement in the development of new materials for solar evaporation applications. Hydrogel is a kind of polymer with hydrophilic groups and three-dimensional network structures. It swells rapidly in water and retains its original structure without being dissolved after swelling.35 Hydrogel has excellent hydrophilicity and a large number of porous structures, which can effectively absorb water.36 In recent years, some high mechanical strength and functional hydrogels have been developed to improve the possibility of practical applications.37,38 Among these hydrogels, double network (DN) hydrogel has remarkable toughness and mechanical strength due to its unique network structures, efficient energy dissipation and firm interpenetrating network entanglement.39 Therefore, DN hydrogel is very suitable for the substrate of seawater evaporation films. However, hydrogel has a higher density than water and cannot float on the water interface. Efficient water evaporation only occurs at the air–liquid interface. Therefore, it is necessary to reduce the density of hydrogel. Silica aerogel as a nanomaterial has low density, low thermal conductivity and high surface area due to its nanoporous network structure.40–42 Introduction of silica aerogel is an effective strategy to reduce the density of hydrogel.
In this work, we have synthesized a floatable composite hydrogel with squid ink nanoparticles, silica aerogel (SA), poly(vinyl alcohol) (PVA) and acrylamide (AM) by physical and chemical cross-linking. This floatable composite hydrogel can significantly improve the evaporation rate of water, which is more than four times as much as the traditional evaporation rate. The hydrophilic properties and porous structure of the floatable composite hydrogel can facilitate the rapid absorption and transfer of water. Moreover, the good mechanical strength and bending flexibility of the floatable composite hydrogel provide a guarantee for its reuse. The simple preparation process of the floatable composite hydrogel is beneficial to the wide application of seawater desalination films.
2. Experimental
2.1. Materials
Squid ink nanoparticles were extracted from a squid obtained from a market. Fresh squid ink was evenly dispersed in deionized water, and was magnetically stirred at room temperature for 4 hours. Then the squid ink nanoparticles were centrifuged and rinsed with deionized water. After freeze-drying, the dried squid ink nanoparticles were obtained. Poly(ethyleneglycol)dimethacrylate (PEGDA, n ≈ 4), ammonium persulfate (analytical reagent) and acrylamide (99%) were provided by Beijing Chemical Industry Group Co., Ltd. Polyvinyl alcohol (PVA, average degree of polymerization of 1750) was supplied by SCR (Shanghai, China). Silica aerogel (hydrophobic, 97%) was purchased from NANO TECH Co., Ltd (Shaoxing, China) with a density of 60 kg m−3. N,N′-Methylenebisacrylamide was provided by Tianjin Aoran Fine Chemical Research Institute. All the chemicals were used without further purification. Deionized water was used in all the experiments.2.2. Preparation of the floatable composite hydrogel
SA powder (0.4 g) and 15 wt% PVA aqueous solution (14 g) were evenly mixed to obtain mixture A. Squid ink nanoparticles (0.68 g), N,N′-methylenebisacrylamide (0.03 g), acrylamide (2 g), poly(ethyleneglycol)dimethacrylate (0.08 g), H2O (4 g) and ammonium persulfate (0.04 g) were mixed with ultrasonic stirring to obtain mixture B. Mixture B was stirred under a nitrogen atmosphere over a period of 10 min. Subsequently, mixture B was added into mixture A with mechanical stirring and the mixture of A and B was heated at 75 °C for 4 h to form the floatable composite hydrogel by chemical crosslinking. Then, the chemical crosslinking hydrogel was frozen at −21 °C for 4 hours and thawed at room temperature for 5 cycles to achieve the physical crystallization of PVA. Finally, the floatable composite hydrogel with a physicochemical double cross-linked network was obtained. A blank hydrogel was prepared in the same way as the floatable composite hydrogel, except that no squid ink nanoparticles were added.2.3. Evaporation experiments
Pure water (or seawater) was put into a 500 ml cylindrical beaker and the beaker was placed on an electronic balance. The floatable composite hydrogel floated on the upper surface of the pure water (or seawater). A full-range light source (CEL-HXF300, UV-vis-NIR) with a 24 W output radiation power was vertically fixed at 15 cm above the floatable composite hydrogel. The area of the light spot was set to be 28.26 cm2 on the hydrogel for local heating. The weight of the pure water (or seawater) in the beaker was recorded using the electronic balance every three minutes. The evaporation rate was calculated from the data recorded in the balance. The dark experiment was to take the same amount of pure water or seawater as the light experiment and put it in a dark environment to measure the evaporation rate of water. The experiments were generally carried out at an ambient temperature of 17 °C and a humidity of ≈36%.2.4. Characterization
The infrared spectrum was analyzed using a Perkin-Elmer Spectrum TWO infrared spectrophotometer. X-ray diffraction (XRD) was performed on a Shimadzu XRD-7000S diffractometer with Cu Kα radiation (200 mA, 50 kV, λ = 0.154 nm). EDS was recorded using an Oxford INCA energy spectrometer at an electron energy of 20 kV. Scanning electron microscopy (SEM) was operated on a JEOL JSM 7800 electron microscope at a primary electron energy of 5.0 kV. Transmission electron microscopy (TEM) was observed on a JEM2100 (UHR) with an electron energy of 200 kV. The contact angles of the samples were recorded using the charge coupled device of a contact angle analyzer (OCA 20, Filderstadt, DataPhysics). Mechanical properties of the floatable composite hydrogel were characterized by a tension test at a speed of 10 mm min−1. The length of the tensile sample was 110 mm, width 19 mm and thickness 5 mm. A white light (CELL-HXF300) source was provided by Beijing Zhongjiao Jinyuan Technology Co. LTD. The temperature of the sample was measured using an infrared thermal imager (FLIR, America E30).3. Results and discussion
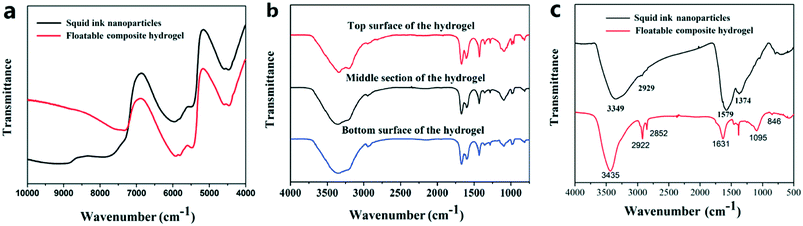
The fabrication process of the floatable composite hydrogel is shown in Scheme 1. Firstly, SA and the PVA aqueous solution were stirred fully and mixed to obtain mixture A. As SA is an ultra-light and ultra-hydrophobic material, it was necessary to add the high viscosity PVA aqueous solution to disperse SA evenly. After adding mixture B (squid ink nanoparticles, N,N′-methylenebisacrylamide, acrylamide, poly(ethyleneglycol)dimethacrylate, H2O and ammonium persulfate), the mixture of A and B was stirred evenly, and the chemical crosslinking hydrogel network was produced with ammonium persulfate as an initiator under heating conditions. To improve the mechanical properties of the floatable composite hydrogel, the physical crystallization of PVA was formed by the freezing–thawing process.For this study, the squid ink nanoparticles and floatable composite hydrogel were characterized via near FT-IR spectroscopy (Fig. 1a). From the near-infrared spectra, it could be found that a typical intense peak appeared at 5940 cm−1. This indicated that the floatable composite hydrogel exhibited strong absorption to near-infrared light, which was conducive to photothermal conversion. Fig. 1b shows the infrared spectra of the upper surface, cross section and bottom surface of the floatable composite hydrogel. It could be seen that the FT-IR spectra of these surfaces were different, indicating that the composition of the hydrogel was not uniform. From the absorption peak at 1095 cm−1, it could be found that the top surface of the hydrogel contained a large amount of SA, the middle section of the hydrogel contained a small amount of SA and the bottom surface contained almost no SA. Fig. 1c shows the FT-IR spectra of the squid ink nanoparticles and floatable composite hydrogel. For the squid ink nanoparticles, the absorption peak at 3349 cm−1 was due to the O–H stretching and the absorption peak at 1579 cm−1 was due to –NH2. A typical broad and intense peak appeared at 3435 cm−1 in the spectrum of the floatable composite hydrogel, which was attributed to the O–H stretching. The characteristic absorption peaks appearing at 2962–2850 cm−1 were associated with the –CH2 (stretching) and –CH3 (stretching) vibrations. The signals for the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ONH2– vibration bands from the floatable composite hydrogel were located at 1630 cm−1. The band at 1095 cm−1 was attributed to the stretching of the Si–O–Si groups of SA. The FT-IR results confirmed the successful preparation of the floatable composite hydrogel. The UV-vis-NIR absorption spectra of the squid ink nanoparticles and the composite hydrogel with and without the squid ink nanoparticles are provided in Fig. S1.† As shown in Fig. S1,† the optical absorption of the floatable composite hydrogel reached 83% in the visible light region. The results suggested that the floatable composite hydrogel could absorb light effectively.
ONH2– vibration bands from the floatable composite hydrogel were located at 1630 cm−1. The band at 1095 cm−1 was attributed to the stretching of the Si–O–Si groups of SA. The FT-IR results confirmed the successful preparation of the floatable composite hydrogel. The UV-vis-NIR absorption spectra of the squid ink nanoparticles and the composite hydrogel with and without the squid ink nanoparticles are provided in Fig. S1.† As shown in Fig. S1,† the optical absorption of the floatable composite hydrogel reached 83% in the visible light region. The results suggested that the floatable composite hydrogel could absorb light effectively.
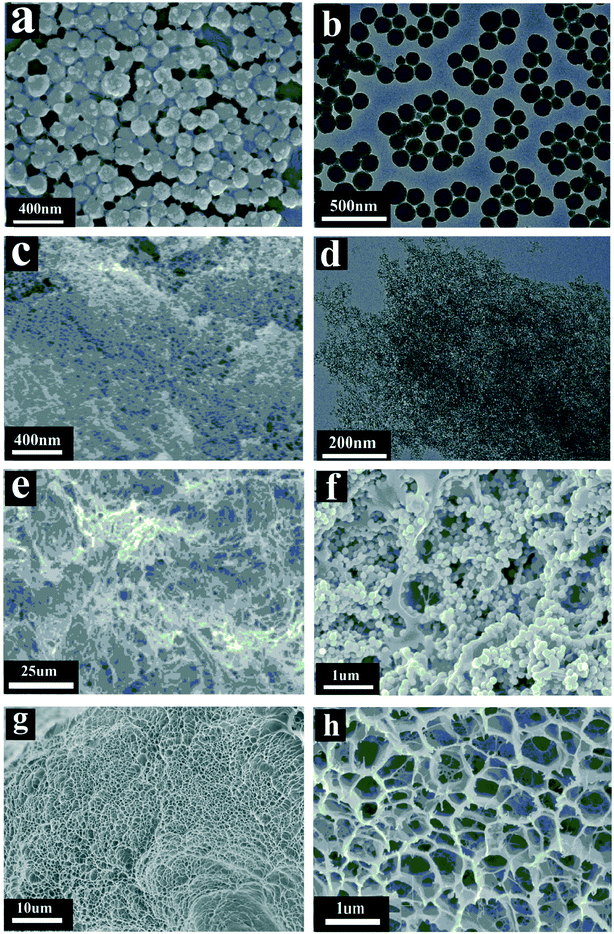
The microstructure of the squid ink nanoparticles after freeze-drying was observed by SEM. The SEM image (Fig. 2a) shows that the squid ink nanoparticles were roughly spherical in shape, with an average diameter of 120 nm (Fig. S2†). The squid ink nanoparticles were uniform in size and the surface of the nanoparticles was rough. The result of the TEM image (Fig. 2b) was consistent with that of the SEM image. As seen in Fig. 2c, the surface of SA was a loose porous structure, which was the reason for its low density and large specific surface area (Fig. S3†). The TEM image (Fig. 2d) shows that the SA was composed of a large number of nanoscale three-dimensional network structures. There were many pores in the network, which had good connectivity. In addition, the sizes of the pores were different, which was a typical disordered porous structure with a diameter of about 5–15 nm (Fig. 2d). As shown in Fig. 2e, a lot of SA appeared on the top surface of the floatable composite hydrogel. Interestingly, there was almost no SA on the bottom surface of the floatable composite hydrogel (Fig. 2g). The main reason for this phenomenon was that the SA density was low, so the SA floated to the top of the hydrogel during the preparation process. As shown in Fig. 2f, the squid ink nanoparticles were uniformly immobilized in the hydrogel network. In Fig. 2g and h, there were many pores with a diameter of 0.5–1 μm on the bottom surface of the hydrogel. The porous structure was not only conducive to the absorption and transmission of water, but also contributed to the reduction of thermal conductivity and local heating of water.
In order to further confirm the existence of SA on the top surface of the floatable composite hydrogel, EDS was carried out on the top surface of the floatable composite hydrogel (Fig. 3a). As could be seen from Fig. 3a, Si elements were evenly dispersed on the surface of the hydrogel, which was consistent with the FT-IR results. Fig. 3b shows the XRD patterns of the PVA hydrogel and the floatable composite hydrogel. The diffraction peaks of the PVA hydrogel were observed clearly at around 2θ = 19.5°, 27.6° and 40.7°, indicating a semi-crystalline polymer hydrogel. The XRD pattern of the floatable composite hydrogel showed the same peaks of the crystalline structures, but the intensity decreased significantly. This suggested that the PVA crystal cross-linking points produced by the freezing–thawing cycles were the second physical cross-linking network of the floatable composite hydrogel. However, the crystallinity of the floatable composite hydrogel was lower than that of the PVA hydrogel, because the addition of other components reduced the number of crystallization of PVA.
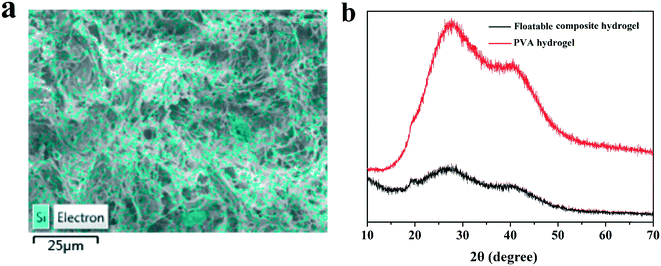 | ||
| Fig. 3 (a) EDS mapping image of Si, and (b) X-ray diffraction patterns of the PVA hydrogel and floatable composite hydrogel. | ||
As hydrophilicity was essential to efficient water supply for solar steam power generation, the wettability of the hydrogel was quantitatively measured with water contact angles. From Fig. 4, it was found that the bottom surface of the water contact angle of the floatable composite hydrogel was 24°, indicating good hydrophilicity. For the top surface of the floatable composite hydrogel, the water droplet could not be captured by the top surface (Movie S1†). The reason for this phenomenon was that superhydrophobic SA was mainly in the top layer of the hydrogel during the preparation of the floatable composite hydrogel. Interestingly, this design had a similar mechanism to the water transmission and evaporation of plants. Plants absorb water through the roots and transport water through the vessels. Due to the transpiration of the surface moisture on the leaves (the osmotic pressure was generated inside the plant), the water is transported from the bottom up, without additional energy input.43 In the evaporation process, the bottom surface of the floatable hydrogel corresponded to the root of the plant, and the top surface corresponded to the leaf of the plant.
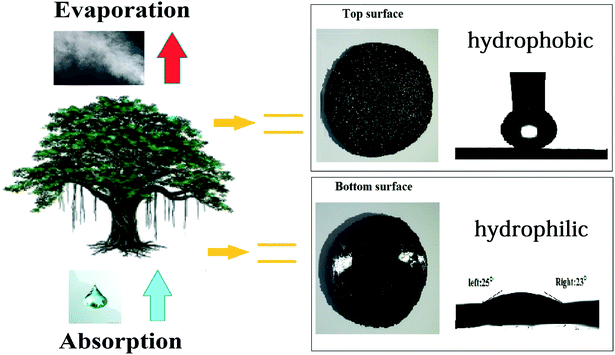 | ||
| Fig. 4 Schematic diagram of the correspondence between the plant and water desalination system and water contact angles of the top and bottom surfaces of the floatable composite hydrogel. | ||
In order to enhance the practicability of hydrogels, it was necessary for the floatable composite hydrogel to have good mechanical properties. Therefore, the mechanical properties of hydrogels were tested. As shown in Fig. 5, the elongation at break and the tensile strength of the blank hydrogel (without squid ink nanoparticles) were 125% and 0.32 MPa. The elongation at break and the tensile strength of the floatable composite hydrogel with 1.5 wt% squid ink nanoparticles were 169% and 0.34 MPa. For the floatable composite hydrogel with the 3 wt% squid ink nanoparticles, the elongation at break was increased to 189%, and the tensile strength was enhanced to 0.35 MPa. The elongation at break and the tensile strength of the floatable composite hydrogel with 4.5 wt% squid ink nanoparticles were 200% and 0.36 MPa. The mechanical properties of the floatable composite hydrogel obviously improved with the increase of the content of squid ink nanoparticles. This is because the squid ink nanoparticles contained a large amount of –OH and –NH– and formed strong hydrogen bonds with the –OH of PVA, which was a key factor for the high mechanical properties of the floatable composite hydrogel. As cross-linking agents, the squid ink nanoparticles could improve the cohesion force between substances and suppress the propagation of cracks, thus enhancing the toughness of the hydrogel. In addition, the hydrogen bond interaction between the squid ink nanoparticles and the polymer chain could dissipate the strain energy in the polymer network of the hydrogel, which was conducive to the tensile strength of the floatable composite hydrogel.
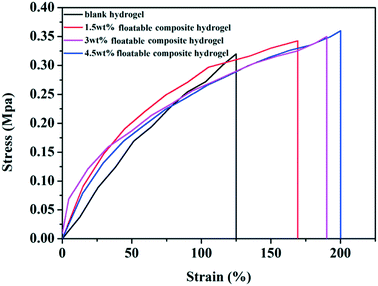 | ||
| Fig. 5 The tensile stress–strain curves of the blank hydrogel and floatable composite hydrogels with different contents of squid ink nanoparticles (1.5 wt%, 3 wt% and 4.5 wt%). | ||
The schematic diagram of water evaporation for the floatable composite hydrogel is shown in Fig. 6a. A beaker with water and the floatable composite hydrogel was placed on the balance. After the light source was turned on, the mass change of water was recorded using the balance. The density of SA was very low which was almost similar to air. Therefore, the introduction of SA could effectively reduce the density of the composite hydrogel and make the hydrogel have floatability (Fig. 6b). The density of the floatable composite hydrogel was measured to be 0.8 g cm−3. Even when the floatable composite hydrogel was pressed into the water, it could immediately float back up (Movie S2†).
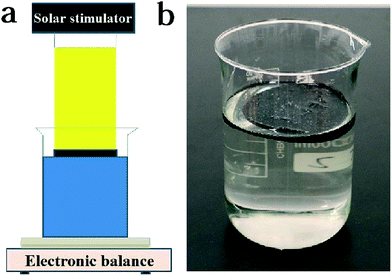 | ||
| Fig. 6 (a) Test method for water evaporation, and (b) image of the floatable composite hydrogel floating on the water surface. | ||
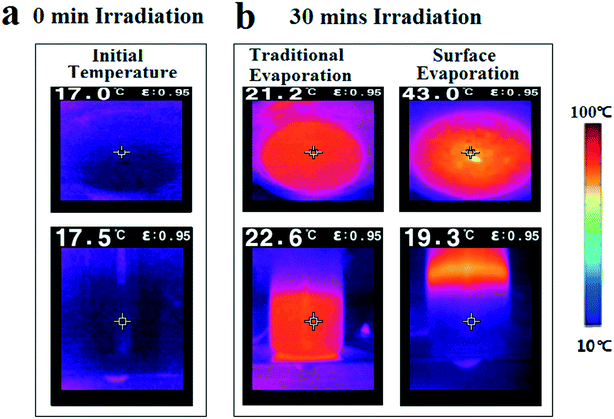
To further study the heat localization caused by the floatable composite hydrogel under 4 kW m−2 intensity, an infrared camera was used to record the temperature (T) variations across the beakers. The initial temperature of the pure water was ∼17.5 °C (Fig. 7a). After 30 min of light illumination, the surface temperature of the floatable composite hydrogel reached 43 °C, while the surface temperature of the traditional evaporation group was 21.2 °C. The bottom temperature of the floatable composite hydrogel was 19.3 °C, while the bottom temperature of the traditional evaporation group reached 22.6 °C (Fig. 7b). For the traditional evaporation group, the temperature of the water showed a negligible variation of 1.4 °C from top to bottom. For the group of floatable composite hydrogel evaporation, the temperature of the water showed a variation of 23.7 °C from top to bottom. By contrast, it was found that the temperature of the floatable composite hydrogel group showed a significant temperature gradient. The result indicated that the floatable composite hydrogel could absorb the energy to heat the water on the top surface without heating the bulk water, which was beneficial to water evaporation efficiency and solar energy utilization.
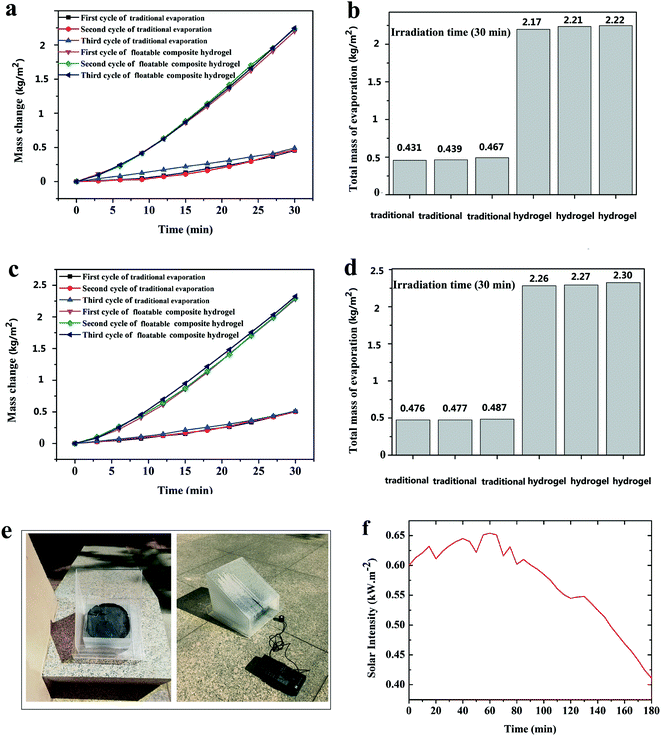
A white-light source with a light density of 4 kW m−2 was used for pure water and seawater evaporation experiments. The pure water evaporation rate in a dark environment was 0.025 kg m−2, and the seawater evaporation rate in a dark environment was 0.026 kg m−2 (Fig. S4†). These dark evaporation rates were subtracted from all the measured evaporation rates under solar illumination, respectively. The seawater used in the evaporation experiment came from the Dalian Bohai Sea, with a salt content of 3.5%. For pure water, the traditional water evaporation rate was around 0.446 kg m−2 for 30 min and the water evaporation rate of the floatable composite hydrogel was around 2.20 kg m−2 for 30 min under 4 kW m−2 solar irradiation. For seawater, the water evaporation rate of the traditional process was around 0.480 kg m−2 for 30 min and the water evaporation rate of the floatable composite hydrogel was around 2.27 kg m−2 for 30 min. The floatable composite hydrogel could achieve an evaporation rate around 4.93 times that of the traditional process for pure water in 30 min and could achieve an evaporation rate 4.73 times that of the traditional process for seawater in 30 min (Fig. 8). For pure water, the evaporation rate of the blank hydrogel (without squid ink nanoparticles) was 0.382 kg m−2 for 30 min (Fig. S4†). For seawater, the evaporation rate of the blank hydrogel (without squid ink nanoparticles) was 0.393 kg m−2 for 30 min (Fig. S4†). As shown in Fig. 8b, for pure water, the traditional total evaporation mass values in 30 min for three cycles were 0.431, 0.439, and 0.467 kg m−2, respectively, and the total evaporation mass values in 30 min of the floatable composite hydrogel for three cycles were 2.17, 2.21, and 2.22 kg m−2, respectively. For seawater, the traditional total evaporation mass values in 30 min for three cycles were 0.476, 0.477, and 0.487 kg m−2, respectively, and the total evaporation mass values in 30 min of the floatable composite hydrogel for three cycles were 2.26, 2.27, and 2.30 kg m−2, respectively (Fig. 8d). From Fig. 8, it could be concluded that the floatable composite hydrogel could effectively enhance the evaporation rate of pure water and seawater. In order to test the reusability of the floatable composite hydrogel, repeatability tests were carried out for 30 min under 4 kW m−2 solar irradiation. The results showed that the floatable composite hydrogel has good reusability (Fig. S5†). Compared to other solar evaporation materials, this floatable composite hydrogel showed better solar evaporation properties (Table S2†). The water evaporation conversion efficiency of the floatable composite hydrogel can be calculated by η = Qe/Qs, where Qe denotes the energy for evaporation of the water and Qs denotes the solar irradiation. Qe can be determined by Qe = m × He, where m is the evaporation rate, and He is the liquid–vapor phase change enthalpy (≈2260 kJ kg−1). According to the above formula, the efficiency of the composite hydrogel was obtained to be 71.38%, which was much higher than that of the traditional evaporation (15.07%) under the same irradiation.
To test the seawater desalination ability of the floatable composite hydrogel under natural sunlight, the hydrogel was placed in the device as shown in Fig. 8e for evaporation. The evaporation experiments were conducted for 3 h (time: 13:00–16:00, environment temperature: 21.5 °C, relative humidity: 60%). The sunlight density in the three hours fluctuated between 0.411 and 0.654 kW m−2 (Fig. 8f). Based on the test results, the freshwater generation rate of the floatable composite hydrogel was calculated to be around 0.444 kg m−2 h−1 under natural solar irradiation (0.411–0.654 kW m−2, environment temperature: 21.5 °C, relative humidity: 60%). Therefore, the floatable composite hydrogel provided a promising application for the solar desalination process.
4. Conclusions
In conclusion, the floatable composite hydrogel was successfully prepared by physicochemical double cross-linking. The as-prepared floatable composite hydrogel exhibited good mechanical properties and excellent heat localization properties of the water surface. The seawater evaporation rate of the floatable composite hydrogel was 4.73 times higher than that of the traditional process under an irradiation of 4 kW m−2. The floatable composite hydrogel exhibited a simple preparation process and low energy consumption. Furthermore, the floatable composite hydrogel had good reuse performance and was suitable for practical application. Therefore, this floatable composite hydrogel is a promising candidate for seawater desalination and water purification.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (31771914, 31800498), the Natural Science Foundation of Liaoning Province of China (20170540076, 20170540079), and the Research on Science and Technology of Education Department of Liaoning Province project (2017J078).References
- B. K. Pramanik, L. Shu and V. Jegatheesan, A review of the management and treatment of brine solutions, Environ. Sci.: Water Res. Technol., 2017, 3, 625–658 RSC
.
- Y. Shi, C. Zhang, R. Li, S. Zhuo, Y. Jin, L. Shi, S. Hong, J. Chang, C. Ong and P. Wang, Solar Evaporator with Controlled Salt Precipitation for Zero Liquid Discharge Desalination, Environ. Sci. Technol., 2018, 52, 11822–11830 CrossRef CAS PubMed
.
- Y. Cai, W. Shen, J. Wei, T. H. Chong, R. Wang, W. B. Krantz, A. G. Fane and X. Hu, Energy-efficient desalination by forward osmosis using responsive ionic liquid draw solutes, Environ. Sci.: Water Res. Technol., 2015, 1, 341–347 RSC
.
- G. N. Tiwari, H. N. Singh and R. Tripathi, Present status of solar distillation, Sol. Energy, 2003, 75, 367–373 CrossRef CAS
.
- X. Li, B. Zhu and J. Zhu, Graphene oxide based materials for desalination, Carbon, 2019, 146, 320–328 CrossRef CAS
.
- A. D. Khawaji, I. K. Kutubkhanah and J.-M. Wie, Advances in seawater desalination technologies, Desalination, 2008, 221(1–3), 47–69 CrossRef CAS
.
- N. S. Lewis, Toward Cost-Effective Solar Energy Use, Science, 2007, 3157, 98–801 Search PubMed
.
- N. S. Lewis, Research opportunities to advance solar energy utilization, Science, 2016, 351(6271), 1920 CrossRef PubMed
.
- M. Romero and A. Steinfeld, Concentrating solar thermal power and thermochemical fuels, Energy Environ. Sci., 2012, 5(11), 9234 RSC
.
- G. Liu, J. Xu and K. Wang, Solar water evaporation by black photothermal sheets, Nano Energy, 2017, 41, 269–284 CrossRef CAS
.
- L. Zhou, Y. Tan, J. Wang, W. Xu, Y. Yuan, W. Cai, S. Zhu and J. Zhu, 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination, Nat. Photonics, 2016, 10(6), 393–398 CrossRef CAS
.
- Y. Liu, J. W. Lou, M. T. Ni, C. Y. Song, J. B. Wu, N. P. Dasgupta, P. Tao, W. Shang and T. Deng, Bioinspired Bifunctional Membrane for Efficient Clean Water Generation, ACS Appl. Mater. Interfaces, 2016, 8, 772 CrossRef CAS PubMed
.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Science and technology for water purification in the coming decades, Nature, 2008, 452(7185), 301–310 CrossRef CAS PubMed
.
- G. N. Tiwari, H. N. Singh and R. Tripathi, Present status of solar distillation, Sol. Energy, 2003, 75, 367–373 CrossRef CAS
.
- O. Neumann, A. D. Neumann, E. Silva, C. Ayala-Orozco, S. Tian, P. Nordlander and N. J. Halas, Nanoparticle-Mediated, Light-Induced Phase Separations, Nano Lett., 2015, 15(12), 7880–7885 CrossRef CAS PubMed
.
- J. Xu, F. Xu, M. Qian, Z. Li, P. Sun, Z. Hong and F. Huang, Copper nanodot-embedded graphene urchins of nearly full-spectrum solar absorption and extraordinary solar desalination, Nano Energy, 2018, 53, 425–431 CrossRef CAS
.
- Y. Li, W. Lu, Q. Huang, M. Huang, C. Li and W. Chen, Copper sulfide nanoparticles for photothermal ablation of tumor cells, Nanomedicine, 2010, 5(8), 1161–1171 CrossRef CAS PubMed
.
- X. Liu, Q. Wang, C. Li, R. Zou, B. Li, G. Song, K. Xu, Y. Zheng and J. Hu, Cu2−xSe@mSiO2–PEG core–shell nanoparticles: a low-toxic and efficient difunctional nanoplatform for chemo-photothermal therapy under near infrared light radiation with a safe power density, Nanoscale, 2014, 6(8), 4361–4370 RSC
.
- Y. Liu, S. Yu, R. Feng, A. Bernard, Y. Liu, Y. Zhang, H. Duan, W. Shang, P. Tao, C. Song and T. Deng, A bioinspired, reusable, paper-based system for high-performance large-scale evaporation, Adv. Mater., 2015, 27(17), 2768–2774 CrossRef CAS PubMed
.
- Q. Tian, M. Tang, Y. Sun, R. Zou, Z. Chen, M. Zhu, S. Yang, J. Wang, J. Wang and J. Hu, Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells, Adv. Mater., 2011, 23(31), 3542–3547 CrossRef CAS PubMed
.
- A. Burke, X. F. Ding, R. Singh, R. A. Kraft, N. Levi-Polyachenko, M. N. Rylander, C. Szot, C. Buchanan, J. Whitney and J. Fisher,
et al. Long-Term Survival Following a Single Treatment of Kidney Tumors with Multiwalled Carbon Nanotubes and Near-Infrared Radiation, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 12897–12902 CrossRef CAS PubMed
.
- C. Finnerty, L. Zhang, D. L. Sedlak, K. L. Nelson and B. Mi, Synthetic Graphene Oxide Leaf for Solar Desalination with Zero Liquid Discharge, Environ. Sci. Technol., 2017, 51, 11701–11709 CrossRef CAS PubMed
.
- J. T. Robinson, K. Welsher, S. M. Tabakman, S. P. Sherlock, H. Wang, R. Luong and H. Dai, High Performance In Vivo Near-IR (>1 mum) Imaging and Photothermal Cancer Therapy with Carbon Nanotubes, Nano Res., 2010, 3(11), 779–793 CrossRef CAS PubMed
.
- L. Sun, J. Liu, Y. Zhao, J. Xu and Y. Li, Highly efficient solar steam generation via mass-produced carbon nanosheet frameworks, Carbon, 2019, 145, 352–358 CrossRef CAS
.
- L. Zhou, Y. L. Tan, D. X. Ji, B. Zhu, P. Zhang, J. Xu, Q. Q. Gan, Z. F. Yu and J. Zhu, Self-assembly of highly efficient, broadband plasmonic absorbers for solar steam generation, Sci. Adv., 2016, 2, e1501227 CrossRef PubMed
.
- Z. H. Wang, Y. M. Liu, P. Tao, Q. C. Shen, N. Yi, F. Y. Zhang, Q. L. Liu, C. Y. Song, D. Zhang, W. Shang and T. Deng, Bio-Inspired Evaporation Through Plasmonic Film of Nanoparticles at the Air–Water Interface, Small, 2014, 10, 3234 CrossRef CAS PubMed
.
- K. Bae, G. Kang, S. K. Cho, W. Park, K. Kim and W. J. Padilla, Flexible thin-film black gold membranes with ultrabroadband plasmonic nanofocusing for efficient solar vapour generation, Nat. Commun., 2015, 6, 10103 CrossRef CAS PubMed
.
- L. Tian, J. Luan, K. K. Liu, Q. Jiang, S. Tadepalli, M. K. Gupta, R. R. Naik and S. Singamaneni, Plasmonic Biofoam: A Versatile Optically Active Material, Nano Lett., 2016, 16(1), 609–616 CrossRef CAS PubMed
.
- J. Ji, R. Li, H. Li, Y. Shu, Y. Li, S. Qiu, C. He and Y. Yang, Phytic acid assisted fabrication of graphene/polyaniline composite hydrogels for high-capacitance supercapacitors, Composites, Part B, 2018, 155, 132–137 CrossRef CAS
.
- L. Cheng, K. Yang, Q. Chen and Z. Liu, Organic Stealth Nanoparticles for Highly Effective In Vivo Near-infrared Photothermal Therapy of Cancer, ACS Nano, 2012, 6, 5605–5613 CrossRef CAS PubMed
.
- K. Yang, H. Xu, L. Cheng, C. Sun, J. Wang and Z. Liu, In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles, Adv. Mater., 2012, 24(41), 5586–5592 CrossRef CAS PubMed
.
- F. Cheng, W. Liu, Y. Zhang, H. Wang, S. Liu, E. Hao, S. Zhao and H. Yang, Squid inks-derived nanocarbons with unique “shell@pearls” structure for high performance supercapacitors, J. Power Sources, 2017, 354, 116–123 CrossRef CAS
.
- Q. Jiang, Y. Liu, R. Guo, X. Yao, S. Sung, Z. Pang and W. Yang, Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors, Biomaterials, 2019, 192, 292–308 CrossRef CAS PubMed
.
- Y. Liu, J. Chen, D. Guo, M. Cao and L. Jiang, Floatable, Self-Cleaning, and Carbon-Black-Based Superhydrophobic Gauze for the Solar Evaporation Enhancement at the Air-Water Interface, ACS Appl. Mater. Interfaces, 2015, 7(24), 13645–13652 CrossRef CAS PubMed
.
- X. Yan, J. Yang, F. Chen, L. Zhu, Z. Tang, G. Qin, Q. Chen and G. Chen, Mechanical properties of gelatin/polyacrylamide/graphene oxide nanocomposite double-network hydrogels, Compos. Sci. Technol., 2018, 163, 81–88 CrossRef CAS
.
- X. Shen, J. L. Shamshina, P. Berton, G. Gurau and R. D. Rogers, Hydrogels based on cellulose and chitin: fabrication, properties, and applications, Green Chem., 2016, 18(1), 53–75 RSC
.
- X. H. Wang, F. Song, D. Qian, Y. D. He, W. C. Nie, X. L. Wang and Y. Z. Wang, Strong and tough fully physically crosslinked double network hydrogels with tunable mechanics and high self-healing performance, Chem. Eng. J., 2018, 349, 588–594 CrossRef CAS
.
- W. Li, H. An, Y. Tan, C. Lu, C. Liu, P. Li, K. Xu and P. Wang, Hydrophobically associated hydrogels based on acrylamide and anionic surface active monomer with high mechanical strength, Soft Matter, 2012, 8(18), 5078 RSC
.
- Q. Chen, H. Chen, L. Zhu and J. Zheng, Fundamentals of double network hydrogels, J. Mater. Chem. B, 2015, 3(18), 3654–3676 RSC
.
- G. Hayase, K. Kanamori, K. Abe, H. Yano, A. Maeno, H. Kaji and K. Nakanishi, Polymethylsilsesquioxane-cellulose nanofiber biocomposite aerogels with high thermal insulation, bendability, and superhydrophobicity, ACS Appl. Mater. Interfaces, 2014, 6(12), 9466–9471 CrossRef CAS PubMed
.
- G. Hayase, K. Kanamori, G. Hasegawa, A. Maeno, H. Kaji and K. Nakanishi, A superamphiphobic macroporous silicone monolith with marshmallow-like flexibility, Angew. Chem., Int. Ed., 2013, 52(41), 10788–10791 CrossRef CAS PubMed
.
- K. Kanamori, M. Aizawa, K. Nakanishi and T. Hanada, New Transparent Methylsilsesquioxane Aerogels and Xerogels with Improved Mechanical Properties, Adv. Mater., 2007, 19(12), 1589–1593 CrossRef CAS
.
- E. Steudle, Water transport across plant tissue: Role of water channels, Biol. Cell, 1997, 89, 259–273 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00661c |
| This journal is © The Royal Society of Chemistry 2020 |