Occurrence of N-nitrosamines and their precursors in Spanish drinking water treatment plants and distribution systems†
Maria José
Farré
 *ab,
Sara
Insa
ab,
Aaron
Lamb
c,
Cristian
Cojocariu
c and
Wolfgang
Gernjak
*ab,
Sara
Insa
ab,
Aaron
Lamb
c,
Cristian
Cojocariu
c and
Wolfgang
Gernjak
 ad
ad
aCatalan Institute for Water Research (ICRA), 17003 Girona, Spain. E-mail: mjfarre@icra.cat; Tel: +34 972 18 33 80
bUniversitat de Girona (UdG), 17003 Girona, Spain
cThermo Fisher Scientific, Runcorn WA7 1TA, UK
dCatalan Institute for Research and Advanced Studies (ICREA), 08010 Barcelona, Spain
First published on 14th November 2019
Abstract
N-Nitrosamines are carcinogenic compounds that can be formed during disinfection processes as by-products in drinking and recycled water systems. Among them, N-nitrosodimethylamine (NDMA) is of particular interest, especially in systems employing chloramines, because its presence is regulated in various countries. Although there is a lot of emphasis on NDMA due to its toxicity, there may be other N-nitrosamines formed during disinfection processes that pose similar or higher toxicities and are currently scarcely studied. This work investigates the presence of NDMA and six additional N-nitrosamines in different drinking water treatment plants (DWTPs) and strategic sampling points from drinking water networks in Spain that employ monochloramines. The other N-nitrosamines investigated are N-nitrosodiethylamine (NDEA), N-nitrosomethylamine (NMEA), N-nitrosodibutylamine (NDBA), N-nitrosopiperidine (NPIP), N-nitrosodipropylamine (NDPA) and N-nitrosopyrrolidine (NPYR). Moreover, the fate of N-nitrosamine precursors was measured across different DWTPs. The actual concentration of NDMA in the final treated water and samples taken from the distribution system was never above 4.2 ± 0.2 ng L−1. NDEA and NDBA were also detected in almost all samples, however, their concentrations did not exceed 1.5 ng L−1 in any case. The maximum concentration of NDMA formation potential following chloramination was 41.5 ± 4.3 ng L−1. The concentration of other N-nitrosamines originating during NDMA formation potential tests was lower than 3 ng L−1. Among the studied DWTPs, those that included ozone followed by granular activated carbon (GAC) in the treatment train removed NDMA formation potential best, showing that this can be an efficient strategy to control NDMA formation during drinking water production when chloramines are used in the distribution systems.
Water impactWe present data on different carcinogenic N-nitrosamines and their precursors in Spanish drinking water and across drinking water treatment plants, obtained with a novel analytical methodology that reduces quantification limits 10 times in comparison to conventional techniques. Although in general concentrations measured are low, we suggest monitoring N-nitrosodiethylamine due to its potency and concentrations measured. |
Introduction
Different treatment methods are employed by public drinking water providers to deliver safe drinking water. In many jurisdictions, treatment is finalized by adding a residual disinfectant before distribution. Disinfection kills or inactivates pathogens that could otherwise cause diseases and for this reason it is one of the greatest technological successes in public health protection in the last century.1 However, during the production of drinking water, dissolved carbon and organic nitrogen may generate disinfection by-products (DBPs) when reacting with chemical disinfectants.2 Despite recognizing the importance and benefits of disinfection, it is also necessary to control and monitor the formation of DBPs below acceptable levels.The formation of DBPs depends on the chemical agent used during disinfection, water quality (including parameters such as the carbon/nitrogen ratio, bromide concentration), disinfectant dose, pH, temperature and contact time.3 Since the discovery of the formation of chloroform in drinking water in 1974,4 a significant number of scientific projects have been carried out to improve our understanding of the formation and control of DBPs. Although it is not clear exactly how many DBPs exist, more than 600 species are known today, although developing a routine analytical methodology has only been possible for about 100 compounds.2
NDMA (N-nitrosodimethylamine) is a DBP that is classified as 2B “possibly carcinogenic to humans”.5 NDMA was detected in 34% of chloraminated drinking water samples (maximum detected concentration 630 ng L−1) and 3% of chlorinated drinking water samples from investigated facilities in the United States (US) as part of the unregulated contaminant monitoring rule (UCMR) and was added to the US Environmental Protection Agency (USEPA) UCMR, requiring many large water utilities to monitor it.6 NDMA is listed in the World Health Organization (WHO) Drinking Water Guidelines at 100 ng L−1 limit7 and is also included in the Contaminant Candidate List 4 of the USEPA.8 California's Department of Public Health set 10 ng L−1 (ref. 9) notification levels for NDMA in drinking water and California's Office of Environmental Health Hazard Assessment established 3 ng L−1 as a public health goal.10 Limits have also been established in Massachusetts11 and Ontario.12 Furthermore, the Australian Guidelines specify a target of 10 ng L−1 during water recycling.13 However, for drinking water the 100 ng L−1 recommendation by the WHO was followed by Australia14 and Japan. Also, a few countries of the European Union have regulated the presence of NDMA in drinking water. The regulatory authorities of the UK and Germany have classified NDMA as a suspected human carcinogen, and in Germany for instance, an observed concentration of 10 ng L−1 concentration will trigger the initiation of remedial actions to reduce NDMA.
The chemistry of NDMA is different from other DBPs since this compound is formed mainly during the disinfection of water by chloramination, which is either added intentionally or formed inadvertently by chlorination in the presence of ammonium.15 More recently, researchers have reported that alternative disinfectants, including chlorine dioxide (ClO2) and ozone (O3), are also able to produce NDMA.16,17 It is believed that NDMA precursors are largely of anthropogenic origin, in contrast to other DBPs, such as trihalomethanes and haloacetic acids, which are derived from organic matter of different environmental sources.18 Therefore, the formation of NDMA is particularly relevant to water recycling practices or water sources with an anthropogenic impact.16 However, NDMA precursors have also been detected in several drinking water sources considered largely without anthropogenic impacts.19,20 To date, precursors still have not been characterized well in drinking water impacted by human activity21 and include tertiary, secondary and quaternary amines. It is also known that the yield of the NDMA formation reaction is generally very low (less than 10% molar conversion),22 although some precursors can have up to ∼90% molar yield (e.g. tertiary amines containing a β-aromatic ring such as ranitidine). In previously published occurrence surveys, NDMA was found at relatively high concentration (>10 ng L−1) in drinking water treatment plants (DWTPs) in the US, Australia and China,5,16,23–26 whereas in the UK and Japan it has been seldom detected and at generally lower concentration levels.16,27,28 One study investigated the occurrence of N-nitrosamines at one specific DWTP plant in Spain employing peroxidation with potassium permanganate followed by coagulation, sedimentation and filtration before disinfection with chloramines.29 The average NDMA concentration in the four samples taken in the distribution system was 7.1 ng L−1, with maximum values measured up to 20 ng L−1. Planas and coauthors30 also measured NDMA in another specific Spanish DWTP using ozonation and found up to 10 ng L−1 after this treatment. This value was reduced after using granular activated carbon (GAC) and the concentration measured in the final treated water was not higher than 5 ng L−1.
Apart from NDMA, the USEPA has included 4 other N-nitrosamines, namely N-nitrosodiethylamine (NDEA), N-nitrosodiphenylamine (NDPhA), N-nitrosodipropylamine (NDPA) and N-nitrosopyrrolidine (NPYR) in the UCMR.8 When other nitrosamines are reported, NDMA concentrations tend to be higher than those.16 However, in the US a concentration of NDEA above 5 ng L−1 was found in 2% of 1198 public waters systems, while in China this proportion was up to 15%.23 NDPhA was found at concentrations between 0.1 and 0.4 ng L−1 in distribution systems in Canada and the US.31 Another study reported concentrations of different N-nitrosamines in drinking water that ranged from 4.6 to 20.5 ng L−1 for NDMA (found in 7/12 samples), 1.9 to 16.3 ng L−1 for NDEA (9/12), 0.4 to 3.4 ng L−1 for NDBA (6/12), 1.1 ng L−1 for NMEA (1/12) and 3.3 ng L−1 for NDPhA (1/12), respectively.32 Much higher concentrations have been detected in China's drinking water.33,34 Jurado-Sánchez and coauthors29 measured 11, 9.4, 1.8 ng L−1 average concentrations of NDBA, NDPhA and NDEA, respectively, in samples taken from a specific distribution system in Spain. Planas and coauthors30 reported values up to 12.9 ng L−1 NDEA in treated water from a specific Spanish plant employing ozonation.
As seen, information about N-nitrosamine occurrence in Spanish drinking water is limited to a couple of specific DWTPs. Also, there is no information on the potential of N-nitrosamine formation in distribution systems. Hence, the first aim of this study is to present broad occurrence data on NDMA and its precursors across eleven DWTPs and their distribution systems in central Spain that employ monochloramine as a disinfectant. Two sampling campaigns were carried out in order to also capture potential seasonal variations. During one of the sampling campaigns, six additional N-nitrosamines and their precursors were also measured with a novel TSQ™ 9000 gas chromatograph coupled to a triple quadrupole mass spectrometer (GC-MS/MS) system equipped with advanced electron ionization (AEI). This method allowed a reporting limit of quantification of 0.1 ng L−1 for NDMA and all additional N-nitrosamines except for NDPA and NPYR, where the limit was 0.5 ng L−1.
Results for NDMA obtained with the AEI mode and the more conventional electron impact (EI) mode, with a limit of quantification of 1 ng L−1, were also compared. Finally, additional samples were taken from a specific drinking water treatment plant employing ozonation followed by GAC to investigate the fate of NDMA precursors across this specific treatment.
Methodology
Chemicals and reagents
The NDMA (CAS 62-75-9) standard for GC-MS analysis (5000 μg mL−1 in methanol, Supelco) had a purity of >99.9%. Deuterated d6-NDMA (>98% Cambridge Isotope Laboratories, Inc.) and d14-NDPA (N-nitrosodipropylamine, >99% Restek) were used as a surrogate and an internal standard, respectively. N-Nitrosomethylamine (NMEA, CAS 10595-95-6), N-nitrosodiethylamine (NDEA, CAS 55-18-5), N-nitrosodibutylamine (NDBA, 924-16-3), N-nitrosopyrrolidine (NPYR, CAS 930-55-2), N-nitrosopiperidine (NPIP, CAS 100-75-4), N-nitrosodipropylamine (NDPA, CAS 621-64-7) were obtained from Supelco as the EPA 521 nitrosamine mix at 2000 μg mL−1 in dichloromethane. Deuterated d10-NDEA was used as a surrogate when NDEA was determined and was also obtained from Cambridge Isotope Laboratories, Inc.NH4Cl (>99.5%, Sigma-Aldrich), NaOH (ACS, ISO, Reag, Scharlau) and NaClO (reagent grade, available chlorine ≥4%, Sigma-Aldrich) were used for the NDMA formation potential test. KH2PO4 (>99%, Sigma-Aldrich) and Na2HPO4 (>99%, Sigma-Aldrich) were used to prepare pH buffer solutions. Na2SO3 (>98%, Sigma-Aldrich) was employed to quench formation potential tests. Commercial DPD test kits (LCK310, Hach Lange) were used for the analysis of free and total chlorine using a Hach DR2800 spectrophotometer.
Sample collection and pretreatment
Samples were taken during January (sampling campaign 1) and October (sampling campaign 2) of 2018 from eleven drinking water treatment plants (DWTP) all of them serving water to the same region but employing different source waters. Table 1 summarizes the characteristics of the DWTPs investigated. As seen, all the DWTPs employ chloramine as a final disinfectant and they can mainly be separated depending on the presence of ozone in the main treatment. Samples were also taken in June and September 2015 from DWTP2. All the DWTPs treat source water from inland dams or rivers. Additional measured parameters are presented in Tables S1 and S2 of the ESI.† Samples were taken in amber glass bottles, refrigerated and shipped to ICRA. Samples used to measure actual NDMA in the system were quenched immediately after sampling with 2.5 g L−1 sodium sulfite. Formation potential tests were started immediately upon reception (less than 24 h from sampling). Additionally, total organic carbon and total nitrogen (TOC, TN Shimadzu TOC-VCSH analyzer with a TNM-1 unit), ammonium (BUCHI B-324 distiller, Titrino 719S Metrohm) and total Kjeldahl nitrogen (BUCHI B-324 distiller, Titrino 719S Metrohm), nitrites (NO2−–N), nitrates (NO3−–N) were measured in all samples, the latest with ion chromatography (ISC5000 from Dionex).| DWTP | Treatment capacity (m3 s−1) | Main treatment steps | Ozone | Activated carbon |
|---|---|---|---|---|
| GAC = granular activated carbon, PAC = powder activated carbon. | ||||
| DWTP1 | 3.8 | Pre-Ox1 (NaOCl, ClO2), pre-Ox2 (O3, KMnO4), Coag (Al), O3, GAC, NH2Cl | Yes | Yes |
| DWTP2 | 12 | Pre-Ox1 (Cl2, O3), pre-Ox2 (ClO2, KMnO4), Coag (Al), O3, GAC, NH2Cl | Yes | Yes |
| DWTP3 | 1 | Pre-Ox1 (Cl2, ClO2), pre-Ox2 (KMnO4), Coag (Al), PAC, NH2Cl | No | Yes |
| DWTP4 | 1.5 | Pre-Ox1 (Cl2, ClO2), pre-Ox2 (KMnO4), Coag (Al), PAC, NH2Cl | No | Yes |
| DWTP5 | 0.4 | Pre-Ox1 (Cl2, ClO2), pre-Ox2 (O3, KMnO4), Coag (Al), O3, GAC, NH2Cl | Yes | Yes |
| DWTP6 | 0.3 | Pre-Ox1 (Cl2, ClO2), pre-Ox2 (O3, KMnO4), Coag (Al), O3, GAC, NH2Cl | Yes | Yes |
| DWTP7 | 16 | Pre-Ox (Cl2, ClO2, KMnO4), Coag (Al), PAC, NH2Cl | No | Yes |
| DWTP8 | 4 | Pre-Ox (NaOCl, ClO2, KMnO4), Coag (Al), PAC, NH2Cl | No | Yes |
| DWTP9 | 6 | Pre-Ox (Cl2, ClO2, KMnO4), Coag (Al), PAC, NH2Cl | No | Yes |
| DWTP10 | 0.5 | Pre-Ox1 (Cl2, ClO2), pre-Ox2 (O3, KMnO4), Coag (Al), PAC, NH2Cl | Yes | Yes |
| DWTP11 | 0.2 | Pre-Ox (Cl2, ClO2, KMnO4), Coag (Al), NH2Cl | No | No |
NDMA formation potential and NDMA analysis
NDMA precursors were quantified by means of NDMA formation potential (FP) tests that followed the protocol published by Mitch et al.15 In short, 10 mM phosphate buffered filtered samples were disinfected with a 140 mg L−1 monochloramine concentration and kept in the dark and under ambient conditions (T = 21 ± 1 °C) for seven days. After this contact time chloramines were quenched with 2.5 g L−1 sodium sulfite prior to NDMA extraction for analysis by GC-MS/MS. The method used for NDMA extraction is based on EPA method 521 (ref. 35) and described elsewhere.36 GC-MS/MS analysis of NDMA was performed using a Trace GC Ultra gas chromatograph equipped with a TriPlus™ autosampler coupled to a TSQ™ Quantum triple quadrupole mass spectrometer system (Thermo Fisher Scientific). Chromatographic separation was performed using a ZB1701 from Phenomenex (30 m × 0.25 mm × 0.25 μm). The injector temperature was 250 °C and was operated in splitless mode. The oven temperature program was as follows: 40 °C held for 1 min, ramp to 65 °C at 5 °C min−1, ramp to 110 °C at 10 °C min−1 held for 1 min and finally ramp to 240 °C at 25 °C min−1 and held for 1 min. Mass spectrometric ionization was carried out in electron impact (EI) ionization mode (EI voltage of 70 eV and a source temperature of 250 °C) as described by Farré et al.37 The method's reporting limit of quantification was 1 ng L−1. The error bar, shown in Fig. 2 and 6, corresponds to the range that was calculated from duplicate injection per sample.Analysis of other N-nitrosamines
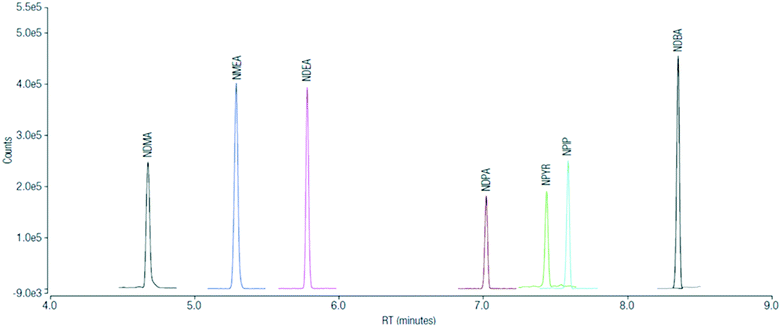
The solid phase extracted samples from sampling campaign 1 were also injected in a Thermo Scientific TSQ™ 9000 triple quadrupole GC-MS/MS system using advanced electron ionization (AEI) to investigate the presence of additional nitrosamines in actual disinfected samples but also after the formation potential tests. A volume of 1 μL of the nitrosamine mixture extract was injected in splitless with surge mode (surge pressure 25 psi for 1.02 min, split flow 80 mL min−1 after 1 min). The injector temperature was set at 240 °C.In order to increase the resolution of chromatographic peaks, the GC separation of extracted components was carried out on a Thermo Scientific TraceGOLD TG-1701MS (30 m × 0.25 mm × 0.5 μm) with the following GC temperature program: initial temperature of 35 °C held for 1 min, increased at a rate of 25 °C min−1 to 130 °C, followed by an increase at a rate of 20 °C min−1 to 250 °C, then held for 2 min (total run 12.8 min). The GC was interfaced with the TSQ™ 9000 instrument via a transfer line heated at 250 °C. The source temperature was set at 300 °C. MS analyses were performed using electron ionization (EI) at 70 eV in time-SRM mode using the AEI and automated system tuned with SmartTune.38Table 2 shows the SRM transitions for the analyzed N-nitrosamines. The method reporting limit of quantification was 0.1 ng L−1 for NDMA and all additional N-nitrosamines except for NDPA and NPYR, where the limit was 0.5 ng L−1. The error bar, shown in Fig. 2–5, corresponds to the interval of confidence that was calculated from the standard deviation of triplicate injection per sample. The method performance was assessed by evaluating the compound recoveries determined from three separate extractions of a 50 ng L−1 nitrosamine fortified HPLC water sample. The results showed that the average recovery values ranged between 80.7% and 111.1% (Table 3). Additionally, d14-NDPA was used as an internal standard for injection error correction. Further details of the method can be found elsewhere.39 Deuterated analogs of NDMA and NDEA were used to correct the recovery of these specific nitrosamines. The remaining ones were not corrected for recovery. Data were processed and reported using Thermo Scientific™ Chromeleon™ Chromatography Data System (CDS) software. Fig. 1 shows the SRM chromatograms of the quantitation transition for nitrosamines in a 1 pg μL−1 solvent standard (equivalent to 1 ng L−1 in sample).
| Name | RT (min) | (SRM) m/z | ||
|---|---|---|---|---|
| Mass (m/z) | Product mass (m/z) | Collision energy V | ||
| d6-NDMA | 4.7 | 80 | 50 | 5 |
| 80 | 46 | 15 | ||
| NDMA | 4.8 | 74 | 42 | 15 |
| 74 | 44 | 5 | ||
| NMEA | 5.5 | 88 | 71 | 5 |
| 88 | 42 | 15 | ||
| d10-NDEA | 5.9 | 112 | 34 | 5 |
| 112 | 50 | 10 | ||
| NDEA | 6 | 102 | 85 | 5 |
| 102 | 44 | 10 | ||
| NDPA-d14 | 7.1 | 78 | 46 | 10 |
| 110 | 78 | 5 | ||
| NDPA | 7.2 | 130 | 113 | 5 |
| 130 | 43 | 10 | ||
| NPYR | 7.6 | 100 | 55 | 5 |
| 100 | 70 | 5 | ||
| NPIP | 7.8 | 114 | 84 | 5 |
| 114 | 97 | 5 | ||
| NDBA | 8.5 | 116 | 99 | 5 |
| 158 | 99 | 5 | ||
| Compound | RT (min) | % recovery |
|---|---|---|
| NDMA | 4.7 | 108.4 |
| NMEA | 5.3 | 83 |
| NDEA | 5.8 | 111.1 |
| NDPA | 7 | 80.7 |
| NPYR | 7.4 | 96.5 |
| NPIP | 7.6 | 90 |
| NDBA | 8.4 | 84.3 |
Results
NDMA and NDMA precursors
The first objective of this study was to determine the NDMA concentration in treated water of different DWTPs as well as in strategic points of a distribution system that employs monochloramine as a residual disinfectant. To address this, two sampling campaigns were carried out during winter (sampling 1, January 2018) and fall (sampling 2, October 2018). Furthermore, NDMA formation potential tests were performed in samples taken at the inlet of the drinking water treatment plants and after treatment just before final disinfection to investigate the fate of NDMA precursors across the various DWTPs. DWTPs 1 to 5 were sampled across the treatment plants, while for DWTP 6–11 samples were only taken after disinfection and/or during distribution. The average values of selected water quality parameters monitored for the different water samples are summarized in Tables S1 and S2 of the ESI.† Total organic carbon (TOC) levels for source waters ranged between 4.9 and 2.3 mg L−1 across the different locations and were similar for each location during the two sampling events. The TOC concentration in final and distributed systems was 1.9 ± 0.2 mg L−1 on average. Total nitrogen (TN) in source and treated waters was always below 1 mg L−1, and the removal across the plants was not significant. DWTP2 and DWTP4 had the highest final TOC values during the two sampling campaigns (3.3 and 2.2 for DWTP2 and 2.2 and 2.4 for DWTP4, for sampling campaigns 1 and 2 respectively). Table 4 shows the NDMA values measured during the two sampling campaigns. The concentration of NDMA in the samples taken after disinfection was always below 2.4 ng L−1, with the highest value observed in the treated sample at DWTP5 during the autumn sampling campaign. This value did almost not increase in the distribution systems (3.3 ± 0.2 ng L−1). The concentration of NDMA in the distribution system of DWTP11 (no ozone treatment) was the highest with 4.2 ± 0.2 ng L−1 NDMA measured. Although there are no established standards for acceptable levels of NDMA in drinking water in the European Union (EU),40 these values are well below the concentrations established in other drinking water guidelines worldwide such as in Australia, where NDMA is regulated at the World Health Organization Drinking Water Guideline level (i.e., 100 ng L−1).7 The maximum values of NDMA measured in this study are also lower than the 10 ng L−1 regulatory limit that authorities of the UK and Germany have stablished to trigger the initiation of remedial actions to reduce NDMA.| DWTP | Sampling 1 (January 18) | Sampling 2 (October 18) |
|---|---|---|
| DWTP1_F | <1 | n.m |
| DWTP1_DIST | 1.2 ± 0.1 | n.m |
| DWTP2_ F | <1 | <1 |
| DWTP2_DIS | <1 | 1.7 ± 0.1 |
| DWTP3_F | n.m | <1 |
| DWTP3_DIS | n.m | 1.9 ± 0.1 |
| DWTP4_F | 1.5 ± 0.2 | <1 |
| DWTP4_DIS | 2.0 ± 0.9 | n.m |
| DWTP5_F | 1.7 ± 0.4 | 2.4 ± 0.1 |
| DWTP5_DIS | 3.5 ± 0.6 | 3.3 ± 0.2 |
| DWTP6_F | <1 | 1.8 ± 0.3 |
| DWTP6_DIS | 2.3 ± 1.4 | 2.9 ± 0.3 |
| DWTP7_F | <1 | <1 |
| DWTP7_DIS | 1.7 ± 1.1 | n.m |
| DWTP8_F | <1 | <1 |
| DWTP8_DIS | 1.2 ± 0.3 | 1.8 ± 0.1 |
| DWTP9_F | <1 | 2.0 ± 0.1 |
| DWTP9_DIS | n.m | 1.9 ± 0.4 |
| DWTP10_DIS | n.m | 1.3 ± 0.1 |
| DWTP11_DIS | 4.2 ± 0.2 | 2.9 ± 0.3 |
In general, NDMA values measured in this study were low and in agreement with the results reported in the UK.28 On the other hand, higher results have been reported in the US, China, and Australia mostly due to the high prevalence of chloramination, wastewater recycling, and effective chloramination resulting from high source water ammonia concentrations.16,23,25,26 One of the reasons for these differences may be that more pristine source waters are used at the investigated DWTPs, as shown by the low TN values measured (see Tables S1 and S2†), but also shown in the indices of ecological status, physical and chemical descriptors which are available at http://www.chtajo.es.
Removing or deactivating NDMA precursors during water treatment before chloramination is one of the strategies to control their formation in distribution systems. In this study, NDMA precursors were measured by means of a 7 day NDMA formation potential test.15 The NDMA FP values are shown in Table 5. The maximum concentration of NDMA formation potential was measured in the inlet water at DWTP2 during sampling campaign 1 and was 41.5 ± 4.3 ng L−1. This concentration was reduced to 16.7 ± 0.1 ng L−1 by the pre-disinfection with ozone (see Table 5). Despite this value, NDMA measured in the distribution system was negligible. The NDMA formation potential at the inlet of the remaining plants ranged from 17.2 ± 0.4 ng L−1 at DWTP5 to 28.5 ± 3.5 ng L−1 at DWTP4. Plants with lower removal of NDMA precursors were DWTP3 (12% removal) and DWTP4 (16% removal), which are the plants with no ozone oxidation treatment involved. On the other hand, the average removal observed for DWTP1 and DWTP2 was 53 and 47%, respectively. These plants include ozonation followed by granular activated carbon (GAC) in the treatment process, which in agreement with the literature proved to be an effective treatment to reduce NDMA precursors.41,42 For DWTP5, a 27% removal of NDMA formation potential was observed during sampling campaign 1. However, the removal was negligible during sampling campaign 2. Again, despite the NDMA formation values measured in the treated samples, the concentration of NDMA measured in the distribution system was below 3.5 ng L−1 in all cases (Tables 4 and 5 and Fig. 2). In contrast, the NDMA formation potential values of the treated samples from DWTP 1 to 5 were 12.6 ± 0.1 ng L−1, 19.1 ± 0.1 ng L−1, 17.5 ± 3.4 ng L−1, 23.7 ± 3.5 ng L−1, 20.5 ± 2.9 ng L−1, respectively, evidencing the conservative overestimation of NDMA in the 7 days of NDMA formation potential as expected due to the higher concentration of chloramines used in the test.43
| DWTP | Sampling 1 (January 18) | Sampling 2 (October 18) |
|---|---|---|
| DWTP1_IN | 26.8 ± 0.5 | n.m |
| DWTP1_TR | 12.6 ± 0.1 | n.m |
| DWTP2_IN | 41.5 ± 4.3 | 32.3 ± 2.1 |
| DWTP2_TR | 16.7 ± 0.1 | 21.5 ± 0.1 |
| DWTP3_IN | n.m | 19.9 ± 0.2 |
| DWTP3_TR | n.m | 17.5 ± 3.4 |
| DWTP4_IN | 17.4 ± 0.9 | 28.5 ± 3.5 |
| DWTP4_TR | n.m | 23.7 ± 3.5 |
| DWTP5_IN | 31.6 ± 1.3 | 17.2 ± 0.4 |
| DWTP5_TR | 23.0 ± 2.7 | 18.0 ± 3.1 |
Additionally, no differences were observed during the two sampling events, neither considering the NDMA formation potential nor actual NDMA formed after treatment. This was also previously observed by Uzun and coauthors44 in 12 surface water samples collected during 21 months where NDMA formation potential in reservoirs remained relatively consistent during the monitoring period and individual rain events around sampling areas did not affect NDMA formation potential levels.
Other N-nitrosamines and their precursors
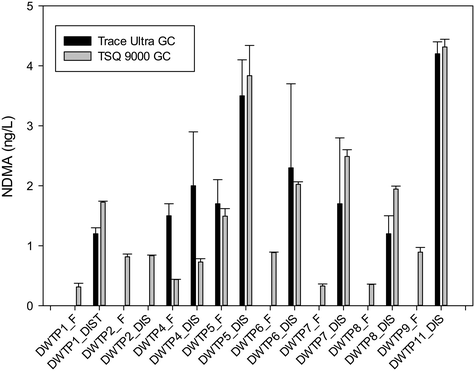
During sampling campaign 1, six additional N-nitrosamines were measured. Apart from these, NDMA was also included in the method and was measured with a TSQ™ 9000 GC system with AEI. The method reporting limit of quantification for NDMA with this new method was 0.1 ng L−1 and therefore it was possible to quantify the concentrations presented as <1 ng L−1 in Table 4. Fig. 2 compares the results for NDMA obtained with the Trace Ultra GC and the TSQ™ 9000 GC systems. The two analytical systems did mutually validate each other as the relative standard deviation of the two values was below 25%, except for the samples from DWTP4 for which the relative standard deviations were higher (1.5 ± 0.2 ng L−1vs. 0.4 ± 0.001 ng L−1 measured at DWTP4 during sampling campaigns 1 and 2, respectively).The results obtained for the additional N-nitrosamines indicated no presence of NPYR, NPIP, NDPA and NMEA above the limit of detection. In contrast, Jurado-Sánchez et al.29 found concentrations as high as 27 ng L−1 for NPIP in the drinking water tanks of a DWTP in Spain, while the other N-nitrosamines listed were not included in the study.
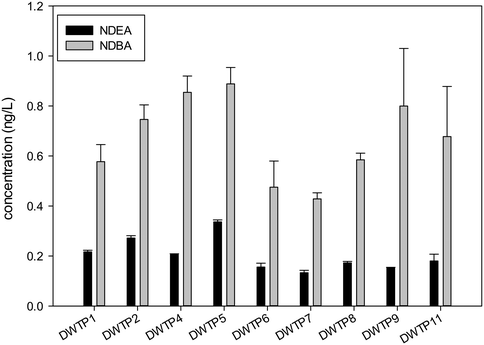
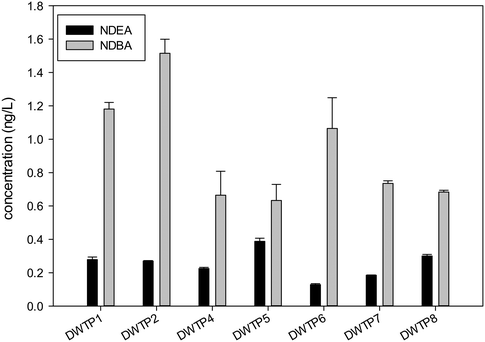
NDEA and NDBA were detected in almost all samples, however the concentrations did not exceed 1.5 ng L−1 in any case, which is in the lower range of previous studies published.23,29,32 However, NDEA specifically is around 10 times more genotoxic than NDMA,45 and therefore it should also be monitored as there is no clear evidence that NDMA and NDEA formation is correlated. Fig. 3 and 4 detail these results for the treated water and distributed water samples, respectively. Precursors for NDBA have been previously related to surface water organic matter of natural or algal origin,43 while NDEA formation has been mostly related to the presence of anthropogenic chemicals such as N,N-diethyl-meta-toluamide (DEET) and lidocaine, which include a diethylamine functional group.22 It is necessary to clarify that during the analysis, deuterated analogs of NDMA and NDEA were used to correct the losses during solid phase extraction (SPE). However, no deuterated analogs were used for the other N-nitrosamines. A recovery value was estimated based on the extraction of samples prepared at a known concentration (Table 3) and recovery values above 80% were obtained for all the nitrosamines measured. The presented values of NDBA are not corrected for losses during extraction which implies that the real value is approximately 10 to 20% higher.
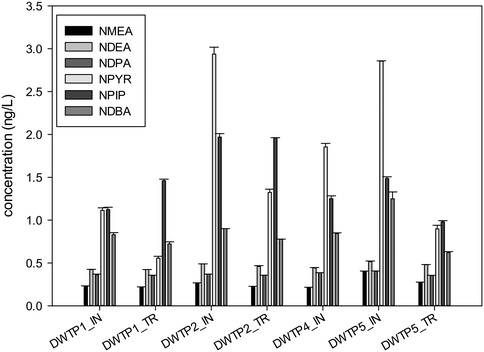
Apart from the N-nitrosamine concentrations measured in the disinfected and distributed samples (reported in Fig. 3 and 4), additional N-nitrosamines were also quantified after the NDMA formation potential test was carried out at the influent and treated samples during sampling campaign 1 for DWTP1, DWTP2, DWTP4 and DWTP5 (Fig. 5). It is obvious that the concentration of all these additional N-nitrosamines is low compared to the NDMA levels measured, which is in agreement with previous results.43 In general, the additional N-nitrosamines were measured below 2 ng L−1, except for NPYR at the inlet of DWTP2 and DWTP5, which was found at 2.9 ± 0.2 and 2.8 ± 0.01 ng L−1, respectively. It is noteworthy that the highest NDMA formation potential was also determined at the inlet of these two DWTPs. While NDBA concentration was similar between the samples taken from the DWTP, distribution system and formation potential tests, a consistently higher concentration of the remaining N-nitrosamines was measured in the formation potential test in comparison to the samples obtained from the DWTPs and distribution systems. This could be explained by the fact that no NDBA precursors were present in these waters or that chloramination is not the mechanism responsible for the formation of this specific compound. Similar to these results, Sacher et al.46 reported that although NDMA concentration in treated drinking water peaked at 4.9 ng L−1 no other N-nitrosamines were found. The laboratory disinfected samples generated NDMA up to 110 ng L−1 and NPYR at a maxima of 7.6 ng L−1, indicating that precursors of this species were also present in raw waters.
The fate of NDMA and NDMA precursors throughout a DWTP with ozonation followed by GAC
DWTP2, which was the DWTP with the highest concentration of NDMA precursors in the source water (DWTP2_IN from Table 5), was studied more in detail by taking samples during three additional sampling events (Fig. 6). During these additional sampling events, the concentration of NDMA formation potential measured at the inlet water of DWTP2 was in the range of 26.5 ± 0.5–45.9 ± 2.4 ng L−1. This concentration was significantly reduced during pre-ozonation to values in the range of 12.7 ± 0.5–14.3 ± 1.3 ng L−1. The following treatment steps including oxidation with chlorine dioxide, coagulation and rapid sand filtration did not reduce this value. During the first and third sampling even a higher NDMA formation potential was measured in this sampling point. Shah et al.47 also found that chlorine dioxide did not effectively reduce NDMA formation, and even increased NDMA in certain waters impacted by wastewater. However, the small observed impact of chlorine dioxide can possibly also be related to the fact that pre-oxidation with ozone had previously been applied. Similarly, the following second ozonation step appeared to have a minor effect. On the other hand, and as expected, the following granular activated carbon filtration had a positive effect on the further elimination of precursors 10.7 ± 1.1–13.3 ± 1.5 ng L−1 in the filtrate. The concentration of NDMA formation potential in the final water remained then in the range of 10.3 ± 0.2–11.9 ± 0.7 ng L−1. In comparison, 1.2 ± 0.3 ng L−1 of NDMA was measured in the final water of the DWTP, similar to the values obtained in the other sampling campaigns (see Table 4). Additional parameters measured can be found in the ESI† (Tables S.I.3–S.I.5).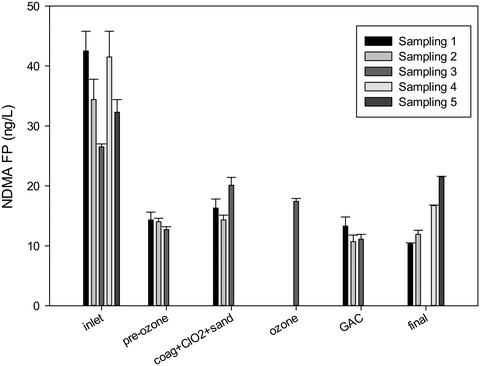 | ||
| Fig. 6 Fate of NDMA precursors across DWTP2 during five sampling events. Error bars correspond to the range (n = 2). | ||
Conclusion
The concentration of several N-nitrosamines (NDMA, NDEA, NMEA, NDBA, NPIP, NDPA, and NPYR) was measured in samples from different drinking water treatment plants (DWTPs) and strategic sampling points from drinking water networks in Spain at a very low limit of quantification (i.e., 0.1–0.5 ng L−1). The maximum concentration of NDMA measured in the final treated water or samples taken from the distribution systems was never above 4.2 ± 0.2 ng L−1. NDEA and NDBA were also detected in almost all samples, though the concentrations did not exceed 1.5 ng L−1 in any case. However, NDEA specifically is around 10 times more genotoxic than NDMA, and therefore it should be monitored as well.Additionally, N-nitrosamine precursors were also measured at the inlet of several DWTPs as well as in the treated water just before disinfection. The maximum concentration of NDMA formation potential measured in raw waters was 41.5 ± 4.3 ng L−1. As expected, plants that better removed NDMA formation potential included ozone followed by granular activated carbon (GAC) in the treatment train. The concentration of other N-nitrosamines formed during the NDMA formation potential test was lower than 3 ng L−1. In general, no seasonal variations were observed.
DWTP2, involving two ozonation steps followed by GAC was sampled three additional times to investigate the formation of NDMA and the removal of their precursors across the different treatment steps. While coagulation followed by oxidation with chlorine dioxide and filtration did not significantly reduced NDMA formation potential, a significant removal was observed after ozone–GAC filtration.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the project funded by the Spanish Ministry of Science, Innovation and Universities AEI-MICIU and the Fondo Europeo de Desarrollo Regional (FEDER) under the National Program for Research Aimed at the Challenges of Society (CTM2017-85335-R AEI-FEDER, UE). Dr. MJF acknowledges her Ramón y Cajal fellowship (RyC-2015-17108 AEI-MICIU/FSE, EU) from the AEI-MICIU. We finally acknowledge Tech 2017 SGR 1318 from the Economy and Knowledge Department of the Catalan Government.References
- G. E. Symons, Water treatment through the ages, J. - Am. Water Works Assoc., 2006, 98, 87–97 CrossRef CAS.
- S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. DeMarini, Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research, Mutat. Res., Rev. Mutat. Res., 2007, 636, 178–242 CrossRef CAS PubMed.
- A. Nikolaou, L. Rizzo and H. Selcuk, Control of disinfection by-products in drinking water systems, 2007 Search PubMed.
- J. J. Rook, Formation of haloforms during chlorination of natural waters, Water Treat. Exam., 1974, 23, 234–243 Search PubMed.
- United States Environmental Protection Agency, Emerging Contaminant- N-Nitrosodimethylamine (NDMA) Fact Sheet, 2008.
- United States Environmental Protection Agency, Emerging Contaminant- N-Nitrosodimethylamine (NDMA) Fact Sheet, 2014.
- World Health Organization International Agency, Guidelines for drinking water quality, World Health Organization, 4th edn, 2011 Search PubMed.
- United States Environmental Protection Agency, Contaminant Candidate List 4-CCL 4, 2016.
- California Environmental Protection Agency, NDMA and other Nitrosamines – California State Water Resources and Control Board., 2014.
- California Office of Environmental Health Hazard Assessment, Public Health Goal for N-Nitrosodimethylamine in Drinking Water, CA, 2006.
- Massachusetts government, Current Regulatory Limit: N-Nitrosodimethylamine. www.mass.gov/eea/agencies/massdep/water/drink, 2004.
- Ontario Ministry of the Environment, Technical Support Document for Ontario Drinking Water Standards, Objectives and Guidelines. http://www.ene.gov.on.ca/envision/gp/4449e01.pdf, 2003.
- Queensland Parliamentary Council, Public Health Regulation 2005, reprinted as at 4 July 2008., 2005.
- Australian Government, National Water Quality Management Strategy, Australian Drinking Water Guidelines 6, 2011 (updated 2018) Search PubMed.
- W. A. Mitch, A. C. Gerecke and D. L. Sedlak, A N-Nitrosodimethylamine (NDMA) precursor analysis for chlorination of water and wastewater, Water Res., 2003, 37, 3733–3741 CrossRef CAS.
- S. W. Krasner, W. A. Mitch, D. L. McCurry, D. Hanigan and P. Westerhoff, Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review, Water Res., 2013, 47, 4433–4450 CrossRef CAS.
- M. Sgroi, F. G. A. Vagliasindi, S. A. Snyder and P. Roccaro, N-Nitrosodimethylamine (NDMA) and its precursors in water and wastewater: A review on formation and removal, Chemosphere, 2018, 191, 685–703 CrossRef CAS.
- T. Bond, E. Goslan, S. Parsons and B. Jefferson, A critical review of trihalomethane and haloacetic acid formation from natural organic matter surrogates, Environ. Technol. Rev., 2012, 1, 93–113 CrossRef CAS.
- Z. Chen and R. L. Valentine, Formation of N-nitrosodimethylamine (NDMA) from humic substances in natural water, Environ. Sci. Technol., 2007, 41, 6059–6065 CrossRef CAS.
- M. J. Farré, A. Jaen-Gil, J. A. Hawkes, M. Petrovic and N. Catalán, Orbitrap molecular fingerprint of dissolved organic matter in natural waters and its relationship with NDMA formation potential, Sci. Total Environ., 2019, 670, 1019–1027 CrossRef.
- D. L. McCurry, S. W. Krasner, U. von Gunten and W. A. Mitch, Determinants of disinfectant pretreatment efficacy for nitrosamine control in chloraminated drinking water, Water Res., 2015, 84, 161–170 CrossRef CAS PubMed.
- R. Shen and S. A. Andrews, Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection, Water Res., 2011, 45, 944–952 CrossRef CAS.
- E. Bei, Y. Shu, S. Li, X. Liao, J. Wang, X. Zhang, C. Chen and S. Krasner, Occurrence of nitrosamines and their precursors in drinking water systems around mainland China, Water Res., 2016, 98, 168–175 CrossRef CAS.
- J. W. A. Charrois, J. M. Boyd, K. L. Froese and S. E. Hrudey, Occurrence of N-nitrosamines in Alberta public drinking-water distribution systems, J. Environ. Eng. Sci., 2007, 6, 103–114 CrossRef CAS.
- W. Wang, J. Yu, W. An and M. Yang, Occurrence and profiling of multiple nitrosamines in source water and drinking water of China, Sci. Total Environ., 2016, 551–552, 489–495 CrossRef CAS.
- D. Liew, J. Culbert, K. Linge, M. J. Farré, N. Knight, J. Morran, D. Halliwell, G. Newcombe and J. W. A. Charrois, National Occurrence of N-Nitrosodimethylamine (NDMA): An investigation of 38 australian drinking water supplies, Recent Advances in Disinfection By-Products, ACS Symposium Series, 2015, vol. 1190, pp. 135–149 Search PubMed.
- M. Asami, M. Oya and K. Kosaka, A nationwide survey of NDMA in raw and drinking water in Japan, Sci. Total Environ., 2009, 407, 3540–3545 CrossRef CAS.
- M. R. Templeton and Z. Chen, NDMA and seven other nitrosamines in selected UK drinking water supply systems, J. Water Supply: Res. Technol.--AQUA, 2010, 59, 277–283 CrossRef CAS.
- B. Jurado-Sánchez, E. Ballesteros and M. Gallego, Occurrence of aromatic amines and N-nitrosamines in the different steps of a drinking water treatment plant, Water Res., 2012, 46, 4543–4555 CrossRef.
- C. Planas, O. Palacios, F. Ventura, J. Rivera and J. Caixach, Analysis of nitrosamines in water by automated SPE and isotope dilution GC/HRMS. Occurrence in the different steps of a drinking water treatment plant, and in chlorinated samples from a reservoir and a sewage treatment plant effluent, Talanta, 2008, 76, 906–913 CrossRef CAS.
- Y. Qian, M. Wu, W. Wang, B. Chen, H. Zheng, S. W. Krasner, S. E. Hrudey and X. F. Li, Determination of 14 nitrosamines at nanogram per liter levels in drinking water, Anal. Chem., 2015, 87, 1330–1336 CrossRef CAS.
- W. Wang, S. Ren, H. Zhang, J. Yu, W. An, J. Hu and M. Yang, Occurrence of nine nitrosamines and secondary amines in source water and drinking water: Potential of secondary amines as nitrosamine precursors, Water Res., 2011, 45, 4930–4938 CrossRef CAS.
- C. Zhao, Q. Lu, Y. Gu, E. Pan, Z. Sun, H. Zhang, J. Zhou, Y. Du, Y. Zhang, Y. Feng, R. Liu, Y. Pu and L. Yin, Distribution of N-nitrosamines in drinking water and human urinary excretions in high incidence area of esophageal cancer in Huai'an, China, Chemosphere, 2019, 235, 288–296 CrossRef CAS.
- Z. Chen, L. Yang, Y. Huang, P. Spencer, W. Zheng, Y. Zhou, S. Jiang, W. Ye, Y. Zheng and W. Qu, Carcinogenic risk of N-Nitrosamines in Shanghai Drinking Water: Indications for the Use of Ozone Pretreatment, Environ. Sci. Technol., 2019, 53, 7007–7018 CrossRef CAS.
- J. W. Munch and M. V. Bassett, Determination of nitrosamines in drinking water by solid phase extraction and capillary column gas chromatography with large volume injection and chemical ionization tandem mass spectrometry (MS/MS), Method 521: Determination of Nitrosamines in Drinking Water by Solid Phase Extraction and Capillary Column Gas Chromatography with Large Volume Injection and Chemical Ionization Tandem Mass Spectrometry (MS/MS), 2004.
- M. J. Farré, J. Keller, N. Holling, Y. Poussade and W. Gernjak, Occurrence of N-nitrosodimethylamine precursors in wastewater treatment plant effluent and their fate during ultrafiltration-reverse osmosis membrane treatment, Water Sci. Technol., 2011, 63, 605–612 CrossRef PubMed.
- M. J. Farré, S. Insa, J. Mamo and D. Barceló, Determination of 15 N-nitrosodimethylamine precursors in different water matrices by automated on-line solid-phase extraction ultra-high-performance-liquid chromatography tandem mass spectrometry, J. Chromatogr. A, 2016, 1458, 99–111 CrossRef.
- T. Anderson, A. Gujar, T. Albertini, P. Silcock, A. Patel and F. Pigozzo, APPLICATION NOTE 10609: SmartTune wizard and easy, vent-free column replacement - two innovations in low-resolution GC-MS technology, Thermo Fisher Scientific, 2018 Search PubMed.
- A. Lamb, C. Cojocariu, S. Insa and M. J. Farré, APPLICATION NOTE 10615: Unparalleled performance of Advanced Electron Ionization GC-MS/MS technology for the determination of nitrosamines in drinking water Thermo Fisher Scientific, 2018 Search PubMed.
- European Comission, Council Directive 98/93 EC of 3 November 1998 on the quality of water intended for human consumption, Off. J. Eur. Comm., 1998, p. L330.
- C. Lee, C. Schmidt, J. Yoon and U. Von Gunten, Oxidation of N-nitrosodimethylamine (NDMA) precursors with ozone and chlorine dioxide: Kinetics and effect on NDMA formation potential, Environ. Sci. Technol., 2007, 41, 2056–2063 CrossRef CAS.
- D. Hanigan, J. Zhang, P. Herckes, S. W. Krasner, C. Chen and P. Westerhoff, Adsorption of N-nitrosodimethylamine precursors by powdered and granular activated carbon, Environ. Sci. Technol., 2012, 46, 12630–12639 CrossRef CAS.
- K. L. Linge, I. Kristiana, D. Liew, C. E. Nottle, A. Heitz and C. A. Joll, Formation of n-nitrosamines in drinking water sources: Case studies from Western Australia, J. - Am. Water Works Assoc., 2017, 109, E184–E196 CrossRef.
- H. Uzun, D. Kim and T. Karanfil, Seasonal and temporal patterns of NDMA formation potentials in surface waters, Water Res., 2015, 69, 162–172 CrossRef CAS.
- European Commission, Scientific Committee on Consumer Products, Opinion on the presence and release of nitrosamines and nitrosatable compounds from rubber balloons, 18, December 2007.
- F. Sacher, C. Schmidt, C. Lee and U. von Gunten, Strategies for Minimizing Nitrosamine Formation during Disinfection, 2008 Search PubMed.
- A. D. Shah, S. W. Krasner, C. F. T. Lee, U. Von Gunten and W. A. Mitch, Trade-offs in disinfection byproduct formation associated with precursor preoxidation for control of N -nitrosodimethylamine formation, Environ. Sci. Technol., 2012, 46, 4809–4818 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00912d |
| This journal is © The Royal Society of Chemistry 2020 |