Membrane fouling in an integrated adsorption–UF system: effects of NOM and adsorbent properties†
Kai
Li
 *ab,
Shu
Li
ab,
Ce
Sun
ab,
Tinglin
Huang
*ab,
Guibai
Li
c and
Heng
Liang
*ab,
Shu
Li
ab,
Ce
Sun
ab,
Tinglin
Huang
*ab,
Guibai
Li
c and
Heng
Liang
 c
c
aKey Laboratory of Northwest Water Resource, Environment and Ecology, MOE, Xi'an University of Architecture and Technology, Xi'an, 710055, PR China. E-mail: likai@xauat.edu.cn; likai@xauat.edu.cn; sisl2018@163.com; wandou1996@126.com; huangtinglin@xauat.edu.cn; Tel: +86 29 82207886
bShaanxi Key Laboratory of Environmental Engineering, Xi'an University of Architecture and Technology, Xi'an, 710055, PR China. Tel: +86 29 82201038
cState Key Laboratory of Urban Water Resource and Environment (SKLUWRE), Harbin Institute of Technology, Harbin 150090, PR China. E-mail: liguibai@vip.163.com; hitliangheng@163.com
First published on 4th November 2019
Abstract
The integration of adsorption with ultrafiltration (UF) is a promising technology for the production of high quality drinking water, but it is still under debate whether the addition of an adsorbent can mitigate membrane fouling. This study was conducted to investigate the impact of properties of the adsorbent and natural organic matter (NOM) in feed water on membrane fouling in an integrated adsorption–UF system. A continuous-flow hollow fiber UF set-up with periodic backwash and aeration was used to imitate the operation of full-scale integrated adsorption–UF systems. Two types of adsorbents, commercial powdered activated carbon (PAC) and homemade mesoporous adsorbent resin (MAR), were examined. As for Songhua River water dominated by allochthonous NOM, membrane fouling of the PAC/UF system was increased by 39.5% compared with that of UF alone, whereas the addition of MAR mitigated membrane fouling by 66.4%. For the synthetic water composed of algal organic matter (AOM), membrane fouling of PAC/UF and MAR/UF systems was reduced by 40.7% and 83.3%, respectively. The results suggested that deposition of adsorbent particles and fouling resistance of the cake layer were determined by the hydrophobicity and molecular weight distribution of NOM as well as the properties of adsorbents. This study highlights the significance of adsorbent selection for the integrated adsorption–UF process according to the source and properties of NOM.
Water impactThe integrated adsorption–UF process is a promising technology for the production of high quality drinking water. In spite of periodic air sparging and backwashing, a dense cake layer can still form on the membrane and result in significant irreversible fouling in some cases, which could be avoided by careful adsorbent selection according to the source and properties of NOM. |
1. Introduction
In the past decades, ultrafiltration (UF) has become an established technology for drinking water production due to the decrease in capital and maintenance costs and its excellent retention towards particulates and pathogens.1–3 However, it has two inherent drawbacks: insufficient rejection of soluble pollutants and membrane fouling.4–6 Therefore, in practical applications, UF is usually combined with other water treatment units, such as coagulation, oxidation and adsorption, to improve the overall performance.7–9 Coagulation can alleviate membrane fouling and improve permeate water quality by increasing the size of contaminants, but some particles formed during coagulation might result in significant irreversible fouling.10 Although chemical oxidation can mitigate organic fouling and biofouling, it might lead to damage of the polymeric membrane and the increase of low molecular weight (MW) organic matter in permeate.4 Adsorption is an efficient technique for the removal of natural organic matter (NOM) and micropollutants, and therefore it is often applied to improve the permeate water quality of UF and/or to control UF membrane fouling.11–14Adsorption can be combined with UF in several configurations, including an adsorption unit placed prior to UF, integration of an adsorption unit with UF, and an adsorption unit following UF.11 For the integrated adsorption–UF process, powdered adsorbents are added to the feed water of UF, and are retained by the membrane, with direct contact between adsorbents and the membrane. In this configuration, the adsorbents can be completely retained by the membrane, and high concentrations of adsorbents can be maintained prior to UF to enhance the removal of contaminants.15,16 Besides, for a submerged UF membrane, the footprint of the process can be reduced by using the membrane tank as the adsorption contactor.17 Therefore, integrated adsorption–UF is a promising technology due to its high purification efficiency and compact footprint.18
However, for the integrated adsorption–UF process, the participation of adsorbent particles makes membrane fouling more complicated.19,20 Although many studies demonstrated that adsorbent particles exerted minor influence on pure water permeability due to their larger size compared with the membrane pore size,21,22 the reported results regarding the impact of adsorbent particles on membrane fouling were controversial. Taking the most common adsorbent in water treatment, powdered activated carbon (PAC), as an example, several studies proved that PAC alleviated organic fouling by removing some NOM,23,24 but many studies indicated that the addition of PAC resulted in serious cake layer fouling and exacerbated membrane fouling ultimately.25,26 Actually, the influence of adsorbent addition on membrane fouling can be regarded as a trade-off between the decrease of organic fouling and the increase of external fouling related to adsorbent particles.20 Adsorbent particles may result in cake/gel layer fouling in combination with organics, and the structure and resistance of the cake/gel layer are closely related to the properties of NOM and the adsorbent.19
Moreover, deposition of adsorbent particles on the membrane surface depends on the configuration and hydrodynamic conditions of the membrane system.27 Many studies regarding the combined fouling of adsorbent particles and organics were conducted using flat-sheet membranes in laboratory filtration cells in dead-end mode or with stirring to simulate the shear stress on the membrane surface.12,28,29 However, in practical application of the integrated adsorption–UF system, hollow fiber membrane systems are extensively utilized, and cross-flow or air sparging is usually applied in pressurized or submerged systems, respectively, to provide shear stress and prevent deposition of particles on the membrane surface.30,31 It is hard to predict to what extent periodic backwashing and cross-flow/air sparging would impact the deposition of adsorbent particles and NOM based on bench-scale short-term filtration tests using flat-sheet membranes. Therefore, it is necessary and meaningful to investigate membrane fouling in integrated adsorption–UF systems using membrane systems mimicking the operation conditions in full scale systems.
Mesoporous adsorbent resin (MAR) is an adsorbent specially developed for membrane fouling control as reported by Clark et al.32 It was made from polymeric membrane materials (e.g., polysulfone) through a process analogous to the non-solvent induced phase separation process. It has been reported to be selective for the adsorption of organic foulants.33,34 In our previous study,19 it has been proved that the structure and resistance of the fouling layer formed by MAR and NOM model foulants during dead-end filtration were different from those formed by PAC and model foulants. The main objective of this study was to obtain a deeper understanding of the effects of characteristics of the adsorbent and NOM on membrane fouling in the integrated adsorption–UF system. A continuous-flow hollow fiber UF set-up with periodic backwashing and aeration was used to imitate the operation of full-scale integrated adsorption–UF systems. Two types of adsorbents, MAR and PAC, and two water samples dominated by allochthonous and autochthonous NOM, respectively, were examined. TMP evolution, fouling resistance distribution and adsorbent particle deposition were quantified, and the roles of adsorbent and NOM properties were highlighted.
2. Materials and methods
2.1 Experimental set-up
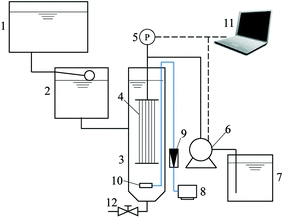
The UF membrane used in this study was a hollow fiber polyvinyl chloride membrane provided by Litree Purifying Technology Co. Ltd (Shenzhen, China). The inside and outside diameters of the fiber were 0.85 and 1.45 mm, respectively. According to the manufacturer, the molecular weight cutoff (MWCO) of the membrane is 100 kDa, and the pure water contact angle is 74 ± 5°. The module was homemade by assembling several fibers together, and the total membrane area of the module was 0.01 m2.A lab-scale submerged hollow fiber UF system with automatic periodic backwashing and aeration was employed to simulate the operational procedure and hydrodynamic conditions in full-scale systems (Fig. 1). The filtration cycle consisted of 118 min filtration and 2 min backwashing, while the aerator was turned on for 0.5 min every 5 min. The filtration flux was kept at 30 L m−2 h−1 using a peristaltic pump (BT-100, Longer Pump, Baoding, China), and backwashing was conducted by reverse rotation of the peristaltic pump. A pressure transducer (PTP708, Tuopu Electric, Foshan, China) was mounted between the membrane module and the peristaltic pump to monitor the trans-membrane pressure (TMP). The transducer was connected to a computer, and the data were automatically logged every five seconds. An aeration intensity of 35 m3 m−2 h−1 (calculated based on the bottom area of the membrane tank) was used to prevent the sedimentation of adsorbent particles at the bottom of the membrane tank. The sludge was discharged every six filtration cycles and a fresh adsorbent was added at the beginning of each sludge discharge cycle at a dose of 50 mg L−1 based on the volume of inflow water in the whole cycle. The system was operated in constant flux mode with automatic backwashing and periodic aeration, and therefore the membrane fouling and adsorbent particle deposition can be regarded as hydraulically irreversible from the perspective of full-scale operation.
At the end of the experiment, the remaining fouling resistance was distinguished as external and internal fouling by determining the filtration resistance before and after wiping the membrane surface with a wet sponge.35 Briefly, after emptying the membrane tank and refilling with pure water, filtration for 5 min was conducted, and the average of TMP values was recorded as TMP1. Then the membrane module was taken out and the surface of each fiber was wiped with a wet sponge carefully. Afterwards, the module was reinstalled and filtration of pure water was carried out again with the average of TMP values recorded as TMP2. Therefore, with the TMP of the new membrane module denoted as TMP2, the resistance of external fouling (Ref) and internal fouling (Rif) can be calculated by eqn (1) and (2), respectively.
| Ref = (TMP1 − TMP2)/(μ·J) | (1) |
| Rif = (TMP2 − TMP0)/(μ·J) | (2) |
2.2 Feed water and adsorbents
Two water samples with different NOM sources, a river water sample taken from Songhua River (Harbin, China) dominated by allochthonous NOM and a synthetic water sample composed of autochthonous NOM (algal organic matter, AOM), were used as feed water. AOM was extracted from Microcystis aeruginosa cultured under axenic conditions with BG 11 medium.36 Algal suspension harvested at the stationary stage was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (11
000 rpm (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 179 g) and 4 °C for 15 min, and the supernatant was filtered using 0.45 μm mixed cellulose filters (Taoyuan, China) to obtain AOM stock solution. The water quality parameters of Songhua River water and AOM-contaminated water are presented in Table 1. The total organic carbon (TOC) of AOM-containing water was adjusted to 3.0 ± 0.4 mg L−1 to obtain a comparable fouling propensity with Songhua River water in the UF process alone.
179 g) and 4 °C for 15 min, and the supernatant was filtered using 0.45 μm mixed cellulose filters (Taoyuan, China) to obtain AOM stock solution. The water quality parameters of Songhua River water and AOM-contaminated water are presented in Table 1. The total organic carbon (TOC) of AOM-containing water was adjusted to 3.0 ± 0.4 mg L−1 to obtain a comparable fouling propensity with Songhua River water in the UF process alone.
| Parameters | Unit | Songhua River water | AOM-containing water |
|---|---|---|---|
| pH | — | 7.8 ± 0.2 | 7.5 ± 0.1 |
| Turbidity | NTU | 6.35 ± 0.24 | 1.53 ± 0.17 |
| TOC | Mg L−1 | 6.2 ± 0.4 | 3.0 ± 0.4 |
| DOC | Mg L−1 | 5.9 ± 0.3 | 2.9 ± 0.4 |
| UV254 | Cm−1 | 0.161 ± 0.005 | 0.042 ± 0.004 |
| SUVA | L (mg−1 m−1) | 2.62 ± 0.03 | 1.38 ± 0.02 |
MAR was made from polyethersulfone (Veradel 3000P, Solvay, USA) by following the method proposed by Clark, et al.32 Wood-based PAC purchased from Bench Chemicals Co. (Tianjin, China) was used without further purification. The particle size and zeta potentials of the two adsorbents were measured using a laser particle size analyzer (MasterSizer 2000, Malvern, UK) and a zeta potential analyzer (Zetasizer Nano S90, Malvern, UK), respectively. The surface areas and pore size distributions of MAR and PAC were measured using a surface area and porosity analyzer (ASAP 2020, Micromeritics, USA) with the N2 adsorption method. The amounts of surface functional groups were determined by Boehm titration. The physical and chemical properties of MAR and PAC are shown in Table S1 in the ESI.† The FTIR spectra of MAR and PAC were acquired by using an infrared spectrometer (Nicolet iS50, Thermo Scientific, MA, USA), and the results are presented in Fig. S1 in the ESI.† It can be seen that MAR and PAC have similar particle sizes (25.2 versus 32.1 μm) and surface zeta potentials (−22.4 versus −23.9 mV), but have distinct specific surface areas and pore structures. The surface area of PAC (1219 m2 g−1) was much larger than that of MAR (108 m2 g−1), and the pore structures of MAR and PAC were dominated by mesopores and micropores, respectively.
2.3 Analytical methods
TOC and dissolved organic carbon (DOC) was measured using a TOC analyzer (multi N/C 2100, Analytik Jena AG, Jena, Germany), and fluorescence excitation–emission matrix (EEM) spectra were obtained using a fluorescence spectrophotometer (F-7000, Hitachi Ltd, Tokyo, Japan). Ultraviolet absorbance at 254 nm (UV254) was determined using a UV/vis spectrophotometer (T6, Puxi, Beijing, China). The MW and hydrophilicity distributions of NOM in feed water samples were determined by a UF separation method and from non-ionic macroporous resins, respectively.36 Each fractionation was conducted in triplicate and quantified by both DOC and UV254 measurements. The distributions were calculated as differences in DOC concentrations and UV254 between permeates of membranes with different MWCOs and adsorption columns filled with different resins.33 Moreover, the distribution of adsorbent particles was quantified by a gravimetric method and mass balance. Briefly, the amount of adsorbent particles suspended in the membrane tank was obtained by determining the total suspended solids through a standard method.37 And then the amount of adsorbent particles deposited on the membrane surface was calculated by subtracting the amount of suspended adsorbent particles from the cumulative adsorbent dosage.3. Results and discussion
3.1 TMP increase in integrated adsorption–UF systems
Effects of the addition of MAR or PAC on the TMP increase during treatment of Songhua River water and AOM-containing water are shown in Fig. 2. In UF alone, filtration of Songhua River water and AOM-containing water resulted in similar TMP increases in the first several filtration cycles. But in the subsequent filtration cycles, the TMP of the system filtering Songhua River water became much higher than that filtering AOM-containing water due to the larger irreversible fouling caused by Songhua River water.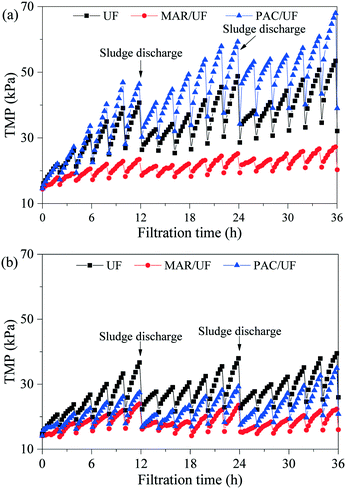 | ||
| Fig. 2 TMP evolution in integrated adsorption–UF systems during treatment of Songhua River water (a) and AOM-containing water (b). | ||
For Songhua River water, both the TMP increase within a filtration cycle and the irreversible TMP increase among filtration cycles were significantly mitigated by the addition of MAR. In contrast, the addition of PAC exerted minor influence on the TMP increase in the first two filtration cycles, but it increased irreversible fouling significantly in the following cycles. At the end of the experiment, compared with UF alone, the irreversible TMP increase of MAR/UF was reduced by 66.4%, whereas that of PAC/UF was increased by 39.5%.
As for AOM-containing water, the TMP increase of both PAC/UF and MAR/UF systems was lower than that of UF alone, and MAR controlled membrane fouling more efficiently. Based on the irreversible TMP increase at the end of the experiment, the addition of MAR and PAC reduced membrane fouling by 83.3% and 40.7%, respectively.
3.2 Distribution of fouling resistance and adsorbent particles
To explain the various influences of MAR and PAC on membrane fouling by two different feed water samples, the distribution of fouling resistance and adsorbent particles at the end of the experiment was examined. Because there was periodic backwashing and aeration in the UF system, the fouling resistance at the end of the experiment was hydraulically irreversible from the perspective of practical application. By determining the filtration resistance before and after wiping the membrane surface with a wet sponge, the fouling resistance can be classified as internal and external fouling. Adsorbent particles can only cause external fouling because their size was much larger than membrane pores. As shown in Fig. 3, external fouling in PAC/UF was more than twice that in UF alone during treatment of Songhua River water, but external fouling was slightly reduced by addition of PAC during treatment of AOM-containing water. With respect to MAR/UF, both external fouling and internal fouling were significantly reduced during filtration of Songhua River water and AOM-containing water in comparison with UF alone.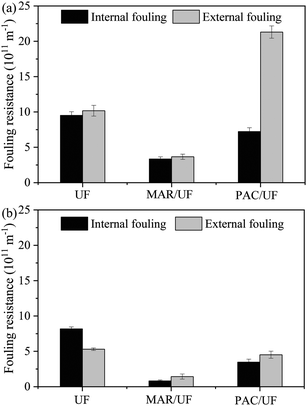 | ||
| Fig. 3 Distribution of irreversible fouling resistance at the end of the experiment for Songhua River water (a) and AOM-containing water (b) (n = 3). | ||
The amount of adsorbent particles suspended in the membrane tank and deposited on the membrane surface at the end of the experiment was determined by a gravimetric method and mass balance, and the ratios are shown in Fig. 4. For Songhua River water, although there was periodic backwashing and aeration, more than 90% of PAC deposited on the membrane surface, while more than 60% of MAR was suspended in the membrane tank. The results were consistent with the significantly higher external fouling for PAC/UF (Fig. 3(a)). With respect to AOM-containing water, though the percentage of deposited PAC was still a little higher than that of MAR, it was much lower than that for Songhua River water.
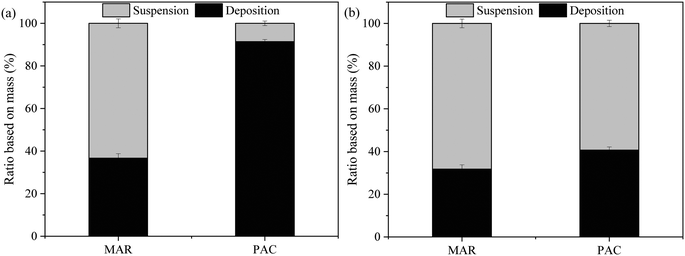 | ||
| Fig. 4 Distribution of adsorbent particles at the end of the experiment for Songhua River water (a) and AOM-containing water (b) (n = 3). | ||
3.3 Properties of NOM in Songhua River water and AOM-containing water
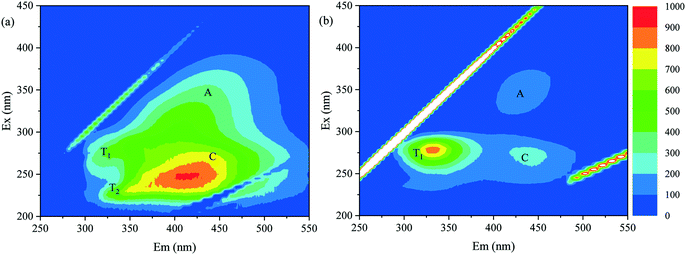
As indicated in Fig. 3 and 4, in the integrated adsorption–UF process, the deposition behavior of adsorbent particles on the membrane surface and the fouling resistance of the cake layer depended on the properties of both the adsorbent and NOM in feed water. Previous studies have elucidated the textural properties and adsorption preference of MAR and PAC in detail,19,28 and the properties of NOM in these two feed water samples were examined herein.The fluorescence EEM spectra of NOM in Songhua River water and AOM-containing water are shown in Fig. 5. The NOM in Songhua River water exhibited four major peaks: peaks A and C representing humic-like substances and peaks T1 and T2 which represent protein-like substances. The intensity of peak C was much higher than the other peaks, suggesting the predominance of allochthonous humic substances.38 In previous studies, it has been demonstrated that the mixture of PAC particles and humic substances can form a dense cake layer on the membrane surface, while the cake layer composed of MAR and humic substances was much porous.19,20 This study suggested that the dense cake layer formed by PAC and humic substances cannot be readily removed by backwashing and shear stress induced by aeration. For AOM-containing water, the intensity of peak T1 was much higher than those of peaks A and C, indicating the dominance of autochthonous proteins.39 Although polysaccharides cannot be detected by EEM, they are usually concomitant with proteins in AOM.36,40 Compared with Songhua River water, the amount of deposited PAC was much lower for AOM-containing water, probably due to the lower hydrophobicity of autochthonous proteins and polysaccharides in comparison with allochthonous humic substances.40
The MW distributions of NOM in Songhua River water and AOM are shown in Fig. 6. It can be observed that most of the NOM in Songhua River water was smaller than 30 kDa, and fractions with MW 10–30 kDa, 3–10 kDa and <3 kDa accounted for 30.2%, 27.2% and 37.3% in terms of DOC, respectively. As shown in Fig. 5(a), the NOM in Songhua River was dominated by humic substances, and therefore the MW distribution in terms of UV254 was in accordance with that based on DOC. As for AOM, the MW distribution exhibited a typical bimodal mode based on DOC, and the MW of more than 47% AOM was higher than 100 kDa. As demonstrated by previous studies,36,40 the high-MW fraction in AOM was mainly attributed to autochthonous proteins and polysaccharides, which was consistent with the result of fluorescence EEM. But the MW distribution in terms of UV254 was distinct from that based on DOC, and most of the UV-absorbing molecules in AOM were smaller than 3 kDa. Because UV-absorption was mainly caused by humic substances, rather than proteins and polysaccharides,41 it can be inferred that most of the humic substances in AOM were smaller than 3 kDa.
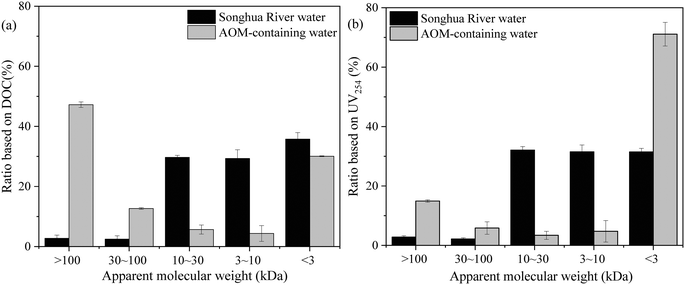 | ||
| Fig. 6 MW distribution of NOM in Songhua River water and AOM-containing water: (a) ratio based on DOC and (b) ratio based on UV254 (n = 3). | ||
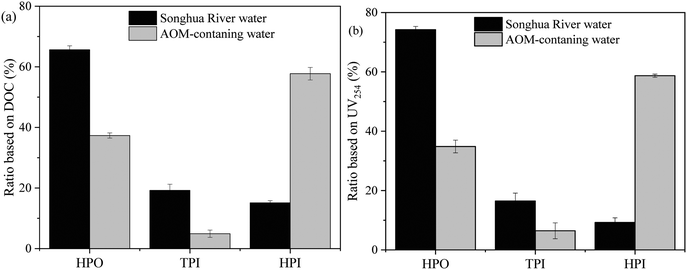
The hydrophobicity distributions of NOM in Songhua River water and AOM are shown in Fig. 7. For both NOM in Songhua River water and AOM, the hydrophilicity distributions based on DOC and UV254 were analogous, suggesting that the hydrophilicity distribution of the UV-absorbing fraction was consistent with that of the non-UV-absorbing fraction. The hydrophobic (HPO) fraction accounted for more than 65% of NOM in Songhua River water in terms of DOC, which was consistent with the nature of allochthonous humic substances as shown in the fluorescence EEM spectra in Fig. 5(a). As for AOM, the hydrophilic (HPI) fraction accounted for 59% in terms of DOC, followed by the HPO fraction accounting for 36%, which was in accordance with the predominance of autochthonous proteins as shown in the fluorescence EEM spectra in Fig. 5(b). In short, there was more HPO organic fraction in Songhua River water than in AOM-containing water.
Based on the analysis of fluorescence EEM, MW distribution and hydrophobicity distribution, it can be concluded that the composition and properties of NOM in the two feed water samples were quite different. The NOM in Songhua River water was dominated by humic substances with low to medium MW and strong hydrophobicity, while there was more HPI fraction in AOM and the MW of most humic substances in AOM was less than 3 kDa. With respect to the properties of adsorbents, the key distinction was the pore structure, and the average pore size of MAR (16.4 nm) was much larger than that of PAC (2.2 nm) (Table S1†). The discrepant influence of MAR and PAC on fouling in the integrated adsorption–UF system for different feed water samples can be explained by the properties of NOM and adsorbents. For Songhua River water, organic molecules with various MWs as well as organic colloids can be removed by entering the internal pores of MAR, and the cake layer formed by MAR particles was porous because there were less organics in the gap between adsorbent particles.19 In contrast, the organics with medium and high MWs as well as organic colloids cannot enter the micropores of PAC due to size exclusion, but their interactions with PAC particles were strong due to the predominance of the HPO fraction, making a dense cake layer composed of adsorbent particles and organics on the membrane surface.26 Therefore, large amounts of PAC particles deposited on the membrane surface and resulted in irreversible fouling, whereas MAR particles deposited on the membrane surface did not lead to obvious cake fouling. With respect to AOM, much less PAC particles were deposited on the membrane surface and the cake layer resistance was small because there was more HPI fraction in AOM and the interactions between the high-MW fraction (i.e., proteins and polysaccharides) and PAC particles were weak.33,40 Meanwhile, the addition of MAR mitigated fouling better because its mesoporous structure favored more removal of high-MW organics.33,34 Overall, in the integrated adsorption–UF system, although periodic air sparging and backwashing can prevent particle deposition to some extent, a dense cake layer composed of adsorbent particles and organics can still form and result in significant irreversible fouling in some cases, which could be avoided by careful adsorbent selection according to the source and properties of NOM.
It should be noted that PAC was more efficient than MAR in reducing DOC concentration in membrane permeate (Fig. S2†), which can be attributed to the much higher specific surface area of PAC compared with MAR (Table S1†). Meanwhile, the mesoporous structure of MAR was the major reason why MAR outperformed PAC in organic fouling control. Therefore, taking into account the mitigation of membrane fouling and the decrease of permeate DOC concentration at the same time, developing novel adsorbents with high specific surface area and abundant large mesopores (10 < w < 50 nm) is of great significance for the integrated adsorption-UF process.
4. Conclusions
The impact of the properties of NOM and adsorbents on membrane fouling in an integrated adsorption–UF system was investigated. Two adsorbents with distinct pore structures and two water samples containing NOM with different origins and characteristics were examined.For surface water dominated by allochthonous NOM (Songhua River water), the addition of PAC exacerbated membrane fouling due to the formation of a dense cake layer composed of PAC particles and hydrophobic organics. In contrast, less MAR particles were deposited on the membrane surface and the cake layer was much more porous due to its mesoporous structure. For the AOM-containing water, the MW and hydrophobicity distribution of AOM prevented the formation of a dense PAC cake layer. The results suggested that the deposition of adsorbent particles and the fouling resistance of the cake layer were influenced by the hydrophobicity and MW distribution of NOM as well as the properties of adsorbents. This study highlights the significance of adsorbent selection for the integrated adsorption–UF process according to the source and properties of NOM.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was jointly supported by the National Natural Science Foundation of China (51608427), the Key Research and Development Program of Shaanxi Province (2019ZDLSF06-01) and The Youth Innovation Team of Shaanxi Universities Funded by the Education Department of Shaanxi Province.References
- S. Chatterjee and S. De, Adsorptive removal of arsenic from groundwater using a novel high flux polyacrylonitrile (PAN)-laterite mixed matrix ultrafiltration membrane, Environ. Sci.: Water Res. Technol., 2015, 1, 227–243 RSC.
- F. Qu, H. Wang, J. He, G. Fan, Z. Pan, J. Tian, H. Rong, G. Li and H. Yu, Tertiary treatment of secondary effluent using ultrafiltration for wastewater reuse: correlating membrane fouling with rejection of effluent organic matter and hydrophobic pharmaceuticals, Environ. Sci.: Water Res. Technol., 2019, 5, 672–683 RSC.
- X. Cheng, W. Zhou, P. Li, Z. Ren, D. Wu, C. Luo, X. Tang, J. Wang and H. Liang, Improving ultrafiltration membrane performance with pre-deposited carbon nanotubes/nanofibers layers for drinking water treatment, Chemosphere, 2019, 234, 545–557 CrossRef CAS.
- K. Li, G. Wen, S. Li, H. Chang, S. Shao, T. Huang, G. Li and H. Liang, Effect of pre-oxidation on low pressure membrane (LPM) for water and wastewater treatment: A review, Chemosphere, 2019, 231, 287–300 CrossRef CAS.
- X. Du, K. Zhang, B. Xie, J. Zhao, X. Cheng, K. Li, J. Nie, Z. Wang, G. Li and H. Liang, Peroxymonosulfate-assisted electro-oxidation/coagulation coupled with ceramic membrane for manganese and phosphorus removal in surface water, Chem. Eng. J., 2019, 365, 334–343 CrossRef CAS.
- K. Gao, T. Li, J. Liu, B. Dong and H. Chu, Ultrafiltration membrane fouling performance by mixtures with micromolecular and macromolecular organics, Environ. Sci.: Water Res. Technol., 2019, 5, 277–286 RSC.
- F. Qu, Z. Yan, H. Wang, X. Wang, H. Liang, H. Yu, J. He and G. Li, A pilot study of hybrid biological activated carbon (BAC) filtration-ultrafiltration process for water supply in rural areas: role of BAC pretreatment in alleviating membrane fouling, Environ. Sci.: Water Res. Technol., 2018, 4, 315–324 RSC.
- S. Shao, Y. Wang, D. Shi, X. Zhang, C. Y. Tang, Z. Liu and J. Li, Biofouling in ultrafiltration process for drinking water treatment and its control by chlorinated-water and pure water backwashing, Sci. Total Environ., 2018, 644, 306–314 CrossRef CAS PubMed.
- J. Tian, C. Wu, H. Yu, S. Gao, G. Li, F. Cui and F. Qu, Applying ultraviolet/persulfate (UV/PS) pre-oxidation for controlling ultrafiltration membrane fouling by natural organic matter (NOM) in surface water, Water Res., 2018, 132, 190–199 CrossRef CAS PubMed.
- Q. Ding, H. Yamamura, N. Murata, N. Aoki, H. Yonekawa, A. Hafuka and Y. Watanabe, Characteristics of meso-particles formed in coagulation process causing irreversible membrane fouling in the coagulation-microfiltration water treatment, Water Res., 2016, 101, 127–136 CrossRef CAS.
- C. Stoquart, P. Servais, P. R. Bérubé and B. Barbeau, Hybrid Membrane Processes using activated carbon treatment for drinking water: A review, J. Membr. Sci., 2012, 411, 1–12 CrossRef.
- S. Shao, L. Cai, K. Li, J. Li, X. Du, G. Li and H. Liang, Deposition of powdered activated carbon (PAC) on ultrafiltration (UF) membrane surface: influencing factors and mechanisms, J. Membr. Sci., 2017, 530, 104–111 CrossRef CAS.
- B. Malczewska and M. M. Benjamin, Efficacy of hybrid adsorption/membrane pretreatment for low pressure membrane, Water Res., 2016, 99, 263–271 CrossRef CAS PubMed.
- A. Ding, J. Wang, D. Lin, X. Cheng, H. Wang, L. Bai, N. Ren, G. Li and H. Liang, Effect of PAC particle layer on the performance of gravity-driven membrane filtration (GDM) system during rainwater treatment, Environ. Sci.: Water Res. Technol., 2018, 4, 48–57 RSC.
- M. Tomaszewska and S. Mozia, Removal of organic matter from water by PAC/UF system, Water Res., 2002, 36, 4137–4143 CrossRef CAS.
- J. Lowenberg, A. Zenker, M. Baggenstos, G. Koch, C. Kazner and T. Wintgens, Comparison of two PAC/UF processes for the removal of micropollutants from wastewater treatment plant effluent: Process performance and removal efficiency, Water Res., 2014, 56C, 26–36 CrossRef.
- J.-y. Tian, H. Liang, Y.-l. Yang, S. Tian and G.-b. Li, Membrane adsorption bioreactor (MABR) for treating slightly polluted surface water supplies: As compared to membrane bioreactor (MBR), J. Membr. Sci., 2008, 325, 262–270 CrossRef CAS.
- M. Campinas and M. J. Rosa, Removal of microcystins by PAC/UF, Sep. Purif. Technol., 2010, 71, 114–120 CrossRef CAS.
- K. Li, H. Liang, F. Qu, S. Shao, H. Yu, Z.-s. Han, X. Du and G. Li, Control of natural organic matter fouling of ultrafiltration membrane by adsorption pretreatment: Comparison of mesoporous adsorbent resin and powdered activated carbon, J. Membr. Sci., 2014, 471, 94–102 CrossRef CAS.
- S. Shao, H. Liang, F. Qu, K. Li, H. Chang, H. Yu and G. Li, Combined influence by humic acid (HA) and powdered activated carbon (PAC) particles on ultrafiltration membrane fouling, J. Membr. Sci., 2016, 500, 99–105 CrossRef CAS.
- S. Mozia, M. Tomaszewska and A. W. Morawski, Studies on the effect of humic acids and phenol on adsorption-ultrafiltration process performance, Water Res., 2005, 39, 501–509 CrossRef CAS.
- M. Campinas and M. J. Rosa, Assessing PAC contribution to the NOM fouling control in PAC/UF systems, Water Res., 2010, 44, 1636–1644 CrossRef CAS PubMed.
- H. Oh, M. Yu, S. Takizawa and S. Ohgaki, Evaluation of PAC behavior and fouling formation in an integrated PAC-UF membrane for surface water treatment, Desalination, 2006, 192, 54–62 CrossRef CAS.
- Y. Zhang, J. Tian, J. Nan, S. Gao, H. Liang, M. Wang and G. Li, Effect of PAC addition on immersed ultrafiltration for the treatment of algal-rich water, J. Hazard. Mater., 2011, 186, 1415–1424 CrossRef CAS PubMed.
- C.-F. Lin, Y.-J. Huang and O. J. Hao, Ultrafiltration processes for removing humic substances: effect of molecular weight fractions and PAC treatment, Water Res., 1999, 33, 1252–1264 CrossRef CAS.
- M. Zhang, C. Li, M. M. Benjamin and Y. Chang, Fouling and Natural Organic Matter Removal in Adsorbent/Membrane Systems for Drinking Water Treatment, Environ. Sci. Technol., 2003, 37, 1663–1669 CrossRef CAS PubMed.
- X. Du, F.-S. Qu, H. Liang, K. Li, L.-M. Bai and G.-B. Li, Control of submerged hollow fiber membrane fouling caused by fine particles in photocatalytic membrane reactors using bubbly flow: Shear stress and particle forces analysis, Sep. Purif. Technol., 2017, 172, 130–139 CrossRef CAS.
- K. Li, T. Huang, F. Qu, X. Du, A. Ding, G. Li and H. Liang, Performance of adsorption pretreatment in mitigating humic acid fouling of ultrafiltration membrane under environmentally relevant ionic conditions, Desalination, 2016, 377, 91–98 CrossRef CAS.
- Z. Cai and M. M. Benjamin, NOM Fractionation and Fouling of Low-Pressure Membranes in Microgranular Adsorptive Filtration, Environ. Sci. Technol., 2011, 45, 8935–8940 CrossRef CAS.
- M. Pourbozorg, T. Li and A. W. K. Law, Effect of turbulence on fouling control of submerged hollow fibre membrane filtration, Water Res., 2016, 99, 101–111 CrossRef CAS.
- X. Liu, Y. Wang, T. D. Waite and G. Leslie, Numerical simulation of bubble induced shear in membrane bioreactors: effects of mixed liquor rheology and membrane configuration, Water Res., 2015, 75, 131–145 CrossRef CAS.
- M. M. Clark, W.-Y. Ahn, X. Li, N. Sternisha and R. L. Riley, Formation of Polysulfone Colloids for Adsorption of Natural Organic Foulants, Langmuir, 2005, 21, 7207–7213 CrossRef CAS.
- K. Li, F. Qu, H. Liang, S. Shao, Z.-s. Han, H. Chang, X. Du and G. Li, Performance of mesoporous adsorbent resin and powdered activated carbon in mitigating ultrafiltration membrane fouling caused by algal extracellular organic matter, Desalination, 2014, 336, 129–137 CrossRef CAS.
- L. C. Koh, W.-Y. Ahn and M. M. Clark, Selective adsorption of natural organic foulants by polysulfone colloids: Effect on ultrafiltration fouling, J. Membr. Sci., 2006, 281, 472–479 CrossRef CAS.
- W. Yu and N. J. D. Graham, Performance of an integrated granular media – Ultrafiltration membrane process for drinking water treatment, J. Membr. Sci., 2015, 492, 164–172 CrossRef CAS.
- F. Qu, H. Liang, Z. Wang, H. Wang, H. Yu and G. Li, Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: Influences of interfacial characteristics of foulants and fouling mechanisms, Water Res., 2012, 46, 1490–1500 CrossRef CAS.
- E. W. Rice, R. B. Baird, A. D. Eaton and L. S. Clesceri, Standard methods for the examination of water and wastewater, American Public Health Association, Washington, DC, 2012 Search PubMed.
- S. Shao, H. Liang, F. Qu, H. Yu, K. Li and G. Li, Fluorescent natural organic matter fractions responsible for ultrafiltration membrane fouling: Identification by adsorption pretreatment coupled with parallel factor analysis of excitation–emission matrices, J. Membr. Sci., 2014, 464, 33–42 CrossRef CAS.
- N. Lee, G. Amy and J.-P. Croué, Low-pressure membrane (MF/UF) fouling associated with allochthonous versus autochthonous natural organic matter, Water Res., 2006, 40, 2357–2368 CrossRef CAS.
- R. K. Henderson, A. Baker, S. A. Parsons and B. Jefferson, Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms, Water Res., 2008, 42, 3435–3445 CrossRef CAS.
- J. L. Weishaar, G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii and K. Mopper, Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon, Environ. Sci. Technol., 2003, 37, 4702–4708 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00843h |
| This journal is © The Royal Society of Chemistry 2020 |