Berry polyphenols metabolism and impact on human gut microbiota and health
Laura
Lavefve
 a,
Luke R.
Howard
a,
Luke R.
Howard
 a and
Franck
Carbonero
a and
Franck
Carbonero
 *ab
*ab
aDepartment of Food Science, University of Arkansas, USA
bDepartment of Nutrition and Exercise Physiology, Elson Floyd School of Medicine, Washington State University-Spokane, USA. E-mail: franck.carbonero@wsu.edu
First published on 15th November 2019
Abstract
Berries are rich in phenolic compounds such as phenolic acids, flavonols and anthocyanins. These molecules are often reported as being responsible for the health effects attributed to berries. However, their poor bioavailability, mostly influenced by their complex chemical structures, raises the question of their actual direct impact on health. The products of their metabolization, however, may be the most bioactive compounds due to their ability to enter the blood circulation and reach the organs. The main site of metabolization of the complex polyphenols to smaller phenolic compounds is the gut through the action of microorganisms, and reciprocally polyphenols and their metabolites can also modulate the microbial populations. In healthy subjects, these modulations generally lead to an increase in Bifidobacterium, Lactobacillus and Akkermansia, therefore suggesting a prebiotic-like effect of the berries or their compounds. Finally, berries have been demonstrated to alleviate symptoms of gut inflammation through the modulation of pro-inflammatory cytokines and have chemopreventive effects towards colon cancer through the regulation of apoptosis, cell proliferation and angiogenesis. This review recapitulates the knowledge available on the interactions between berries polyphenols, gut microbiota and gut health and identifies knowledge gaps for future research.
1. Introduction
The consumption of fruits and vegetables is part of dietary recommendations for a healthy diet, due to their contribution in providing fibers, vitamins, minerals and phytochemicals to the body. Several epidemiological and clinical studies have demonstrated that regular consumption of fruits and vegetables lowers the risks of developing cancers, cardiovascular diseases or obesity, and demonstrates antioxidant and anti-inflammatory properties (reviewed in ref. 1 and 2). Amongst fruits, berries are of particular interest due to their generally higher content in bioactive compounds.Botanically, berries are defined as fleshy fruits produced from a single ovary. The definition excludes strawberries or raspberries. However, this review will focus on fruits commonly considered as berries, including those from the genera Rubus (blackberries, raspberries), Vaccinium (blueberries, cranberries, lingonberries), Fragaria (strawberries), Ribes (black currant) or Lycium (goji berries). Despite fitting the botanical definition of berry, grapes will not be discussed in detail here as their impact human health and gut microbiota have already been reviewed extensively elsewhere.3–5
Because of their purported health properties,6 the use of berries or berry extracts as ingredients in functional foods or supplements is currently a growing market.7 This increasing interest of the impact of berries on health is mainly due to their rich content in bioactive molecules, and particularly phenolic compounds such as phenolic acids, anthocyanins, flavonols, flavanols, or tannins.8 The wide variety of phytochemicals present in the plants leads to disparate profiles and concentrations of polyphenols, resulting in the presence of different major compounds within each berry genera and species.9,10 Many studies have investigated the beneficial effects of berries on health and the association between berry type and health conditions. The use of cranberry to treat urinary tract health as an alternative to antibiotics has been extensively studied and the most accepted mechanism of action is through the antiadhesive properties of the berry (reviewed in ref. 11). Accumulating evidences suggest a preventive action of cranberry against intestinal diseases and cardiovascular health.11,12 A wide number of studies have focused on the preventive effect of blueberries towards cardiovascular diseases (reviewed in ref. 13 and 14), as well as strawberries, due to their ellagitannin content.15 Blackcurrants are mainly being investigated for their anti-inflammatory, immune-modulatory and antioxidant properties (reviewed in ref. 16). More recently, an increasing number of studies have explored the potential anticancer effects of berries (reviewed in ref. 17 and 18). The high levels of vitamin C, carotenoids and phenolic compounds in berries grants them high antioxidant potential, and they are considered to be the most important source of antioxidant molecules in the human diet.18 These properties are suggested to contribute to the positive health properties attributed to berries, as well as potential beneficial impacts on apoptosis, proliferation of cancerous cells, reduction of inflammation, inhibition of angiogenesis or protection from DNA damage.17
A recent but expanding field of research aims to demonstrate the significance of the gut and gut microbiome in health and disease. The gut exerts many functions, including a protective role, and metabolic and physiologic processes. The resident microorganisms in the gut protect the host against the colonization by pathogens, metabolize non-digestible compounds and interact with the epithelial cells. This interplay provides the host with nutrients and helps build the immune system.19 Probiotic and prebiotics are designed with the aim to confer benefits to the gut microbiome and gut health. Specifically, prebiotics are, by definition, able to resist the digestion by human enzymes and reach the gut, where they are fermented by the microbiota and positively stimulate the growth of beneficial bacteria.20 This definition mainly includes dietary fibers and sufficient scientific evidences are needed to support other compounds classification as prebiotics. More recently, the scientific community focused on the interaction between polyphenols, which have generally low digestibility, and the gut microbiota. Particularly, the positive modulation of the microbiota by these compounds, potentially presenting prebiotic-like properties is of interest.21 Current research shows a large interest in the possible effects of berries and their phenolic compounds on human health, particularly in cardiovascular health, aging, inflammation and cancer prevention. Gut health and microbiota are emerging topics due to the recognition of the multiple roles of the gut in maintaining health,22 as well as the greater availability of affordable high-throughput DNA sequencing technologies.23 Whole berries, due to their high content and diverse phenolic profiles, are good candidates to study the effect of dietary polyphenols on the gut microbiota as well as potential synergies that cannot be studied by extracts alone.6 This review aims to recapitulate the available knowledge on the fate of dietary polyphenols from a wide range of berries in the digestive tract as well as the potential modulation of the gut microbiota and their effects on gut health. The literature search was mainly focused on studies that used whole berries, juice, freeze-dried powder or fractions and compounds directly extracted from the berries. Keywords used for searches in PubMed included “berries”, “strawberries”, “blueberries”, “raspberries”, “blackberries” and other berries in combination with “gut microbiome”, “metabolome”, “metabolites” and other related terms.
2. Berries polyphenol profiles
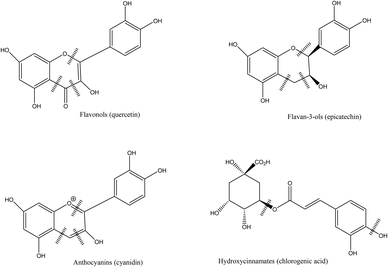
Berries contain sugars (mainly glucose and fructose), carotenoids, vitamins (A, C, E folic acid), minerals and phenolic compounds.7,24 Phytochemicals, comprising phenolic compounds are widely found in fruits and vegetables, although their profile and concentration are influenced by genotypes, environmental conditions, cultivation site, processing and storage.10 The roles of phenolic compounds are diverse and they are mainly involved in the plant growth and defense, by providing antimicrobial or antifungal actions, UV radiation protection, chelation of toxic metal or antioxidant action on free radicals.25 Berry fruits are particularly rich in phenolic acids, flavonols (quercetin and kaempferol), flavanols, proanthocyanidins, stilbenes, tannins, but the most studied compounds are anthocyanins. The latest have different profile in berries, and have been used for taxonomic purposes.8 Phenolic compounds are usually found in higher concentration in the skin of berries.7,26Phenolic acids, particularly hydroxycinnamic acids and hydroxybenzoic acids are common in berries. Hydroxycinnamic acids are mainly represented in berries by caffeic and ferulic acids while the benzoic derivatives commonly found are p-hydroxybenzoic, gallic, salicylic, vanillic and ellagic acids.8,27 Hydrolysable and condensed tannins are also found. Most berries contain condensed tannins, in particular cranberries, a particularly rich source of proanthocyanidins with A type linkage.28 Hydrolysable tannins are mainly represented by esters of gallic acids (gallotannins) and ellagic acids (ellagitannins), mostly found in strawberry, raspberry and blackberry.7,8 Flavonols, flavanols and anthocyanins are sub-groups of flavonoids. The most common flavonols found in berries are quercetin, myricetin and kaempferol, while flavanols are represented by catechin, epicatechin, gallocatechin and epigallocatechin.8 Anthocyanins are glycosides of anthocyanidins and are the major flavonoids found in berries. They provide the fruits with their red, blue or purple colors. These pigments have strong antioxidant properties and have been extensively studied for their potential benefits on human health.29 The main anthocyanins found in berries are derived from cyanidin, pelargonidin, delphinidin, petunidin, peonidin and malvidin anthocyanidins.30 The profile of anthocyanins of berries is mainly determined by the species and is particularly diversified.8,27 Some contain one major type of anthocyanin (cyanidin in blackberries31) while other species contain a wide array of different compounds (blueberry, blackcurrant).10
3. Fate of polyphenols during berry consumption
a. General mechanisms of the bioavailability and metabolism of dietary polyphenols
Bioavailability of dietary polyphenols in the digestive tract is highly variable. Their concentration in food sources does not influence their bioavailability, which is rather determined by their chemical structure and the presence of functional groups. Due to a poor absorption, but also their high metabolization rate and rapid elimination, the dietary polyphenols may not be the most biologically active compounds in the body. Instead, the bioactive molecules are suspected to be their metabolites, which are able to reach the blood circulation and the target organs.32–36Chemical structure influences bioavailability, and aglycones, monomeric and dimeric structures can be absorbed in the small intestine.32,37 The best absorbed flavonoids are proanthocyanidins, while anthocyanins are mainly described as poorly available in the small intestine,34,38,39 although the bioavailability of cyanidin-3-glucoside have been reported to be similar as one of the other flavonoids.40 Generally, the polyphenols are present in ester, glycoside and polymer forms that requires hydrolyzation to be absorbed, either by intestinal enzymes or the gut microbiota. Hydrolyzation may already occur in the oral cavity, through the action of β-glucosidase present in the saliva or produced by the oral microbiota, however reports are contradictory on this metabolic process.38,39,41 The most widely assumed mechanism takes place in the small intestine, where the Lactase Phlorizin Hydrolase (LPH), present in the brush border of the epithelial cells, hydrolyzes the molecules and releases aglycones.32 The aglycones may enter the intestinal cell by passive diffusion.33,42 Once in the enterocyte, they undergo phase I biotransformation (oxidation, reduction or hydrolysis) before reaching the liver. The polyphenols not absorbed in the small intestine (estimated around 90–95% of the initial intake) reaches the colon.43 Once in contact, the gut microbiota hydrolyzes glycosides into aglycones by opening the heterocycle. This catabolism leads to the production of smaller molecules that can be absorbed34,35,44,45 (Fig. 1 and 2). There is an interindividual variation of the bioavailability of the polyphenols in the general population, due to the difference in the composition of the gut microbiota that affects the resulting produced metabolites.32,46
 | ||
| Fig. 1 Potential ring cleavage sites of berry flavonoids and chlorogenic acid by gut microbiota (Adapted from M. V. Selma, J. C. Espín and F. A. Tomás-Barberán, J. Agric. Food Chem., 2009, 57, 6485–6501. Copyright (2019) American Chemical Society).81 | ||
 | ||
| Fig. 2 Ellagitannin and their hydrolysis products metabolized by the gut microbiota (Adapted M. V. Selma, J. C. Espín and F. A. Tomás-Barberán, J. Agric. Food Chem., 2009, 57, 6485–6501. Copyright (2019) American Chemical Society).81 | ||
Once absorbed, the molecules reach the liver. In the hepatocytes, they are subjected to phase II transformations and particularly conjugation, resulting in methyl, glucuronide or sulfate derivatives.39,45,47 The goal of this conjugation step is to increase the hydrophilicity of the molecules and facilitates their elimination.32 The metabolites are then liberated in the blood circulation where they enter other organs, however the distribution of the molecules between the plasma and the target organs does not seem directly related.48–50 The elimination of these molecules happens through the bile and urine. Highly conjugated molecules are carried back to the small intestine through bile excretion, and may then reach the colon while the small conjugates are mainly excreted through the urine.39,44,45,47
b. Bioavailability and metabolism of berry anthocyanins in vitro and in vivo
Whole berries have a more complex polyphenol profile than extracts used to study the general mechanisms of bioavailability and metabolism of these compounds. Interactions between these compounds and the fruit matrix may influence their fate in the digestive tract. The following parts address the fate of polyphenols from whole berry extracts (Table 1), and not individual polyphenol compounds.| Polyphenol class | Berry used in study | Type of study | Possible gut microbial metabolites | Potential microorganisms involved | Ref. |
|---|---|---|---|---|---|
| Dietary polyphenolics | Cranberry, blueberry, black raspberry | Animal study | Gallic acid, 4-hydroxybenzoic, 3-hydroxybenzoic, protocatechuic, vanillic, chlorogenic, p-coumaric, ferulic, caffeic, 4-hydroxyphenylacetic, 3-hydroxyphenylacetic, homoprocatechuic, homovanillic, gentisic, 3-hydroxycinnamic, 4-hydroxycinnamic, phloretic, hydrocinnamic, hippuric acids | Rat gut microbiota (not specified) | 85 |
| Urine samples | |||||
| Cranberry | Human study | Cinnamic, dihydrocinnamic, benzoic, phenylacetic, hippuric acid derivatives, benzaldehydes, valerolactones, catechols, pyrogallols | Human gut microbiota (not specified) | 89 | |
| Plasma samples | |||||
| Cranberry | Human study | Protocatechuic, 4-hydroxybenzoic, p-coumaric, vanillic, salicylic, homovanillic, ferulic, sinapic, gentisic, caffeic, homoprotocatechuic | Human gut microbiota (not specified) | 90 | |
| Plasma and urine samples | Benzoic acid, 3-hydroxybenzoic acids | ||||
| Urine samples | |||||
| Bound phenolic acids | Blueberry | In vitro colonic fermentation | Benzoic, phenylacetic, cinnamic, phenylpropionic acid derivatives | Human gut microbiota (not specified) | 83 |
| Anthocyanins | Bilberry | Human study | Gallic, protocatechuic, syringic, vanillic acids, 2,4,6-trihydroxybenzaldehyde | Human gut microbiota (not specified) | 80 |
| Plasma samples | |||||
| Blackberry | In vitro colonic fermentation | Gallic, p-coumaric, ferulic, chlorogenic acids, catechol, rutin, caffeic acid, coumarin, protocatechuic acid, 2,4,6-trihydroxybenzoic acid, 2,4,6-trihydroxybenzaldehyde | Human gut microbiota (not specified) | 57 | |
| Black raspberry | Animal study | Protocatechuic, p-coumaric, 3-Hydroxybenzoic, caffeic, ferulic, homoprocatechuic, homovanillic, 3-(3,4-dihydroxyphenyl)-propionic, 4-hydroxyphenylacetic, 3-hydroxyphenylacetic, 3-(3-hydroxyphenyl)-propionic acids | Pig gut microbiota (not specified) | 84 | |
| Blueberry | In vitro colonic fermentation | Rhamnetin, syringic, hippuric, cinammic, protocatechuic, caffeic acids, kaempferol rhamnoside | Human gut microbiota (not specified) | 53 | |
| Blueberry | Animal study | Rat gut microbiota (not specified) | 66 | ||
| Plasma samples | Protocatechuic, 4-hydroxybenzoic, benzoic, hippuric acids | ||||
| Urine samples | Gallic, syringic, protocatechuic, 4-hydroxybenzoic, hippuric acids | ||||
| Feces samples | Gallic, protocatechuic, 4-hydroxybenzoic, benzoic, hippuric acids | ||||
| Liver and brain samples | Benzoic acid | ||||
| Mulberry | In vitro batch culture of specific probiotic strains | Chlorogenic, cryptochlorogenic, caffeic, ferulic acids | Lactobacillus acidophilus, Lactobacillus bulgaricus, Bifidobacterium animalis, Lactobacillus plantarum and Streptococcus thermophiles | 58 | |
| Mulberry | In vitro colonic fermentation | Main metabolites: Protocatechuic, vanillic, p-coumaric, gallic, syringic acids, 2,4,6-trihydroxybenzaldehyde | Rat gut microbiota (not specified) | 59 | |
| Minor metabolites: Protocatechuic acid-glucoside, caffeic, tartaric, p-hydroxybenzoic, ferulic acids, pyrogallol, catechol | |||||
| Raspberry | Animal study urine samples | n-Glycine, 4-hydroxybenzoic, 4-hydroxyphenylacetic, benzenepropanoic, hippuric, phenylacetylaminoacetic, 1,2-benzenedicarboxylic, ferulic acids | Rat gut microbiota (not specified) | 65 | |
| Raspberry | Human study | Human gut microbiota (not specified) | 68 | ||
| Urine samples | 4-Hydroxybenzoic, protocatechuic, vanillic acids, 4-hydroxybenzoic acid-3-sulfate, 3-hydroxybenzoic acid-4-sulfate, 3′-methoxy-4′-hydroxyphenylacetic acid (traces), caffeic acid-3′-sulfate, dihydroxycaffeic acid-3′-sulfate, ferulic acid, isoferulic acid-3′-sulfate, hippuric, 3′-4′-dihydroxyphenylacetic acids | ||||
| Urine and plasma samples | Ferulic acid-4′-sulfate, ferulic acid-4′-O-glucuronide, isoferulic acid-3′-O-glucuronide, 4′-hydroxyhippuric acid | ||||
| Sea buckthorn | In vitro colonic fermentation | Quercetin, caffeic, ferulic acids | Human gut microbiota Bacteroides (can convert rutin to quercetin), Escherichia coli, Bifidobacterium lactis, Lactobacillus gasseri (can convert chlorogenic acid to caffeic acid) | 60 | |
| Ellagitannins | Black raspberry | Animal study (mice) | Urolithin A, urolithin C, protocatechuic acid | Mouse gut microbiota (not specified) | 88 |
| Plasma samples | |||||
| Raspberry | Human study | Human gut microbiota (not specified) | 68 | ||
| Urine samples | Urolithin A and urolithin B (traces) | ||||
| Urine and plasma samples | Urolithin A-O-glucuronide, isourolithin A-O-glucuronide, urolithin A-sulfate, (Iso)urolithin A-sulfate-O-glucuronide, urolithin B-3-O-glucuronide, dimethylellagic acid-O-glucuronide | ||||
| Raspberry | Human study | Ellagic acid, ellagic acid-O-glucuronide | Human gut microbiota (not specified) | 79 | |
| Urine samples | |||||
| Proanthocyanidins | Cranberry | In vitro colonic fermentation | Benzoic, 2-phenylacetic, 3-phenylpropionic, 2-(3′-hydroxyphenyl)acetic, 2-(4′-hydroxyphenyl)acetic, 3-(3′-hydroxyphenyl)propionic, 2-(3′,4′-dihydroxyphenyl)acetic, hydroxyphenylvaleric acids | Human gut microbiota (not specified) | 82 |
Small intestine compartment models have a higher pH (6.5–7.5) and the presence of a pancreatic and bile enzymatic mixture. All studies described here reported a large effect of these conditions on anthocyanins, in agreement with the previous assertion that pH impacts the stability of anthocyanins. Under in vitro conditions, about half of chokeberry anthocyanins and less than 20% of blueberry and Chilean currant anthocyanins were recovered in the duodenum compartment.51,53,54 The cleavage of most anthocyanins initially present in the fruits to form a chalcone pseudobase in alkaline conditions could explain this decline. However, acylated anthocyanins from blueberry, delphinidin- and malvidin-acetoyl-glucosides, appeared to have a higher stability.53 The bioaccessibility of the polyphenolic compounds in the small intestine was assessed with the use of a dialysis membrane. Less than 0.5% of mulberry anthocyanins were recovered in the absorbed material, while 4% remained in the compartment and would therefore be available in the colon.52 A similar experiment conducted on blackberry showed an intestinal bioaccessibility of less than 2% for the major anthocyanin, cyanidin-3-glucoside. This low bioavailability is explained by the unstable ring fraction of the molecules under the conditions present in the small intestine. Acylated anthocyanins present in the raw blackberry showed a similar bioaccessibility, even though they were detected at lower concentration in the berries.55 This could be due to their higher stability to the intestinal conditions mentioned above.
An increase in relative abundance from 18 compounds have been reported after the in vitro gastro-intestinal digestion of mulberry, among which flavonoids (myricetin, epicatechin gallate, quercetin derivative) and phenolic acids (tartaric acid, caffeic acid, ferulic acid) predominate. These candidate metabolites of anthocyanins breakdown could be formed after the B ring fracture of the anthocyanins, generating phenolic molecules or result from the degradation of other molecules present in the mulberry extracts.52 Part of the anthocyanins are recovered after intestinal digestion and reach the colon, where they can be catabolized by the gut microbiota into smaller molecules to be absorbed. After colonic fermentation of blueberry extracts, the presence of new phenolic compounds has been reported: syringic acid, rhamnetin, hippuric acid, cinammic acid, protocatechuic acid, caffeic acid, and kaempferol rhamnoside.53 Syringic acids have been reported as a major product of the fermentation of malvidin-3-glucoside, while the fermentation of various anthocyanins by the gut microbiota can lead to the formation of gallic, syringic and p-coumaric acids.56 The fecal fermentation of digesta of blackberry resulted in the recovery of very low concentration of cyanidin-3-glucoside and its derivatives after 24–48 hours of fermentation. Most of anthocyanins disappear during the first few hours of colonic fermentation, due to metabolization by the gut microbiota.55,57 In the gut compartment too the stability of the anthocyanins is influenced by their structure, with simpler molecules being less stable.
Depending on the composition of the microbiota, different metabolites may be produced from the fermentation of berry anthocyanins (Table 1). The conversion of mulberry anthocyanins by probiotic strains has been reported and the highest conversions were exercised by L. plantarum and S. thermophiles, respectively degrading cyanidin-3-glucoside and cyanidin-3-rutinoside. The recovered metabolites were mainly chlorogenic acid, cryptochlorogenic acid, caffeic acid and ferulic acid.58 When fermented in vitro with rats’ gut microbiota, cyanidin-3-glucoside and cyanidin-3-rutinoside from mulberry extracts were mainly metabolized into protocatechuic, vanillic and p-coumaric acids.59 Fermentation of sea buckthorn juice produced an increase in quercetin and caffeic acid, but a decrease in rutin and chlorogenic acid. These observations may be explained by the conversion of some compounds into others, such as rutin into quercetin possibly through the metabolism carried by some Bacteroides species.60 Microbial fermentation led to the formation of a wide range of compounds, as well as their catabolism at some time of the process, resulting in a highly dynamic profile of some compounds.57
The digestion of berries anthocyanins has mainly focused on cyanidin derivatives, but the structure of the compounds (simple or acylated, structure of aglycone or sugar moiety) was found to impact the stability of anthocyanins in the digestive tract.53 The pH in the different compartment is often reported as the reason for the high loss of anthocyanins, while other mechanisms like metabolism, oxidation or degradation could also play a role.52 Very few anthocyanins are recovered after microbial fermentation in vitro, suggesting catabolism into smaller molecules by the microbiota, principally phenolic acids.
After the injection of an acute dose of anthocyanins extracts from blackberries directly into the small intestine, only anthocyanins glycosides were detected in the plasma, urine and bile of rats. The low concentration of native and methylated cyanidin-3-glucoside reported in the plasma shows a low bioavailability. Cyanidin-3-glucoside and metabolites were reported in the bile 25 minutes after the injection, indicating a rapid absorption and metabolism of the anthocyanins.61 The low bioavailability of bilberry anthocyanins in rats was reported to be between 0.6 and 1.8%, and differences between anthocyanins were explained by the methylation state of the aglycone and the attached sugar type. Arabinoside disappeared more quickly from the plasma than glucoside and galactoside, while among anthocyanins with the same sugar attached, delphinidin and cyanidin remained longer in the plasma than petunidin, peonidin or malvidin. In excretion fluids, the profile of anthocyanins was very different from the bilberries’, being as a result of metabolization in the tissues.62
In pigs, the fate of anthocyanins from black raspberries have been studied in the different compartment of the gastrointestinal tract, as well as their excretion. Again, anthocyanins’ structure impacted the recovery of the molecules in the gastrointestinal tract. 98% of cyanidin-3-glucoside disappeared from the gastrointestinal tract after 4 hours ingestion, however the lack of metabolites recovered from the urine indicated a possible degradation of the molecule rather than its direct absorption. The other cyanindin derivatives carrying more complex sugars (sambubiose and rutinose) showed higher recovery in the gut and were identified as parent compounds of the majority of metabolites found in urine.64 These observations were also reported in the rat model, following the consumption of red raspberries anthocyanins. Only traces of cyanidin sophoroside and glucosylrutinoside were recovered in the digestive tract but their level in the plasma increased, while cyanidin-3-glucoside decreased in the intestine but did not increase in plasma or tissue, indicating metabolism in the gastrointestinal tract.65
The previous studies all used acute doses of berry extract or direct perfusion in the intestine, which may not reflect accurately the normal consumption of berry anthocyanins. Long term exposure of rats to blueberry anthocyanins have been conducted to provide more insights on the absorption, metabolism and excretion under more realistic conditions.66 Cyanidin, petunidin, peonidin and malvidin derivatives were found in urine after 4 weeks, and their content increased after 8 weeks, possibly resulting from a saturation of storage. In the feces, the content remained stable during the study and the profile of anthocyanins detected was composed of acylated forms of anthocyanins. The authors reported increased contents of some anthocyanins in both urine and feces compared to the berries, that may result from the methylation of anthocyanins during metabolism. Hippuric acid was reported as the main metabolite from the anthocyanins consumption and the ultimate product of their degradation. The rapid absorption of anthocyanins was also confirmed in this study as no such compounds were identified in plasma, liver or brain after 3–4 hours of last meal consumption.
The absorption in the gastro-intestinal tract of anthocyanins in vivo seems to be mainly dictated by the sugar attached to the aglycone, as well as the aglycone structure itself. The bioavailability and distribution of these polyphenols remains very low and is influenced by the same factors as well as hydrophobicity of the molecule (methylated compounds versus compounds carrying free OH groups). Overall, the absorption and excretion of anthocyanins in vivo do not seem to be associated with extensive metabolism in animal models.67
The form under which anthocyanins are absorbed is not fully understood, even though it remains clear that the nature of the sugar moiety and the aglycone are determinant in the absorption and excretion of the molecules.70 However, results are contradictory regarding the absorption of native anthocyanins or their metabolized form. Cyanidin-3-glucoside and cyanidin-3-sambubioside are very often found in plasma or urine.40,71–73 After the consumption of strawberries, pelargonidin-3-glucosides present in the berries were recovered in the plasma under their glucuronated form, with transformation explained by the action of LPH or β-glucosidase in the small intestine, or in urine under their glucuro- or sulfo-conjugated forms.74,75 Following the consumption of blackberries, most of the anthocyanins were excreted as monoglucuronides, but also as methylated glycosides and a sulfoconjugate of cyanidin.76 The methylation of the cyanidin- derivatives into peonidin and glucuronide conjugates after the consumption of anthocyanins from elderberry was reported,73 but another report did not find any glucuronates or sulfates of the same anthocyanin from elderberry.72 Aglycones or conjugates of anthocyanins in plasma were not identified after the consumption of red fruits.71 McGhie et al. (2003)70 detected intact and unmetabolized anthocyanins in urine of humans following the consumption of diverse berries, but reported both intact and metabolized anthocyanins (after methylation) in a later study on boysenberry anthocyanins.70,77
The low absorption of anthocyanins in the upper gut suggests the availability of these molecules in the gut, where they can be subjected to further degradation. Up to 85% of blueberry anthocyanins (depending on their structure) were recovered in ileostomy bags of participants and would therefore be able to reach the gut in patients with an intact gastrointestinal tract.78 The role of the intestine in the absorption of anthocyanins has been further studied by comparing a population with ileostomy and a population with intact upper gut following the ingestion of raspberries and bilberries, in two separate studies. González_Barrio et al. (2010)79 confirmed the low bioavailability of anthocyanins from raspberries by no detection of the compounds nor their metabolites in the plasma. However, 40% of anthocyanins were recovered in ileal fluid of ileostomists, indicating the possibility for a large amount of anthocyanins to reach the large intestine in people with an intact gut. The recovered phenolic compounds in the ileal fluid were associated with the metabolism of native anthocyanins from the fruits. This indicates a possible metabolism by the enterocytes or could be the products of a metabolism happening in the liver with the products returning to the gastrointestinal tract via biliary excretion.79 Mueller et al. (2017),80 also on bilberries, showed that anthocyanins recovery was about 80% higher in the plasma and 40% higher in the urine of the population with an intact gut in respect to the ileostomists population, indicating a better bioavailability of these molecules in the healthy subjects. These results showed that some anthocyanins could be absorbed from the small intestine (primarily), with the stomach also being another potential site of absorption. The amount of metabolites was higher in the plasma and urine of the population with an intact gut, suggesting that the absorbed anthocyanins can form degradation products in the plasma.80
Absorption of anthocyanins in humans is generally low, confirming findings in vitro and in animal models, with a large amount being able to reach the gut although conflicting reports exist.33,36,40 The bioavailability is highly variable between subjects, generally below 1% in most studies, but sometimes with values reported as high as 3%. Their metabolism seems to depend on the native compounds and their structures, the food matrix and the individual ingesting the anthocyanins. Once in the gut, they can be catabolized by specific genera producing enzymes necessary to the reactions. Most of the studies regarding the metabolism of anthocyanins by the gut microbiota have been conducted in vitro, as the environment and parameters in vivo studies are complicated to control and lead to several confounding factors that could influence the observations.
c. Bioavailability and metabolism of other phenolic compounds from berries in vitro and in vivo
The in vitro gastric digestion of chokeberry (pH = 2) showed no significant effects on the content of flavonols, caffeic acid derivatives, neochlorogenic acid and chlorogenic acids or flavanols found in the berries.51 However, in vitro gastric digestion with incremental pH increases resulted in the decrease of phenolic acids and the loss of about 12% of the total flavonoids in Chilean currants.54 The acidic conditions seem to play a role in the absorption of polyphenols in general. After intestinal digestion, the flavonols, neochlorogenic and chlorogenic acid contents of chokeberry digesta decreased by about 25%, and the flavanols content by 19%.51 The authors compared these results with control samples incubated without digestive enzymes, and concluded that the degradation was mainly due to the chemical conditions. In Chilean currants previously digested in the gastric compartment, the remaining total flavonoids decreased again in the small intestine. The slight increase in the content of phenolic acids could be due to isomerization reactions among hydroxycinnamic acids under the intestinal conditions.54
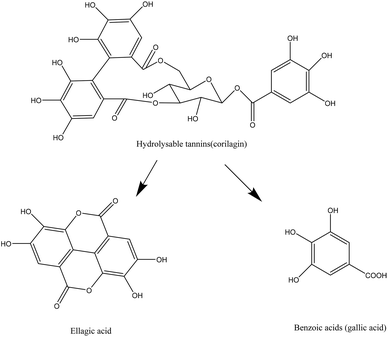
Van de Velde et al. (2018)55 reported the bioaccessibility of ellagitannins from blackberry in the intestine with values inferior to 1% for lambertianin A and C. Less than 5% of these two compounds remained in the intestinal tract. However, consistent amount of free ellagic acid were recovered in the intestinal tract, suggesting the breakdown of ellagitannins into free ellagic acids in this compartment. The fermentation of ellagitannins by the gut microbiota showed no recovery of lambertianin A and C, indicating the hydrolysis of these compounds in this compartment as well. Free ellagic acids were not found after the fermentation either, suggesting further metabolization of ellagic acid into urolithin derivatives.55,81
The results of an in vitro colonic fermentation with human microbiota of proanthocyanidin from cranberry, but without previous digestion, suggested a metabolization of the proanthocyanidins A found in the fresh cranberry into phenolic acids. However, a decreased access by the microbiota when the molecules had a higher degree of polymerization was reported.82 In blueberry, a majority of the phenolic acids present in the fruits were bound to plant cell-wall molecules, suggesting their availability in the colon. After fermentation, only a few parent compounds were recovered, but most of the molecules were metabolites, derivatives of benzoic, phenylacetic and phenylpropionic acids.83
In vitro studies on the digestion of phenolic compounds from berries suggest poor degradation of complex molecules in the stomach. The availability of these compounds remains low in the intestine; however, they are intensively metabolized by the gut microbiota in vitro.
Phenolic acids excreted by rats’ urine after the consumption of cranberry, blueberry or blackberry were not only excreted in free form, but also conjugated forms. The profile of recovered phenolic acids differ among the berries ingested, due to their initial profile in polyphenols. Cranberry and blueberry, initially rich in proanthocyanidins, mainly led to the formation of 4-hydroxycinnamic acid for the former and chlorogenic, ferulic and 3,4-dihydroxycinnamic acids for the latter. Black raspberry, which contains primarily anthocyanins in the form of cyanidins in the fresh fruits, led to the formation of 3-hydroxyphenylpropionic, 3-hydroxybenzoic and 3-hydroxycinnamic acids.85 The recovery of phenolic acids depends on the profile of polyphenols in the gastrointestinal tract and their structure, as well as the composition of the gut microbiota.
The proanthocyanidins from grape pomace has been recovered in the plasma of rats after 4 hours feeding. Catechin, epicatechin, dimer and trimer were identified, as well as their metabolites, catechin and epicatechin glucuronide, methyl catechin and epicatechin glucuronide and methyl catechin and epicatechin sulphate.86 These results are consistent with metabolization of certain polyphenols via methylation, glucuronidation and sulfoconjugation. Proanthocyanidins, and more specifically catechin and epicatechin metabolites also include valerolactone derivatives.87
The study of raspberry ellagitannins fate in the gastrointestinal tract of rats showed that from the two molecules initially present in the fruit juice, lambertianin C and sanguiin H-6, they were not recovered in the entire gastrointestinal tract nor the plasma, urine or feces an hour after ingestion. The acidic conditions of the pH are probably responsible for the rapid breakdown of these molecules. Ellagic acid were recovered in the stomach (9.6% of their initial amount in the juice), but not in the organs or fluids.65 These results do not reflect the observations made in vitro by Van de Velde et al. (2018) since the breakdown of ellagitannins did not lead to substantial increase in the content of ellagic acid.55
Black raspberries, a berry rich in ellagitannins, were fed to mice in order to study the colonic metabolites produced after the berries consumption, as well as the impact of the phytochemicals on the microbial populations. Ellagitannins metabolites were found in the mice plasma, liver, prostate and colon under the form of urolithin A (dominantly), and C, produced by the mice microbiota. Protocatechuic acid was also recovered in the plasma and indicated as an anthocyanins metabolite.88
Quercetin from berries have been shown to be bioavailable. After the ingestion of black currant juice, quercetin in plasma was higher than in the control group. A long term study in which the participants ate 100 g of a berry mix per day also lead to an increase of 30 to 50% in the plasma of volunteer after 8 weeks.91 However, another study evaluating the fate of compounds from cranberry juice, sauce and fruits did not recover quercetin or myricetin in the plasma of volunteers following an acute dose of product.92 In this same study, most of the other phenolics were recovered in the plasma, but also had smaller molecular weights. Some of the phenolics even had two absorption peaks, suggesting a reabsorption of the compounds excreted in the bile, or the metabolism of high weight molecules not absorbed in the stomach by the gut microbiota.
The role of the large intestine in the metabolism of ellagitannins has been investigated by comparing ileostomists and volunteers with an intact gut. From the three ellagitannins found in the raspberry (sanguiin H-6, sanguiin H-10 and lambertianin C), only sanguiin H-6 was recovered in ileal fluid, representing a fourth of the initial intake. The ellagic acids were recovered in the same compartment as 241% of their initial intake. They may originate from the breakdown of ellagitannins.79 No raspberry parent compounds were recovered in the plasma of the healthy subjects, and the timing of recovery in urine indicates a low level of absorption in the small intestine. In the urine samples, trace of ellagic acids were found while urolithins were recovered in the urine of healthy subjects. Ellagic acids are an intermediate to the production of urolithin. The recovery of urolithin seems to depend on the gut microbiota composition as the recovery in urine between subjects varied from 0.3 to 8.6% of the ellagitannins/ellagic acids intake.68,79 The recovery of ellagitannins also varied with the food matrix they originated from, with a higher recovery found for raspberry than strawberry, but there again a high variability between subjects suggested that their bioavailability depends on gut microbiota, food matrix and possible interaction with other constituents of the diet.93
In all human studies, large inter-individual variabilities have been reported, implying the individual's ability to generate specific metabolites. In addition to difference in gender and genetic polymorphism of transporters or metabolizing enzymes, the influence of the gut microbiota composition is becoming evident. Due to the complexity of the berry matrices as well as the gut microbiota, studies on the role played by specific microorganisms in the metabolism of berry polyphenols are scarce. However, Williamson & Clifford have reviewed the microorganisms action on individual polyphenols commonly found in berries.94
4. Modulation of the gut microbiota by berry polyphenols
A large amount of polyphenols can reach the colon and be subjected to degradation there, leading to the production of metabolites. On the other hand, these polyphenols and their metabolites can also modulate the bacterial population in the gut.21 It is estimated that 500 to 1000 different microbial species inhabit the gut, reaching a concentration of up to 1012 cells per gram of feces. Among these bacteria, Bifidobacterium, Lactobacillus, Akkermansia can confer benefits to the host, and together with Bacteroides and Eubacterium they have the ability to catalyze polyphenols. Other genera, however, can be human pathogens such as Pseudomonas, Salmonella, Staphylococcus or Bacillus.21,95 Modulation of the gut microbiota by berry consumption is detailed in the following sections and in Table 2. The phenolic compounds of berries affect the bacteria selectively, and the microorganisms do not exert the same sensitivity towards the berry compounds.| Berry used in study | Type of study | Dosage | Treatment duration | Analytical method | Changes in microbiota | No effect in microbiota | Ref. |
|---|---|---|---|---|---|---|---|
| qPCR: Quantitative polymerase chain reaction; DGGE: denaturing gradient gel electrophoresis; HTS: high throughput sequencing; FISH: fluorescent in situ hybridization. | |||||||
| Blackberry, black raspberry | Animal study (mice, high fat diet) | 400 μg anthocyanins (from berry powder) | 12 weeks | qPCR | Firmicutes, Bacteroidetes, Actinobacteria | 99 | |
| Blueberry, blackcurrant | (+) Bacteroidetes, Actinobacteria | ||||||
| Blueberry and blackcurrant anthocyanins and prebiotic blend | Human study (obese volunteers) | 215 mg anthocyanins | 8 weeks | HTS (Illumina Miseq) | (+) Bacteroidetes | 101 | |
| (−) Firmicutes, Actinobacteria, Firmicutes to Bacteroidetes ratio | |||||||
| Cloudberry raspberry, strawberry | In vitro | 0.5, 1 and 5 mg mL−1 (extracts) | 24 and 48 hours | Culture methods | (−) of Gram-negative bacteria, Salmonella | Gram-positive bacteria | 109 |
| Bilberry, cloudberry, strawberry, raspberry, blackcurrant, lingonberry, buckthorn berry | In vitro | 2 and 10 mg ml−1 (dry berry powder) | 24 hours | Culture methods | (−) Salmonella enterica, Staphylococcus aureus | Listeria monocytogenes, Lactobacillus rhamnosus | 110 |
| Cranberry | (−) Salmonella enterica, Staphylococcus aureus | Lactobacillus rhamnosus | |||||
| (−) Listeria monocytogenes | |||||||
| Cranberry, grape seeds | In vitro (batch-culture fermentation) | 500 mg l−1 (extracts) | 48 hours | qPCR | (−) Bacteroides, Prevotella, Blautia coccoides-Eubacterium rectale | 102 | |
| (−) Lactobacillus, Bifidobacterium, Enterobacteriaceae, Clostridium leptum, Ruminococcus | |||||||
| Açai | In vitro (batch-culture fermentation) | 1 g (dry berry powder) | 24 hours | FISH | (−) Clostridium histolyticum, Bacteroides-Prevotella spp. | Bifidobacterium spp., Lactobacillus/Enterococcus spp., Clostridium coccoides-Eubacterium rectale | 103 |
| Blackberry | Animal study (rats, high fat diet) | 25 mg kg−1 day−1 | 17 weeks | HTS (Illumina Miseq) | Normal diet: (+) Pseudoflavonifractor, Oscillobacter | 98 | |
| High fat diet: (+) Oscillobacter, (−) Rumminococcus | |||||||
| Black currant | Animal study (rats, healthy) | 2 ml (berry extracts) | 4 weeks | FISH | (+) Bifidobacteria, lactobacilli | 105 | |
| (−) Bacteroides, clostridia | |||||||
| Human study (healthy volunteers) | 672 mg dry berry powder per day | 2 weeks | FISH | (+) Lactobacillus spp., Bifidobacterium spp. | 107 | ||
| (−) Clostriudium spp., Bacterioides spp. | |||||||
| Black raspberry | Animal study (rats, healthy) | 5% (dry berry powder) | 6 weeks | Pyrosequencing | (+) Akkermansia, Desulfovibrio, Anaerostipes | 121 | |
| Animal study (mice, healthy) | 10% (dry berry powder) | 6 weeks | HTS (Illumina Miseq) | (+) Barnesiella | 88 | ||
| (−) Clostridium, Lactobacillus | |||||||
| Blueberry | Animal study (mice, inflammation bowel disease model) | 10% (dry berry powder) | 21 weeks | qPCR | (−) Clostridium perfringens, Enterococcus spp., Lactobacillus spp., Escherichia coli | Bacteroides-Prevotella-Porphyromonas, Bifidobacterium spp., Bacteroides vulgatus, Faecalibacterium prausnitzii | 111 |
| Animal study (rats, healthy) | 8% (dry berry powder) | 6 weeks | HTS (Illumina Hiseq) | (−) Lactobacillus and Enterococcus | 115 | ||
| (+) Bacteria from the orders Actinomycetales, Bifidobacteriales, Coriobacteriales | |||||||
| In vitro | 10 and 25% (berry extracts) | 5 days | Culture methods | (+) Lactobacillus rhamnosus, Bifidobacterium breve | 114 | ||
| In vitro (batch-culture fermentation) | 5, 10 and 25% (berry extracts) | 48 hours | FISH | (+) Lactobacilli, Bifidobacteria populations | |||
| Animal study (rats, healthy) | 4 ml berry extracts per kg per day | 6 days | FISH | (+) Lactobacilli, Bifidobacteria populations | |||
| Human study (healthy volunteers) | 250 ml drink (10% dry berry powder in water) | 6 weeks | qPCR | (+) Bifidobacterium spp., Lactobacillus acidophilus | Bacteroides spp., Prevotella spp., Enterococcus spp., Clostridium coccoides | 123 | |
| Human study (healthy volunteers) | 250 ml drink (10% dry berry powder in water) | 6 weeks | qPCR | (+) Bifidobacterium longum subsp. infantis | Bifidobacterium adolescentis, B. longum subsp. longum, B. catenulatum, B. breve. B. bifidum | 124 | |
| Cranberry | Animal study (mice, high fat diet) | 200 mg kg−1 (berry extracts) | 9 weeks | Pyrosequencing | (+) Akkermansia | 97 | |
| Goji berry | Animal study (mice, colitis model) | 1% (dry berry powder) | 10 weeks | HTS (Illumina Miseq) | (+) Bifidobacteria, Clostridium leptum, Fecalibacterium prazusnitzii | 117 | |
| Juçara | In vitro (batch-culture fermentation) | 1% (dry berry powder) | 24 hours | FISH | (+) Bifidobacterium, Eubacterium rectale-Clostridium coccoide, Bacteroides spp.-Prevotella | Lactobacillus/Enterococcus spp. | 112 |
| Animal study (rats, high fat diet) | 0.5 and 0.25% (dry berry powder) | 7 days | qPCR | (+) Bifidobacterium | 119 | ||
| Lingonberry | Animal study (mice, high fat diet) | 44% (dry berry powder) | 8 weeks | HTS (Illumina Miseq) | (+) Bacteroides, Parabacteroides, Clostridium | 106 | |
| (−) Mucispirillum, Oscillospira | |||||||
| Lonicera cerula L. | Animal study (mice, high fat diet) | 1% (dry berry powder) | 45 days | HTS (Illumina Miseq) | (+) Bacteroides, Parabacteroides, 2 genera from the order Bacteroidales | 100 | |
| (−) Staphylococcus, Lactobacillus, Ruminococcus, Oscillospira | |||||||
| Lycium ruthenicum | In vitro (batch-culture fermentation) | 1 g L−1 anthocyanins | 24 hours | HTS (Illumina Miseq) | (+) Bifidobacterium, Allisonella | 104 | |
| (−) Prevotella, Dialister, Megamonas, Clostridium | |||||||
| Plinia jaboticaba | Animal study (rats, healthy) | Ad libitum, juice | 2 and 7 weeks | Culture methods | (+) Lactobacillus, Bifidobacterium, Enterobacteriaceae | 116 | |
| Schisandra chinensis | Human study (obese volunteers) | 6.7 g dry berry powder per day | 12 weeks | qPCR DGGE | (+) Bacteroides, Akkermansia, Roseburia, Prevotella. Bifidobacterium | 108 | |
| (−) Ruminococcus | |||||||
| Sea buckthorn | In vitro (batch-culture fermentation) | 250 mg lyophilized fraction of small intestine digested berries | 72 hours | PCR-DGGE | (+) Bacteroides/Prevotella, Bifidobacteria | 60 | |
| In vitro (batch-culture fermentation) | 10% berry juice | 1 week (continuous gut model) | PCR-DGGE | (+) Lactobacilli, Bacteroides/Prevotella, Bifidobacteria | 113 | ||
| Strawberry | Animal study (mice, diabetic model) | 2.35% (dry berry powder) | 10 weeks | HTS (Illumina Miseq) | (+) Bifidobacterium | Dehalobacterium, Dorea, Lactobacillus, Turicibacter | 118 |
| (−) Bacteroides, Akkermansia | |||||||
a. General modulation of colonic bacteria
Modulation of the gut microbiota can be observed at the phylum level. Firmicutes and Bacteroidetes are the two most abundant phyla in the human and mice gut microbiota. High fat diets have been found to reduce the abundance of Bacteroidetes and favor the growth of Firmicutes. A ratio in favor of Firmicutes is associated with several conditions, including obesity.96 In animal models consuming high fat diet (with no berry extracts), this shift in the Firmicutes/Bacteroidetes ratio has been observed.97–100 Blackberry and black raspberry supplements did not inverse this trend when analyzed by qPCR,99 however the ratio was decreased after the consumption of Lonicera caerulea (or honeyberry), with a greater impact when the berries were included at a higher proportion in the diet, observed by HTS (high throughput sequencing) methods.100 Blueberry and blackcurrant significantly increased the Bacteroidetes and Actinobacteria populations, two obligate anaerobes. A potential reduction in the oxygen tension in the gut due to the addition of anthocyanins may benefit the growth of oxygen-sensitive bacteria.99 Similar observations were reported in obese human subjects using HTS methods. The consumption of an anthocyanin blend from blueberry, blackcurrant and black rice associated with a prebiotic blend (inulin) by obese subjects reduced the populations of Firmicutes and Actinobacteria and increases the Bacteroidetes, therefore decreasing the Firmicutes/Bacteroidetes ratio.101The modulation of the gut microbiota at the genus level has also been observed. The antimicrobial effect of cranberry extracts was assessed using fermentation batch culture incubated with human colonic bacteria, but without previous digestion of the berry extracts. Observation with qPCR shows antimicrobial effects towards Bacteroides, Prevotella and Blautia coccoides-Eubacterium rectale populations.102 Similar antimicrobial effects towards Bacteroides/Prevotella populations were reported when the batch cultures were treated with açai pulp and Lycium ruthenicum Murray (commonly known as Russian box thorn) anthocyanin extract, respectively analyzed by FISH (Fluorescent In Situ Hybridization) and HTS.103,104
In animal models, blackcurrant extracts have been found to reduce the population of Bacteroides, and the addition of blackcurrant and black raspberry extracts decreased Clostridium spp. in murine models,88,105 therefore suggesting that berry compounds can exert antimicrobial effects in vivo as well. The consumption of honeyberry extracts by mouse fed with high-fat diet reduced the Ruminococcus and Oscillospira populations, and the addition of blackberry extracts impacted the gut microbiota of diet-induced obese rats by increasing Oscillobacter and Sporobacter and decreasing Rumminococcus.98 Lingonberries fed to atherosclerosis-prone mice increased the relative abundance of Bacteroides, Parabacteroides and Clostridium, while Musispirillum and Oscillospira were decreased. This study also showed a positive association between Musispirillum and the number of plaques. Thus, lingonberry may be beneficial towards vascular conditions.106
In human, the consumption of blackcurrant products led to a decrease in Clostridium spp. and Bacteroides.107 The analysis of the gut microbiota of obese volunteers consuming Schisandra chinensis fruits, also called magnolia berry, showed an increase in Roseburia, Bacteroides, Prevotella and Bifidobacterium.108
b. Modulation of potentially pathogenic bacteria at the genus level
Berries have been studied for their potential antimicrobial effect on human intestinal pathogens by agar diffusion methods and were found to be, in general, a better inhibitor of Gram-negative bacteria than Gram-positive.109 More particularly, Staphylococcus and Salmonella were the most sensitive to the berries. Staphylococcus was strongly inhibited by ellagitannins of cloudberry and raspberry, whereas Listeria was not affected by the phenolic extracts nor the whole berries, except by cranberry, as shown with culture-based methods.110 Still in vitro, the pathogen Clostridium histolyticum, associated with tumor promoting properties and inflammatory bowel disease, was reduced by gallic acid (a common anthocyanin metabolite)56 and açai pulp.103 In mice fed a high fat diet, honeyberry (Lonicera cerula L.) extracts led to a decrease of Staphylococcus reported by HTS methods.100 Inflammatory bowel disease (IBD) has been associated with microbiota enteric population. Particularly, Clostridium perfringens and Enterococcus faecalis have shown capability to induce IBD in mice.111 The supplementation of IBD mouse model with blueberry decreased the population of C. perfringens, Enterococcus spp. and E. coli observed by qPCR, suggesting the ability of the berries to alter the composition of the microbiota in a positive way.111c. Modulation of potentially beneficial bacteria at the genus level
Animal studies have also focused on the effects of berry polyphenols when the gut microbiota is disturbed by a high fat diet or health concerns such as obesity, gut inflammation or other chronic diseases. These conditions generally lead to a dysbiosis (unbalanced gut microbiota). In the following studies, berry extracts were used to evaluate their potential effect in restoring a balanced gut microbiota. In mice fed with a high-fat diet, the addition of honey berry (Lonicera cerula L.) led to a decrease in Lactobacillus population.100 In a mice IBD model, Lactobacillus spp. was also decreased by the consumption of blueberries, while no effect as reported on Bifidobacterium.111 Goji berries tested in another IBD mouse model were found to significantly increase Bifidobacterium as well as several butyrate-producing bacteria such as Fecalibacterium prazusnitzii. These bacteria show protective effects against IBD.117 In the case of diabetic mouse model, their population of Bifidobacterium was lower than in healthy mice. Supplementation with strawberry helped increase this genus in the diabetic animals.118 Rats fed with juçara pulp following a high fat diet had their gut microbiota reshaped by a build-up of Bifidobacterium spp., supporting the observation made in vitro with this same fruit.119 When mice were fed with trans fatty acid during pregnancy and lactation, the population of Bifidobacterium in their offsprings’ colon was reduced, however the supplementation of the mothers’ diet with juçara pulp restored Bifidobacterium population in the offsprings, suggesting protective effects of polyphenol-rich fruits in maternal diets.120
Black raspberry extracts in the diet of rats increased the relative abundance of Akkermensia and butyrate producing bacteria. Akkermansia have been associated with a generally healthy gut, being found in lower amount in patients with inflammatory bowel diseases and metabolic disorders.121Akkermansia muciniphila is a bacterium associated with the maintenance of gut health, and is inversely related to body fat mass in mice.122 Increasing its growth through the inclusion of prebiotics in the diet could be a way to promote a healthy gut state. The addition of cranberry extract in the diet-induced obese mice led to an increase of the Akkermansia population by 30% and helped prevent the negative metabolic phenotype linked to obesity (reduction of visceral obesity, triglyceride accumulation and intestinal triglyceride content).97Akkermansia abundance also increased in high fat diet mice fed with honeyberry, but only for the highest concentration of treatment used (1%).100 The mechanisms behind the ability of Akkermansia to restore the gut balance can be hypothesized because of its capacity to grow quickly after disruption, such as antibiotic therapy. This initiates competition for resources rapidly, thus influencing the ecology of the gut.97 However, in a mouse diabetic model, the addition of strawberry in the diet led to a decrease of Akkermansia.118
In conclusion, berry constituents can reach the gut and modulate the microbiota by decreasing the growth of some genera and increasing the populations of others. In a healthy gut, these effects were mainly beneficial. In challenged microbiota due to health conditions, the supplement with berries or their compounds often resulted in an improvement in terms of the gut composition. Direct associations between the consumption of berries and the regulation of the gut microbiota are hard to draw due to scarce knowledge on the role of specific genus in health or disease, the interindividual differences between subjects in term of microbiota composition, the diversity of compounds from which the effect could originate and the diversity of methods used in the studies (plate count, fingerprinting or sequencing methods).
5. Berry polyphenols impact on gut inflammation and colon cancer
a. Berries and ulcerative colitis
In 2015, about 3 million adults in the US reported being diagnosed with Inflammatory Bowel Disease (IBD).125 IBD includes Crohn's disease and ulcerative colitis, two chronic inflammation disorders affecting the gut. Particularly, ulcerative colitis can affect the rectum and progress to the entire colon. Symptoms are diarrhea, abdominal pain, gastrointestinal bleeding and weight loss. If a genetic basis has been established, environmental factors are of importance too in the prevalence of IBD, and particularly the gut microbiota. Chronic inflammation of the gut can increase the risk of developing colorectal cancer.126 During ulcerative colitis, inflammation in colonic epithelial cells and immune cells increases. The main mechanism involves an increase in NF-κB, promoting the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1b (IL-1b), interferon-c and interleukin-23 (IL-23).127,128 Treatments for ulcerative colitis are not ideal, with a large portion of patients experiencing side effects or being non responsive to more advanced therapy.129 Thus, alternative strategies have been studied, among which the use of polyphenols for their antioxidant and anti-inflammatory effects. Berries, due to their content in these phytochemicals and fibers have potential prevention properties towards intestinal health.130 IBD is often linked to an abnormal microbial colonization or disequilibrium,131 and a beneficial modulation of the gut microbiota by berries can also partly explain their positive impact on gut health. The effects of berry treatments on gut inflammation are summarized (Table 3).| Berry used as treatment | Type of study | Dosage | Treatment duration | Benefits at the end of treatment | Accompanied effects | Ref. |
|---|---|---|---|---|---|---|
| COX: Cyclooxygenase, IFN: interferon, IL: interleukin, MPO: myeloperoxidase, NO: nitric oxide, TNF: tumor necrosis factor. | ||||||
| Bilberry | Cell culture | 2.5, 5, 10 and 25 μg ml−1 extracts | Reduction of inflammation | Inhibition of pro-inflammatory mediators | 138 | |
| Animal model | 20% dried berries or 1 or 10% anthocyanins extracts | 8 days (acute model), 2 months (chronic model) | Reduction of inflammation | Reduction of IFN-γ, tumor necrosis factor, cytokines secretion | 137 | |
| Improve histological score | ||||||
| Bilberry | Human study | 160 g berry preparation | 6 weeks | Remission in 63.4% and response in 90.9% of patients | Decrease in Mayo score and Riley index | 129 |
| Reduction in fecal calprotectin levels | ||||||
| Culture (human samples) | 10 μg ml−1 of anthocyanin bilberry extract | 6 weeks | Reduction of inflammation | Inhibition of IFN-γ-receptor 2 | 139 | |
| Reduction of IFN- γ, TNF-α and activated NF-κB p65 | ||||||
| Black raspberry | Animal model | 5–10% berry powder | 14 days | Reduction of inflammation | Reduction of pro-inflammatory cytokines, phosphor-IκBα, COX-2 | 135 |
| Animal model | 5% dry berry powder | 28 days | Reduction of inflammation | Reduction of macrophages and neutrophils | 127 | |
| Inhibition of nuclear localization of NF-κB p65 | ||||||
| Regulation proteins expression | ||||||
| Reduction of promoter methylation in the Wnt pathway | ||||||
| Blueberry | Animal model | 5 g berry | 10 days | Reduction of inflammation | Decrease in disease activity index | 132 |
| Reduction of colonic myeloperoxidase activity and bacterial translocation | ||||||
| Gut microbiota modulation | ||||||
| Animal model | 10, 20 or 40 mg kg−1 anthocyanin extracts | 6 days | Protection against colonic damage for higher dose | Prevention of body weight loss | 133 | |
| Improve score of diarrhea, morphology and histology tests | ||||||
| Increase of IL-10 and reduction of NO, MPO, IL-12, TNF-α and IFN-γ | ||||||
| Animal model | 50 mg extract per kg body weight | 7 days | Reduction of inflammation | Decrease disease activity index | 134 | |
| Improve macroscopic and histological scores | ||||||
| Decrease MPO activity, malondialdehyde and prostaglandin E2 | ||||||
| Increase superoxide dismutase and catalase | ||||||
| Decrease of expression of COX-2 and IL-1β | ||||||
| Reduction of nuclear translocation of NF-κB | ||||||
| Cranberry | Animal model (high fat diet) | 200 mg extract per kg | 8 weeks | Reduction of inflammation | Prevent decrease of superoxide dismutase | 97 |
| Reduction of COX2 and TNF-α expression and normalization of NFκB/IκB ratio | ||||||
| Animal model | 0.1% or 1% extract or 1.5% whole berry powder | 6 weeks | Reduction of inflammation | Attenuation of colon shortening | 128 | |
| Reduction of colonic MPO activity | ||||||
| Reduction of pro-inflammatory cytokines production | ||||||
Black raspberry showed positive effects on induced-colitis in murine models, by suppressing levels of key pro-inflammatory cytokines, and particularly NF-κB. A proposed mechanism is that black raspberry compounds prevent the phosphorylation of Iκβα in the colon, thus resulting in the inhibition of NF-κB and its target genes playing a role in inflammation (COX-2, TNF and IL-1b).135 Dysregulation of the Wnt pathway during ulcerative colitis is usually observed, due to the methylation of its negative regulator. After treatment with black raspberry and the decrease in NF-κB expression, the promoter methylation in the Wnt pathway decreased as well, therefore prohibiting its activation.127,136 Unlike the results observed with blueberry, black raspberry did not seem to prevent inflammatory cell infiltration nor suppress markers of oxidative stress.135
The ingestion of cranberry also had beneficial effects on MPO activity and production of proinflammatory cytokines, however the results were only significant for the cranberry product containing fibers and not the cranberry polyphenols extracts. Both products decreased the disease activity index. The authors hypothesized a regulatory effect of the fermented products from the gut microbiota to contribute to the positive effects of cranberry on ulcerative colitis.128 Cranberry reduced TNF-α levels and COX-2 expression.97,128 Bilberry and their anthocyanins had positive effects on several parameters in acute induced-colitis in mice, by reducing disease severity and secretion of IFN-γ and TNF-α. Bilberries and anthoycanins also significantly reduced the apoptosis of epithelial cells.137
In conclusion, ingestion of berries seems to ameliorate the symptoms of colitis in colitis-induced murine models. The main reported effect was the downregulation of proinflammatory cytokines through the inhibition of NF-κB expression.
Bilberry was efficient in improving the conditions of patients with ulcerative colitis, however the disease activity increased again after the end of the treatment.129 The anti-inflammatory mechanisms of the bilberry compounds rely on the modulation of T-cell cytokine signaling.
b. Berries and colon cancer
Colorectal cancer is one of the most common cancers in men and women, and about 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new cases were estimated in the US in 2019.140 This type of cancer is the third leading cause of cancer related deaths in men and women. Most cases start with polyps that may become cancerous or not. The effects of berry treatments on colon cancer are summarized (Table 4).
000 new cases were estimated in the US in 2019.140 This type of cancer is the third leading cause of cancer related deaths in men and women. Most cases start with polyps that may become cancerous or not. The effects of berry treatments on colon cancer are summarized (Table 4).
| Berry used as treatment | Type of study | Dosage | Treatment duration | Benefits at the end of treatment | Accompanied effects | Ref. |
|---|---|---|---|---|---|---|
| COX: Cyclooxygenase, DNMT1: DNA methyltransferase, IL: interleukin. | ||||||
| Blueberry, bilberry, cowberry, cranberry, black currant, red currant, raspberry, blackberry, strawberry | In vitro (cell culture) | 2 or 4 mg ml−1 of berry extract | 48 hours | Inhibition of the cell growth (bilberry had the strongest effect) | 141 | |
| Grape, bilberry, chokeberry | In vitro (cell culture) | 10–75 μg extract per ml | 72 hours | Inhibition of the cell growth (chokeberry had the strongest effect) | 142 | |
| Blackberry, black raspberry, blueberry, cranberry, red raspberry, strawberry | In vitro (cell culture) | 25 to 200 μg ml−1 | 48 hours | Increasing inhibition of the cells with increasing concentration of the berry extact | Induction of apoptosis (strongest effect for black raspberry and strawberry) | 145 |
| Grape, bilberry and chokeberry | Animal study | 3.85 g anthocyanin extracts per kg | 14 weeks | Protective role towards colon cancer | Reduction of total aberrant crypt foci | 148 |
| Decrease of colonic cellular proliferation (bilberry and chokeberry extracts) | ||||||
| Decrease of COX2 mRNA expression (grape and bilberry) | ||||||
| Black raspberry | Human study | 60 g berry powder per day | 1–9 weeks | Protective role towards colon cancer | Modulation of methylation of inhibitors of the Wnt pathway (at least 4 weeks of treatment) | 150 |
| Decrease expression of DNMT1 | ||||||
| Human study | 20 g berry powder per 100 ml drink | 1–9 weeks | Attenuation of mechanism involve in colon cancer | Increase of granulocyte macrophage colony stimulating factor and markers of apoptosis | 152 | |
| Decrease in IL-8 and marker of proliferation | ||||||
| Human study | 9 months | Regression of rectal polyps | Decrease of burden of rectal polyps, cellular proliferation, DNA methylation methyl transferase 1 protein expression and p16 promoter methylation | 153 | ||
| Human study | 60 g berry powder per day | 1–9 weeks | Metabolic changes associated with beneficial regulation against colon cancer | 151 | ||
| Blueberry | In vitro (cell culture) | Concentration depends on fraction of berry extract tested | 48 hours | Inhibition of the cell growth depends on the extract (anthocyanin extract had the strongest effect) | Induction of apoptosis | 144 |
| In vitro (cell culture) | 10 to 100 μg ml−1 (polyphenol concentration) | 48 hours | Inhibition of the cell growth (dose dependent manner) | 53 | ||
| Cranberry | In vitro (cell culture) | 100–500 mg extract per L | 4 days | Inhibition of the cell growth | 143 | |
| In vitro (cell culture) | 8 to 500 μg ml−1 of whole berry and proanthocyanidin fraction | 48 hours | Inhibition of the cell growth | Inhibition of matrix metalloproteinases | 146 | |
The active compound and the mechanisms behind these potential effects remain to be elucidated, but the induction of apoptosis in the cancer cells has been reported. Different blueberry extracts tested against HT-29 and Caco-2 cancer cell lines showed that the anthocyanin fractions had the most important antiproliferative effect, while the phenolic acids fraction had little effect. The anthocyanins fraction also induced cancer cells apoptosis.144 Several berry extracts were shown to stimulate apoptosis in the HT-29 cell line expressing COX-2, with black-raspberry and strawberry extracts having the most pro-apoptotic effects. These observations suggest an association between inflammation, induced by COX-2, and cancer. Of all the berries tested in this study, all showed some pro-apoptotic effects on the HT-29 cells, except cranberry extracts.145 However, cranberry proanthocyanidin extracts tested against 8 tumor cell lines were effective in inhibiting the growth of a lung cancer line, leukemia cells and HT-29 colon cells.146 Interestingly, apoptosis of colon tumor cell lines was reported when treated with cranberry proanthocyanidin extracts, but not with cranberry polyphenolic extract.147
More recent studies have looked at the effect of berry extracts on colon cancer lines following the in vitro digestion and fermentation of these extracts. The compounds from raspberries, strawberries and blackcurrants were broken down into metabolites during in vitro digestion and fermentation, but their products retained anti-genotoxic, anti-mutagenic and anti-invasive activities on colon cells. However, they had an anti-migration effect on the cells tested.96 However, when comparing the growth inhibition of blueberry extracts before and after in vitro digestion and fermentation, the digested extracts retained the same antiproliferative activity, but the catabolites resulting from the fermentation showed lower growth inhibition activity on the human colon cancer cells.53 Therefore, the fermentation by the gut microbiota may alter the biological activity of blueberry polyphenols, contradicting the hypothesis that polyphenol metabolites are the active compounds, while no adverse effects were reported with raspberry, strawberry and blackcurrant extracts.
Black raspberries show promising action against colon cancer in patients, through modulation of apoptosis, proliferation and angiogenesis in cancerous cells. However, not all patients responded to the treatments. Similar effects in other berries remain to be studied.
6. Conclusion
Berries have widely been studied for their effects on human health, mainly attributed to their content in fibers and polyphenols (with a focus on anthocyanins). Their polyphenol profile is different from a species to another, and the study of the effect of the whole berries instead of each compound is necessary, due to potential synergies between compounds and interaction with the fruit matrix. The reported low bioavailability of polyphenols, and particularly anthocyanins has led to the hypothesis that the metabolites resulting from the digestion of these compounds are the main bioactive molecules in the body. A large amount of the polyphenols from the berries reach the gut and are metabolized into smaller molecules there. These polyphenols and their metabolites, once in the gut, can modulate the microorganism populations. A potential prebiotic-like effect of berries is of current interest and has been reported in several studies, with the berry compounds promoting the growth of the beneficial bacteria Bifidobacterium, Lactobacillus and Akkermansia. Finally, beneficial effects of the consumption of berries have been observed in the cases of chronic inflammation and cancer in the colon, mainly through anti-inflammatory activity and promotion of apoptosis. However, the mechanisms remain to be fully explained, and studies should also focus on berry compounds other than anthocyanins, that could also be beneficial, alone or in synergy with anthocyanins. More dietary intervention-based studies, ideally clinical trials, using whole berries are necessary to fully comprehend the bioavailability and metabolism of compounds ingested with the berry matrix, as to elucidate potential candidate for the positive effect on gut health. Personalized nutrition, specifically through metabotypes determination, appears as a realizable goal for maximizing any health benefits provided by berries and their dietary polyphenols.Conflicts of interest
There are no conflicts to declare.References
- M. A. S. Van duyn and E. Pivonka, J. Am. Diet. Assoc., 2000, 100, 1511–1521 CrossRef CAS.
- J. L. Slavin and B. Lloyd, Adv. Nutr., 2012, 3, 506–516 CrossRef CAS.
- J. Yang and Y.-Y. Xiao, Crit. Rev. Food Sci. Nutr., 2013, 53, 1202–1225 CrossRef CAS.
- C. K. Singh, X. Liu and N. Ahmad, Ann. N. Y. Acad. Sci., 2015, 1348, 150–160 CrossRef CAS.
- V. Nash, C. S. Ranadheera, E. N. Georgousopoulou, D. D. Mellor, D. B. Panagiotakos, A. J. McKune, J. Kellett and N. Naumovski, Food Res. Int., 2018, 113, 277–287 CrossRef CAS.
- C. Govers, M. Berkel Kaşikci, A. A. van der Sluis and J. J. Mes, Nutr. Rev., 2018, 76, 29–46 CrossRef.
- S. H. Nile and S. W. Park, Nutrition, 2014, 30, 134–144 CrossRef CAS.
- M. E. Valverde and O. Paredes-Lopez, in Berries: Properties, Consumption and Nutrition, Carlo Tuberoso, Nova Biomedical Books, New York, 2012, p. 19 Search PubMed.
- R. A. Moyer, K. E. Hummer, C. E. Finn, B. Frei and R. E. Wrolstad, J. Agric. Food Chem., 2002, 50, 519–525 CrossRef CAS PubMed.
- J. Beattie, A. Crozier and G. Duthie, Curr. Nutr. Food Sci., 2005, 1, 71–86 CrossRef CAS.
- J. B. Blumberg, T. A. Camesano, A. Cassidy, P. Kris-Etherton, A. Howell, C. Manach, L. M. Ostertag, H. Sies, A. Skulas-Ray and J. A. Vita, Adv. Nutr., 2013, 4, 618–632 CrossRef CAS.
- E. Pappas and K. M. Schaich, Crit. Rev. Food Sci. Nutr., 2009, 49, 741–781 CrossRef CAS.
- X. Wu and J. Kang, in Berries: Properties, Consumption and Nutrition, Carlo Tuberoso, Nova Biomedical Books, New York, 2012, p. 23 Search PubMed.
- W. Kalt, J. A. Joseph and B. Shukitt-Hale, J. Am. Pomol. Soc., 2007, 61, 151–160 Search PubMed.
- F. Giampieri, S. Tulipani, J. M. Alvarez-Suarez, J. L. Quiles, B. Mezzetti and M. Battino, Nutrition, 2012, 28, 9–19 CrossRef CAS.
- A. Gopalan, S. C. Reuben, S. Ahmed, A. S. Darvesh, J. Hohmann and A. Bishayee, Food Funct., 2012, 3, 795–809 RSC.
- A. S. Kristo, D. Klimis-Zacas and A. K. Sikalidis, Antioxidants, 2016, 5, 37 CrossRef.
- B. Baby, P. Antony and R. Vijayan, Crit. Rev. Food Sci. Nutr., 2018, 58, 2491–2507 CrossRef CAS.
- F. Guarner and J.-R. Malagelada, Lancet, 2003, 361, 512–519 CrossRef.
- M. Roberfroid, J. Nutr., 2007, 137, 830S–837S CrossRef CAS.
- F. Cardona, C. Andrés-Lacueva, S. Tulipani, F. J. Tinahones and M. I. Queipo-Ortuño, J. Nutr. Biochem., 2013, 24, 1415–1422 CrossRef CAS.
- N. P. Seeram and B. Burton-Freeman, Food Funct., 2018, 9, 20–21 RSC.
- J. B. Blumberg, A. Basu, C. G. Krueger, M. A. Lila, C. C. Neto, J. A. Novotny, J. D. Reed, A. Rodriguez-Mateos and C. D. Toner, Adv. Nutr., 2016, 7, 759S–770S CrossRef PubMed.
- M. Mikulic-Petkovsek, V. Schmitzer, A. Slatnar, F. Stampar and R. Veberic, J. Food Sci., 2012, 77, C1064–C1070 CrossRef CAS.
- D. C. Close and C. McArthur, Oikos, 2002, 99, 166–172 CrossRef CAS.
- A. Szajdek and E. J. Borowska, Plant Foods Hum. Nutr., 2008, 63, 147–156 CrossRef CAS.
- K. R. Määttä-Riihinen, A. Kamal-Eldin, P. H. Mattila, A. M. González-Paramás and A. R. Törrönen, J. Agric. Food Chem., 2004, 52, 4477–4486 CrossRef.
- R. L. Prior, S. A. Lazarus, G. Cao, H. Muccitelli and J. F. Hammerstone, J. Agric. Food Chem., 2001, 49, 1270–1276 CrossRef CAS.
- T. Tsuda, Mol. Nutr. Food Res., 2012, 56, 159–170 CrossRef CAS.
- Ø. M. Andersen and M. Jordheim, in eLS, American Cancer Society, 2010 Search PubMed.
- M. J. Cho, L. R. Howard, R. L. Prior and J. R. Clark, J. Sci. Food Agric., 2004, 84, 1771–1782 CrossRef.
- M. D'Archivio, C. Filesi, R. Di Benedetto, R. Gargiulo, C. Giovannini and R. Masella, Ann. Ist. Super. Sanita, 2007, 43, 348–361 Search PubMed.
- A. Crozier, D. Del Rio and M. N. Clifford, Mol. Aspects Med., 2010, 31, 446–467 CrossRef CAS.
- N. C. Ward, K. D. Croft, I. B. Puddey and J. M. Hodgson, J. Agric. Food Chem., 2004, 52, 5545–5549 CrossRef CAS.
- K. Gao, A. Xu, C. Krul, K. Venema, Y. Liu, Y. Niu, J. Lu, L. Bensoussan, N. P. Seeram, D. Heber and S. M. Henning, J. Nutr., 2006, 136, 52–57 CrossRef CAS.
- G. Annunziata, M. Maisto, C. Schisano, R. Ciampaglia, P. Daliu, V. Narciso, G. C. Tenore and E. Novellino, Nutrients, 2018, 10, 1711 CrossRef PubMed.
- J. L. Donovan, V. Crespy, C. Manach, C. Morand, C. Besson, A. Scalbert and C. Rémésy, J. Nutr., 2001, 131, 1753–1757 CrossRef CAS.
- M. Dueñas, I. Muñoz-González, C. Cueva, A. Jiménez-Girón, F. Sánchez-Patán, C. Santos-Buelga, M. V. Moreno-Arribas and B. Bartolomé, BioMed Res. Int., 2015, 2015, 850902 Search PubMed.
- A. Stalmach, S. Troufflard, M. Serafini and A. Crozier, Mol. Nutr. Food Res., 2009, 53(Suppl 1), S44–S53 CrossRef.
- C. Czank, A. Cassidy, Q. Zhang, D. J. Morrison, T. Preston, P. A. Kroon, N. P. Botting and C. D. Kay, Am. J. Clin. Nutr., 2013, 97, 995–1003 CrossRef CAS.
- C. Laurent, P. Besançon and B. Caporiccio, Food Chem., 2007, 100, 1704–1712 CrossRef CAS.
- A. Rzepecka-Stojko, J. Stojko, A. Kurek-Górecka, M. Górecki, A. Kabała-Dzik, R. Kubina, A. Moździerz and E. Buszman, Molecules, 2015, 20, 21732–21749 CrossRef CAS.
- M. Monagas, M. Urpi-Sarda, F. Sánchez-Patán, R. Llorach, I. Garrido, C. Gómez-Cordovés, C. Andres-Lacueva and B. Bartolomé, Food Funct., 2010, 1, 233–253 RSC.
- L. Y. Rios, M.-P. Gonthier, C. Rémésy, I. Mila, C. Lapierre, S. A. Lazarus, G. Williamson and A. Scalbert, Am. J. Clin. Nutr., 2003, 77, 912–918 CrossRef CAS.
- M. Urpi-Sarda, M. Monagas, N. Khan, R. Llorach, R. M. Lamuela-Raventós, O. Jáuregui, R. Estruch, M. Izquierdo-Pulido and C. Andrés-Lacueva, J. Chromatogr. A, 2009, 1216, 7258–7267 CrossRef CAS.
- C. Atkinson, S. Berman, O. Humbert and J. W. Lampe, J. Nutr., 2004, 134, 596–599 CrossRef CAS.
- L. Actis-Goretta, A. Lévèques, F. Giuffrida, F. Romanov-Michailidis, F. Viton, D. Barron, M. Duenas-Paton, S. Gonzalez-Manzano, C. Santos-Buelga, G. Williamson and F. Dionisi, Free Radicals Biol. Med., 2012, 53, 787–795 CrossRef CAS.
- S. M. Henning, W. Aronson, Y. Niu, F. Conde, N. H. Lee, N. P. Seeram, R.-P. Lee, J. Lu, D. M. Harris, A. Moro, J. Hong, L. Pak-Shan, R. J. Barnard, H. G. Ziaee, G. Csathy, V. L. W. Go, H. Wang and D. Heber, J. Nutr., 2006, 136, 1839–1843 CrossRef CAS.
- E. M. Janle, M. A. Lila, M. Grannan, L. Wood, A. Higgins, G. G. Yousef, R. B. Rogers, H. Kim, G. S. Jackson, L. Ho and C. M. Weaver, J. Med. Food, 2010, 13, 926–933 CrossRef CAS.
- X. Meng, S. Sang, N. Zhu, H. Lu, S. Sheng, M.-J. Lee, C.-T. Ho and C. S. Yang, Chem. Res. Toxicol., 2002, 15, 1042–1050 Search PubMed.
- M.-J. Bermúdez-Soto, F.-A. Tomás-Barberán and M.-T. García-Conesa, Food Chem., 2007, 102, 865–874 CrossRef.
- L. Liang, X. Wu, T. Zhao, J. Zhao, F. Li, Y. Zou, G. Mao and L. Yang, Food Res. Int., 2012, 46, 76–82 CrossRef CAS.
- J. Correa-Betanzo, E. Allen-Vercoe, J. McDonald, K. Schroeter, M. Corredig and G. Paliyath, Food Chem., 2014, 165, 522–531 CrossRef CAS.
- A. Burgos-Edwards, F. Jiménez-Aspee, S. Thomas-Valdés, G. Schmeda-Hirschmann and C. Theoduloz, Food Chem., 2017, 237, 1073–1082 CrossRef CAS.
- F. Van de Velde, M. E. Pirovani and S. R. Drago, J. Food Compos. Anal., 2018, 72, 22–31 CrossRef CAS.
- M. Hidalgo, M. J. Oruna-Concha, S. Kolida, G. E. Walton, S. Kallithraka, J. P. E. Spencer and S. de Pascual-Teresa, J. Agric. Food Chem., 2012, 60, 3882–3890 CrossRef CAS.
- V. Gowd, T. Bao, L. Wang, Y. Huang, S. Chen, X. Zheng, S. Cui and W. Chen, Food Chem., 2018, 269, 618–627 CrossRef CAS.
- J.-R. Cheng, X.-M. Liu, Z.-Y. Chen, Y.-S. Zhang and Y.-H. Zhang, Food Chem., 2016, 213, 721–727 CrossRef CAS PubMed.
- Y. Chen, Q. Li, T. Zhao, Z. Zhang, G. Mao, W. Feng, X. Wu and L. Yang, Food Chem., 2017, 237, 887–894 CrossRef CAS.
- S. Attri, K. Sharma, P. Raigond and G. Goel, Food Res. Int., 2018, 105, 324–332 CrossRef CAS.
- S. Talavéra, C. Felgines, O. Texier, C. Besson, C. Manach, J.-L. Lamaison and C. Rémésy, J. Nutr., 2004, 134, 2275–2279 CrossRef.
- T. Ichiyanagi, Y. Shida, M. M. Rahman, Y. Hatano and T. Konishi, J. Agric. Food Chem., 2006, 54, 6578–6587 CrossRef CAS.
- G. Baron, A. Altomare, L. Regazzoni, V. Redaelli, S. Grandi, A. Riva, P. Morazzoni, A. Mazzolari, M. Carini, G. Vistoli and G. Aldini, J. Pharm. Biomed. Anal., 2017, 144, 112–121 CrossRef CAS.
- X. Wu, H. E. Pittman and R. L. Prior, J. Agric. Food Chem., 2006, 54, 583–589 CrossRef CAS PubMed.
- G. Borges, S. Roowi, J.-M. Rouanet, G. G. Duthie, M. E. J. Lean and A. Crozier, Mol. Nutr. Food Res., 2007, 51, 714–725 CrossRef CAS PubMed.
- C. Del Bò, S. Ciappellano, D. Klimis-Zacas, D. Martini, C. Gardana, P. Riso and M. Porrini, J. Agric. Food Chem., 2010, 58, 2491–2497 CrossRef.
- D. Del Rio, G. Borges and A. Crozier, Br. J. Nutr., 2010, 104(Suppl 3), S67–S90 CrossRef CAS.
- I. A. Ludwig, P. Mena, L. Calani, G. Borges, G. Pereira-Caro, L. Bresciani, D. Del Rio, M. E. J. Lean and A. Crozier, Free Radicals Biol. Med., 2015, 89, 758–769 CrossRef CAS.
- P. E. Milbury, J. A. Vita and J. B. Blumberg, J. Nutr., 2010, 140, 1099–1104 CrossRef CAS.
- T. K. McGhie, G. D. Ainge, L. E. Barnett, J. M. Cooney and D. J. Jensen, J. Agric. Food Chem., 2003, 51, 4539–4548 CrossRef CAS.
- T. Miyazawa, K. Nakagawa, M. Kudo, K. Muraishi and K. Someya, J. Agric. Food Chem., 1999, 47, 1083–1091 CrossRef CAS PubMed.
- P. E. Milbury, G. Cao, R. L. Prior and J. Blumberg, Mech. Ageing Dev., 2002, 123, 997–1006 CrossRef CAS.
- X. Wu, G. Cao and R. L. Prior, J. Nutr., 2002, 132, 1865–1871 CrossRef CAS PubMed.
- C. Felgines, S. Talavéra, M.-P. Gonthier, O. Texier, A. Scalbert, J.-L. Lamaison and C. Rémésy, J. Nutr., 2003, 133, 1296–1301 CrossRef CAS PubMed.
- W. Mullen, C. A. Edwards, M. Serafini and A. Crozier, J. Agric. Food Chem., 2008, 56, 713–719 CrossRef CAS PubMed.
- C. Felgines, S. Talavera, O. Texier, A. Gil-Izquierdo, J.-L. Lamaison and C. Remesy, J. Agric. Food Chem., 2005, 53, 7721–7727 CrossRef CAS PubMed.
- J. M. Cooney, D. J. Jensen and T. K. McGhie, J. Sci. Food Agric., 2004, 84, 237–245 CrossRef CAS.
- K. Kahle, M. Kraus, W. Scheppach, M. Ackermann, F. Ridder and E. Richling, Mol. Nutr. Food Res., 2006, 50, 418–423 CrossRef CAS PubMed.
- R. González-Barrio, G. Borges, W. Mullen and A. Crozier, J. Agric. Food Chem., 2010, 58, 3933–3939 CrossRef PubMed.
- D. Mueller, K. Jung, M. Winter, D. Rogoll, R. Melcher and E. Richling, Food Chem., 2017, 231, 275–286 CrossRef CAS PubMed.
- M. V. Selma, J. C. Espín and F. A. Tomás-Barberán, J. Agric. Food Chem., 2009, 57, 6485–6501 CrossRef CAS PubMed.
- K. Ou, P. Sarnoski, K. R. Schneider, K. Song, C. Khoo and L. Gu, Mol. Nutr. Food Res., 2014, 58, 2196–2205 CrossRef CAS PubMed.
- W. R. Russell, A. Labat, L. Scobbie and S. H. Duncan, Mol. Nutr. Food Res., 2007, 51, 726–731 CrossRef CAS PubMed.
- X. Wu, H. E. Pittman Iii, T. Hager, A. Hager, L. Howard and R. L. Prior, Mol. Nutr. Food Res., 2009, 53(Suppl 1), S76–S84 CrossRef PubMed.
- R. Khanal, L. R. Howard and R. L. Prior, J. Agric. Food Chem., 2014, 62, 3987–3996 CrossRef CAS PubMed.
- M.-P. Martí, A. Pantaleón, A. Rozek, A. Soler, J. Valls, A. Macià, M.-P. Romero and M.-J. Motilva, J. Sep. Sci., 2010, 33, 2841–2853 CrossRef PubMed.
- A. Serra, A. Macià, M.-P. Romero, N. Anglés, J.-R. Morelló and M.-J. Motilva, Food Chem., 2011, 126, 1127–1137 CrossRef CAS.
- J. Gu, J. M. Thomas-Ahner, K. M. Riedl, M. T. Bailey, Y. Vodovotz, S. J. Schwartz and S. K. Clinton, Mol. Nutr. Food Res., 2019, 63, e1800636 CrossRef PubMed.
- R. P. Feliciano, C. E. Mills, G. Istas, C. Heiss and A. Rodriguez-Mateos, Nutrients, 2017, 9, 268 CrossRef PubMed.
- D. L. McKay, C.-Y. O. Chen, C. A. Zampariello and J. B. Blumberg, Food Chem., 2015, 168, 233–240 CrossRef CAS PubMed.
- I. Erlund, R. Freese, J. Marniemi, P. Hakala and G. Alfthan, Nutr. Cancer, 2006, 54, 13–17 CrossRef CAS PubMed.
- C. Wang, Y. Zuo, J. A. Vinson and Y. Deng, Food Chem., 2012, 132, 1420–1428 CrossRef CAS PubMed.
- B. Cerdá, F. A. Tomás-Barberán and J. C. Espín, J. Agric. Food Chem., 2005, 53, 227–235 CrossRef PubMed.
- G. Williamson and M. N. Clifford, Biochem. Pharmacol., 2017, 139, 24–39 CrossRef CAS PubMed.
- G. Jamar, D. Estadella and L. P. Pisani, BioFactors, 2017, 43, 507–516 CrossRef CAS PubMed.
- K. Brown, D. DeCoffe, E. Molcan and D. L. Gibson, Nutrients, 2012, 4, 1095–1119 CrossRef CAS PubMed.
- F. F. Anhê, D. Roy, G. Pilon, S. Dudonné, S. Matamoros, T. V. Varin, C. Garofalo, Q. Moine, Y. Desjardins, E. Levy and A. Marette, Gut, 2015, 64, 872–883 CrossRef PubMed.
- C. Marques, I. Fernandes, M. Meireles, A. Faria, J. P. E. Spencer, N. Mateus and C. Calhau, Sci. Rep., 2018, 8, 11341 CrossRef PubMed.
- J. Overall, S. A. Bonney, M. Wilson, A. Beermann, M. H. Grace, D. Esposito, M. A. Lila and S. Komarnytsky, Int. J. Mol. Sci., 2017, 18, 422 CrossRef PubMed.
- S. Wu, R. Hu, H. Nakano, K. Chen, M. Liu, X. He, H. Zhang, J. He and D.-X. Hou, Molecules, 2018, 23, 3213 CrossRef PubMed.
- S. N. Hester, A. Mastaloudis, R. Gray, J. M. Antony, M. Evans and S. M. Wood, J. Nutr. Metab., 2018, 2018, 7497260 Search PubMed.
- F. Sánchez-Patán, E. Barroso, T. van de Wiele, A. Jiménez-Girón, P. J. Martín-Alvarez, M. V. Moreno-Arribas, M. C. Martínez-Cuesta, C. Peláez, T. Requena and B. Bartolomé, Food Chem., 2015, 183, 273–282 CrossRef PubMed.
- R. M. Alqurashi, S. N. Alarifi, G. E. Walton, A. F. Costabile, I. R. Rowland and D. M. Commane, Food Chem., 2017, 234, 190–198 CrossRef CAS PubMed.
- Y. Yan, Y. Peng, J. Tang, J. Mi, L. Lu, X. Li, L. Ran, X. Zeng and Y. Cao, J. Funct. Foods, 2018, 48, 533–541 CrossRef CAS.
- A.-L. Molan, Z. Liu and M. Kruger, World J. Microbiol. Biotechnol., 2010, 26, 1735–1743 CrossRef.
- C. Matziouridou, N. Marungruang, T. D. Nguyen, M. Nyman and F. Fåk, Mol. Nutr. Food Res., 2016, 60, 1150–1160 CrossRef CAS PubMed.
- A.-L. Molan, Z. Liu and G. Plimmer, Phytother. Res., 2014, 28, 416–422 CrossRef CAS PubMed.
- M. Song, J. Wang, T. Eom and H. Kim, Nutr. Res., 2015, 35, 655–663 CrossRef CAS PubMed.
- R. Puupponen-Pimiä, L. Nohynek, C. Meier, M. Kähkönen, M. Heinonen, A. Hopia and K. M. Oksman-Caldentey, J. Appl. Microbiol., 2001, 90, 494–507 CrossRef PubMed.
- R. Puupponen-Pimiä, L. Nohynek, S. Hartmann-Schmidlin, M. Kähkönen, M. Heinonen, K. Määttä-Riihinen and K.-M. Oksman-Caldentey, J. Appl. Microbiol., 2005, 98, 991–1000 CrossRef PubMed.
- G. Paturi, T. Mandimika, C. A. Butts, S. Zhu, N. C. Roy, W. C. McNabb and J. Ansell, Nutrition, 2012, 28, 324–330 CrossRef CAS PubMed.
- K. B. Guergoletto, A. Costabile, G. Flores, S. Garcia and G. R. Gibson, Food Chem., 2016, 196, 251–258 CrossRef CAS PubMed.
- S. Attri and G. Goel, Food Res. Int., 2018, 111, 314–323 CrossRef CAS PubMed.
- A. L. Molan, M. A. Lila, J. Mawson and S. De, World J. Microbiol. Biotechnol., 2009, 25, 1243–1249 CrossRef CAS.
- A. Lacombe, R. W. Li, D. Klimis-Zacas, A. S. Kristo, S. Tadepalli, E. Krauss, R. Young and V. C. H. Wu, PLoS One, 2013, 8, e67497 CrossRef CAS PubMed.
- J. K. da Silva-Maia, A. G. Batista, L. C. Correa, G. C. Lima, S. B. Junior and M. R. M. Junior, J. Food Biochem., 2019, 43, e12705 CrossRef PubMed.
- Y. Kang, G. Yang, S. Zhang, C. F. Ross and M.-J. Zhu, Mol. Nutr. Food Res., 2018, 62, e1800535 CrossRef PubMed.
- C. Petersen, U. D. Wankhade, D. Bharat, K. Wong, J. E. Mueller, S. V. Chintapalli, B. D. Piccolo, T. Jalili, Z. Jia, J. D. Symons, K. Shankar and P. V. A. Babu, J. Nutr. Biochem., 2019, 66, 63–69 CrossRef CAS PubMed.
- G. Jamar, A. B. Santamarina, L. V. Mennitti, H. de C. Cesar, L. M. Oyama, V. V. de Rosso and L. P. Pisani, J. Funct. Foods, 2018, 46, 212–219 CrossRef CAS.
- C. A. Morais, L. M. Oyama, R. de Moura Conrado, V. V. de Rosso, C. O. do Nascimento and L. P. Pisani, Food Res. Int., 2015, 77, 186–193 CrossRef CAS.
- P. Pan, V. Lam, N. Salzman, Y.-W. Huang, J. Yu, J. Zhang and L.-S. Wang, Nutr. Cancer, 2017, 69, 943–951 CrossRef CAS.
- M. C. Dao, A. Everard, J. Aron-Wisnewsky, N. Sokolovska, E. Prifti, E. O. Verger, B. D. Kayser, F. Levenez, J. Chilloux, L. Hoyles, MICRO-Obes Consortium, M.-E. Dumas, S. W. Rizkalla, J. Doré, P. D. Cani and K. Clément, Gut, 2016, 65, 426–436 CrossRef CAS PubMed.
- S. Vendrame, S. Guglielmetti, P. Riso, S. Arioli, D. Klimis-Zacas and M. Porrini, J. Agric. Food Chem., 2011, 59, 12815–12820 CrossRef CAS PubMed.
- S. Guglielmetti, D. Fracassetti, V. Taverniti, C. Del Bo’, S. Vendrame, D. Klimis-Zacas, S. Arioli, P. Riso and M. Porrini, J. Agric. Food Chem., 2013, 61, 8134–8140 CrossRef.
- CDC, Inflammatory Bowel Disease (IBD), https://www.cdc.gov/ibd/data-statistics.htm, (accessed May 13, 2019).
- D. C. Rubin, A. Shaker and M. S. Levin, Front. Immunol., 2012, 3, 107 Search PubMed.
- L.-S. Wang, C.-T. Kuo, K. Stoner, M. Yearsley, K. Oshima, J. Yu, T. H.-M. Huang, D. Rosenberg, D. Peiffer, G. Stoner and Y.-W. Huang, Carcinogenesis, 2013, 34, 2842–2850 CrossRef CAS PubMed.
- X. Xiao, J. Kim, Q. Sun, D. Kim, C.-S. Park, T.-S. Lu and Y. Park, Food Chem., 2015, 167, 438–446 CrossRef CAS PubMed.
- L. Biedermann, J. Mwinyi, M. Scharl, P. Frei, J. Zeitz, G. A. Kullak-Ublick, S. R. Vavricka, M. Fried, A. Weber, H.-U. Humpf, S. Peschke, A. Jetter, G. Krammer and G. Rogler, J. Crohns Colitis, 2013, 7, 271–279 CrossRef PubMed.
- G. D. Stoner, Cancer Prev. Res., 2009, 2, 187–194 CrossRef CAS PubMed.
- C. Manichanh, N. Borruel, F. Casellas and F. Guarner, Nat. Rev. Gastroenterol. Hepatol., 2012, 9, 599–608 CrossRef CAS PubMed.
- N. Osman, D. Adawi, S. Ahrné, B. Jeppsson and G. Molin, Dig. Dis. Sci., 2008, 53, 2464–2473 CrossRef PubMed.
- L.-H. Wu, Z.-L. Xu, D. Dong, S.-A. He and H. Yu, J. Evidence-Based Complementary Altern. Med., 2011, 2011, 525462 Search PubMed.
- M. Pervin, M. A. Hasnat, J.-H. Lim, Y.-M. Lee, E. O. Kim, B.-H. Um and B. O. Lim, J. Nutr. Biochem., 2016, 28, 103–113 CrossRef CAS PubMed.
- D. C. Montrose, N. A. Horelik, J. P. Madigan, G. D. Stoner, L.-S. Wang, R. S. Bruno, H. J. Park, C. Giardina and D. W. Rosenberg, Carcinogenesis, 2011, 32, 343–350 CrossRef CAS PubMed.
- L.-S. Wang, C.-T. Kuo, T. H.-M. Huang, M. Yearsley, K. Oshima, G. D. Stoner, J. Yu, J. F. Lechner and Y.-W. Huang, Cancer Prev. Res., 2013, 6, 1317–1327 CrossRef PubMed.
- H. Piberger, A. Oehme, C. Hofmann, A. Dreiseitel, P. G. Sand, F. Obermeier, J. Schoelmerich, P. Schreier, G. Krammer and G. Rogler, Mol. Nutr. Food Res., 2011, 55, 1724–1729 CrossRef CAS PubMed.
- S. Triebel, H.-L. Trieu and E. Richling, J. Agric. Food Chem., 2012, 60, 8902–8910 CrossRef CAS PubMed.
- S. Roth, M. R. Spalinger, C. Gottier, L. Biedermann, J. Zeitz, S. Lang, A. Weber, G. Rogler and M. Scharl, PLoS One, 2016, 11, e0154817 CrossRef PubMed.
- American Cancer Society, Key Statistics for Colorectal Cancer, https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html, (accessed May 13, 2019).
- N. Katsube, K. Iwashita, T. Tsushida, K. Yamaki and M. Kobori, J. Agric. Food Chem., 2003, 51, 68–75 CrossRef CAS PubMed.
- C. Zhao, M. M. Giusti, M. Malik, M. P. Moyer and B. A. Magnuson, J. Agric. Food Chem., 2004, 52, 6122–6128 CrossRef CAS PubMed.
- P. J. Ferguson, E. Kurowska, D. J. Freeman, A. F. Chambers and D. J. Koropatnick, J. Nutr., 2004, 134, 1529–1535 CrossRef CAS PubMed.
- W. Yi, J. Fischer, G. Krewer and C. C. Akoh, J. Agric. Food Chem., 2005, 53, 7320–7329 CrossRef CAS PubMed.
- N. P. Seeram, L. S. Adams, Y. Zhang, R. Lee, D. Sand, H. S. Scheuller and D. Heber, J. Agric. Food Chem., 2006, 54, 9329–9339 CrossRef CAS PubMed.
- C. C. Neto, C. G. Krueger, T. L. Lamoureaux, M. Kondo, A. J. Vaisberg, R. A. Hurta, S. Curtis, M. D. Matchett, H. Yeung, M. I. Sweeney and J. D. Reed, J. Sci. Food Agric., 2006, 86, 18–25 CrossRef CAS.
- C. C. Neto, J. W. Amoroso and A. M. Liberty, Mol. Nutr. Food Res., 2008, 52(Suppl 1), S18–S27 Search PubMed.
- G. Lala, M. Malik, C. Zhao, J. He, Y. Kwon, M. M. Giusti and B. A. Magnuson, Nutr. Cancer, 2006, 54, 84–93 CrossRef CAS PubMed.
- P. Pan, C. W. Skaer, H.-T. Wang, S. M. Stirdivant, M. R. Young, K. Oshima, G. D. Stoner, J. F. Lechner, Y.-W. Huang and L.-S. Wang, Carcinogenesis, 2015, 36, 1245–1253 CrossRef CAS PubMed.
- L.-S. Wang, M. Arnold, Y.-W. Huang, C. Sardo, C. Seguin, E. Martin, T. H.-M. Huang, K. Riedl, S. Schwartz, W. Frankel, D. Pearl, Y. Xu, J. Winston, G.-Y. Yang and G. Stoner, Clin. Cancer Res., 2011, 17, 598–610 CrossRef CAS PubMed.
- P. Pan, C. W. Skaer, S. M. Stirdivant, M. R. Young, G. D. Stoner, J. F. Lechner, Y.-W. Huang and L.-S. Wang, Cancer Prev. Res., 2015, 8, 743–750 CrossRef CAS PubMed.
- R. A. Mentor-Marcel, G. Bobe, C. Sardo, L.-S. Wang, C.-T. Kuo, G. Stoner and N. H. Colburn, Nutr. Cancer, 2012, 64, 820–825 CrossRef CAS PubMed.
- L.-S. Wang, C. A. Burke, H. Hasson, C.-T. Kuo, C. L. S. Molmenti, C. Seguin, P. Liu, T. H.-M. Huang, W. L. Frankel and G. D. Stoner, Cancer Prev. Res., 2014, 7, 666–674 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2020 |
