Selenium alleviates lipopolysaccharide-induced endometritis via regulating the recruitment of TLR4 into lipid rafts in mice
Yu
Chen†
,
Yi-fan
Zhao†
,
Jing
Yang
,
Hong-yuan
Jing
,
Wan
Liang
,
Miao-yu
Chen
,
Mei
Yang
,
Ying
Wang
and
Meng-yao
Guo
 *
*
Department of Clinical Veterinary Medicine, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, People's Republic of China. E-mail: gmy1985@163.com
First published on 25th November 2019
Abstract
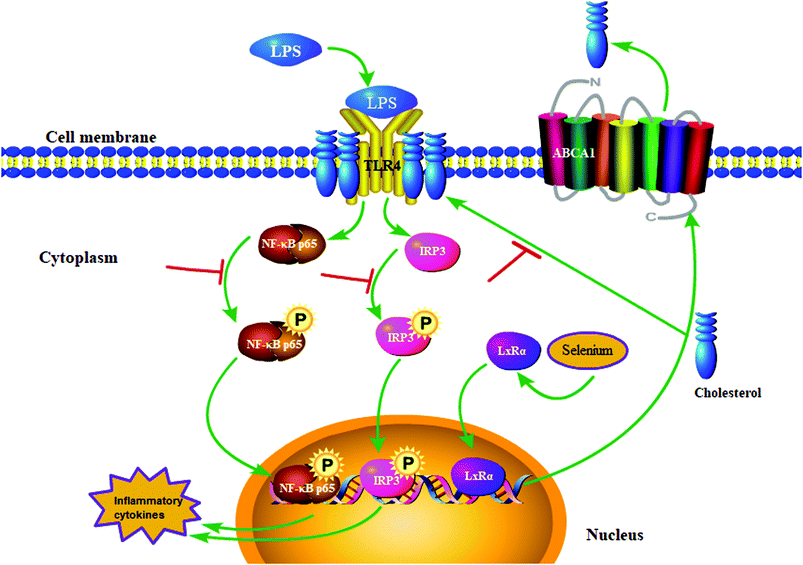
Selenium (Se) is an essential trace element for living organisms and plays diverse biological roles. Endometritis is a common reproductive disorder in dairy cows, causing huge economic losses. In this study, we explored the effects of Se on lipopolysaccharide (LPS)-induced endometritis in mice and expounded its underlying mechanism of action. We validated the anti-inflammatory effects of Se in vivo by establishing a mouse model of endometriosis induced by LPS. Se significantly reversed the LPS-induced uterine histopathological changes, MPO activity and inflammatory cytokine levels in vivo. Simultaneously, TLR4 and its downstream signaling pathways, lipid rafts and cholesterol levels in the tissues were also attenuated by Se under LPS stimulation. In addition, the molecular mechanism of the Se anti-inflammatory effect was clarified in mouse endometrial epithelial cells. Se inhibited TLR4-mediated NF-κB and IRF3 signal transduction pathways to reduce the production of inflammatory factors. We found that Se promoted the consumption of cholesterol to suppress the lipid rafts coming into being and inhibited the TLR4 positioning to the lipid raft to prevent the inflammatory response caused by LPS. Meanwhile, Se activated the LxRα-ABCA1 pathway to cause the outflow of cholesterol in cells. The anti-inflammatory effect of Se was disrupted by silencing LxRα. In conclusion, Se exerted anti-inflammatory effects most likely by the LxRα-ABCA1 pathway activation, which inhibited lipid rafts by depleting cholesterol and ultimately impeded the migration of TLR4 to lipid rafts.
1 Introduction
Endometritis is a reproductive disorder that causes enormous economic losses in animal husbandry. It occurs in uterine tissue, resulting in a decline in productivity and fertility in cows and other mammals.1 Endometritis is usually caused by a variety of pathogen infections after parturition, especially Escherichia coli.2,3 Lipopolysaccharide (LPS), triggering inflammatory response consistent with E. coli, have been demonstrated to have a function of mediating the activation of the toll like receptor 4 (TLR4) pathway.4 Under LPS stimulation, TLR4 was recruited to lipid rafts, causing the activation of a series of downstream signaling pathways and eventually inducing the production of inflammatory mediators.5 Lipid raft-mediated signaling pathways are associated with the development of inflammation.6,7 The breaking of lipid rafts may have an influence on the recognition of pathogens and blocking TLR4-mediated inflammatory signaling pathways.Selenium (Se) is a vital trace element for nutrition with various biological functions, such as immune protection,8 anti-injury activity,9,10 muscle metabolism, apoptosis,11,12 neurobiology,13 and reproduction.14 It has been confirmed that Se deficiency could lead to many diseases such as reproductive disorders and inflammatory infiltration.15,16 The generation of the biological effects of Se is mediated by the construction of selenoproteins, and about 25 kinds of selenoproteins have been identified currently.17 SelS, located on the ER membrane, was discovered to participate in the release of pro-inflammatory mediators and also plays an essential role in the reproductive health of humans and animals.18 Se could modulate the occurrence and severity of inflammatory responses by regulating TLR4-assiociated signaling pathways.1
Lipid rafts, an acceptor of TLR4, provide microdomains for the ligand receptor recognition and complex production to anchor in the TLR4 signaling cascade.7,19 Lipid rafts are micro-structure areas on the membrane that are rich in cholesterol,20 while some studies have demonstrated that Se has a function of reducing the cholesterol levels.21,22 However, it has not been elucidated whether Se can regulate the metabolism of lipid rafts to exert anti-inflammatory effects.
The aim of this work was to explore whether Se can adjust lipid raft formation and further influence the occurrence of inflammation and the potential mechanism in the process of inflammation.
2 Materials and methods
2.1 Reagents
The myeloperoxidase (MPO) detection kits were bought from the Jiancheng Bioengineering Institute of Nanjing (Nanjing, Jiangsu, China). The reverse transcriptase reagent and the SYBR Green Plus mix were obtained from Vazyme Biotech Co., Ltd (Nanjing, Jiangsu, China). A cholesterol quantitation kit was purchased from Sigma-Aldrich (St Louis, Missouri, USA). The mouse TNF-α, IL-1β, and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were purchased from BioLegend (Camino Santa Fe, CA, USA). All antibodies used in this study were provided by Cell Signaling Technology (Beverly, MA, USA). All chemical reagents were of the reagent standard in this study.2.2 Animal experiments
All female c57 mice (total of 60, age 4–5 weeks, weight 10–15 g) were housed in Huazhong Agricultural University Experimental Animal Center and the experimental operations followed the guidelines provided by the Laboratory Animal Research Center of Hubei Province and were approved by the Ethical Committee on Animal Research at Huazhong Agricultural University (HZAUMO-2015-12).All animals were divided into three groups (n = 20) at random. The three groups of mice were fed a diet purchased from the Nantong Trophic Diet Technology Company (Jiangsu, China) with different Se contents: low (0.015 mg Se per kg), normal (0.15 mg Se per kg) and high (1.5 mg Se per kg). All mice were fed a diet with different Se contents for 8 weeks. Double distilled water was given to the mice in the low Se dietary group, and high Se content water was provided to the high Se dietary group. Furthermore, half of the mice were randomly selected in each diet group to challenge with 50 μl 1 mg ml−1 LPS by intrauterine infusion and others without LPS stimulation (given normal saline infusions without LPS). All mice were anesthetized and sacrificed after 24 h of treatment with LPS. The uterine samples were obtained and saved at −80 °C for the next study.
2.3 Se concentration test
The uterine tissues were treated with an acid and then reacted with an aromatic ortho-diamine to form 4,5-benzodiazepine which exhibited bright lime-green fluorescence in cyclohexane at an excitation wavelength of 366 nm. The fluorescence intensity was measured at an Ex/Em of 366/520 nm using a fluorescence spectrophotometer (PerkinElmer, MA, USA). The results were calculated according to the standard curve.2.4 Histopathological examination
The uterine tissue was fixed in 10% formalin, embedded with paraffin and then sectioned. Paraffin sections were sequentially treated with xylene and different concentrations of ethanol for dyeing. Then haematoxylin and eosin (H&E) staining was performed to examine the histopathological changes by optical microscopy.2.5 Myeloperoxidase (MPO) analysis
The homogenate of uterine tissues was obtained by using a glass homogenizer, and centrifuged to collect the supernatant. The MPO activity of uterine tissues was tested with an MPO kit following the manufacturer's instructions, and the result was examined using a spectrophotometer at 460 nm.2.6 TdT-mediated dUTP Nick-End Labeling (TUNEL) assay
Paraffin sections were dewaxed with xylene, then treated with proteinase K and finally washed with PBS three times. A mixture of TUNEL dyes (TdT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dUTP, 1
dUTP, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was added to the sections for 60 min at 37 °C. Finally, the images were collected with a fluorescence microscope (Nikon, Japan) at an Ex/Em of 530/630 nm.
9) was added to the sections for 60 min at 37 °C. Finally, the images were collected with a fluorescence microscope (Nikon, Japan) at an Ex/Em of 530/630 nm.
2.7 Cell isolation and culture
Mouse uterine tissue was aseptically harvested, minced, and digested with a mixture of trypsin and collagenase for 1 h at 37 °C. The digestion solution was filtered through a cell sieve, then centrifuged and washed several times. Mouse uterine epithelial cells were purified by the differential adherence method. The cells were treated with medium that contains Se in a low (0 μM), normal (2.5 μM) and high (5 μM) level. Each Se content group was randomly classified into two parts: one part was stimulated with LPS (1 mg ml−1) and the other was untreated. After 12 h of cultivation, both cells and the supernatant were collected for later experiment.2.8 Cell viability assay
The cells were digested, resuspended, and transferred to a 96-well plate. When the cells were attached to 60%–70% of the wall, each well was supplemented with 20 μl of MTT (5 mg ml−1) for 4 h. Then DMSO was used to decompose formazan. The results were demonstrated at 570 nm with a microplate reader.2.9 Extraction and calibration of lipid rafts
Uterine tissue homogenates and cells were treated with MBS buffer. The lysate was transferred to an SW41 ultracentrifuge tube after being mixed with an equal volume of 80% sucrose solution. Subsequently, 30% and 5% sucrose solutions were sequentially added to the ultracentrifuge tube, which was centrifuged at 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 h at 4 °C. Lipid rafts were shown indirectly by labeling cholesterol in lipid rafts.
000 rpm for 20 h at 4 °C. Lipid rafts were shown indirectly by labeling cholesterol in lipid rafts.
2.10 Cholesterol concentration determination
Cholesterol levels in tissues and cells were examined by using a cholesterol quantitation kit according to the instruction manual.2.11 Enzyme linked immunosorbent assay (ELISA)
The tissues were weighed, homogenized, and then centrifuged in order to collect the supernatant. Meanwhile, the supernatant of cells was also harvested. The inflammatory cytokine level was measured by using an ELISA kit according to the instructions, and then the OD value was measured using a microplate reader.2.12 Quantitative real time polymerase chain reaction (qRT-PCR) assay
The cells were used to extract RNA with the TRIquick reagent (Solarbio, Beijing, China). The concentrations and purity of total RNA were examined with a UV-vis spectrophotometer (Quawell, USA). Then total RNA was reverse transcribed into cDNA with reverse transcriptase reagents. The primers used in the study are displayed in Table 1. The expression of IL-6, IL-1β and TNF-α mRNA levels was measured using qRT-PCR. The result was calculated by the 2−ΔΔCt method while GAPDH served as an internal reference gene.| Name | Primer sequence (5′–3′) | GenBank accession number | Product size (bp) |
|---|---|---|---|
| TNF-α | CTTCTCATTCCTGCTTGTG ACTTGGTGGTTTGCTACG | NM_013693.3 | 198 |
| IL-1β | CCTGGGCTGTCCTGATGAGAG TCCACGGGAAAGACACAGGTA | NM_008361.4 | 131 |
| IL-6 | GGCGGATCGGATGTTGTGAT GGACCCCAGACAATCGGTTG | NM_031168.1 | 199 |
| GAPDH | CAATGTGTCCGTCGTGGATCT GTCCTCAGTGTAGCCCAAGATG | NM_001289726.1 | 124 |
2.13 Western blot analysis
The protein was isolated with RIPA in uterine tissues and cells, and the total protein concentration was detected with a BCA protein assay kit. The samples that have the same amount of protein (60 μg) were separated by SDS-PAGE and transferred onto a PVDF membrane. Then 5% skimmed milk was used to block the membrane at room temperature for 2 h. The primary antibody in dilution buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilutions) was used to soak the membrane overnight at 4 °C. Furthermore, the membranes were incubated with secondary antibody for 1 h at room temperature. After being washed with TBST three times, the membrane was developed under an enhanced chemiluminescence detection system to display protein expression.
1000 dilutions) was used to soak the membrane overnight at 4 °C. Furthermore, the membranes were incubated with secondary antibody for 1 h at room temperature. After being washed with TBST three times, the membrane was developed under an enhanced chemiluminescence detection system to display protein expression.
2.14 Statistical analysis
The data were analyzed with SPSS software. All data were expressed as mean ± S.E.M. One-way ANOVA was used to evaluate the difference within the same dietary group and among different dietary groups. A significant difference was noted at p < 0.05.3 Results
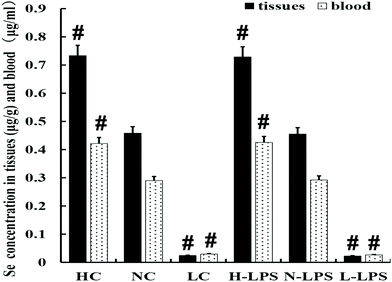
3.1 Diets with different Se concentrations affect Se content in mice
The Se content was detected in the blood and uterus of mice. As shown in Fig. 1, the concentration of Se in the blood and uterine tissues obviously increased with the increase of Se content in the feed. Meanwhile, LPS stimulation had no effect on the Se content of tissues and blood in each dietary group.3.2 Effects of Se on LPS-induced mouse endometritis
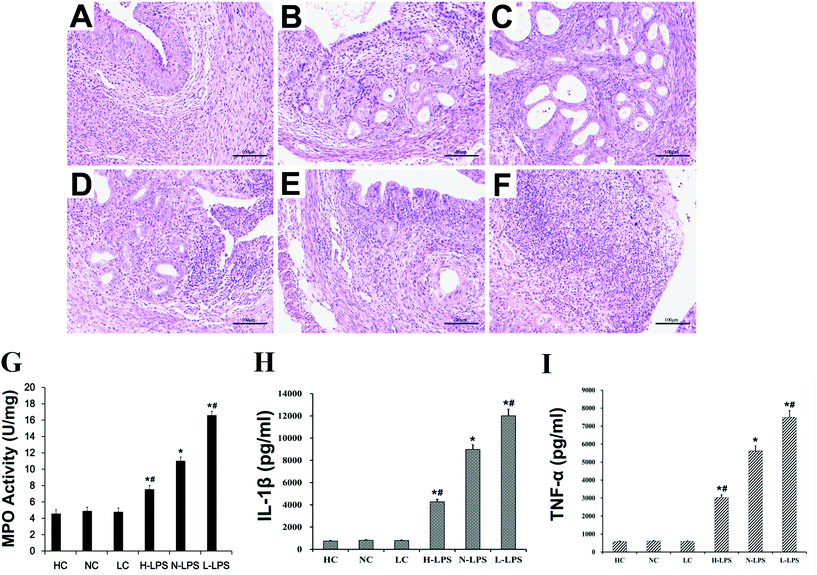
HE staining observation, the MPO activity test and inflammatory cytokine detection were used to evaluate the endometritis model. The uterus without LPS stimulation showed no obvious structural damage (Fig. 2A–C). But the uterus stimulated with LPS showed significant inflammatory histopathological changes, such as endometrial epithelial shedding, inflammatory cell filling and serious damage to the structure. Moreover, under LPS stimulation, it was obvious that the tissues from the high-Se group were injured the lightest (Fig. 2D); the low-Se group had the most serious inflammatory injury (Fig. 2F); and the normal-Se group was between the two (Fig. 2E). In addition, MPO activity was significantly higher in the LPS-stimulated mouse uterus than that of mice without LPS stimulation. And this also revealed that Se played a role in down-regulating MPO activity after LPS treatment (Fig. 2G). The MPO activity of the uterine tissue decreased gradually with the increase of tissue Se concentration. Furthermore, the pro-inflammatory cytokine level in the uterus was detected by using an ELISA kit. The ELISA result displayed that the IL-1β and TNF-α level was increased after challenging with LPS, while Se relieved inflammatory response in a dose-dependent way (Fig. 2H & I).3.3 Effects of Se on apoptosis in uterine tissue
TUNEL staining is a classic method for illustrating the degree of apoptosis in tissues at present. Apoptosis is a cell death process associated with inflammation. As shown in Fig. 3, almost no TUNEL stain occurred in the three groups without LPS stimulation (Fig. 3A–C). Apoptotic cells obviously increased in the LPS-stimulated tissues. In contrast, the number of apoptotic cells was sharply decreased with the Se concentration in a dose-dependent manner (Fig. 3D–F).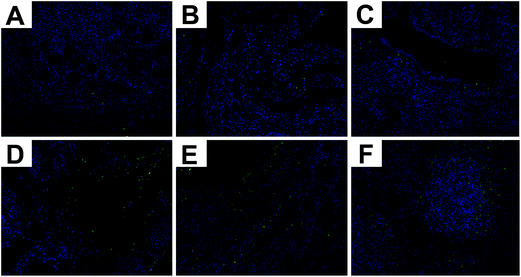 | ||
| Fig. 3 TUNEL staining of uterine tissue. (a) High-Se group, (b) normal-Se group, (c) low-Se group, (d) LPS + high-Se group, (e) LPS + normal-Se group, and (f) LPS + low-Se group. | ||
3.4 Effects of Se on the lipid raft concentration in the mouse uterus
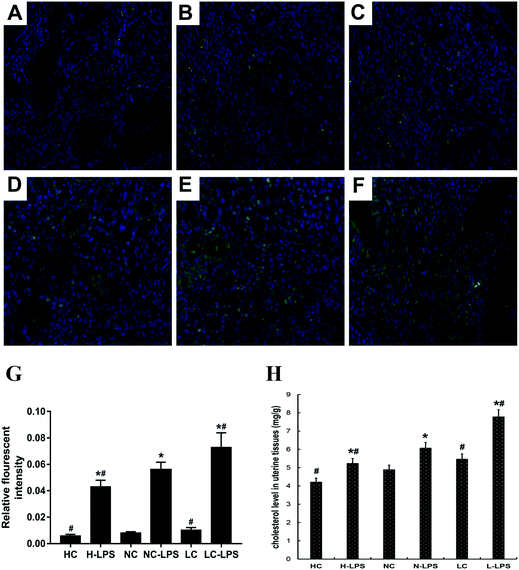
As is known to all, TLR4 is associated with the inflammatory process by translocation to lipid rafts to trigger the downstream signaling pathway. The content of lipid rafts in uterine tissues was determined by immunofluorescence staining. Lipid raft content in uterine tissues challenged with LPS aggressively increased compared to those without LPS stimulation (Fig. 4A–F). In addition, as the concentration of Se increased, the content of lipid rafts on the membrane dropped dramatically under LPS stimulation conditions (Fig. 4G). Moreover, the lipid rafts extracted from the low-Se group had the highest concentration of cholesterol, and the cholesterol content of the normal-Se group and high-Se group was respectively below it (Fig. 4H). Thus, Se had a negative regulatory effect on tissue lipid raft concentrations.3.5 Primary cell identification and effects of Se on cell viability
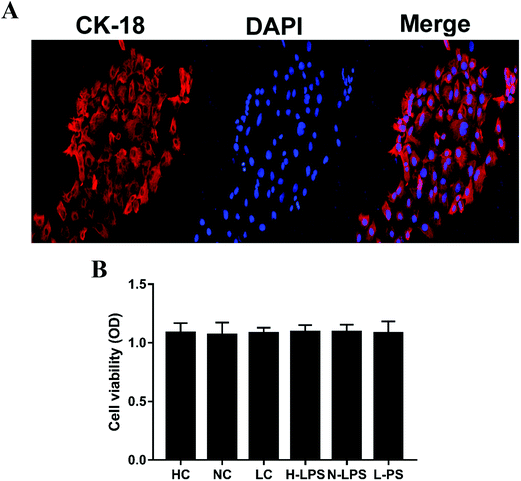
The mouse endometrial epithelial cells could be adherently grown after 4 or 5 hours of culture and 24 hours later, the cells could generate a monolayer. The endometrial epithelial cells could be labeled with keratin 18. Keratin 18 showed red fluorescence while the nucleus was stained blue under a laser confocal microscope (Fig. 5A). Likewise, the effect of Se on potential cytotoxicity was measured by MTT assay. As shown in Fig. 5B, cell viability was not affected in different Se concentration groups relative to the control group, illustrating that Se might have no cytotoxicity at the used dosage.3.6 Effects of Se on inflammatory cytokines in cells
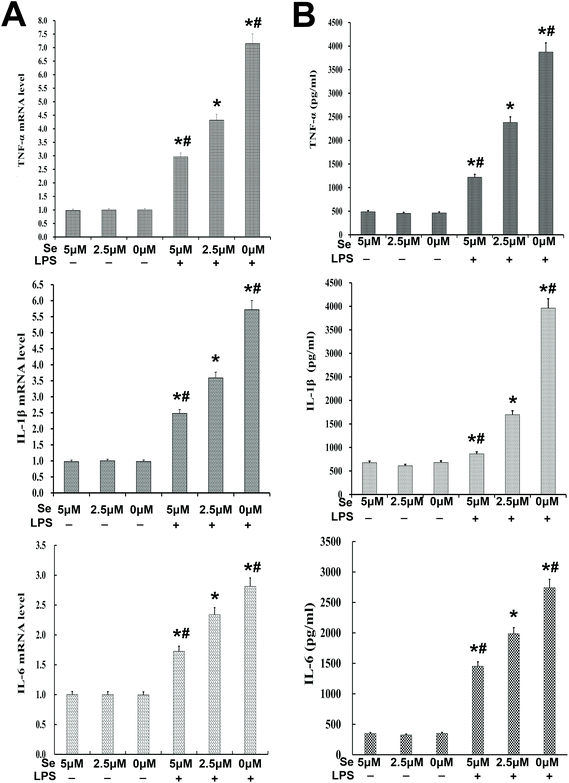
The expression of inflammatory cytokines, including IL-6, IL-1β and TNF-α, was examined with qPCR and ELISA. The results revealed that their production was reversed by Se in a dose-dependent manner (Fig. 6A and B).3.7 Effects of Se on TLR4 and its down-stream signaling pathway
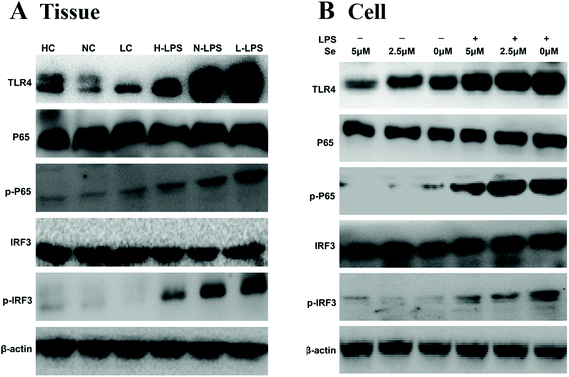
TLR4 recognizes and combines LPS to initiate its downstream signaling pathway, such as IRF3 and NF-κB. In order to investigate whether the anti-inflammatory effect shown by Se regulated the IRF3 and NF-κB pathway, the key proteins in the pathway were measured by western blot. The results in Fig. 7A showed a significant increase in TLR4, IRF3 and NF-κB expression levels under LPS stimulation in tissues. Meanwhile, Se restrained the activity of IRF3 and NF-κB with the challenge with LPS. In addition, TLR4 in lipid rafts was expressed little in the groups without LPS treatment regardless of the Se content in cells (Fig. 7B). The expression of IRF3 and NF-κB levels in cells was consistent with the result for the uterus.3.8 Effects of Se on the LxRα/ABCA1 pathway
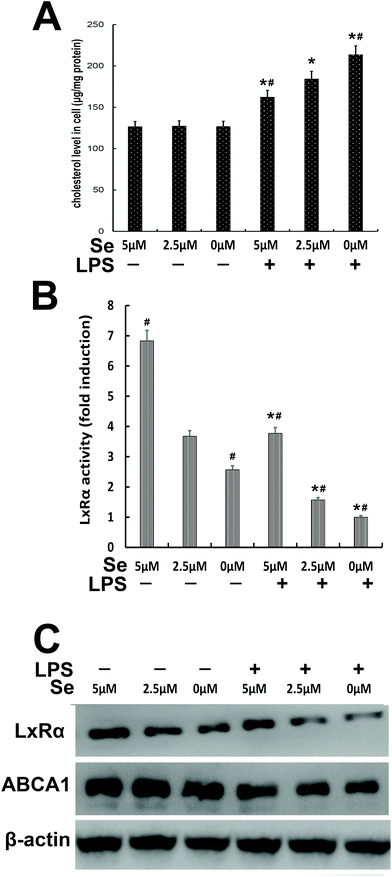
The content of cholesterol was measured in cells treated with different Se concentrations. The cholesterol level in the high Se group obviously decreased compared to the no-Se medium group when the cells were stimulated with LPS (Fig. 8A). The activity of LXRα was determined by luciferase reporter gene assay, whose activity significantly decreased under LPS challenge, and the more the amount of Se was supplied to the cells, the more the LxRα luciferase reporter gene was expressed (Fig. 8B). Moreover, western blot was performed to detect LXRα and ABCA1, and their level increased with the increase of Se-treatment concentration (Fig. 8C).3.9 Knockdown of LxRα to resist the inhibitory effects of Se on LPS-induced inflammation
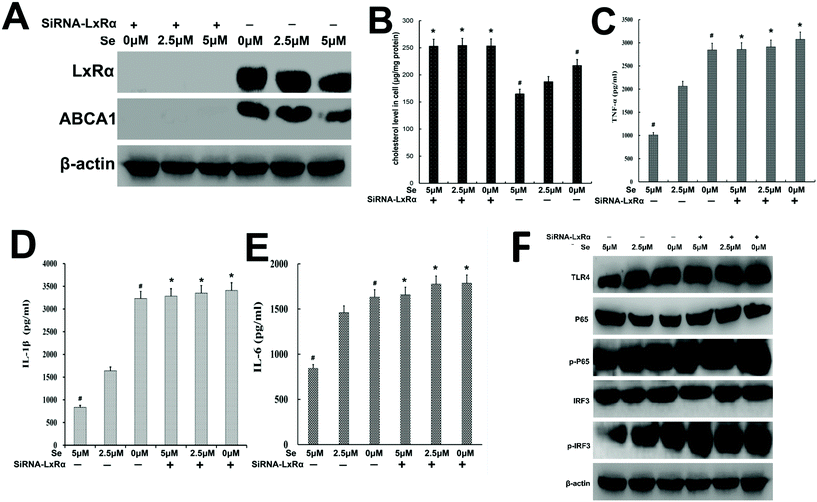
In order to explore whether Se exerted the anti-inflammatory function depending on the presence of LxRα, specific siRNA was used to silence the LxRα. We found that LxRα was strongly suppressed by siRNA (Fig. 9A). Furthermore, knockout of LxRα reversed ABCA1 expression, cholesterol content, inflammatory cytokines level, and TLR4-mediated pathway-related protein expression (Fig. 9B–F).4 Discussion
Endometritis is an inflammation that takes place in the endometrial tissue whose occurrence is closely related to the inflammatory factors released.1 Many previous research studies have reported that Se has a certain inhibitory effect on inflammatory response,17 and at the same time, it also shows an ability to degrade cholesterol.21 Our study indicated that Se cut down the inflammatory cytokine production by blocking TLR4 locating to lipid rafts and ultimately protected mouse endometritis from LPS-induced injury.Histological tests suggested that the normal physiological structure of the uterus was destroyed after LPS stimulation. And the degree of apoptosis was increased in the tissue. The addition of Se protects the uterus tissues from the above inflammatory injuries. MPO activity is used to demonstrate the neutrophil quantification in tissues to indirectly reflect the severity of inflammation. We found that MPO activity was significantly suppressed by Se in LPS-treated groups. We inferred that Se had an inhibitory effect on endometritis.
When the animal's uterus is invaded or stimulated by certain pathogens, the activated macrophages will secrete lots of inflammatory factors, promoting the body's defense function against the pathogens. The immune mechanism of the animal body will be activated and eventually, the inflammatory injury can be controlled or even eliminated. Many previous studies illustrated that the LPS treatment can increase the secretion of IL-6, IL-1β, and TNF-α, and these inflammatory cytokines are also of great importance in endometriosis.5,23 Our results suggested that Se could reduce their production in LPS-challenged mice and cells.
After TLR4 is stimulated and activated by LPS, NF-κB and IRF3 will be released24 as TLR4-mediated downstream signaling pathways and play an irreplaceable role in regulating inflammatory cytokines.25 We examined the influence of Se on p65 and IRF3 activation and the expression of TLR4 to clarify the anti-inflammatory mechanism of Se. The results in Fig. 7 indicated that Se suppressed the activation of p65 and IRF3.
TLR4, which acts as the primary receptor for LPS, will be transferred to the lipid raft after the stimulation with LPS, interact with the molecules located on the lipid raft and cause the activation of a series of downstream signaling pathways.26,27 Cholesterol and sphingomyelin-rich lipid rafts are dynamically variable microdomains on the cell membrane that play important roles in the TLR4 signaling pathway.19,20 The result in Fig. 8 indicated that Se can prevent TLR4 from recruiting and locating to lipid rafts, thus interfering with the development of inflammation. Meanwhile, we already know that Se can degrade cholesterol (Fig. 4 and 8). Once cholesterol, an important component of lipid rafts, is degraded, the structure of the lipid raft will also be disintegrated. Due to lipid raft reduction TLR4 cannot be anchored to the cell membrane and will not be able to recruit molecules to initiate signal transduction. Thus, blocking TLR4 can effectively inhibit the dramatic expansion of inflammatory response.
LxR regulates the proteins related to the formation and degradation of cholesterol to affect cholesterol metabolism in the body,28 including the plasma membrane protein ABCA1 in macrophages.29 Based on the results in Fig. 9, we can speculate that Se activated the LxRα-ABCA1 pathway to reduce the cholesterol content in cells and conversely regulated lipid raft production to alleviate inflammatory responses.
5. Conclusion
This study verified that Se inhibited inflammatory cytokine secretion induced by LPS treatment (Fig. 10). The specific anti-inflammatory effect of Se involved the degradation of cholesterol and upregulation of the LxRα-ABCA1 pathway, which led to the disruption of lipid raft formation and the impediment of TLR4 migration to lipid rafts. Furthermore, blocking of TLR4-mediated IRF3 and NF-κB signaling pathways downregulated the secretion of inflammatory cytokines, thereby assuaging inflammation caused by LPS stimulation. The results provided the basis for our idea of using Se to prevent or treat endometritis.Author contributions
Y. C., Y. Z. and M. G. conceived and designed the experiments. Y. C., Y. Z., M. Y., Y. W., J. Y. and H. J. carried out the experiments. Y. C., W. L., M. Y., Y. W. and J. Y. processed the data. Y. C., Y. Z., M. Y. and M. G. wrote the paper. The final manuscript was read and approved by all authors.Conflicts of interest
The authors declare that there is no conflict of interests.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 31502130) and the Fundamental Research Funds for the Central Universities (No. 2662014BQ024).References
- Z. Zhang, X. Gao, Y. Cao, H. Jiang, T. Wang, X. Song, M. Guo and N. Zhang, Selenium Deficiency Facilitates Inflammation Through the Regulation of TLR4 and TLR4-Related Signaling Pathways in the Mice Uterus, Inflammation, 2015, 38, 1347–1356 CrossRef CAS PubMed.
- E. J. Williams, D. P. Fischer, D. U. Pfeiffer, G. C. England, D. E. Noakes, H. Dobson and I. M. Sheldon, Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle, Theriogenology, 2005, 63, 102–117 CrossRef PubMed.
- A. Werner, V. Suthar, J. Plontzke and W. Heuwieser, Relationship between bacteriological findings in the second and fourth weeks postpartum and uterine infection in dairy cows considering bacteriological results, J. Dairy Sci., 2012, 95, 7105–7114 CrossRef CAS PubMed.
- K. Takeda, T. Kaisho and S. Akira, Toll-like receptors, Curr. Protoc. Immunol., 2003, 21, 335–376 CAS.
- Z. Wei, J. Wang, M. Shi, W. Liu, Z. Yang and Y. Fu, Saikosaponin a inhibits LPS-induced inflammatory response by inducing liver X receptor alpha activation in primary mouse macrophages, Oncotarget, 2016, 7, 48995 Search PubMed.
- T. Murai, Lipid Raft-Mediated Regulation of Hyaluronan-CD44 Interactions in Inflammation and Cancer, Front. Immunol., 2015, 6, 420 Search PubMed.
- J. Fu, Q. Shi, X. Song, Z. Liu, Y. Wang, Y. Wang, E. Song and Y. Song, From the Cover: Tetrachlorobenzoquinone Exerts Neurological Proinflammatory Activity by Promoting HMGB1 Release, Which Induces TLR4 Clustering within the Lipid Raft, Toxicol. Sci., 2016, 153, 303–315 CrossRef CAS PubMed.
- T. Zhuang, H. Xu, S. Hao, F. Ren, X. Chen, C. Pan and K. Huang, Effects of selenium on proliferation, interleukin-2 production and selenoprotein mRNA expression of normal and dexamethasone-treated porcine splenocytes, Res. Vet. Sci., 2015, 98, 59–65 CrossRef CAS PubMed.
- J. L. Li, R. Gao, S. Li, J. T. Wang, Z. X. Tang and S. W. Xu, Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium, BioMetals, 2010, 23, 695–705 CrossRef CAS PubMed.
- G. I. Harisa, O. M. Abo-Salem, S. M. El-sayed el and G. Shazly, Effects of nutritional and excessive levels of selenium on red blood cells of rats fed a high cholesterol diet, Biol. Trace Elem. Res., 2013, 152, 41–49 CrossRef CAS PubMed.
- M. Guo, X. Gao, N. Zhang, C. Qiu, C. Li and G. Deng, Effects of Se on the Diversity of SelT Synthesis and Distribution in Different Smooth Muscle Tissues in Rats, Biol. Trace Elem. Res., 2016, 170, 340–347 CrossRef CAS PubMed.
- H. D. Yao, Q. Wu, Z. W. Zhang, J. L. Zhang, S. Li, J. Q. Huang, F. Z. Ren, S. W. Xu, X. L. Wang and X. G. Lei, Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks, J. Nutr., 2013, 143, 613–619 CrossRef CAS PubMed.
- U. Schweizer, L. Schomburg and N. E. Savaskan, The neurobiology of selenium: lessons from transgenic mice, J. Nutr., 2004, 134, 707–710 CrossRef CAS PubMed.
- H. D. Mistry, F. Broughton Pipkin, C. W. Redman and L. Poston, Selenium in reproductive health, Am. J. Obstet. Gynecol., 2012, 206, 21–30 CrossRef CAS PubMed.
- T. Pan, X. Hu, T. Liu, Z. Xu, N. Wan, Y. Zhang and S. Li, MiR-128-1-5p regulates tight junction induced by selenium deficiency via targeting cell adhesion molecule 1 in broilers vein endothelial cells, J. Cell. Physiol., 2018, 233, 8802–8814 CrossRef CAS PubMed.
- X. Jin, T. Jia, R. Liu and S. Xu, The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-gamma/PI3K/Akt pathway in chicken pancreas, J. Hazard. Mater., 2018, 357, 355–362 CrossRef CAS PubMed.
- L. A. Seale and M. J. Berry, Selenium in human health and disease, Antioxid. Redox Signaling, 2011, 14, 1337–1383 CrossRef PubMed.
- M. Roman, P. Jitaru and C. Barbante, Selenium biochemistry and its role for human health, Metallomics, 2014, 6, 25–54 RSC.
- K. Nakahira, H. P. Kim, X. H. Geng, A. Nakao, X. Wang, N. Murase, P. F. Drain, X. Wang, M. Sasidhar, E. G. Nabel, T. Takahashi, N. W. Lukacs, S. W. Ryter, K. Morita and A. M. Choi, Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts, J. Exp. Med., 2006, 203, 2377–2389 CrossRef CAS PubMed.
- S. Rauch and O. T. Fackler, lipid rafts and signal transduction, Signal Transduction, 2007, 7, 53–63 CrossRef CAS.
- M. P. Rayman, S. Stranges, B. A. Griffin, R. Pastor-Barriuso and E. Guallar, Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial, Ann. Intern. Med., 2011, 154, 656–665 CrossRef PubMed.
- [Unkown], The Effects of Selenium Supplements on Blood Cholesterol Levels, Ann. Intern. Med., 2011, 154, I-32 Search PubMed.
- J. C. Kagan and R. Medzhitov, Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling, Cell, 2006, 125, 943–955 CrossRef CAS PubMed.
- Y. C. Lu, W. C. Yeh and P. S. Ohashi, LPS/TLR4 signal transduction pathway, Cytokine, 2008, 42, 145–151 CrossRef CAS PubMed.
- X. Hu, Q. Chi, Q. Liu, D. Wang, Y. Zhang and S. Li, Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-kappaB pathway and NLRP3 inflammasome in broiler thymus, Chemosphere, 2019, 237, 124427 CrossRef CAS PubMed.
- S. Olsson and R. Sundler, The role of lipid rafts in LPS-induced signaling in a macrophage cell line, Mol. Immunol., 2006, 43, 607–612 CrossRef CAS PubMed.
- S. Wang, Q. Chi, X. Hu, Y. Cong and S. Li, Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia, Ecotoxicol. Environ. Saf., 2019, 183, 109578 CrossRef CAS PubMed.
- P. A. Edwards, H. R. Kast and A. M. Anisfeld, BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis, J. Lipid Res., 2002, 43, 2–12 CAS.
- X. Zhu, J. S. Owen, M. D. Wilson, H. Li, G. L. Griffiths, M. J. Thomas, E. M. Hiltbold, M. B. Fessler and J. S. Parks, Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol, J. Lipid Res., 2010, 51, 3196–3206 CrossRef CAS PubMed.
Footnote |
| † These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |