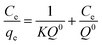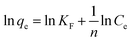Carotenoids, chlorophylls and phycocyanin from Spirulina: supercritical CO2 and water extraction methods for added value products cascade†
Stefania
Marzorati
 *a,
Andrea
Schievano
*a,
Andrea
Schievano
 a,
Antonio
Idà
b and
Luisella
Verotta
a,
Antonio
Idà
b and
Luisella
Verotta
 a
a
aDepartment of Environmental Science and Policy, Università degli Studi di Milano, via Celoria 2, 20133 Milano, Italy. E-mail: stefania.marzorati@unimi.it; Tel: +390250314114
bAlgaria srl, via Tucidide 61, 20133 Milano, Italy
First published on 21st November 2019
Abstract
In the last decade, the cyanobacterium Spirulina has gained a high commercial interest as a food supplement, mainly due to its high protein content as well as high amounts of pigments, such as carotenoids, chlorophylls and phycocyanins. In particular, phycocyanin has been widely considered as a precious food-dye because of its protein-based structure and the rare intense-blue color. Different strategies were developed for the isolation and purification of phycocyanin. The main drawback of such processes is that carotenoids and chlorophylls are generally wasted together with the residual biomass. In this study, a different approach is proposed, suggesting an integrated pigment extraction chain. The body of the strategy involves two consecutive steps of the supercritical-CO2 extraction of carotenoids and chlorophylls, before phycocianin extraction. The total carotenoid, chlorophyll a and chlorophyll b contents in the extracts were equal to 3.5 ± 0.2 mg g−1, 5.7 ± 0.2 mg g−1 and 3.4 ± 0.3 mg g−1, respectively (by dry Spirulina weight). The biomass residue, exhausted in terms of carotenoids and chlorophylls, was then extracted in water to yield phycocyanin. Consecutive steps were developed in order to ehance the phycocyanin purity, including electrocoagulation, dialysis and protein salting-out. These processes yielded 250 mg g−1 of phycocyanin (by dry Spirulina weight). A potentially scalable strategy to obtain the blue pigment with high purity (A620/A280 = 2.2) was developed. The practical application of the extracted blue phycocyanin pigment as a cotton-based tissue colorant was also experimented.
Introduction
In the ninth century, the Kanem empire in Chad already discovered the benefits of Arthrospira platensis.1,2 Nowadays, the blue-green algae of the genus Arthrospira, commonly known as Spirulina, are commercially grown all around the world for their nutritional properties.3 The popularity of Spirulina as a food supplement is mainly due to its high protein content (up to about 70% by dry weight) and its richness in minerals, vitamins and provitamins, phytochemicals, essential amino acids, fibers and pigments.4 Spirulina contains distinctive natural orange, green and blue pigments, namely carotenoids, chlorophylls and phycocyanins, respectively. Spirulina commercialization has aligned year by year with the consumer awareness regarding the importance of natural agents.5 Obtaining pigments from natural sources is in fact a well-known strategy due to legislation restrictions in terms of synthetic dyes.6Carotenoids are natural lipophilic pigments responsible for the red, yellow, and orange colors found in many living organisms. They are popular as food and feed dyes and flavorings as well as nutritional supplements.5 Moreover, Spirulina may contain about 2 wt% of chlorophylls, which is ten times more than that found in ordinary terrestrial plants and hence accounts for an enhanced photosynthetic conversion efficiency equal to 8–10% in comparison to land plants, which possesses just a 3% conversion efficiency.7 Since chlorophyll derivatives are heat-, light-, acid- and base-stable, they are potentially applicable in food, cosmetics, and pharmaceutical fields as additives or colorants.8 According to numerous studies, the free radical-scavenging and strong antioxidant activities of Spirulina are mostly attributable to the blue-colored phycocyanin. The extraction, isolation and purification of phycocyanin have been the focus for many years, resulting in diverse strategies leading to different results.4,9–14
Many works in literature have been focusing specifically on the phycocyanin extraction from Spirulina biomass4,9–17 due to the high commercial value of phycocyanin.18,19 The main drawback of these processes is the discard of the remaining added-value pigment content, such as carotenoids and chlorophylls as waste in the biomass residues.
In this study, a different approach is proposed, suggesting an extraction chain that only leads to phycocyanin at the end. The body of the strategy involves a double-step extraction of carotenoids and chlorophylls using supercritical-CO2 (scCO2) with minimized modifications on the biomass. The biomass residue, exhausted in terms of lipophilic compounds and chlorophylls, is ready to undergo the extraction of phycocyanin.
The absence of any heating or destructive steps allows the reuse of the scCO2-treated Spirulina biomass for a further extraction step in aqueous media, aiming at obtaining phycocyanin. On the basis of recent and past literature on the topic, a strategy to yield the blue pigment with high purity was finally developed, keeping an eye on the scalability of the overall process in terms of cost and time consumption.9,20,21 The practical application of the extracted blue phycocyanin pigment as a cotton-based tissue colorant was also experimented.
Materials and methods
Chemicals and starting materials
Milli-Q water was obtained from an Elix Essential Millipore SAS water system. NH4Cl (BioUltra, for molecular biology, ≥99.5%), (NH4)2SO4, KAl(SO4)·12H2O, Na2CO3 and ethanol (HPLC grade) were purchased from Sigma-Aldrich. A carbon dioxide tube (CO2 purity 5.0) was purchased from Sapio, Italy. A dialysis tubing cellulose membrane was purchased from Sigma-Aldrich. All the extracts were analyzed as soon as they were obtained and then kept at 4 °C in the dark.Spirulina platensis dried flakes (Spireat®) were obtained from Algaria slr (Italy) and stored at 4 °C in the dark. Phycocyanin obtained from Sigma-Aldrich (12.3 mg ml−1, suspended in 150 mM sodium phosphate, 60% ammonium sulfate, 1 mM EDTA, 1 mM sodium azide, pH 7.0), labelled as PCstd, was used as the standard to generate a calibration curve in the PCstd concentration range: 20–150 μg ml−1 (water solutions).
Supercritical CO2 extractions of carotenoids and chlorophylls
Supercritical fluid extractions were performed using a pilot unit SFT110XW System supplied by Supercritical Fluid Technologies, Inc. (USA). It consisted of a 100 cm3 stainless steel extractor inserted in an oven, an SFT10 CO2 pump with a Peltier Cooler, a Waters 515 HPLC pump for the co-solvent and a collection vessel.Spirulina platensis dried flakes were ground into powder (about 50 μm particle size) using a kitchen blender for 1 min at the maximum velocity. To avoid powder heating during blending and the consequent degradation of thermolabile species, liquid nitrogen was added.
The extraction vessel was filled with 31.4 g of spirulina powder. The system was sealed, and the oven and restrictor block temperatures were set at 45 °C and 70 °C, respectively. Fig. 1 displays the picture of the scCO2 extraction instrument. Some experimental trials, reported in Table S1,† based on literature studies22,23 were performed in order to assess the best procedure. This was performed in consecutive steps, keeping the biomass inside the vessel. A larger scale extraction would need the support of a mathematical model in order to really optimize the parameters.24
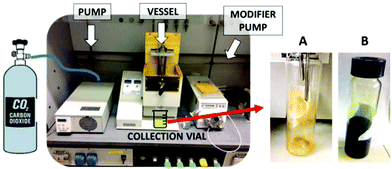 | ||
| Fig. 1 Pictures of the instrumental setup (left) and collection flasks during (A) scCO2 extraction and (B) scCO2 + 10% ethanol extraction. | ||
Carotenoid extraction by scCO2: the pump pressurized the sample vessel at 300 bar at a flow rate of 15 ml min−1. Once the set pressure was reached, after a static period of 30 min, the valves were opened to collect the sample for 1 h in dynamic conditions (CO2 flow rate of 15 ml min−1). The vessel was then depressurized. The biomass was kept inside the vessel for the next extraction of chlorophylls.
Chlorophyll extraction by scCO2 + 10% EtOH: 10 ml of ethanol was loaded into the vessel before the pressurization at 300 bar at a flow rate of 15 ml min−1. Once the set pressure was reached, after a static period of 30 min, valves were opened to collect the sample for 1 h in dynamic conditions. During the dynamic extraction, an ethanol flow rate of 1.5 ml min−1 was maintained (CO2 flow rate of 13.5 ml min−1). The vessel was then depressurized and the residual biomass was collected.
The following eqn (1), (2) and (3), proposed by Wellburn,25 were used to determine the concentration of total carotenoids, chlorophyll a and chlorophyll b in the samples from visible spectra acquired through a Varian Cary 50 Bio UV-Vis spectrophotometer:
 | (1) |
| Cchlorophyll a (μg ml−1) = 15.65A666 − 7.34A653 | (2) |
| Cchlorophyll b (μg ml−1) = 27.05A653 − 11.21A666 | (3) |
UPLC-MS methods for carotenoids identification
The scCO2 extract composition was analyzed via UPLC using a Dionex Ultimate 300 equipped with a UV detector. The separation was performed using an ACQUITY UPLC® BEH C18 column (1.7 μm, 2.1 × 50 mm). The mobile phase was a mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 70
methanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 at a flow rate of 0.2 ml min−1 in an isocratic mode for 10 min. 10 μl of a methanolic solution of the extract was filtered (0.2 μm nylon filters), injected and monitored at 445 nm. Chromatographic separation was followed by a mass spectrometry (LCQ Fleet Thermofisher) analysis. A positive electrospray mode was used for the ionization of molecules with a capillary voltage of 80 V, at a capillary temperature of 275 °C. The heater temperature was set at 80 °C, the gas flow rate was 35 (arb) and the spray voltage was 5.50 kV. The monitored mass range was from m/z 50 to 1000. Before sample injection, mass spectrometry parameters were optimized using a standard of beta-carotene (purity >97%) from Sigma.
30 at a flow rate of 0.2 ml min−1 in an isocratic mode for 10 min. 10 μl of a methanolic solution of the extract was filtered (0.2 μm nylon filters), injected and monitored at 445 nm. Chromatographic separation was followed by a mass spectrometry (LCQ Fleet Thermofisher) analysis. A positive electrospray mode was used for the ionization of molecules with a capillary voltage of 80 V, at a capillary temperature of 275 °C. The heater temperature was set at 80 °C, the gas flow rate was 35 (arb) and the spray voltage was 5.50 kV. The monitored mass range was from m/z 50 to 1000. Before sample injection, mass spectrometry parameters were optimized using a standard of beta-carotene (purity >97%) from Sigma.
Phycocyanin extraction
30.0 g of the biomass residue in the vessel, post scCO2 extraction, underwent phycocyanin extraction optimization trials in water (see ESI†).Then, in order to eliminate the suspended biomass residues, an electrocoagulation method was performed on 50 ml of the prepared suspension by immersing two Al electrodes (3 cm × 3 cm) at a distance of 3 cm inside the liquid. A potential difference of 14 V (0.8 A) was set between the electrodes for 2 min by a power supply (Tech Star®, TPR3005-2D). An ice bath was used to avoid any temperature increase. A cotton tissue was enough to filter away the vegetal residue out of the blue solution.
The blue filtrate was forced to pass through a 12 mesh paper by a peristaltic pump in order to get rid of the remaining cell debris suspended in the solution.
Phycocyanin purification methods
Dialysis: A lab-scale dialysis tubing cellulose membrane with a cutoff at 15 kDa was employed. The membrane was pre-treated as follows: tubes were washed in running water for 3–4 hours, then with hot water (60 °C) for 2 minutes, followed by acidification with a 0.2% (v/v) solution of sulfuric acid; then, they were rinsed with hot water to remove the acid.1 ml of the sample solution was loaded inside the membrane. 50 ml of Milli-Q water was placed in external contact with the dialysis membrane and replaced three times every 24 hours. External solutions and the solution inside the dialysis membrane were lyophilized (Edwards, Pirani 1001).
Ammonium sulfate precipitation: the previously lyophilized powder was dissolved in a minimum amount of Milli-Q water (45 mg ml−1). Ammonium sulfate powder was slowly added to the solution with continuous stirring until it reached 24%, 39% or 50% of its saturation. After standing in the fridge for 2 h, the solution got turbid and it was centrifuged for 30 min at 6000 rpm (Hettich Rotofix 32A). A blue pellet was collected while the light blue supernatant was discarded.
Phycocyanin purity determination
The purity of phycocyanin was evaluated spectrophotometrically on the basis of the ratio between the phycocyanin absorbance at 620 nm (A620) and the absorbance from aromatic amino acids in all proteins at 280 nm (A280). As described by Rito-Palomares et al.,18 phycocyanin preparations with A620/A280 lower than 0.7 are considered to be food grade, while those with A620/A280 between 0.7 and 3.9 are reagent grade and those with A620/A280 greater than 4.0 are considered to be analytical grade.Application of Phycocyanin extract as blue dye for tissues
Two different cotton fibers were experimented: commercial sterile gauzes and a cotton bed linen. In separate beakers, a weighed amount of each tissue was immersed in Milli-Q water containing 20% w/w of KAl(SO4)·12H2O (calculated on the basis of the weight of the tissue) as a mordant and 6% w/w of Na2CO3. The temperature was increased up to 100 °C and kept for 1 h. Then, tissues were rapidly rinsed with Milli-Q water and dried at room temperature.Six weighed pieces of each type of tissue were put in contact for 1 h with aqueous solutions of different amounts of phycocyanin to develop adsorption isotherms (from 24 to 1 μgphycocyanin g−1tissue). After this time, tissues were extracted from the solution, rinsed and dried at room temperature. The absorbance at t = 0 and t = 1 h was measured at 620 nm for each solution and the absorption isotherms were developed. TIMCAL ENSACO activated carbon was employed as a reference adsorption material.
Colored tissues were rinsed using tap water after 1, 2 and 3 weeks after the dying procedure.
Results and discussion
sc-CO2 extraction of carotenoids
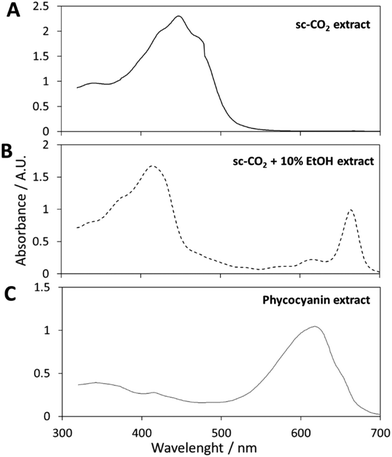
High contents of carotenoids (β-carotene, cryptoxanthin, and zeaxanthin among others) were reported for Spirulina, as compared to other natural sources.26 Their relative percentages may vary with Spirulina growth, environmental conditions, processing, etc. El-Baky et al. grew Spirulina in the presence of different concentrations of nitrogen while varying the ionic strength of the growth medium. These factors were responsible for the variations in the relative percentages of carotenoids.27 scCO2 has been successfully used for the isolation of carotenoids from various fruit and vegetables matrices in the past and in more recent literature.28–31 This technique is able to provide extracts that are totally free of organic solvents (avoiding oxygen contact) at a relatively low critical temperature and pressure. Since various parameters potentially affect the scCO2 extraction process, the optimization of the experimental conditions represents a critical step in the development of the method. Based on preliminary experiments (reported in Table S1†) and literature data,22,23 some operational parameters were optimized. 300 bar and 45 °C were set as optimized process parameters. Pressures lower than 300 bar were not high enough to yield carotenoids-enriched extracts. A temperature increase from 45 °C to 55 °C did not allow any significant yield improvement. No organic modifier was added since scCO2 alone displays a solvating power similar to n-hexane, hence being able to dissolve lipophilic compounds, such as carotenoids.32After the static period, the deep orange extract was collected in a weighed flask in dynamic conditions. The extraction has been performed under supercritical CO2 and the extracts have been analysed as soon as they were obtained, implying that oxygen has never influenced the biomass or the extracts. This is also valid for light and temperature, since the extracts have been stored at 4 °C in the dark.
Fig. 1A shows the picture of the obtained extract: a deep orange viscous extract (with red, darker spots) was collected after 30 min, corresponding to an extraction yield of around 0.5%. The visible spectrum of carotenoids-enriched extract is displayed in Fig. 2A. By applying the previously mentioned eqn (1), (2) and (3), Ca and Cb were approximated to zero, in agreement with the hypothesis that no chlorophylls were extracted under these conditions. The concentration of carotenoids was equal to 3.5 ± 0.2 mg g−1 of dry spirulina biomass. These results are in agreement, in terms of orders of magnitude, with the data reported by Careri et al. They employed supercritical fluid extraction of carotenoids in Spirulina pacifica algae.33 1.7 mg g−1 of carotenoids (zeaxanthin, β-cryptoxanthin and β-carotene) could be extracted from the biomass through a chemometric approach. The same authors obtained similar values via a comparison with a standard method based on conventional solvent extraction, confirming the efficacy of selected supercritical conditions. While assessing the suitability of scCO2 to extract carotenoids, similar results were obtained by Macías-Sánchez et al.34 They performed a conventional methanol extraction and their results were in agreement with what obtained though scCO2 optimization trials.
 | ||
| Fig. 2 (A) Visible spectrum of the carotenoid extract; (B) visible spectrum of the chlorophyll extract; (C) visible spectrum of the phycocyanin extract. | ||
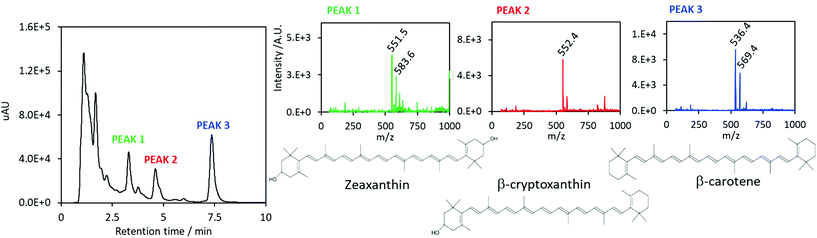
The composition of the carotenoids-enriched extract has been further investigated by UPLC-MS analysis. The UV-Vis chromatogram is displayed in Fig. 3. The identified peaks, which are highlighted in the figure, correspond to zeaxanthin (a retention time of 3.3 min), β-cryptoxanthin (a retention time of 4.6 min) and β-carotene (a retention time of 7.4 min). The assignments were confirmed by mass spectrometry results. Peak 1 is characterized by two signals at m/z = 551.5 and m/z = 583.6, corresponding to zeaxanthin [M − H2O + H]+ and [M − H2O + MeOH + H]+ species. Regarding peak 2, the main signal at m/z = 552.4 corresponds to the β-cryptoxanthin [M]+˙ molecular species; about peak 3, the main signal at m/z = 536.4 corresponds to β-carotene [M]+˙, the secondary signal at m/z = 569.4 might correspond to the coordination of methanol molecule. Results are in agreement with literature data.35,36
 | ||
| Fig. 3 Left: UV-Vis chromatogram of the carotenoids-containing extract by scCO2. Left: Mass spectrometry analyses and molecular structures of assigned carotenoids. | ||
sc-CO2 extraction of chlorophylls
Usually, chlorophyll a is extracted from raw materials based on solvent extraction.37 Although the conventional process has been well investigated, drawbacks, like the high extraction temperature, long extraction time, large amounts of solvents, and low extraction yield claim the need for alternative techniques. Nevertheless, only few investigations are available on the extraction of chlorophyll a using scCO2. Some works in the literature employed a co-solvent to enhance the supercritical fluid's solvating power towards the molecule.38,39Carotenoids-free spirulina biomass underwent scCO2 extraction with the addition of ethanol as a co-solvent, to enhance the extraction yield towards more polar compounds.
The experimental parameters, such as temperature and pressure, as well as the static and dynamic periods in supercritical conditions, have been kept equal to those of the scCO2 carotenoid extraction process.
This approach was adopted to shift the target compounds to be extracted from carotenoids to chlorophylls. 10% of ethanol was selected as the co-solvent amount. A further increase of the ethanol percentage did not improve the extraction yield, meaning that the chlorophylls have been completely extracted by using 10% of co-solvent (Table S1†). The collected extract solution (in ethanol) was dark green in color, as displayed in Fig. 1B. A visible spectrum was recorded and it is displayed in Fig. 2B. Chlorophyll a displays maxima at wavelengths of 400–450 nm and 650–700 nm, while the absorption spectra of chlorophyll b are at 450–500 nm and 600–650 nm.40
The total concentration of carotenoids and chlorophylls was determined by measuring the absorbance of a weighed amount of sample in the range of 320–700 nm and applying the Wellburn equation. As expected, this extract did not contain any carotenoid, and Ctotal carotenoids was approximated to zero. This interesting result confirmed the high selectivity of the method.
Chlorophyll a content was calculated to be equal to 5.7 ± 0.2 mg g−1 (by dry Spirulina weight). Chlorophyll b content was calculated to be equal to 3.4 ± 0.3 mg g−1.
Tong et al. ran single-factor and response surface analysis experiments for the supercritical fluid extraction of chlorophyll from Spirulina platensis. Our work is in agreement with their results: they found out the necessity of adding a modifier in order to obtain the desired product. Their comparison experiments demonstrated that the scCO2 extraction with a modifier was more efficient than the conventional solvent methods. Their results are also in numerical agreement with the present work (their extraction yield was 6.84 mg g−1).38 Macías-Sánchez et al. recovered only a low amount of chlorophylls as compared to the results obtained in this work due to the absence of any modifier in the extraction process.39
All the mentioned works that are already published in literature specifically focused on a class of target compounds, such as chlorophylls or carotenoids. For them, methanol was also suggested as a modifier. However, solvents like methanol, which are prohibited in industrial processes, has been disregarded in this work in the view of potential scale-up applications.
Table 1 summarizes the results in terms of carotenoid and chlorophyll a and b contents from Sprirulina biomass by supercritical carbon dioxide (first) with and (then) without ethanol as a modifier. Results confirm the selectivity of the technique: supercritical CO2 alone was able to selectively extract carotenoids, while the addition of a co-solvent under the same operational conditions exclusively provided chlorophylls (a and b). By adjusting the successive operational parameters, the technique is able to extract valuable compounds one by one from the biomass, without the need for purification steps.
| scCO2 operative conditions | C total carotenoids/mg g−1 (by dry Spirulina weight) | C chlorophyll a/mg g−1 | C chlorophyll b/mg g−1 |
|---|---|---|---|
| T vessel = 45 °C | 3.5 ± 0.2 (86% purity) | — | — |
| p = 300 bar | |||
| T vessel = 45 °C | — | 5.7 ± 0.2 (63% purity) | 3.4 ± 0.3 (37% purity) |
| p = 300 bar | |||
| +10% ethanol |
For carotenoid and chlorophyll extraction, supercritical CO2 (scCO2) has to be preferred for its immediate advantages over traditional solvent-based techniques.41
It allows the efficient and selective extraction of lipophilic compounds with no need of concentration steps, providing high purity products while avoiding the use of potentially harmful organic solvents.41
The addition of a co-solvent (for example ethanol) in a relatively low mixing ratio with scCO2 is able to modify its polarity, allowing the extraction of more polar molecules.42,43 Moreover, the operative temperatures are low enough to avoid the degradation of thermolabile substances. Finally, CO2 is easily removed from the solid at ambient conditions and/or, in an industrial scale, it can be recovered through a specific apparatus for its fresh reuse. Literature results show a substantial advantage with respect to the conventional extraction in terms of the ease of recovery, selectivity, compounds’ stability, time, and overall energy saving.22
Extraction of phycocyanin
Starting from a commercial standard product (PCstd), a calibration curve was generated in order to calculate the phycocyanin content in further extraction solutions. The best fit of experimental data in the plot of “A620vs. PCstd concentration” (A620 is the absorbance at 620 nm and PCstd concentration is in μg ml−1) was, as expected, a straight-line, represented by the following mathematical equation: y = 0.0086x − 0.0246, resulting in a R2 equal to 0.999. The slope m and intercept q of the regression line with their respective standard deviations were (0.0086 ± 0.0001) and (−0.025 ± 0.006), respectively. The calculated equation of the regression line was then employed to determine the phycocyanin concentration in sample solutions (see Fig. S10† for experimental data).Fundamental units of phycocyanin protein are α and β subunits, arranged in (αβ) protomers. These structures, in turn, can be associated into trimers (α β)3 and hexamers (α β)6. For this reason, phycocyanin molecular weight lies between 18 kDa (ref. 44) (considering single units) and 210 kDa (ref. 9) (considering the hexamers). By using an average of the most frequently encountered molar masses of phycocyanin, which is equal to 120 kDa, the molar extinction coefficient ε was calculated to be equal to 1.0 × 106 cm−1 M−1 at 620 nm, which is in agreement with the literature.45
The two-step scCO2 process, first yielding carotenoids and then chlorophylls, was able to keep the phycocyanin content of the Spirulina biomass unchanged. In fact, the grinded powder was deprived of its orange and green pigments, appearing enriched in blue color after scCO2, mainly due to the removal of chlorophylls, which is responsible of its typical green aspect. Fig. 4A shows the Spirulina powder before and after the scCO2 extraction step.
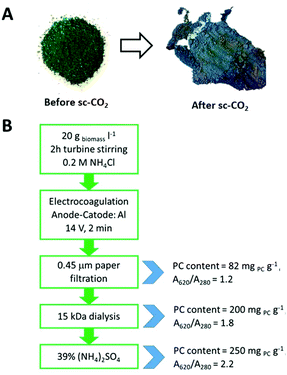 | ||
| Fig. 4 (A) Grinded Spirulina powder before and after scCO2 extraction processes; (B) scheme of the optimized steps in phycocyanin extraction and purification. | ||
Spirulina biomass that was recovered after scCO2 was then re-used to extract phycocyanin by aqueous methods. Optimization trials are reported in the ESI.† Briefly, water stirring and sonication have been first experimented and compared: ultrasound was found to excessively disrupt cells, hence yielding less pure extracts (see Fig. S4†). The freezing and thawing method did not provide any advantage compared to water stirring and hence was abandoned, being less simple and more time-consuming. pH variations seemed to be the key point: stirring the suspension in an ammonium chloride solution not only enhanced the yield (in comparison to alkaline pH solutions), but was also able to provide purer extracts, lacking in the species absorbing between 400 and 500 nm (Fig. S7†) that could badly affect the intense blue color of the phycocyanin solution.
For these reasons, turbine stirring for 2 hours in 0.2 M NH4Cl of 20 g l−1 biomass dispersion was set as the best extraction strategy. Ammonium chloride was found to be an effective and suitable salt solution for enhancing the phycocyanin purity in crude extracts. Furthermore, it reduces the extraction cost because it is cheaper than other salts.11
Separation of suspended solids
After extraction, in order to develop a scalable procedure that could help in removing a great part of suspended solid residues, one of the most cost-effective techniques in algae harvesting was tested: electrocoagulation. In electrocoagulation, flocculation is induced through the electrolytic release of metal ions from a sacrificial anode and avoids the contaminations of anions. The operating cost of such a technique is typically below 0.04 € m3.46 The key for this technology is the application of a current between a sacrificial anode (in this case aluminum) and an inert cathode (made of aluminum as well as the anode). Aluminum ions are released from the anode, and they are able to neutralize the negative surface charge of the alga. In addition, oxygen and hydrogen bubbles are also produced by the electric current, thus promoting turbulence and helping the flocculation process. Electrocoagulation was indeed effective and a majority of cell fragments could be easily removed simply by filtration on a gauze.However, electrocoagulation was not exhaustive in removing the smallest fraction of particulate residues of the cell walls from the solution. Depending on the specific use of the pigment (for example if the pigment application is related to food printing, where a channeling inside a needle is necessary), a further filtration might be necessary. Notwithstanding the large number of publications in the field of phycocyanin extraction, filtration topic has never been comprehensively explained in literature. Many works employed ultrafiltration16,18,47 on lab-scale experiments. However, this method is expensive and hence hardly applicable in large scale. Here, the balance between effectiveness, large scale applicability and costs was realized using a 12-mesh paper filtration procedure under pressure. Paper filters are in fact the cheapest disposable material in commerce and can be easily replaced when clogged.
After the filtration, the obtained solution showed a phycocyanin content and a purity of 82 mg g−1 (by dry weight) and A620/A280 = 1.2, respectively. A visible spectrum of the phycocyanin solution is displayed in Fig. 2C.
A non-negligible amount of the blue fraction was lost during the filtration procedure due to the adsorption on membranes, which was about 8% of the phycocyanin content.
Phycocyanin purification
The consecutive steps performed to achieve high purity phycocyanin are effective but also time consuming. With this awareness, different levels of purification are proposed, so that once the purity is enough to satisfy the needs of a specific application, the next steps can be omitted.The next step involved the protein purification by a dialysis membrane. Dialysis is not frequently adopted in literature, however it displays many advantages compared to other purification methods in terms of cost saving.48 It could be an interesting option to concentrate phycocyanin, while washing out ammonium chloride and other solutes with molecular dimensions below 15 kDa. Dialysis was performed against Milli-Q water to maximize the osmotic pressure and without the addition of other chemicals. From the UV-Vis spectrum, the phycocyanin content and the purity were 200 mg PC g−1 (calculated on dry weight) and A620/A280 = 1.8, respectively. The phycocyanin content was enhanced by more than a double, while increasing the purity by 50%.
As a further step to improve PC purification, ammonium sulfate precipitation was employed for protein salting-out. The mechanism of salting-out is based on preferential solvation due to the exclusion of the salt ions from the layer of water closely associated with the hydration layer of the protein. When a salt is added to the solution, the surface tension of the water increases, enhancing the hydrophobic interactions between the protein and water. The protein reacts by decreasing its surface area in an attempt to minimize its contact with the solvent, resulting in folding and self-association, leading to precipitation.49
Different ammonium sulfate concentrations were experimented: 24%, 39% or 50% of ammonium sulfate saturation. After centrifugation, the blue pellet was collected and characterized. Table 2 summarizes the results. The optimized percentage of ammonium sulfate was identified as 39% of its saturation, since reaching 50% of its saturation did not significantly improve either the phycocyanin content or its purity.
| % Saturation (NH4)2SO4 | PCcontent/mg PC g−1 (by dry weight) | A 620/A280 |
|---|---|---|
| 24 | 217 | 1.9 |
| 39 | 250 | 2.2 |
| 50 | 252 | 2.2 |
Overview on extractions
In the first two steps of the process, carotenoids and chlorophylls were extracted in supercritical conditions in 180 min (90 min for carotenoids and 90 min for chlorophylls). The use of supercritical CO2 is intrinsically a time-saving procedure since it is more selective compared to traditional extraction methods with organic solvents.Fig. 4B shows the operational scheme summarizing the procedure steps for the phycocyanin extraction. The overall diagram leads to a phycocyanin product with a middle-high grade content and purity, as described by Rito-Palomares et al.18 In fact, phycocyanin preparations with A620/A280 between 0.7 and 3.9 are considered of reagent grade. Hence, the proposed extraction strategy is suitable for larger scale applications. When the purity requirement is specified, the goal of the process design is to obtain the maximum recovery at the lowest cost.50
Depending on the need for the purity level of the phycocyanin extract for a specific application, some of the proposed steps can be omitted, which would save time and energy costs. The 12-mesh paper filtration is enough to provide a purity of about 1.2, which lies in the reagent grade range; also, the final product can undergo dialysis and protein salting-out as additional steps. If the procedure stops after electrocoagulation, a blue water solution of phycocyanin is obtained in 120 min. If the purity needs to be further enhanced by dialysis, this needs three more days; if the ammonium sulfate precipitation is performed, this adds 150 minutes more. The time needed to achieve a satisfying purity extract strongly depends on the specific application.
Talking with numbers, the procedure presented in this work started with 31.4 g of Spirulina powder and provided a blue powder with a phycocyanin content of 7.85 g.
The added value of the process presented in this work is first related to the preliminary extraction of orange and green pigments, which are usually wasted in the discarded biomass. Second, the chosen processes in the phycocyanin extraction were selected setting apart, or at least minimizing costly steps. Moreover, the overall process, yielding middle grade phycocyanin, lasts only few hours. The cost of phycocyanin is frequently determined by the quality (measured in terms of purity and concentration), which is strongly dependent on the steps used in the process. The minimization of production steps contributes to a reduction in process costs and an increase in product yield. For instance, electrocoagulation and dialysis are already widely applied in industry and known to be easy-to-perform steps.46,51,52 The paper filtration is preferred over ultrafiltration and other more technological methods in terms of time and cost saving. The commercial price of phycocyanin varies with the increasing purity index; reagent grade phycocyanin, as the one prepared in this study, was reported to cost from US 1–5 $ per mg.11 Through the strategy proposed here, phycocyanin can be obtained without a considerable product loss, maintaining a high protein recovery while potentially being able to reduce both processing cost and time. The finally discarded biomass, containing only vegetable residues and salts, has no more any added-value content.
Few works in literature have claimed the development of large-scale methods for phycocyanin extraction from algal biomass. Some authors reported the extraction by osmotic shock, followed by purification by chromatographic techniques.9,20,21 Their final products displayed purities around 5. The use of chromatography was avoided in the present work for the sake of robustness, cost-effectiveness and to keep the procedure as easy applicable as possible.
As a last mention, Gupta et al. employed chitosan or charcoal for further phycocyanin purification steps.53 This method was also experimented in the present work. Although chitosan and charcoal are effective for the purification procedure, they are responsible for the loss of large amounts of phycocyanin due to the adsorption on the material itself. For this reason, chitosan and charcoal have not been further considered in the present work.
Application of Phycocyanin extract as blue dye for tissues
The first regulation in the field for dye additives was introduced in 1904 by the United States. Since then, a strict evaluation process of dyes was adopted by the Food and Drug Administration (FDA).8 The European Union followed a similar approach through the European Food Safety Authority (EFSA). The law is very restrictive concerning color additives but it has been favoring the use of natural or “nature identical” colorants.54 The resulting growing awareness of the importance of natural colors, especially in food and cosmetics colorants, accounts for the tendency in food manufacturing to use natural additives.Due to their renowned pigment contents, Spirulina and microalgae in general are perfectly able to answer these commercial expectations with the added value of displaying health-beneficial effects, in many cases.
The need to overcome the potential toxicological effect of synthetic dyes is a key issue in the coloring processes. Rose et al. extracted anthocyanins from blackcurrant waste from the fruit pressing industry and studied their adsorption on hair.55 In this study, a similar approach was adopted to investigate the sorption properties of the blue phycocyanin pigment as a tissue dye. The dye absorption from the phycocyanin solution onto the tissue fiber was measured before and after the dyeing procedure by recording visible spectra, monitoring the absorbance at 620 nm, which is typical of the phycocyanin chromophore.
Adsorption results were applied to the Langmuir and Freundlich isotherm models.
The Langmuir isotherm is graphically characterized by a plateau, an equilibrium saturation point where once a molecule occupies a site, no further adsorption can take place. It considers a monolayer coverage, and that all the adsorption sites are equally probable; its linearized form is characterized by the following equation:
where Q0 is the maximum adsorption capacity for forming a single layer (in mg gadsorbent−1), Ce is the adsorbate concentration in solution at equilibrium (in mg l−1) and qe indicates the quantity adsorbed on the adsorbent at equilibrium (in mg g adsorbent−1). If the plot Ce/qevs. Ce displays a linear trend, it means that the adsorption process can be described by the Langmuir model.
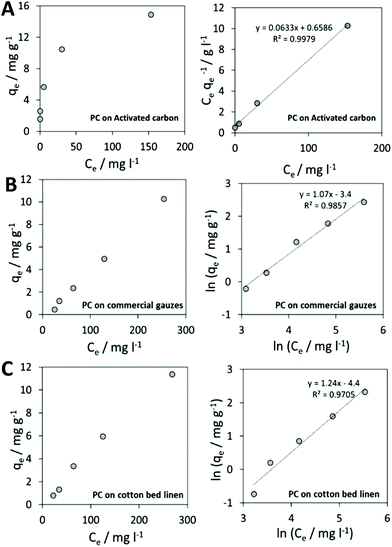
Results on activated carbon displayed a behavior that could be described by the Langmuir isotherm. In Fig. 5A, experimental results on activated carbon are shown.
The Langmuir isotherm linearization for the adsorption of phycocyanin on activated carbon provided a value of maximum adsorption capacity of Q0 = 15.8 mg gadsorbent−1. This could also be visually derived from the plateau reached by experimental data in Fig. 5A (right). Langmuir isotherm in fact considers a monolayer coverage: once a molecule occupies a site, no further adsorption is allowed.56
Among the experimented cotton-based materials, none of them could be fitted by a Langmuir model isotherm for the phycocyanin adsorption process. In fact, many practical cases cannot be described using the Langmuir model. Freundlich isotherm is empirical and is very widely used to describe the adsorption characteristics of a heterogeneous surface. The Freundlich equation implies that the energy of adsorption on a homogeneous surface is independent of surface coverage. Its linearized form is described by the following equation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qevs. ln
qevs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce displays a linear trend, it means that the adsorption process can be described by the Freundlich model.56
Ce displays a linear trend, it means that the adsorption process can be described by the Freundlich model.56
Fig. 4B and C display experimental results and the Freundlich model linearization obtained for phycocyanin adsorption on commercial sterile gauzes and colorless cotton bed linen.
The linear regression lines in Fig. 5B and C (right) demonstrate that the linearization of the Freundlich isotherm is able to fit experimental data.
Fig. 5 displays the coloring result with varying ratios of phycocyanin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) amount of tissue.
amount of tissue.
By calculating the 1/n from the slopes of regression lines, 1.06 and 1.24 values were obtained for phycocyanin adsorption on commercial gauzes and cotton bed linen, respectively. This suggests that the adsorption is more favorable on cotton bed linen, as also visible by the more pronounced coloring of bed linen tissues displayed in Fig. 6A in comparison to Fig. 6B, relative to commercial gauzes. This might be explained by the presence of polymeric additives in commercial gauzes, hindering the phycocyanin adsorption process.
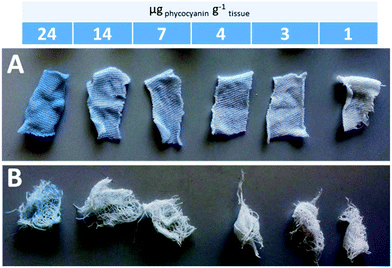 | ||
Fig. 6 Pictures of the colored bed linen (A) and commercial gauzes (B) after phycocyanin adsorption experiments. Each colored tissue is the result of a different ratio phycocyanin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) amount of tissue. amount of tissue. | ||
These experiments are meant exclusively as preliminary results to develop potential applications of phycocyanin as a stable dye after some rinsing steps. In fact, all colored tissues underwent three rinsing steps in three successive weeks, and the color did not significantly dissolve in water.
To translate into real tissue dye processing, the extracted blue pigment needs to be incorporated into a base formulation.
Conclusions
Distinctive natural orange, green and blue pigments of Spirulina were extracted from the dry biomass through an integrated chain. First, supercritical CO2 alone was able to selectively extract carotenoids. Then, the addition of ethanol as a co-solvent exclusively provided chlorophylls.A strategy to finally extract high purity phycocyanin (A620/A280 = 2.2) from the biomass residue was developed and its potential application as a tissue dye was studied.
The benefit of the suggested extraction cascade is the approach, where almost all the relevant contents have been efficiently recovered, stage by stage, in a potentially scalable process with no loss of pigments or added-value compounds.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by Algaria srl. Authors thank Professor Giangiacomo Beretta and Dr Fabrizio Gelmini for UV-Vis facilities, Dr Graziano Colombo for dialysis experiments, Dr Marco Parolini for lyophilizations and Dr Ermelinda Falletta for UPLC-MS analyses.References
- G. Clement, C. Giddey and R. Menzi, J. Sci. Food Agric., 1967, 18, 497–501 CrossRef CAS.
- L. Vernès, P. Granvillain, F. Chemat and M. Vian, Curr. Biotechnol., 2016, 4, 481–491 CrossRef.
- C. Safi, A. V. Ursu, C. Laroche, B. Zebib, O. Merah, P.-Y. Pontalier and C. Vaca-Garcia, Algal Res., 2014, 3, 61–65 CrossRef.
- G. Chamorro-Cevallos, Int. J. Food Nutr. Sci., 2016, 3, 1–10 Search PubMed.
- W. S. Park, H.-J. Kim, M. Li, D. H. Lim, J. Kim, S.-S. Kwak, C.-M. Kang, M. G. Ferruzzi and M.-J. Ahn, Molecules, 2018, 23, 2065 CrossRef.
- M. A. Rostagno and J. M. Prado, Natural product extraction: principles and applications, RSC Pub, 2013 Search PubMed.
- H. Qiang and A. Richmond, J. Appl. Phycol., 1996, 8, 139–145 CrossRef.
- F. Delgado-Vargas and O. Paredes-Lopez, Natural Colorants for Food and Nutraceutical Uses, CRC Press, 2002 Search PubMed.
- W. Song, C. Zhao and S. Wang, Int. J. Biosci., Biochem. Bioinf., 2013, 3, 293–297 CAS.
- K. M. Minkova, A. A. Tchernov, M. I. Tchorbadjieva, S. T. Fournadjieva, R. E. Antova and M. C. Busheva, J. Biotechnol., 2003, 102, 55–59 CrossRef CAS.
- E. Manirafasha, T. Murwanashyaka, T. Ndikubwimana, Q. Yue, X. Zeng, Y. Lu and K. Jing, J. Appl. Phycol., 2017, 29, 1261–1270 CrossRef CAS.
- W. Pan-utai, W. Kahapana and S. Iamtham, J. Appl. Phycol., 2018, 30, 231–242 CrossRef CAS.
- G. Patil, S. Chethana, A. S. Sridevi and K. S. M. S. Raghavarao, J. Chromatogr. A, 2006, 1127, 76–81 CrossRef CAS.
- S. T. Silveira, L. K. De Menezes Quines, C. A. V. Burkert and S. J. Kalil, Bioprocess Biosyst. Eng., 2008, 31, 477–482 CrossRef CAS.
- J. M. Doke, Int. J. Food Eng., 2005, 1, 1 Search PubMed.
- R. Chaiklahan, N. Chirasuwan, V. Loha, S. Tia and B. Bunnag, Bioresour. Technol., 2011, 102, 7159–7164 CrossRef CAS.
- H. A. Tavanandi, R. Mittal, J. Chandrasekhar and K. S. M. S. Raghavarao, Algal Res., 2018, 31, 239–251 CrossRef.
- M. Rito-Palomares, L. Nuez and D. Amador, J. Chem. Technol. Biotechnol., 2001, 76, 1273–1280 CrossRef CAS.
- G. Martelli, C. Folli, L. Visai, M. Daglia and D. Ferrari, Process Biochem., 2014, 49, 154–159 CrossRef CAS.
- A. Ramos, F. G. Acién, J. M. Fernández-Sevilla, C. V. González and R. Bermejo, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 511–519 CrossRef CAS PubMed.
- R. Bermejo and A. Ramos, Chromatographia, 2012, 75, 195–204 CrossRef CAS.
- J. A. Mendiola, L. Jaime, S. Santoyo, G. Reglero, A. Cifuentes, E. Ibañez and F. J. Señoráns, Food Chem., 2007, 102, 1357–1367 CrossRef CAS.
- A. P. R. F. Canela, P. T. V. Rosa, M. O. M. Marques and M. A. A. Meireles, Ind. Eng. Chem. Res., 2002, 41, 3012–3018 CrossRef CAS.
- C. Da Porto and A. Natolino, J. Supercrit. Fluids, 2017, 130, 239–245 CrossRef CAS.
- A. R. Wellburn, J. Plant Physiol., 1994, 144, 307–313 CrossRef CAS.
- H. H. A. El-Baky, F. K. El Baz and S. E.-B. Gamal, Biotechnology, 2003, 2, 222–240 CrossRef.
- W. Miki, K. Yamaguchi and S. Konosu, Bull. Jpn. Soc. Sci. Fish., 1986, 52, 1225–1227 CrossRef CAS.
- E. Cadoni, M. Rita De Giorgi, E. Medda and G. Poma, Dyes Pigm., 1999, 44, 27–32 CrossRef CAS.
- G. A. Spanos, H. Chen and S. J. Schwartz, J. Food Sci., 1993, 58, 817–820 CrossRef CAS.
- T. Baysal, S. Ersus and D. A. J. Starmans, J. Agric. Food Chem., 2000, 48, 5507–5511 CrossRef CAS.
- M. Durante, M. S. Lenucci and G. Mita, Int. J. Mol. Sci., 2014, 15, 6725–6740 CrossRef.
- A. Janghel, S. Deo, P. Raut, D. Bhosle, C. Verma, S. S. Kumar, M. Agrawal, N. Amit, M. Sharma, T. Giri, D. K. Tripathi, A. Ajazuddin and A. Alexander, Res. J. Pharm. Technol., 2015, 8, 775–786 CrossRef.
- M. Careri, L. Furlattini, A. Mangia, M. Musci, E. Anklam, A. Theobald and C. Von Holst, J. Chromatogr. A, 2001, 912, 61–71 CrossRef CAS.
- M. D. Macías-Sánchez, C. Mantell, M. Rodríguez, E. M. de la Ossa and L. M. Lubián, J. Supercrit. Fluids, 2007, 39, 323–329 CrossRef.
- S. M. Rivera, P. Christou and R. Canela-Garayoa, Mass Spectrom. Rev., 2014, 33, 353–372 CrossRef CAS.
- H. Li, S. T. Tyndale, D. D. Heath and R. J. Letcher, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2005, 816, 49–56 CrossRef CAS.
- H. K. Lichtenthaler and C. Buschmann, Curr. Protoc. Food Anal. Chem., 2001, 1, F4.2.1–F4.2.6 CrossRef.
- Y. Tong, L. Gao, G. Xiao and X. Pan, Chem. Eng. Technol., 2011, 34, 241–248 CrossRef CAS.
- M. D. Macías-Sánchez, C. Mantell, M. Rodríguez, E. M. de la Ossa, L. M. Lubián and O. Montero, Talanta, 2009, 77, 948–952 CrossRef.
- A. J. Meléndez-Martínez, G. Britton, I. M. Vicario and F. J. Heredia, Food Chem., 2006, 101, 1145–1150 CrossRef.
- R. M. Couto, J. Fernandes, M. D. R. G. da Silva and P. C. Simões, J. Supercrit. Fluids, 2009, 51, 159–166 CrossRef CAS.
- K. Ameer, H. M. Shahbaz and J.-H. Kwon, Compr. Rev. Food Sci. Food Saf., 2017, 16, 295–315 CrossRef.
- G. Ferrentino, K. Morozova, O. K. Mosibo, M. Ramezani and M. Scampicchio, J. Cleaner Prod., 2018, 186, 253–261 CrossRef CAS.
- D. S. Berns, E. Scott and K. T. O'Reilly, Science, 1964, 145, 1054–1056 CrossRef CAS.
- S. Benedetti, F. Benvenuti, S. Pagliarani, S. Francogli, S. Scoglio and F. Canestrari, Life Sci., 2004, 75, 2353–2362 CrossRef CAS PubMed.
- F. L. Souza, S. Cotillas, C. Saéz, P. Cañizares, M. R. V. Lanza, A. Seco and M. A. Rodrigo, J. Chem. Technol. Biotechnol., 2014, 91, 82–87 CrossRef.
- A. Reis, A. Mendes, H. Lobo-Fernandes, J. A. Empis and J. M. Novais, Bioresour. Technol., 1998, 66, 181–187 CrossRef CAS.
- T. Janoschka, N. Martin, U. Martin, C. Friebe, S. Morgenstern, H. Hiller, M. D. Hager and U. S. Schubert, Nature, 2015, 527, 78–81 CrossRef CAS.
- P. T. Wingfield, Curr. Protoc. Protein Sci., 2016, 1–10 Search PubMed.
- C. C. Moraes and S. J. Kalil, Bioresour. Technol., 2009, 100, 5312–5317 CrossRef CAS PubMed.
- G. Chen, Sep. Purif. Technol., 2004, 38, 11–41 CrossRef CAS.
- Industrial Membrane Separation Technology, ed. K. Scott and R. Hughes, Springer Science+Business Media, Dordrecht, 1996 Search PubMed.
- A. Gupta and J. K. Sainis, J. Appl. Phycol., 2010, 22, 231–233 CrossRef CAS.
- M. Oplatowska-Stachowiak and C. T. Elliott, Crit. Rev. Food Sci. Nutr., 2017, 57, 524–548 CrossRef CAS.
- P. M. Rose, V. Cantrill, M. Benohoud, A. Tidder, C. M. Rayner and R. S. Blackburn, J. Agric. Food Chem., 2018, 66, 6790–6798 CrossRef CAS.
- A. Dada, A. Olalekan, A. Olatunya and A. Dada, J. Appl. Chem., 2012, 3, 38–45 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9gc03292d |
| This journal is © The Royal Society of Chemistry 2020 |