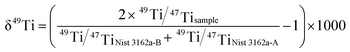A new procedure for titanium separation in geological samples for 49Ti/47Ti ratio measurement by MC-ICP-MS†
Xinyue
He
 ab,
Jinlong
Ma
ab,
Jinlong
Ma
 *a,
Gangjian
Wei
*a,
Gangjian
Wei
 a,
Le
Zhang
a,
Le
Zhang
 a,
Zhibing
Wang
a and
Qiaoshan
Wang
ab
a,
Zhibing
Wang
a and
Qiaoshan
Wang
ab
aState Key Laboratory of Isotope Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China. E-mail: jlma@gig.ac.cn; Fax: +86-20-85290130; Tel: +86-20-85290116
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 12th November 2019
Abstract
Here we present a convenient and efficient procedure for separating Ti from matrix elements in geological samples using a dual-column loaded with Ln-spec and AG50W-X12 resins. Boric acid was adopted to reduce the use of HF and to avoid precipitation of Ti. Ln-spec resin was used to remove most of the matrix elements. The residual matrix elements, such as Mo, V, Cr and trace Fe, were further removed using a column filled with AG50W-X12 resin. This procedure results in a Ti recovery near 100% with very low matrix element concentrations in the final Ti solution which has little influence on the measurement of Ti isotopes. Ti isotope measurement was carried out on a Neptune Plus multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS) with a standard-sample-standard bracketing technique. The intermediate precision of the NIST SRM 3162a solution for δ49Ti was 0.047‰ (2SD, N = 130). The δ49TiOL-Ti values (recalculated to the OL-Ti standard) of 14 geological reference materials and six kinds of minerals selected from a quartz monzonite were measured using this method, and the results of the reference materials were in good agreement with the published results within analytical error. The 14 geological reference materials display large δ49Ti variations up to 1.7‰. Meanwhile, the variations of δ49TiOL-Ti values of the six minerals are up to 1.5‰, indicating significant Ti isotope fractionation during the mineral crystallization process. Therefore, the analytical procedure established here provides a powerful tool to investigate Ti isotope variations. The results of the geological samples in this study show the potential application of Ti isotopes in tracing geological processes.
1 Introduction
Titanium (Ti) isotopes are a powerful tool in tracing extraterrestrial and geological processes. The mass-independent fractionations or Ti isotope anomalies found in extraterrestrial materials have been widely applied to determine the mixing level of nucleosynthetic anomalies in the initial solar nebula,1–5 the origin of presolar materials and the nucleosynthesis they have undergone.6–8 However, mass-dependent Ti isotope fractionation in Earth materials had been less studied until the development of high precision Ti isotope measurements with MC-ICP-MS.9,10 To date, significant mass-dependent Ti isotope fractionations have been detected in terrestrial rocks with δ49TiOL-Ti values varying from −0.005‰ to +2.012‰.11–13 It is suggested that the fractional crystallisation of Ti-bearing oxides mainly controls the Ti isotope fractionation during magmatic processes.11,14 Besides Ti isotopes might provide evidence for the time of formation of the felsic crust and the start of plate tectonics.12,15 Similar studies, however, are scarce as high-precision Ti isotope measurement is still very difficult; in particular, Ti separation from matrix elements is not easy.Many isobaric interferences overlap the masses of Ti isotopes, such as 46Ca on 46Ti, 48Ca on 48Ti, and 50V and 50Cr on 50Ti. In addition, it's imperative that the sample and standard have the same analyte concentration and sample matrices when standard-sample-standard bracketing is used to correct for instrumental mass bias.16 It is necessary to separate Ti from these matrix elements prior to isotope measurement. In previous studies, methods have been developed to purify Ti, such as dual-column methods using AG1-X8 + AG1-X8 resins17 and AG1-X8 + DGA resins,5 three-column methods using AG1-X8 + U/TEVA + AG1-X8 resins10,18 and TODGA + AG1-X8 + AG1-X8 resins,4,14,19 and even four-column methods using AG50W-X8/DGA + AG1-X8 + DGA + AG50W-X8 resins.20 In these methods, AG1-X8 anion resin was generally adopted, and HF acid was used to separate Ti from matrix elements. However, if samples have high Ca, Mg and Al contents, using HF leads to precipitation of CaF2 or CaAlF5, which may induce Ti isotope fractionation.9,10 In addition, HF acid is a high risk reagent. Minimization or elimination of HF use would be of great advantage.
Here we present a new chemical separation procedure to purify Ti from matrix elements in geological samples via a dual-column packed with Ln-spec resin and AG50W-X12 cation resin. It will be shown that this method can remove matrix elements completely, requires a shorter operation time and consumes smaller amounts of reagents with a full Ti recovery and no Ti isotope fractionation during column chemistry. Moreover, much less HF was used in the whole chemical procedure. With this chemical purification technique, high precision Ti isotopes of 14 geological references and a batch of minerals separated from a quartz monzonite were reported.
2 Experimental
2.1 Reagents and resins
All the reagent purification was performed in a class 100 clean hood at the State Key Laboratory of Isotope Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences (GIG-CAS). Ultra-pure water (Milli-Q water) was produced using a Millipore system (MilliporeSigma Corp. St. Louis, MO, USA) yielding a resistivity of 18.2 MΩ cm. Hydrofluoric acid (HF) and hydrochloric acid (HCl) were distilled from BVIII grade reagents (electronic grade) using a Savillex DST-1000 system (Savillex, Eden Prairie, Minnesota, USA). Nitric acid (HNO3) was twice distilled from the GR grade reagent with the same system. H2O2 (electronic grade, Beijing Institute of Chemical Reagents, Beijing, China) and H3BO3 powder (99.99% pure grade, Macklin Reagent company, Shanghai, China) were diluted in Milli-Q water to appropriate concentrations for use in this experiment.Ln-spec resin (50–100 μm, Eichrom Technologies, Darin, USA) and AG50W-X12 cation-exchange resin (200–400 mesh, Bio-Rad Laboratories, Inc., Hercules, California, USA) were chosen to purify Ti from matrix elements in this study.
2.2 Sample preparation
A series of international geological reference materials, including basalts (BIR-1a, BHVO-2, JB-1, JB-2, and BCR-2), a diabase (W-2a), andesites (JA-2, AGV-2, and AGV-1), granodiorites (GSP-2 and JG-1a), rhyolite (JR-2), granite (JG-2) and a polymetallic nodule (GBW07295), were selected to conduct Ti purification and isotope measurement. In addition, a set of minerals and whole rocks were selected from a quartz monzonite, collected from Qinghu (QH) Guangxi, China, which consists of 27.7% K-feldspar, 47.2% plagioclase (An17), 18.2% hornblende and 5.4% quartz and minor chlorite, as well as some accessory minerals including zircon, titanite, apatite, ilmenite and magnetite.21–23 XRD analysis of the QH whole-rock was performed at the Guangzhou Institute of Geochemistry, Chinese Academy of Sciences (GIG-CAS). The minerals were picked under a binocular microscope and the samples were washed with Milli-Q water and then crushed into 200 mesh powder.About 5–50 mg of rock, silicate mineral and ilmenite samples were weighed into pre-cleaned 7 mL PFA Savillex vials. 1 mL of 8 M HNO3 and 2 mL of 24 M HF were added into the vials, which were then capped tightly and kept on a hotplate at a temperature of 120 °C for 7 days. Then, the samples were evaporated to dryness, during which Si was removed,24 and re-dissolved in 16 M HNO3 and evaporated to dryness again. Then, 1 mL of aqua regia was added to dissolve the samples, keeping the solutions on a hotplate at a temperature of 115 °C for 6 hours. The solutions were dried again and dissolved with 6 M HCl and then evaporated to dryness. The samples were finally dissolved in 1 mL of a mixed acid of 3 M HCl + 2 wt% H3BO3 for further chemical treatment.
For oxide samples (magnetite and the polymetallic nodule), about 5 mg samples were weighed into pre-cleaned 7 mL vials. 2 mL aqua regia were added and kept on a hotplate at 115 °C for 5 days. Then, the solutions were dried and re-dissolved in 1 mL of 6 M HCl. After evaporating to dryness, 1 mL of a mixed acid of 3 M HCl + 2 wt% H3BO3 was added for further chemical treatment.
2.3 Column chemistry
Ti purification in this experiment was conducted via a dual-column packed with Ln-spec resin and AG50W-X12 resin. In the first stage, 0.5 g Ln-spec resin was packed into a polypropylene column with 0.7 cm diameter × 6 cm length. Prior to loading the samples, Ln-spec resin was cleaned with 10 mL of a mixed acid of 6 M HCl + 0.5 M HF, 10 mL of Milli-Q water and 5 mL of 3 M HCl. After calculating the Ti content in each solution, about 10 μg of Ti were extracted from each solution and loaded onto Ln-spec resin and all the loaded solutions had volumes less than 0.3 mL. The main matrix elements such as Si, K, Ca, Na, Mg, Al, Mn, V, Cr and Fe were subsequently removed with 12 mL of 3 M HCl. Then 4 mL of 6 M HCl was added into the column to elute Yb. Finally, Ti, together with Mo and trace Fe, V and Cr, was collected with 6 mL of 3 M HCl +1 wt% H2O2. This Ti-containing solution was evaporated to dryness, and then redissolved with 0.25 mL of 1 M HCl to prepare for further Ti purification.In the second stage, 0.6 mL of AG50W-X12 cation exchange resin was packed into a polypropylene column with 0.7 cm diameter × 3.5 cm length. 8 mL of 6 M HCl and 4 mL of Milli-Q water were used sequentially to clean the resin. After the Ti-containing solution was loaded, Mo and trace V and Cr were eluted with 4 mL of 0.1 M HCl + 1 wt% H2O2. After this, Ti was collected with 3 mL of a mixture acid of 0.2 M HCl + 0.2 M HF. The detailed chemical column procedures are listed in Table 1 and the leaching curves are plotted in Fig. 1a and b.
| Acid | Volume (mL) | Procedures and eluted elements |
|---|---|---|
| 1.35 mL Ln-spec; 0.7 cm diameter × 6 cm length column | ||
| 6 M HCl + 0.5 M HF | 10 | Clean |
| Milli-Q | 10 | Clean |
| 3 M HCl | 5 | Precondition |
| 3 M HCl + 2 wt% H3BO3 | 0.1 | Load |
| 3 M HCl | 12 | Rinse matrix (Li, B, Ca, V, Cr, Fe) |
| 6 M HCl | 4 | Rinse Fe and Yb |
| 3 M HCl + 1 wt% H2O2 | 6 | Collect Ti and Mo |
| 2 M HF | 6 | Collect Nb, Ta, Zr, Hf, U and Th |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| 0.6 mL AG50W-X12; 0.7 cm diameter × 3.5 cm length column | ||
| 6 M HCl | 8 | Clean |
| Milli-Q | 4 | Precondition |
| 1 M HCl | 0.25 | Load |
| 0.1 M HCl + 1 wt% H2O2 | 4 | Mo, Fe and trace V and Cr |
| 0.2 M HCl + 0.2 M HF | 3 | Collect Ti |
| 6 M HCl + 0.2 M HF | 4 | Rinse Fe |
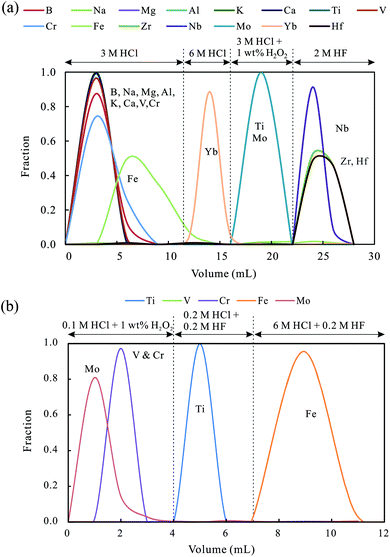 | ||
| Fig. 1 The elution curves of Ti purification procedures using two columns with (a) Ln-spec resin and (b) AG50W-X12 resin. | ||
The collected Ti solution was evaporated to dryness, and then a few drops of concentrated HNO3 were added and dried twice to remove Cl−. Finally the sample was redissolved with 0.32 M HNO3 + 0.0024 M HF for isotope analysis by MC-ICP-MS. The whole procedural blank of Ti was typically <1 ng, which is negligible considering that about 10 μg Ti were loaded. The Ti recoveries were assessed through loading 10 μg of a NIST SRM 3162a Ti solution. After treatment using our chemical columns, the total Ti recovery is >99.5% and the average value of δ49Ti is 0.000 ± 0.051‰ (2SD, n = 10), indicating that no artificial Ti isotope fractionation was introduced in our purification process.
2.4 Mass spectrometry and Ti isotope measurement
The Ti isotope measurements were carried out on a Thermo Fisher Scientific Neptune Plus MC-ICP-MS at GIG-CAS. A detailed description of this instrument was reported by Zhang et al.25 and Wei et al.26 The signals of 46Ti (46Ca), 47Ti, 48Ti (48Ca), 49Ti, 50Ti (50V and 50Cr) and 52Cr were collected in six Faraday cups, respectively. In theory, the signals of 42Ca and 51V should also be collected in Faraday cups to monitor the interferences of 48Ca on 48Ti and 50V on 50Ti. However, the two high Faraday cups (H3 and H4) are mounted with ion counters in our instrument, so all the masses of interest in our experiment cannot be adjusted to suitable Faraday cup positions to monitor these interferences in one measuring procedure. Thus, only 52Cr was monitored for the possible interference of 50Cr on 50Ti. High-precision ratio measurements of 46Ti/47Ti, 48Ti/47Ti and 50Ti/47Ti without monitoring 42Ca and 51V during Ti isotope measurement require separating Ti from Ca and V completely in geological materials through chemical purification. The Ti solution in 0.32 M HNO3 (2% m m−1) + 0.0024 M HF (0.01% m m−1) was introduced through a Micromist PFA nebulizer with a 50 μL min−1 self-aspiration capillary and buffered in a glass cyclonic spray chamber. The minor HF is likely to decompose the glass spray chamber and to produce possible molecular interference of 28Si19F on 47Ti. However, a very low signal of <0.001 V for m/z28Si was produced and no detectable ion interferences from silicon compounds were observed on all masses of Ti during the long-term run of 0.32 M HNO3 + 0.0024 M HF.The typical instrument parameters during Ti isotope measurement are shown in Table S1.† Each data acquisition contains 1 block of 60 cycles. The integration time for each cycle is 4.194 s, and the total 60 scans take about 4.2 min. The intensity of 48Ti was generally optimized to about 8 V by using the NIST SRM 3162a Ti standard of about 250 ng g−1 at medium resolution before each measurement session. The background of 0.32 M HNO3 + 0.0024 M HF on m/z 48 was generally below 2 mV. Prior to measurement, all the samples were adjusted to a 48Ti intensity of 8 V in 1 mL of 0.32 M HNO3 + 0.0024 M HF, matching that of the bracketed NIST SRM 3162a Ti standard solution. This enables a perfect intensity match between the sample and the bracketing standards, to avoid possible influences from mis-match between the standard and sample solution.16 After one measurement, all the signals of the Ti isotope dropped to lower than 2 mV within 4 min of washing by using 0.32 M HNO3 + 0.0024 M HF as the rinse. The signal-to-noise ratio for each measurement was generally >4000 but decreased gradually due to increasing blank which was influenced by minor Ti memory during long Ti isotope measurements. Thus, the influence of the background was subtracted after the blank solution (0.32 M HNO3 + 0.0024 M HF) was measured.
The Ti isotope measurement was carried out in the sequence of NIST SRM 3162a Ti standard solution-sample-NIST SRM 3162a Ti standard solution. Given that the variations of the Ti isotope composition in terrestrial samples obey mass-dependent fractionation9,19 and 47Ti and 49Ti are less interfered among the five Ti isotopes, the value of δ49Ti was only considered in this study. The δ49Ti was calculated from the measured Ti isotope ratios of the sample and the averages of the bracketed NIST SRM 3162a Ti standard as below:
Some recent studies11,19,27 suggest that the OL-Ti standard from SARM (Service d’Analyse des Roches et des Minéraux, Nancy, France) is more suitable as the Ti isotope reference because its isotope composition is similar to the Ti composition of natural geological samples. In order to precisely compare the interlaboratory results, repeated measurements of the OL-Ti standard were also carried out in our laboratory and the δ49Ti of the OL-Ti standard is 1.070 ± 0.050‰ (2SD, n = 12) relative to NIST SRM 3162a, which is in good agreement with the 1.056 ± 0.026‰ measured by Greber et al.14 within analytical error. The forthcoming δ49TiOL-Ti values of the geological samples studied are reported relative to the OL-Ti standard calculated to be 1.070.
3 Results and discussion
3.1 Optimization of Ti chemical purification
In previous methods, the geological samples were re-dissolved in a medium of 2 M HF or 4 M HF acid before column chemistry treatments. There is a great risk of the formation of insoluble fluorides, such as CaF2, MgF2 and/or CaAlF4 if the concentrations of Ca, Mg and Al are high. This may cause some loss of Ti. Even though a previous study suggested limited Ti isotope fractionation between insoluble fluorides and the supernatant by using a synthetic solution,10 its influence on Ti isotopes of natural geological samples with complicated matrices has not been evaluated yet.Here we evaluate Ti isotope fractionation between insoluble fluorides and the supernatant by using two natural geological samples, BHVO-2 and GSP-2. They were first digested using the method mentioned in Section 2, but in the last step, 1 mL of 2 M HF rather than a mixed acid of 3 M HCl + 2 wt% H3BO3 was added to extract the sample. Then the samples were placed on a hotplate at a temperature of 25 °C for 8 h enabling insoluble fluoride deposition. After this, the supernatant solution and the precipitate were separated after centrifugation. Then both were dried and re-dissolved in concentrated HNO3 twice. After further evaporation, 1 mL of aqua regia was added to dissolve the samples, and they were kept on a hotplate for 6 hours. Finally they were dried again and dissolved with 1 mL of 3 M HNO3.
0.1 mL solution was extracted from these solutions and diluted to an appropriate concentration for Ti content measurement by ICP-MS. The residual solution was evaporated to dryness and 1 mL of 6 M HCl was added and dried again. Finally, a mixed acid of 3 M HCl + 2 wt% H3BO3 was added to redissolve the samples for further chemical treatment, and Ti isotopes were measured following the above mentioned methods.
As shown in Table 2, the Ti isotope composition of the supernatant was heavier than that of the precipitate for both the BHVO-2 and GSP-2 samples, with δ49TiOL-Ti differing up to 0.40‰. This indicates significant Ti isotope fractionation between the supernatant and precipitate, and suggests that full digestion would be necessary for geological samples.
| Sample | Ti/μg | δ49Ti | δ49TiOL-Ti | 2SD | Ti fraction |
|---|---|---|---|---|---|
| a BHVO-2* and GSP-2* were calculated by mass balance, and they are in good agreement with the measured data within the error. δ49TiOL-Ti values have been calculated relative to the OL-Ti standard with δ49TiOL-Ti = δ49Ti + 1.070‰. | |||||
| BHVO-2 supernatant | 713 | −1.022 | 0.048 | 0.008 | 0.87 |
| BHVO-2 precipitate | 106 | −1.105 | −0.035 | 0.056 | 0.13 |
| BHVO-2* | −1.033 | 0.037 | |||
| GSP-2 supernatant | 176 | −0.661 | 0.409 | 0.002 | 0.89 |
| GSP-2 precipitate | 22 | −1.079 | −0.009 | 0.009 | 0.11 |
| GSP-2* | −0.707 | 0.363 | |||
In our method, a mixture of 3 M HCl + 2 wt% H3BO3 instead of HF was used to re-dissolve the samples before the first column chemistry using Ln-spec resins. This HF free procedure can avoid precipitation of insoluble fluorides and achieve high Ti recovery. Here, the amount of H3BO3 is critical for high Ti recovery. Table 3 shows the details of the test on Ti recovery by using a rock sample, GSP-2. When the amount of H3BO3 is up to 2 wt%, the Ti recovery is close to 100%. This will enable accurate Ti isotope results for geological samples.
| Loading media | Recovery |
|---|---|
| a The sample was GSP-2 containing 10 μg Ti when loading onto the Ln-spec resin. | |
| 3 M HCl | 95.9% |
| 3 M HCl + 0.5 wt% H3BO3 | 94.0% |
| 3 M HCl + 1 wt% H3BO3 | 97.5% |
| 3 M HCl + 2 wt% H3BO3 | 99.9% |
| 3 M HCl + 2.5 wt% H3BO3 | 99.8% |
3.2 The efficiency of our dual-column purification for Ti
Our purification procedure involving a dual-column packed with Ln-spec resin and AG50W-X12 resin can remove nearly all the matrix elements, but some trace matrix elements, such as Ca, V, Cr, Zr and Mo, will remain. Here we use two rock samples, BHVO-2 basalt and JG-2 granite, and a Ti bearing mineral, ilmenite, containing 2.7 wt%, 0.044 wt% and 44.81 wt% TiO2, respectively, to evaluate the efficiency of this procedure. They were treated by using the dual-column procedure, and the cut Ti solutions were measured for the Ca/Ti, V/Ti, Cr/Ti, Zr/Ti and Mo/Ti ratios by using ICP-MS. The results indicate that the ratios of Ca/Ti, V/Ti, Cr/Ti, Zr/Ti and Mo/Ti in the cut Ti solution of all samples were less than 0.02, 0.0005, 0.005, 0.0006 and 0.002, respectively.The influence of such matrix elements on Ti isotope measurement by MC-ICP-MS in SSB mode was evaluated by the following doping tests. Ca, V, Cr, Zr and Mo were added to the NIST SRM 3162a solution, respectively, to obtain variable X/Ti ratios (Table 4). Then, they were measured for Ti isotopes (δ49Ti) using the method mentioned above. The results indicate that the δ49Ti values were not affected when the ratios of Ca/Ti, V/Ti, Cr/Ti, Zr/Ti and Mo/Ti were less than 0.1, 0.05, 0.05, 0.001 and 0.01, respectively (Fig. S1†). The corresponding ratios in the cut Ti solution purified by our dual-column procedure are far less than these critical points, indicating that our purification procedure is effective for geological samples.
| Doping test | δ49Ti | 2SE |
|---|---|---|
| a The data errors are represented as internal precision calculated on the basis of a typical 60 integration cycle analysis of each measurement. The bracketing standard was NIST SRM 3162a. | ||
| Ca/Ti 0.001 | 0.007 | 0.019 |
| Ca/Ti 0.01 | −0.001 | 0.017 |
| Ca/Ti 0.1 | 0.010 | 0.017 |
| V/Ti 0.0001 | 0.010 | 0.015 |
| V/Ti 0.001 | 0.005 | 0.018 |
| V/Ti 0.01 | −0.051 | 0.018 |
| V/Ti 0.05 | 0.002 | 0.016 |
| Cr/Ti 0.0001 | −0.014 | 0.015 |
| Cr/Ti 0.001 | −0.014 | 0.017 |
| Cr/Ti 0.01 | −0.007 | 0.016 |
| Cr/Ti 0.05 | −0.049 | 0.017 |
| Mo/Ti 0.01 | 0.011 | 0.015 |
| Mo/Ti 0.05 | 0.112 | 0.015 |
| Zr/Ti 0.0005 | −0.008 | 0.014 |
| Zr/Ti 0.001 | −0.118 | 0.017 |
| Zr/Ti 0.01 | −0.126 | 0.014 |
3.3 Long-term δ49Ti stability of Ti standard solutions
In this experiment, the NIST SRM 3162a Ti standard in 0.32 M HNO3 + 0.0024 M HF was measured continuously for 6 hours and produced a repeatability of δ49Ti of 0.000 ± 0.036‰ (2SD, N = 30). For long-term stability (9 month measurements), the intermediate precision of NIST SRM 3162a is 0.000 ± 0.047‰ (2SD, n = 130) (Fig. S2†), which is similar to the precision of the recent SSB method.20 This is slightly poorer than a precision of 0.020‰ measured by the double-spike method,19 but it is still good enough to identify the Ti isotope compositions of geological samples.3.4 Ti isotope compositions of geological samples
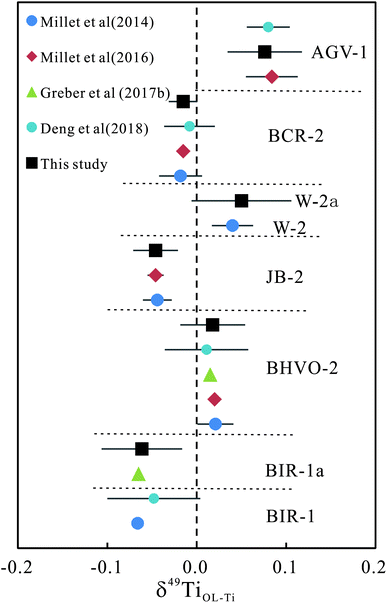
Using the method established here, the δ49TiOL-Ti values of a series of rock standard materials were measured and the results, together with the previous reported results, are listed in Table 5. Our results for BIR-1a, BHVO-2, JB-2, BCR-2 and AGV-1 are in good agreement with the previously published data5,11,14,19 within analytical error, demonstrating the robustness of the method in this study (Fig. 2). The δ49TiOL-Ti values in basites show limited variation, ranging from −0.061 ± 0.045‰ (BIR-1a, 2SD) to 0.050 ± 0.056‰ (W-2a, 2SD). The δ49TiOL-Ti values in andesites range from 0.040 ± 0.051‰ (JA-2, 2SD) to 0.109 ± 0.017‰ (AGV-2, 2SD). Both granodiorites (GSP-2 of 0.373 ± 0.060‰ and JG-1a of 0.231 ± 0.019, respectively, 2SD) show significantly higher δ49TiOL-Ti values than basites and andesites. JG-2 (granite) contains the least TiO2, but has a very high δ49TiOL-Ti value of 0.758 ± 0.014‰ (2SD, n = 2). JR-2 shows the highest δ49TiOL-Ti value of 1.696 ± 0.011‰ (2SD, n = 4) among all the samples. The δ49TiOL-Ti value in one polymetallic nodule (GBW07295) is 0.438 ± 0.031‰ (2SD, n = 4).| Sample | Type | SiO2 wt% | δ49Ti | δ49TiOL-Ti | 2SD | n | References |
|---|---|---|---|---|---|---|---|
| a The data of δ49Ti were represented as parts per mil deviation relative to the NIST SRM 3162a Ti standard. For interlaboratory comparison, the values have been scaled to the OL-Ti standard with δ49TiOL-Ti = δ49Ti + 1.070‰. The data errors for Greber et al. (2017b) are represented as 95% confidence interval. | |||||||
| BIR-1a | Basalt | 47.8 | −1.131 | −0.061 | 0.045 | 5 | This study |
| −0.065 | 0.025 | 40 | Greber et al. (2017b) | ||||
| BIR-1 | Basalt | 47.8 | −0.066 | 0.006 | 3 | Millet et al. (2014) | |
| −0.048 | 0.052 | 27 | Deng et al.(2018) | ||||
| BHVO-2 | Basalt | 49.6 | −1.052 | 0.018 | 0.036 | 18 | This study |
| 0.021 | 0.020 | 9 | Millet et al. (2014) | ||||
| 0.020 | 0.019 | 12 | Millet et al. (2016) | ||||
| 0.015 | 0.025 | 44 | Greber et al. (2017b) | ||||
| 0.011 | 0.047 | 18 | Deng et al.(2018) | ||||
| JB-1 | Basalt | 52.5 | −1.106 | −0.036 | 0.051 | 4 | This study |
| JB-2 | Basalt | 53.3 | −1.116 | −0.046 | 0.025 | 3 | This study |
| −0.044 | 0.016 | 4 | Millet et al. (2014) | ||||
| −0.046 | 0.007 | 3 | Millet et al. (2016) | ||||
| W-2a | Diabase | 52.6 | −1.020 | 0.050 | 0.056 | 9 | This study |
| W-2 | Diabase | 52.4 | 0.040 | 0.023 | 7 | Millet et al. (2014) | |
| BCR-2 | Basalt | 54.0 | −1.085 | −0.015 | 0.016 | 8 | This study |
| −0.018 | 0.024 | 7 | Millet et al. (2014) | ||||
| −0.015 | 0.016 | 12 | Millet et al. (2016) | ||||
| −0.008 | 0.028 | 8 | Deng et al.(2018) | ||||
| JA-2 | Andesite | 56.4 | −1.030 | 0.040 | 0.051 | 4 | This study |
| AGV-1 | Andesite | 58.8 | −0.994 | 0.076 | 0.042 | 8 | This study |
| 0.084 | 0.006 | 2 | Millet et al. (2016) | ||||
| 0.080 | 0.024 | 4 | Deng et al.(2018) | ||||
| AGV-2 | Andesite | 59.4 | −0.961 | 0.109 | 0.017 | 6 | This study |
| GSP-2 | Granodiorite | 66.6 | −0.697 | 0.373 | 0.060 | 9 | This study |
| JG-1a | Granodiorite | 72.3 | −0.839 | 0.231 | 0.019 | 2 | This study |
| JR-2 | Rhyolite | 75.7 | 0.626 | 1.696 | 0.011 | 4 | This study |
| JG-2 | Granite | 76.8 | −0.312 | 0.758 | 0.014 | 2 | This study |
| GBW07295 | Polymetallic nodule | 15.5 | −0.632 | 0.438 | 0.031 | 4 | This study |
| OL-Ti standard | −1.070 | 0.000 | 0.050 | 12 | This study | ||
| Processed NIST SRM 3162a | 0.000 | 1.070 | 0.051 | 10 | This study | ||
We also measured the Ti isotope compositions of six minerals separated from the Qinghu monzonite. The δ49TiOL-Ti of the whole-rock of the Qinghu monzonite is 0.309 ± 0.020‰ (n = 2). However, the δ49TiOL-Ti values of the minerals exhibit large variations, up to 1.5‰ (Table S3† and Fig. 3). Hornblende shows a δ49TiOL-Ti value similar to that of the whole-rock of 0.345 ± 0.044‰ (2SD), whereas K-feldspar and plagioclase have higher δ49TiOL-Ti values, 0.529 ± 0.042‰ (2SD) and 0.613 ± 0.064‰ (2SD), respectively. Magnetite shows the heaviest Ti isotope composition of δ49TiOL-Ti = 1.656 ± 0.050‰ (2SD). Both titanite and ilmenite show lower δ49TiOL-Ti values of 0.146 ± 0.008‰ (2SD) and 0.121 ± 0.038‰ (2SD), respectively, consistent with the assumption that Fe–Ti oxides mainly host lighter Ti isotopes likely because Ti is 6-coordinated in Fe–Ti oxides whereas it is 4 and 6-coordinated in silicate minerals.11 The significant variations of the Ti isotope in the minerals demonstrate that mineral crystallization should play a pivotal role in controlling the Ti isotope composition in different magmatic processes.
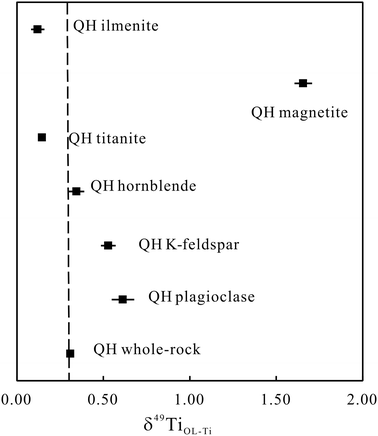 | ||
| Fig. 3 The Ti isotope compositions of QH minerals. The dashed line represents the Ti isotope composition of the QH whole-rock. The error bars are repeatability (2SD). | ||
4 Conclusions
A new procedure for Ti purification in geological samples was developed via a dual-column method. Much less HF acid was introduced and almost all the matrix elements were effectively removed and the recovery of Ti was close to 100%. Based on this method, Ti isotopes in a series of geological standard materials were measured and the results are in good agreement with the data published previously within analytical error, indicating that this is a new robust method to measure Ti isotopes. The significant variations of Ti isotopes in these geological standard materials and a set of minerals from a quartz monzonite indicate that Ti isotopes are potentially powerful tools to trace geological processes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Xianglin Tu of the State Key Laboratory of Isotope Geochemistry, GIG-CAS for his assistance with ICP-MS measurements. We are also grateful to Marc-Alban Millet and Christophe Cloquet for providing the OL-Ti standard. This work was financially supported by the National Key Research and Development Project of China (2016YFA0601204), the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB18000000), the GIGCAS 135 Project (135PY201605), and the National Natural Science Foundation of China (Grants 41573003 and 41703023). This paper is contribution No. IS-2764 from the GIG-CAS.Notes and references
- H. R. Heydegger, J. J. Foster and W. Compston, Nature, 1979, 278, 704–707 CrossRef CAS.
- I. Leya, M. Schönbächler, U. Wiechert, U. Krähenbühl and A. N. Halliday, Earth Planet. Sci. Lett., 2008, 266, 233–244 CrossRef CAS.
- A. Trinquier, T. Elliott, D. Ulfbeck, C. Coath, A. N. Krot and M. Bizzarro, Science, 2009, 324, 374–376 CrossRef CAS.
- J. Zhang, N. Dauphas, A. M. Davis and A. Pourmand, J. Anal. At. Spectrom., 2011, 26, 2197 RSC.
- Z. B. Deng, F. Moynier, K. van Zuilen, P. A. Sossi, E. A. Pringle and M. Chaussidon, Geochim. Cosmochim. Acta, 2018, 239, 409–419 CrossRef CAS.
- T. R. Ireland, E. K. Zinner and S. Amari, Astrophys. J., 1991, 376, L53–L56 CrossRef CAS.
- E. Zinner, S. Amari, R. Guinness, C. Jennings, A. F. Mertz, A. N. Nguyen, R. Gallino, P. Hoppe, M. Lugaro, L. R. Nittler and R. S. Lewis, Geochim. Cosmochim. Acta, 2007, 71, 4786–4813 CrossRef CAS.
- A. N. Nguyen, L. R. Nittler, C. M. O. D. Alexander and P. Hoppe, Geochim. Cosmochim. Acta, 2018, 221, 162–181 CrossRef CAS.
- X. K. Zhu, A. Makishima, Y. Guo, N. S. Belshaw and R. K. O'Nions, Int. J. Mass Spectrom., 2002, 220, 21–29 CrossRef CAS.
- A. Makishima, X.-K. Zhu, N. S. Belshaw and R. K. O'Nions, J. Anal. At. Spectrom., 2002, 17, 1290–1294 RSC.
- M.-A. Millet, N. Dauphas, N. D. Greber, K. W. Burton, C. W. Dale, B. Debret, C. G. Macpherson, G. M. Nowell and H. M. Williams, Earth Planet. Sci. Lett., 2016, 449, 197–205 CrossRef CAS.
- N. D. Greber, N. Dauphas, A. Bekker, M. P. Ptacek, I. N. Bindeman and A. Hofmann, Science, 2017, 357, 1271–1274 CrossRef CAS.
- Z. Deng, M. Chaussidon, P. Savage, F. Robert, R. Pik and F. Moynier, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 1132–1135 CrossRef CAS.
- N. D. Greber, N. Dauphas, I. S. Puchtel, B. A. Hofmann and N. T. Arndt, Geochim. Cosmochim. Acta, 2017, 213, 534–552 CrossRef CAS.
- Z. Deng, F. Moynier, P. A. Sossi and M. Chaussidon, Geochemical Perspectives Letters, 2018, 11–15, DOI:10.7185/geochemlet.1831.
- F. Albarède, P. Telouk, J. Blichert-Toft, M. Boyet, A. Agranier and B. Nelson, Geochim. Cosmochim. Acta, 2004, 68, 2725–2744 CrossRef.
- I. Leya, M. Schönbächler, U. Wiechert, U. Krähenbühl and A. N. Halliday, Int. J. Mass Spectrom., 2007, 262, 247–255 CrossRef CAS.
- S. Tang, J. Li, J.-X. Ma, X.-M. Zhao and X.-K. Zhu, Chin. J. Anal. Chem., 2018, 46, 1618–1627 CAS.
- M.-A. Millet and N. Dauphas, J. Anal. At. Spectrom., 2014, 29, 1444 RSC.
- K. K. Larsen, D. Wielandt and M. Bizzarro, J. Anal. At. Spectrom., 2018, 33, 613–628 RSC.
- X. Li, W. Li, X. Wang, Q. Li, Y. Liu and G. Tang, Sci. China, Ser. D: Earth Sci., 2009, 52, 1262–1278 CrossRef CAS.
- X. Li, G. Tang, B. Gong, Y. Yang, K. Hou, Z. Hu, Q. Li, Y. Liu and W. Li, Chin. Sci. Bull., 2013, 58, 4647–4654 CrossRef CAS.
- L. Xu and Z. Yuan, Guangxi. Acta Petrol. Mineral., 1992, 11, 289–298 CAS.
- A. Makishima, in Thermal Ionization Mass Spectrometry (TIMS), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016, pp. 1–40, DOI:10.1002/9783527696413.ch1.
- Z. Zhang, J. Ma, L. Zhang, Y. Liu and G. Wei, J. Anal. At. Spectrom., 2018, 33, 322–328 RSC.
- G. Wei, J. Wei, Y. Liu, T. Ke, Z. Ren, J. Ma and Y. Xu, J. Anal. At. Spectrom., 2013, 28, 606–612 RSC.
- F. Teng, N. Dauphas and J. M. Watkins, Rev. Mineral. Geochem., 2017, 82, 1–26 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ja00316a |
| This journal is © The Royal Society of Chemistry 2020 |