Open Access Article
Open Access ArticleA three-dimensional porous MoS2–PVP aerogel as a highly efficient and recyclable sorbent for oils and organic solvents†
Pin
Song
 *a,
Jun
Di
*a,
Jun
Di
 a,
Haiping
Chen
b,
Sirui
Zhao
c,
Cao
Wu
d,
Xun
Cao
a,
Haiping
Chen
b,
Sirui
Zhao
c,
Cao
Wu
d,
Xun
Cao
 a,
Meiling
Wang
a,
Meiling
Wang
 e,
Jun
Xiong
*f and
Xinli
Ye
d
e,
Jun
Xiong
*f and
Xinli
Ye
d
aSchool of Materials Science & Engineering, Nanyang Technological University, Singapore 639798, Singapore. E-mail: songpin@ntu.edu.sg
bSchool of Mechanics, and Optoelectronic, Physics, Anhui, University, of Science, and Technology, Huainan 232001, P. R. China
cCollege of Resources and Environment, Anhui Agricultural University, Hefei 230036, P. R. China
dCollege of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 211106, P. R. China
eInstitute of New Carbon Materials, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
fInstitute for Energy Research, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: xiongjun@ujs.edu.cn
First published on 5th June 2020
Abstract
Three-dimensional (3D) aerogels have attracted more and more attention in oil–water separation, due to their advantages of low density, high porosity, and large specific surface area. However, their application is greatly limited due to their hydrophilic and low adsorption properties. In this work, we report a 3D MoS2–polyvinylpyrrolidone (PVP) aerogel, prepared by a freeze-drying method, where PVP was used as a skeleton to support the aerogel. As a surfactant, PVP can easily attach to the surface of MoS2 nanosheets and facilitate the interconnection between nanosheets. The 3D MoS2–PVP aerogel exhibits low density, high porosity, good hydrophobicity, and excellent adsorption capacity (195–649 times). Moreover, after 30 cycles, the structure of the 3D MoS2–PVP aerogel is well kept and the adsorption capacity is still retained, at 93.5% and 92.9%, by squeezing and distillation, respectively. Therefore, the obtained 3D MoS2–PVP aerogel is a promising adsorption material and has great practical application potential in oil–water separation.
Introduction
With the development of society, the problem of water pollution will become more and more serious. Oil leakage, industrial wastewater discharge, and organic solvent leakage are the main causes of water pollution. These problems have caused a serious ecological crisis, which has been the focus of wide attention in basic and applied research all over the world.1–4 Some traditional approaches were adopted to solve this serious problem, for example, including dispersants, adsorbent materials, oil skimmer vessels, and oil containment booms.5–13 Among the above methods, using an adsorbent material is one of the most promising approaches because of its simple process and excellent adsorption efficiency.Porous materials have been widely used as absorbents because they can separate oil and water through a simple and effective absorption process.14–17 Generally, the ideal absorbent material should have low density, high adsorption capacity, excellent recyclability, and environmental friendliness. Thus, a large number of adsorbent materials have been used to treat sewage, including wool fibers,18 activated carbon,19 expanded graphite,20 and BN nanosheets because of their microporosity.17,21–23 Although these adsorbent materials are effective to a certain extent, practical application is severely limited due to their low adsorption capacity and poor recyclability. Therefore, it is highly desirable to develop adsorption materials with high adsorption capacity and excellent recyclability.
Recently, the preparation of porous polymer aerogels by a freeze-drying method has been investigated.24–28 This method exhibits great advantages of simplicity and controllability, which has been widely studied and applied in various fields.29–33 It has been reported that a GO-PVA aerogel was successfully obtained,34 as PVA can be used as the skeleton to support GO. A boron nitride-modified PVA aerogel was obtained via a freeze-drying method, and it possesses low density, high porosity, and outstanding adsorption capacity for various solvents.35 MoS2 is a layered transition metal disulfide with a graphene-like structure.36 Its unique structure advantages gives it great prospects in the fields of catalysis, batteries, and sensors.37–42 Due to the extensive study of three-dimensional (3D) aerogels, the demand for MoS2 aerogels is increasing to realize its potential application.
Herein, a 3D MoS2–PVP aerogel has been successfully fabricated using a freeze-drying method. As a surfactant, PVP could easily attach to the surface of MoS2 nanosheets and facilitate the interconnection between nanosheets. The obtained 3D MoS2–PVP aerogel exhibits great advantages of high adsorption capacity and strong recyclability. In addition, after 30 cycles, the structure of the 3D MoS2–PVP aerogel is well kept and the adsorption capacity still retained 93.5% and 92.9%, by squeezing and distillation, respectively. It can be used as a promising adsorption material for environmental remediation.
Results and discussion
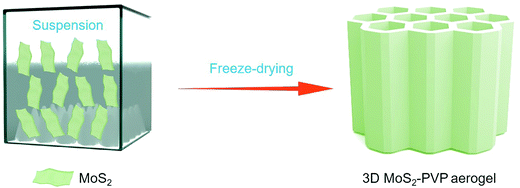
Fig. 1 depicts the assembling process of the 3D MoS2–PVP aerogel. Here, amphiphilic PVP can be used as a framework to support the aerogel (Fig. S1, ESI†). The MoS2 shows a lateral size of ∼200–600 nm (Fig. S2a, ESI†). The specific surface area (SSA) of the pure PVA aerogel is 44.5 m2 g−1, with the addition of MoS2, the specific surface area of 3D MoS2–PVP increased to 75.2, 79.6, 83.5 and 90.4 m2 g−1, respectively (Table S1, ESI†).Fig. 2a and b show the XRD patterns of the samples. Two diffraction peaks at 11.3° and 19.7° confirm the amorphous nature of PVP (Fig. 2b). The diffraction peaks of the MoS2–PVP aerogel match well with those of MoS2 (Fig. 2a). Nevertheless, it can be seen that the intensity ratio of (002)/(103) of MoS2–PVP aerogels (7.3) is higher than that of MoS2 (1.6), confirming that the PVP promotes the exfoliation of the MoS2 nanosheets.37,43
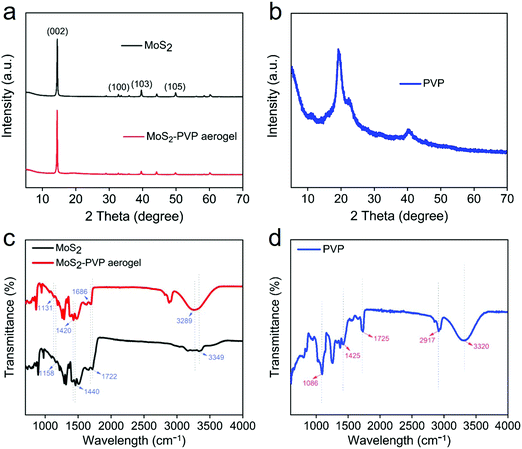 | ||
| Fig. 2 (a and b) XRD patterns of MoS2, MoS2–PVP aerogel and PVP. (c and d) FTIR spectra of MoS2, MoS2–PVP aerogel and PVP. | ||
The morphologies of these samples are shown in Fig. S2 (ESI†). Fig. S2a (ESI†) shows that the MoS2 had a 2D sheet-shaped morphology. In particular, when MoS2 is combined with PVP, the irregular porous structure of the MoS2 nanosheets can be obtained (Fig. S2b, ESI†). Furthermore, PVP can easily insert into the MoS2 nanosheets to generate thinner nanosheets.43
The FTIR spectra in Fig. 2c and d further indicate the interaction between MoS2 and PVP. These peaks of PVP at 3320, 2917 and 1425, 1725, 1086 cm−1 are attributed to O–H, –CH3, C = O and C–O functional groups, respectively. The peaks of MoS2 at 3349, 1722, 1440 and 1158 cm−1 correspond to O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –CH3 and C–O functional groups, respectively. However, the characteristic peaks of the MoS2–PVP aerogel blueshift to 3289, 1686, 1420 and 1131 cm−1, indicating the interaction between MoS2 and PVP.44
O, –CH3 and C–O functional groups, respectively. However, the characteristic peaks of the MoS2–PVP aerogel blueshift to 3289, 1686, 1420 and 1131 cm−1, indicating the interaction between MoS2 and PVP.44
Fig. S3 (ESI†) contains optical photos of the 3D MoS2–PVP aerogel with different concentrations of MoS2, which have basically the same macroscopic morphology. Moreover, the PVP aerogel can also be obtained by the freeze-drying method, which indicates its potential as the 3D framework to support the aerogel. Importantly, our method is widely applicable to the preparation of other 1D or 2D material-PVP aerogels. Taking BN-PVP and CNTs-PVP aerogels as examples, they were also successfully obtained by this method (Fig. S4, ESI†).
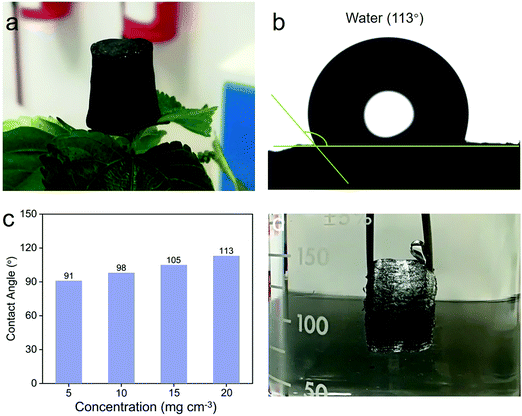
It has been reported that a high specific surface area is beneficial for the improvement of adsorption performance.45 Therefore, we selected the 3D MoS2–PVP aerogel (20 mg cm−3) with a high specific surface area as the research object. The 3D MoS2–PVP aerogel can stand stably on the surface of a flower (Fig. 3a). Fig. S5 (ESI†) shows that the 3D MoS2–PVP aerogel can support water droplets on its surface, ascribed to its good hydrophobicity. The hydrophobicity of the adsorption materials has great significance for the application of oil–water separation. Fig. 3b and c exhibit the water contact angles (WCAs) of the 3D MoS2–PVP aerogel. The WCAs of the 3D MoS2–PVP aerogel increase from 91° to 113° with the increase of MoS2 (Fig. 3c), indicating the hydrophobicity of the 3D MoS2–PVP aerogel. As shown in Fig. 3d, the mirror-reflection is observed, ascribed to the formation of a new interface between the aerogel and the surrounding water,46 which further confirms the hydrophobicity of the 3D MoS2–PVP aerogel.
Furthermore, the 3D MoS2–PVP aerogel shows excellent mechanical performance. Fig. S6 (ESI†) shows that the compressive curves have good hysteresis loops, which are consistent with the typical behavior of porous materials.47 With the increase of MoS2, the mechanical properties improve. When the concentration of MoS2 is 5 mg cm−3, the compression strength is 4 kPa. The compression strength of MoS2–PVP was reinforced to 25, 45, and 63 kPa at the concentrations of 10, 15 and 20 mg cm−3, respectively. The result shows that the MoS2–PVP aerogel had an outstanding compression performance, which may be attributed to the high contribution of the ordered structure.
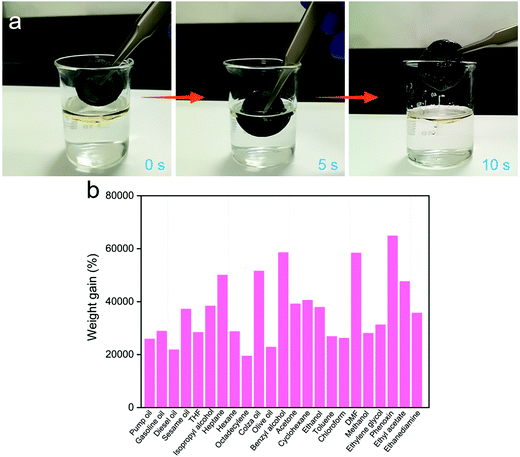
The 3D MoS2–PVP aerogel is one of the ideal adsorption materials for removing various oils and organic solvents due to its good hydrophobicity and excellent mechanical stability. As shown in Fig. 4a and Movie S1 (ESI†), when the 3D MoS2–PVP aerogel is placed on the surface of an oil–water mixture, it can absorb the oil completely and quickly. These results confirm that it has great prospects in the field of oil–water separation.
To explore the adsorption efficiency of the 3D MoS2–PVP aerogel, the weight gain (wt%) was defined as the adsorbed weight of the solvent per unit weight by the dry aerogel. Herein, Table S1 (ESI†) shows that the SSA of the 3D MoS2–PVP aerogel is increased with the increasing MoS2. Moreover, the adsorption capacity of the 3D MoS2–PVP aerogel increases with the increasing SSA (Fig. S7, ESI†).
The adsorption capability of the 3D MoS2–PVP aerogel of various oils and organic solvents were measured. The result shows that the 3D MoS2–PVP aerogel has excellent adsorption capacity and can absorb solvents 195–649 times its own weight (Fig. 4b).
In addition, the adsorption capacity of the 3D MoS2–PVP aerogel is higher than that of the previously reported adsorption materials (Table 1),46,48–73 such as exfoliated graphite (60–90 times),74 carbon nanotube sponge (80–180 times),50 graphene sponge (60–160 times),54 and reduced graphite oxide foam (5–40 times).55 Moreover, the method to prepare the 3D MoS2–PVP aerogel is relatively simple and its precursor is relatively cheap. Therefore, the obtained 3D MoS2–PVP aerogel is regarded as the most promising adsorbent for environmental remediation.
| Adsorbent materials | Absorbed substances | Sorption capacity (g g−1) | Cost | Ref. |
|---|---|---|---|---|
| SMF: superhydrophobic melamine-formaldehyde, BCM: biomass-decorated carbonaceous melamine, CMB: carbon microbelt, GMF: graphene modified foam, OCA: oleophilic carbon aerogel, MCF: microfibrillated cellulose fibers, SMS: superoleophilic MOS2 nanosheet sponge, MCGA: modified cellulose/graphene aerogels, FGN/PU: functionalized graphene/PU, HAP: hydroxyapatite, EVOH NFAs: poly(vinyl alcohol-co-ethylene) nanofiber aerogels, GCTs: giant carbon tubes, TCF: twisted carbon fibers, 3C: compressible and conductive carbon, CF: carbon foam, and CMA: carbon microtube aerogel. | ||||
| Wool-based nonwoven | Diesel, crude oil, SN 150 | 9–15 | Low | 48 |
| Graphene/CNT foam | Compressor oil, organic solvents | 80–140 | High | 53 |
| Vegetable fiber | Crude oil | 1–100 | Low | 49 |
| Graphene sponge | Oils and organic solvents | 60–160 | High | 54 |
| Exfoliated graphite | Heavy oil | 60–90 | Low | 51 |
| Carbon nanotube sponge | Oils and organic solvents | 80–180 | High | 50 |
| Magnetic exfoliated graphite | Oils | 30–50 | High | 52 |
| Graphene-based sponge | Oils and organic solvents | 60–160 | High | 46 |
| Reduced graphite oxide foam | Oils and organic solvents | 5–40 | High | 55 |
| SMF foam | Oils and organic solvents | 78–172 | High | 79 |
| CNT sponge-doped graphene foam | Oils and organic solvents | 25–125 | High | 57 |
| BCM sponge | Oils and organic solvents | 86–201 | High | 80 |
| CMB aerogel | Oils and organic solvents | 56–188 | Low | 59 |
| GMF aerogel | Oils and organic solvents | 60–140 | Low | 60 |
| OCA aerogel | Oils and organic solvents | 81–171 | Low | 61 |
| MCF aerogel | Oils and organic solvents | 88–228 | Low | 62 |
| SMS sponge | Oils and organic solvents | 82–159 | Low | 63 |
| MCGA | Oils and organic solvents | 80–197 | High | 64 |
| FGN/PU sponge | Oils and organic solvents | 25–44 | High | 81 |
| HAP nanowire aerogel | Oils and organic solvents | 83–156 | Low | 65 |
| Silylated wood sponge | Oils and organic solvents | 16–41 | Low | 66 |
| Graphene foams | Oils and organic solvents | 120–250 | High | 67 |
| EVOH NFAs | Oils and organic solvents | 45–102 | Low | 68 |
| GCTs | Oils and organic solvents | 250–400 | High | 69 |
| TCF aerogel | Oils and organic solvents | 50–192 | Low | 70 |
| Carbon aerogel | Oils and organic solvents | 80–161 | High | 71 |
| 3C aerogels | Oils and organic solvents | 33–70 | High | 72 |
| Co–C/CF sponge | Oils and organic solvents | 85–200 | High | 73 |
| CMA | Oils and organic solvents | 78–348 | Low | 45 |
| 3D MoS2–PVP aerogel | Oils and organic solvents | 195–649 | Low | This work |
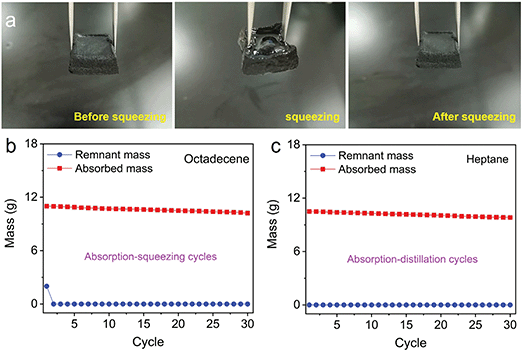
The key for oil–water separation is the recyclability of the contaminants, as most contaminants contain both valuable and harmful materials. Two typical methods have been reported for recovering contaminants: squeezing and distillation. As observed in Fig. 5a, the octadecene absorbed by the 3D MoS2–PVP aerogel can be recovered by squeezing. When the pressure is released, the aerogel can return to its original shape (Fig. 5a).
Moreover, the 3D MoS2–PVP aerogel was used in recycle tests by squeezing and distillation (Fig. 5b and c). Fig. 5b shows the absorption–squeezing cycle process of the 3D MoS2–PVP aerogel. The structure of the 3D MoS2–PVP aerogel was well maintained and the gel still retained an adsorption capacity of 93.5% after 30 cycles (Fig. S8a and b, ESI†). As illustrated in Fig. 5c, the 3D MoS2–PVP aerogel still retained an adsorption capacity of 92.9% after 30 cycles, which suggests its good recyclability. In addition, the structure of 3D MoS2–PVP aerogel was well maintained after 30 cycles of the adsorption–distillation process (Fig. S9a and b, ESI†). Therefore, the obtained 3D MoS2–PVP aerogel can be used to remove contaminants by squeezing, distillation, or a combination of the two methods. It shows a great potential practical application value in the field of oil–water separation.
Conclusions
In conclusion, a 3D MoS2–PVP aerogel with low density, good hydrophobicity, high adsorption capacity, and excellent recyclability was fabricated by the freeze-drying method. The adsorption capacity of the 3D MoS2–PVP aerogel can be as high as 195–649 times its own weight. Moreover, after 30 cycles, the structure of the 3D MoS2–PVP aerogel is well maintained and the gel still retains an adsorption capacity of 93.5% and 92.9% by squeezing and distillation, respectively. Therefore, the obtained 3D MoS2–PVP aerogel has extremely good potential for applications in oil–water separation.Experimental section
Materials
MoS2 powder was purchased from Nanjing XFNANO Materials Tech Co., Ltd. PVP and various organic solvents were from Shanghai Aladdin Biochemistry Technology Co., Ltd. Gasoline oil was provided by the local gas station. Pump oil, sesame oil, colza, and olive oil were obtained in the local market.Preparation of the 3D MoS2–PVP aerogel
The 3D MoS2–PVP aerogel was prepared by the freeze-drying method.45,75–78 Briefly, MoS2 powder was uniformly dispersed in PVP solutions (1 mg cm−3) under stirring and the concentration of MoS2 was set to 5, 10, 15 and 20 mg cm−3, respectively. Then the suspension was poured into a rubber mold placed at the top of a cold steel rod, which was cooled by adding liquid nitrogen and kept at −70 °C for 1 h. Then the obtained frozen sample was further freeze-dried by using a Labconco-195 freeze-drier for 72 h. Finally, the 3D MoS2–PVP aerogel was obtained.Characterization
The morphology was observed by SEM (S-4800, Hitachi) at an accelerating voltage of 10 kV. The X-ray diffraction pattern was obtained using an XRD-6000 (Shimadzu) system with Cu Kα radiation (λ = 1.54178 Å). Fourier transform infrared spectroscopy (FTIR) was characterized from 4000 to 400 cm−1 wavenumber by using a Nicolet 6700 instrument. The contact angle with water was characterized by using an OCA 15 Pro instrument. The specific surface area was studied using an ASAP 2020 system at 77 K. The compression tests were carried out using an Instron 5565A system at the speed of 0.1 mm min−1.Absorption of oils and organic solvents
During the adsorption tests, these 3D MoS2–PVP aerogels were required to be completely filled with oils or organic solvents. To avoid evaporation of solvents with low boiling points, the weight measurements should be made quickly. The weights of the 3D MoS2–PVP aerogels before and after adsorption were recorded.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21606113).References
- J. Aurell and B. K. Gullett, Environ. Sci. Technol., 2010, 44, 9431–9437 CrossRef CAS PubMed.
- K. Jayaramulu, K. K. Datta, C. Rosler, M. Petr, M. Otyepka, R. Zboril and R. A. Fischer, Angew. Chem., Int. Ed., 2016, 55, 1178–1182 CrossRef CAS PubMed.
- K. Yin, D. Chu, X. Dong, C. Wang, J. A. Duan and J. He, Nanoscale, 2017, 9, 14229–14235 RSC.
- Y. Yang, X. Li, X. Zheng, Z. Chen, Q. Zhou and Y. Chen, Adv. Mater., 2018, 30, 1704912–1704922 CrossRef PubMed.
- A. Bayat, S. F. Aghamiri, A. Moheb and G. R. Vakili-Nezhaad, Chem. Eng. Technol., 2005, 28, 1525–1528 CrossRef CAS.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, J. Porous Mater., 2003, 10, 159–170 CrossRef CAS.
- V. Rajakovic, G. Aleksic, M. Radetic and L. Rajakovic, J. Hazard. Mater., 2007, 143, 494–499 CrossRef CAS PubMed.
- J. Xiong, P. Song, J. Di, H. M. Li and Z. Liu, J. Mater. Chem. A, 2019, 7, 25203–25226 RSC.
- P. Song, J. Cui, J. Di, D. Liu, M. Xu, B. Tang, Q. Zeng, J. Xiong, C. Wang, Q. He, L. Kang, J. Zhou, R. Duan, B. Chen, S. Guo, F. Liu, J. Shen and Z. Liu, ACS Nano, 2020, 14, 595–602 CrossRef CAS PubMed.
- T. Chen, M. Li, L. Zhou, X. Ding, D. Lin, T. Duan, G. Yang, R. He and W. Zhu, ACS Sustainable Chem. Eng., 2020, 8, 6458–6465 CrossRef CAS.
- X. Wang, Z. Han, Y. Liu and Q. Wang, Appl. Surf. Sci., 2020, 505, 144577–144587 CrossRef.
- M. Yu, P. Xu, J. Yang, L. Ji and C. Li, Adv. Mater. Interfaces, 2020, 7, 1901671–1901680 CrossRef CAS.
- J. Gu, H. Fan, C. Li, J. Caro and H. Meng, Angew. Chem., Int. Ed., 2019, 58, 5297–5301 CrossRef CAS PubMed.
- X. Zhang, Z. Li, K. Liu and L. Jiang, Adv. Funct. Mater., 2013, 23, 2881–2886 CrossRef CAS.
- H. Bi, Z. Yin, X. Cao, X. Xie, C. Tan, X. Huang, B. Chen, F. Chen, Q. Yang, X. Bu, X. Lu, L. Sun and H. Zhang, Adv. Mater., 2013, 25, 5916–5921 CrossRef CAS PubMed.
- X. Gui, Z. Zeng, Z. Lin, Q. Gan, R. Xiang, Y. Zhu, A. Cao and Z. Tang, ACS Appl. Mater. Interfaces, 2013, 5, 5845–5850 CrossRef CAS PubMed.
- W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777–1783 CrossRef PubMed.
- T. R. Annunciado, T. H. Sydenstricker and S. C. Amico, Mar. Pollut. Bull., 2005, 50, 1340–1346 CrossRef CAS PubMed.
- M. A. Lillo-Ródenas, D. Cazorla-Amorós and A. Linares-Solano, Carbon, 2005, 43, 1758–1767 CrossRef.
- M. Inagakl, H. Konno, M. Toyoda, K. Moriya and T. Kihara, Desalination, 2000, 128, 213–218 CrossRef.
- Y. Yu, H. Chen, Y. Liu, V. Craig, L. H. Li and Y. Chen, Adv. Mater. Interfaces, 2014, 1, 1300002–1300006 CrossRef.
- L. Ci, L. Song, C. Jin, D. Jariwala, D. Wu, Y. Li, A. Srivastava, Z. F. Wang, K. Storr, L. Balicas, F. Liu and P. M. Ajayan, Nat. Mater., 2010, 9, 430–435 CrossRef CAS PubMed.
- L. Song, L. J. Ci, H. Lu, P. B. Sorokin, C. H. Jin, J. Ni, A. G. Kvashnin, D. G. Kvashnin, J. Lou, B. I. Yakobson and P. M. Ajayan, Nano Lett., 2010, 10, 3209–3215 CrossRef CAS PubMed.
- H. F. Zhang, I. Hussain, M. Brust, M. F. Butler, S. P. Rannard and A. I. Cooper, Nat. Mater., 2005, 4, 787–793 CrossRef CAS PubMed.
- R. Y. Zhang, W. C. Wan, L. J. Qiu and Y. Zhou, Mater. Lett., 2016, 181, 321–324 CrossRef CAS.
- I. Lee, S.-M. Kang, S.-C. Jang, G.-W. Lee, H. E. Shim, M. Rethinasabapathy, C. Roh and Y. S. Huh, J. Mater. Chem. A, 2019, 7, 1737–1748 RSC.
- Y. A. Haleem, D. B. Liu, W. X. Chen, C. D. Wang, C. H. Hong, Z. He, J. W. Liu, P. Song, S. H. Yu and L. Song, Composites, Part B, 2015, 78, 480–487 CrossRef CAS.
- M. Xu, R. Yu, Y. Guo, C. Chen, Q. Han, J. Di, P. Song, L. Zheng, Z. Zhang, J. Yan, W. Zhao, J. Yun, C. Liu, Q. Li, Y. Wang, X. Wang and Z. Liu, Inorg. Chem. Front., 2019, 6, 2801–2809 RSC.
- B. Cao, J. Yin, S. F. Yan, L. Cui, X. S. Chen and Y. T. Xie, Macromol. Biosci., 2011, 11, 427–434 CrossRef CAS PubMed.
- P. Song, Z. J. Peng, Y. L. Yue, H. Zhang, Z. Zhang and Y. C. Fan, eXPRESS Polym. Lett., 2013, 7, 546–553 CrossRef CAS.
- J. Di, C. Chen, C. Zhu, P. Song, J. Xiong, M. X. Ji, J. D. Zhou, Q. D. Fu, M. Z. Xu, W. Hao, J. X. Xia, S. Z. Li, H. M. Li and Z. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 30786–30792 CrossRef CAS PubMed.
- J. Xiong, P. Song, J. Di and H. Li, Appl. Catal., B, 2019, 256, 117788–117802 CrossRef CAS.
- Y. A. Haleem, P. Song, D. B. Liu, C. D. Wang, W. Gan, M. F. Saleem and L. Song, Materials, 2016, 9, 507–518 CrossRef PubMed.
- J. Dai, T. Huang, S. Q. Tian, Y. J. Xiao, J. H. Yang, N. Zhang, Y. Wang and Z. W. Zhou, Mater. Des., 2016, 107, 187–197 CrossRef CAS.
- R. Zhang, W. Wan, L. Qiu, Y. Wang and Y. Zhou, Appl. Surf. Sci., 2017, 419, 342–347 CrossRef CAS.
- X. Zhang, X. F. Qiao, W. Shi, J. B. Wu, D. S. Jiang and P. H. Tan, Chem. Soc. Rev., 2015, 44, 2757–2785 RSC.
- D. Cao, K. Ye, O. A. Moses, W. Xu, D. Liu, P. Song, C. Wu, C. Wang, S. Ding, S. Chen, B. Ge, J. Jiang and L. Song, ACS Nano, 2019, 13, 11733–11740 CrossRef CAS PubMed.
- H. Xu, J. Yi, X. She, Q. Liu, L. Song, S. Chen, Y. Yang, Y. Song, R. Vajtai, J. Lou, H. Li, S. Yuan, J. Wu and P. M. Ajayan, Appl. Catal., B, 2018, 220, 379–385 CrossRef CAS.
- Q. Liu, X. Li, Q. He, A. Khalil, D. Liu, T. Xiang, X. Wu and L. Song, Small, 2015, 11, 5556–5564 CrossRef CAS PubMed.
- Q. Liu, Q. Fang, W. Chu, Y. Wan, X. Li, W. Xu, M. Habib, S. Tao, Y. Zhou, D. Liu, T. Xiang, A. Khalil, X. Wu, M. Chhowalla, P. M. Ajayan and L. Song, Chem. Mater., 2017, 29, 4738–4744 CrossRef CAS.
- W. Zheng, Y. Xu, L. Zheng, C. Yang, N. Pinna, X. Liu and J. Zhang, Adv. Funct. Mater., 2020, 30, 2000435–2000444 CrossRef CAS.
- Y. Tian, X. Liu, X. Cao, D. Zhang, S. Xiao, X. Li, Z. Le, X. Li and H. Li, Chem. Eng. J., 2019, 374, 429–436 CrossRef CAS.
- J. Liu, Z. Zeng, X. Cao, G. Lu, L. H. Wang, Q. L. Fan, W. Huang and H. Zhang, Small, 2012, 8, 3517–3522 CrossRef CAS PubMed.
- R. Zhang, W. Wan, L. Qiu and Y. Zhou, Mater. Lett., 2016, 181, 321–324 CrossRef CAS.
- P. Song, J. W. Cui, J. Di, D. B. Liu, M. Z. Xu, B. J. Tang, Q. S. Zeng, J. Xiong, C. D. Wang, Q. He, L. X. Kang, J. D. Zhou, R. H. Duan, B. B. Chen, S. S. Guo, F. C. Liu, J. Shen and Z. Liu, ACS Nano, 2020, 14, 595–602 CrossRef CAS PubMed.
- D. D. Nguyen, N. H. Tai, S. B. Lee and W. S. Kuo, Energy Environ. Sci., 2012, 5, 7908–7912 RSC.
- Y. Tang, K. L. Yeo, Y. Chen, L. W. Yap, W. Xiong and W. L. Cheng, J. Mater. Chem. A, 2013, 1, 6723 RSC.
- M. M. Radetić, D. M. Jocić, P. M. Jovančić, Z. L. J. Petrović and H. F. Thomas, Environ. Sci. Technol., 2003, 37, 1008–1012 CrossRef PubMed.
- T. R. Annunciado, T. H. D. Sydenstricker and S. C. Amico, Mar. Pollut. Bull., 2005, 50, 1340–1346 CrossRef CAS PubMed.
- X. C. Gui, J. Q. Wei, K. L. Wang, A. Y. Cao, H. W. Zhu, Y. Jia, Q. K. Shu and D. H. Wu, Adv. Mater., 2010, 22, 617–621 CrossRef CAS PubMed.
- M. Toyoda and M. Inagaki, Carbon, 2000, 38, 199–210 CrossRef CAS.
- G. L. Wang, Q. R. Sun, Y. Q. Zhang, J. H. Fan and L. M. Ma, Desalination, 2010, 263, 183–188 CrossRef CAS.
- X. C. Dong, J. Chen, Y. W. Ma, J. Wang, C. P. Mary, B. X. Liu, L. Wang, W. Huang and P. Chen, Chem. Commun., 2012, 48, 10660 RSC.
- J. P. Zhao, W. C. Ren and H. M. Cheng, J. Mater. Chem., 2012, 22, 20197–20202 RSC.
- Z. Q. Niu, J. Chen, H. H. Hng, J. Ma and X. D. Chen, Adv. Mater., 2012, 24, 4144–4150 CrossRef CAS PubMed.
- Y. Zhao, C. G. Hu, Y. Hu, H. H. Cheng, G. Q. Shi and L. T. Qu, Angew. Chem., Int. Ed., 2012, 124, 11533–11537 CrossRef.
- D. P. Hashim, N. T. Narayanan, J. M. Romo-Herrera, D. A. Cullen, M. G. Hahm, P. Lezzi, J. R. Suttle, D. Kelkhoff, E. Muñoz-Sandoval and S. Ganguli, Sci. Rep., 2012, 2, 363 CrossRef PubMed.
- H. Y. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- H. C. Bi, X. Huang, X. Wu, X. H. Cao, C. L. Tan, Z. Y. Yin, X. H. Lu, L. T. Sun and H. Zhang, Small, 2014, 10, 3544–3550 CrossRef CAS PubMed.
- H. G. Zhu, D. Y. Chen, A. Wei, N. J. Li, Q. F. Xu, H. Li, J. H. He and J. M. Lu, Small, 2015, 11, 5222–5229 CrossRef CAS PubMed.
- S. Y. Gao, X. G. Li, L. Y. Li and X. J. Wei, Nano Energy, 2017, 33, 334–342 CrossRef CAS.
- S. Wang, X. W. Peng, L. X. Zhong, J. W. Tan, S. S. Jing, X. F. Cao, W. Chen, C. F. Liu and R. C. Sun, J. Mater. Chem. A, 2015, 3, 8772–8781 RSC.
- X. J. Gao, X. F. Wang, X. P. Ouyang and C. Wen, Sci. Rep., 2016, 6, 27207–27214 CrossRef CAS PubMed.
- H. Y. Mi, X. Jing, A. L. Politowicz, E. Chen, H. X. Huang and L.-S. Turng, Carbon, 2018, 132, 199–209 CrossRef CAS.
- Y. G. Zhang, Y. J. Zhu, Z. C. Xiong, J. Wu and F. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 13019–13027 CrossRef CAS PubMed.
- H. Guan, Z. Y. Cheng and X. Q. Wang, ACS Nano, 2018, 12, 10365–10373 CrossRef CAS PubMed.
- L. R. Shi, K. Chen, R. Du, A. Bachmatiuk, M. H. Rummeli, K. W. Xie, Y. Y. Huang, Y. F. Zhang and Z. F. Liu, J. Am. Chem. Soc., 2016, 138, 6360–6363 CrossRef CAS PubMed.
- J. W. Lu, D. D. Xu, J. K. Wei, S. Yan and R. Xiao, ACS Appl. Mater. Interfaces, 2017, 9, 25533–25541 CrossRef CAS PubMed.
- J. F. Chen, X. C. Shen, Y. B. Pan, C. Liu, S. Y. Hwang, Q. Xu and Z. M. Peng, J. Mater. Chem. A, 2018, 6, 3996–4002 RSC.
- H. C. Bi, Z. Y. Yin, X. H. Cao, X. Xie, C. L. Tan, X. Huang, B. Chen, F. T. Chen, Q. L. Yang, X. Y. Bu, X. H. Lu, L. T. Sun and H. Zhang, Adv. Mater., 2013, 25, 5916–5921 CrossRef CAS PubMed.
- L. X. Li, T. Hu, H. X. Sun, J. P. Zhang and A. Q. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 18001–18007 CrossRef CAS PubMed.
- L. Li, B. Li, H. Sun and J. Zhang, J. Mater. Chem. A, 2017, 5, 14858–14864 RSC.
- X. Ge, W. Qin, H. Zhang, G. Wang, Y. Zhang and C. Yu, Nanoscale, 2019, 11, 12161–12168 RSC.
- M. Toyodaa and M. Inagakib, Carbon, 2000, 38, 199–210 CrossRef.
- P. Song, J. Di, L. X. Kang, M. Z. Xu, B. J. Tang, J. Xiong, J. W. Cui, Q. S. Zeng, J. D. Zhou, Y. M. He, Q. D. Fu, J. Peng, S. S. Guo, B. Lin, J. Y. Zhang, P. Meng and Z. Liu, Nanoindentation Mater. Sci., 2019, 1, 310–317 Search PubMed.
- D. Sylvain, S. Eduardo, N. Ravi K and T. Antoni P, Science, 2006, 311, 515–518 CrossRef PubMed.
- P. Song, H. L. Qin, H. L. Gao, H. P. Cong and S. H. Yu, Nat. Commun., 2018, 9, 2786–2794 CrossRef PubMed.
- S. Deville, E. Maire, G. Bernard-Granger, A. Lasalle, A. Bogner, C. Gauthier, J. Leloup and C. Guizard, Nat. Mater., 2009, 8, 966–972 CrossRef CAS PubMed.
- M. Li, C. Bian, G. Yang and X. Qiang, Chem. Eng. J., 2019, 368, 350–358 CrossRef CAS.
- Z. Lei, P. Zheng, L. Niu, Y. Yang, J. Shen, W. Zhang and C. Wang, Appl. Surf. Sci., 2019, 489, 922–929 CrossRef CAS.
- S. Zhou, G. Hao, X. Zhou, W. Jiang, T. H. Wang, N. Zhang and L. H. Yu, Chem. Eng. J., 2016, 302, 155–162 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00219d |
| This journal is © The Royal Society of Chemistry 2020 |